Abstract
Covert spatial attention allows us to prioritize visual processing at relevant locations. A fast growing literature suggests that alpha-band (8–12 Hz) oscillations play a key role in this core cognitive process. It is clear that alpha-band activity tracks both the locus and timing of covert spatial orienting. There is limited evidence, however, for the widely embraced view that alpha oscillations suppress irrelevant visual information during spatial selection. Extant evidence is equally compatible with an account in which alpha activity enables spatial selection through signal enhancement rather than distractor suppression. Thus, more work is needed to characterize the computational role of alpha activity in spatial attention.
Keywords: spatial attention, alpha, oscillations, suppression, inhibition
Alpha-band activity tracks covert spatial attention
Our capacity to process visual information is limited. Thus, we must prioritize processing at relevant locations. Covert spatial attention allows us to select relevant locations without moving our eyes, enhancing processing at the attended location [1]. Human electroencephalogram (EEG) studies have long linked alpha-band (8–12 Hz) oscillations with covert spatial attention [2–7]. When attention is deployed to one side of space, posterior alpha-band power is reduced at electrodes over the contralateral hemisphere, which processes the attended side of space [4– 6]. Further work has shown that the scalp topography of alpha power tracks more than just the relevant side of space [4,7]. For example, Rihs and colleagues [7] showed that the topography of alpha power on the scalp covaried with the specific location that was cued when observers were instructed to attend one of eight possible locations around a fixation point.
Recently, multivariate analysis techniques have enabled a more refined quantification of the spatial information present in alpha activity. We and others have used an inverted encoding model (IEM) [8–10] to track the spatial and temporal dynamics of covert attention [11,12]. This approach (Figure 1) assumes that alpha power at each electrode reflects the combined activity of a number of spatially selective channels (or neuronal populations). By first estimating the relative contribution of each of these spatial channels, it is then possible to invert the model to estimate the profile of activity across the channels from the pattern of alpha power across electrodes. This results in a graded profile of activity (a channel tuning function or CTF) that peaks at the channel tuned for the attended location. These alpha CTFs reflect the spatial selectivity of the population-level activity that is measured with EEG [13]. In spatial-cueing tasks, alpha CTFs track covert spatial orienting to the precise location that is cued, starting several hundred milliseconds after the onset of a central cue (Figure 2a) [11,12]. During visual search, the time-course of alpha CTFs tracks trial-by-trial variations in the latency of target selection, as measured with response times (Figure 2b) [11]. Thus, alpha activity is tightly linked with both the locus and timing of covert spatial selection (also see Box 1).
Figure 1. Reconstructing alpha channel tuning functions with an inverted encoding model.
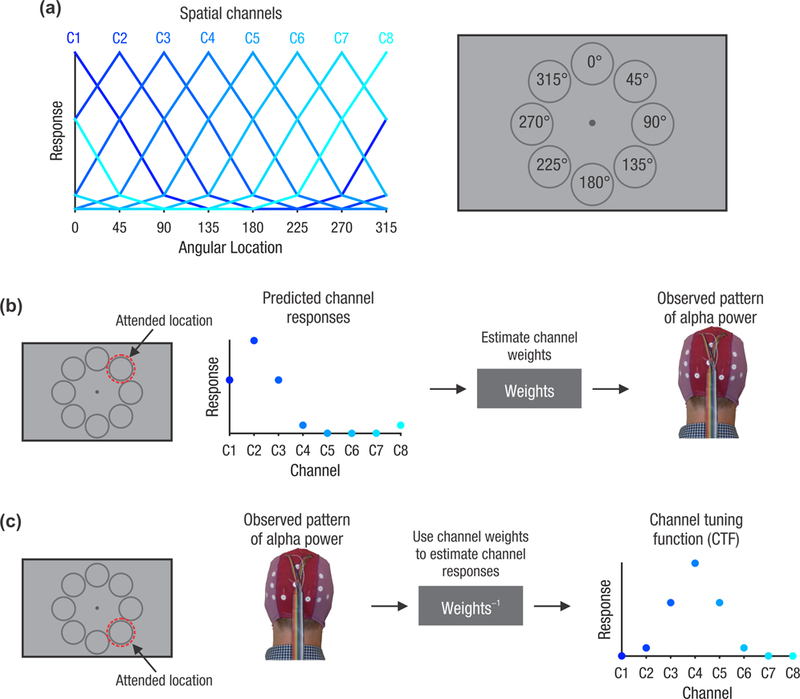
Inverted encoding models (IEMs) are a powerful tool for reconstructing population-level representations from aggregate measures of neural activity (e.g. EEG or fMRI). We and others have used the IEM approach to reconstruct spatially selective channel tuning functions (CTFs) from the scalp distribution of alpha-band power [11,12]. This approach assumes that alpha-band power at each electrode reflects the combined activity of a number of spatially tuned channels (or neuronal populations), each tuned for a different spatial position. Each curve shown in (a) shows the predicted response of each of eight spatially selective channels (C1-C8) across eight possible attended locations (right). The IEM analysis proceeds in two stages. In the training phase (b), we estimate the relative contribution of each channel to the response measured at each electrode (called channel weights). For a given attended location, the predicted response of each channel can be derived from the functions in (a). The example shown here is for an attended location at 45°. Because the predicted channel responses vary as a function of the attended location, by varying the attended location it is possible to estimate how strongly each channel contributes to activity measured at each electrode (i.e., the channel weights). In the test phase (c), using an independent set of data, we use the channel weights obtained in the training phase to estimate the profile of channel responses given the observed pattern of activity across the scalp. The example shown here is for an attended location at 135°. The resulting CTF reflects the spatial selectivity of population-level alpha-band activity. Adapted from [11].
Figure 2. Alpha-band oscillations enable spatially and temporally resolved tracking of covert spatial attention.
(a) Alpha CTFs precisely track where attention is deployed following an attentional cue. Observers performed a spatial-cueing task (left). A central cue (a cross with one uniquely colored arm) directed observers to attend one of eight place holders. After delay, a target digit was presented among distractor letters and then masked with a pound sign. The plot on the right shows the reconstructed alpha CTFs across time for each of eight locations. A channel offset of 0° corresponds to the channel tuned for the cued location. The yellow band in each subplot shows the peak channel response, which tracked the cued location start around 300 ms after cue onset. (b) The time course of alpha-band CTFs track the latency of target selection during visual search. Observers searched for a target (a vertical or horizontal bar) among distractors and reported the orientation of the target (left). The plots on the right show the selectivity of target-related CTFs as for easy and hard search (upper) and as a function of response times regardless of search condition (lower). Spatially selective activity that tracked the target position emerged earlier during easy search than during hard search, and earlier on trials with fast RTs than on trials with slow RTs. Adapted from [11].
Box 1. Alpha-band activity tracks spatial working memories.
Working memory allows us to hold goal-relevant information in an “online” state. It is thoughts that there is considerable functional overlap between spatial attention and spatial working memory [14,15]. Consistent with this view, recent work has shown that alpha activity also precisely tracks spatial locations maintained in working memory [16–20]. Interestingly, alpha activity encodes the location of memoranda even when spatial position is irrelevant to the task [17,20], suggesting that space may be an integral component of visual working memories. These findings provide further evidence that alpha oscillations play a central role in spatial cognition.
Does alpha-band activity suppress irrelevant visual inputs? The jury is out
What computational role does alpha activity play in covert attention? The modal view is that alpha activity mediates the suppression or gating of irrelevant visual inputs [2,3,21–23]. This view falls in line with the consensus that distractor exclusion is a critical component of visual attention [24]. However, it is broadly acknowledged that improved perception at a relevant location can also occur via signal enhancement, which directly improves processing at attended locations [1,25]. While many studies have shown that alpha activity tracks the attended positions in the visual field [4–6], even in the absence of irrelevant distractors [5,7,26], recent work has cast doubt on whether alpha activity tracks locations at which distractors are expected [27] At first glance, this may seem to point towards a role in signal enhancement rather than distractor suppression. However, a neural signal that suppresses interference at all unattended locations may also enable precise tracking of target position. Thus, the fact that alpha activity precisely tracks the selected locations – but not the locations of distractors – still leaves open the question of how alpha activity supports selective attention (also see Box 2).
Box 2. Pre-stimulus alpha power and perception.
To directly examine how alpha-band power influences visual processing, researchers have tested whether pre-stimulus alpha power predicts perceptual outcomes. Early studies found that near-threshold stimuli are more readily detected when alpha power is low [28,29], which was taken as evidence that low alpha power improves stimulus perception [28,30]. However, recent work has challenged this view. Two recent studies [31,32] used a signal detection analysis to show that fluctuations in alpha power predict response bias (i.e., how likely the observer is to report a stimulus) instead of perceptual sensitivity (i.e., the degree to which signal and noise can be discriminated during perception). Thus, alpha power predicted a shift in response thresholds rather than changes in the sensitivity of visual encoding. Consistent with this view, others have found that alpha power does not predict performance in visual discrimination tasks [32–35]. A key limitation of these findings, however, is that they focused on fluctuations in power without manipulating the attended location, in contrast to the work we reviewed above that focused on spatially selective modulations of alpha power following spatial cues. These two approaches may not tap into the same process, given the likelihood that alpha oscillations reflect more than a single aspect of cognitive processing (e.g. [36]). For example, fluctuations in pre-stimulus alpha power could be tracking global changes in visual processing rather than something directly linked with spatial attention per se. Future work that combines these approaches to examine pre-stimulus fluctuations in spatially selective alpha activity could help to determine whether these pre-stimulus effects are directly linked with changes in spatial selection.
In support of the distractor suppression account, past work has emphasized the finding in spatial-cueing tasks that alpha power decreases contralateral to the cued location and/or increases contralateral to the uncued location [4–7]. This finding has been presented as suggesting that higher alpha power contralateral to the uncued location reflects increased suppression of irrelevant stimuli [2,3,21–23]. But this empirical pattern is equally compatible with the view that reduced alpha-band power reflects signal enhancement. Another finding that has motivated a suppression account of alpha activity is the inverse relationship between alpha power and other neural signals such as spiking activity [37] and gamma-band oscillations [38]. However, these findings do not establish whether alpha activity influences the quality of sensory representations because the information content of neural activity can be disconnected from the overall amount of neural activity (e.g. [39]). For example, inhibition of specific neural units could improve the fidelity of a sensory representation. Thus, it is unclear whether these inverse relationships reflect an inverse relationship between alpha power and the strength of sensory representations. Even though there is some evidence for an inverse relationship between alpha power and the strength of sensory representations measured via BOLD responses in visual cortex [40], these data are still compatible with the hypothesis that decreased alpha power reflects a relative increase in signal enhancement over the attended regions.
In our view, a more diagnostic approach for distinguishing between suppression and enhancement accounts is to use experimental designs that selectively manipulate the process of interest. If the degree of distractor suppression can be manipulated while signal enhancement is held constant, this provides an opportunity to link specific neural signals with suppression per se. For example, Serences and colleagues [41] used this approach to test whether preparatory activity measured with fMRI reflected distractor exclusion. Spatial attention increases baseline activity measured with fMRI in visual cortex tuned for the attended location [42,43]. To test whether this preparatory activity reflects distractor exclusion, Serences et al. varied the probability that distractors accompanied visual targets, a manipulation that has been shown to increase resistance to distractor interference without affecting performance with distractor-free displays [44]. The selective effect of the probability manipulation suggests a specific effect on distractor exclusion, because changes in that process should not affect performance when there are no distractors to exclude. Critically, Serences et al. found that preparatory activity, measured via retintopic changes in the amplitude of the BOLD signal, was greater when the probability of distractors was higher, suggesting that this preparatory activity plays a specific role in distractor exclusion. Therefore, this work provides a clear example of how preparatory activity can be unambiguously linked with distractor exclusion.
Of the substantial body of work that links alpha-band power with covert attention, only a few studies have attempted to selectively manipulate distractor suppression [26,27,45,46]. In one study, Kelly and colleagues [26] cued the location of an upcoming target. In some blocks, the target appeared alone, while in others a distractor appeared in the uncued hemifield. Kelly et al. reasoned that if spatially specific alpha-band power reflects distractor exclusion, then lateralized alpha-band should be stronger when observers expect a distractor than when no distractor is expected. Interestingly, lateralization of alpha-band power was weaker when distractors were expected (also see [45], which found no effect of the strength of distractors on the lateralization of alpha-band power in a somatosensory task). However, one caveat here is that there was no behavioral evidence that distractor exclusion was increased in blocks that contained distractors. Thus, the null effect on alpha laterality may not provide strong evidence against a distractor exclusion account.
Händel and colleagues [47] took a different approach. Rather than manipulating the degree of distractor exclusion, they sought to test whether the degree of lateralization of alpha-band activity predicted individual differences in distractor exclusion. The authors reasoned that if lateralized alpha-band activity predicted processing of the stimulus in the uncued hemifield (on invalidly cued trials) but not processing of the stimulus in the cued hemifield (on validly cued trials), then this would provide evidence in favor of the distractor exclusion account of alpha-band power. Their results followed this pattern, but there was considerably more variability in performance for the uncued stimulus than for the cued stimulus. Thus, the failure to detect a relationship between alpha-band lateralization and processing of the cued stimulus may reflect a restriction of range for performance at the cued position.
In another study, Noonan and colleagues [27] successfully manipulated distractor exclusion. Observers responded to a target stimulus. Noonan et al. varied whether or not the target was accompanied by a distractor in different blocks of trials, and found that the presence of a distractor reliably slowed response times. In some blocks, Noonan et al. cued the location of the target or the distractor in advance. Unsurprisingly, a spatial cue indicating the target location speeded responses (compared to trials with no cue). Interestingly, a spatial cue indicating the distractor location also speeded responses (again, compared to trials with no cue). Critically, this distractor-cueing benefit was not seen when the distractor was absent, providing clear evidence that cues indicating the location of a distractor selectively enabled distractor exclusion. Strikingly, Noonan et al. found that alpha - band activity tracked the cued location when the target location was cued but not when the distractor location was cued, suggesting that alpha-band activity plays a role in signal enhancement but providing no evidence for a role in distractor exclusion.
To summarize, while it is clearly the modal view that alpha activity reflects the suppression of irrelevant visual information, the evidence is equivocal. Extant work linking alpha activity with spatial attention is compatible with an account in which alpha supports target selection via signal enhancement. Thus, there is strong motivation for further work in which alpha-band activity is assessed during selective manipulations of different aspects of spatial selection, preferably with analytic approaches that focus on spatially-selective alpha-band activity rather than overall power in that frequency band (see Box 2). For example, given prior work that has reported covariations of alpha power and BOLD activity [48], it may be worthwhile to use an approach similar to that of Serences et al. [41] to examine how alpha activity is affected by selective changes in distractor exclusion. Until such work is conducted, our view is that the jury is still out regarding the computational role played by alpha activity during spatial selection.
Highlights:
Alpha-band (8–12 Hz) oscillations precisely track the locus covert spatial attention.
Alpha-band activity tracks the temporal dynamics of covert orienting.
Evidence is lacking for the view that alpha-band oscillations suppress irrelevant input during spatial selection.
Alpha-band oscillations may mediate direct enhancement of input at attended locations.
Acknowledgements:
This work was supported by National Institute of Mental Health [grant number 2R01MH087214-06A1].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Of outstanding interest:
[3**] Jensen and Mazaheri (2010)
A highly influential review that outlined the gating by inhibition hypothesis, which posits that high alpha-band power is responsible for suppressing the processing of irrelevant information. In the case of spatial attention, this model proposes that alpha-band activity inhibits processing in retinotopic cortex responsible for processing unattended locations.
[11**] Foster et al. (2017)
This study used an inverted encoding model (IEM) to reconstruct alpha channel tuning functions (CTFs) that precisely tracked where spatial attention was deployed. During visual search, the time course of alpha CTFs tracked trial-by-trial variations in the latency of covert orienting toward the search target. This work highlights the potential of alpha CTFs as a method for spatially and temporally resolved tracking of covert attention.
[16**] Foster et al. (2016)
Foster et al. showed that alpha-band power precisely tracks spatial representations held in working memory.
[27**] Noonan et al. (2016)
Noonan et al. showed that observers can suppress processing of an irrelevant distractor when provided with a pre-cue indicating the distractors locations. Interestingly, Noonan et al. found evidence for distractor suppression only when the location of the distractor was fixed across a block of trials (and not when the distractor location varied trial-to-trial), suggesting that selection history is critical to this form of distractor suppression. Furthermore, the authors found that alpha-band activity tracked the cued location when the cue indicated the location of the forthcoming target but not when it indicated the location of the forthcoming distractor. This finding clearly links alpha-band activity with spatial selection, but provides no evidence for a link with distractor suppression.
[31**] Limbach and Corballis (2016)
Using signal detection theory, Limbach and Corballis found that pre-stimulus alpha-band power predicts response criterion but not sensitivity in visual detection task. This finding is incompatible with an earlier view that low alpha-band power indexes a state of enhanced visual processing.
[32**] Iemi et al. (2017)
Consistent with Limbach and Corballis [31], Iemi et al. found that pre-stimulus alpha power predicts response criterion but not visual sensitivity in a visual detection task. They also found that pre-stimulus alpha-band power did not predict performance in visual discrimination tasks, further challenging the earlier view that low alpha-band power is linked with improved visual processing.
Of special interest:
[12*] Samaha et al. (2016)
Samaha et al. used an inverting encoding model to show that alpha oscillations precisely tracks orienting of spatial attention following spatial cues.
[17*] Foster et al. (2017)
This paper showed that alpha-band activity encodes the precise location of a stimulus maintained in visual working memory when spatial positon was entirely irrelevant to the task. This result contrasts with prior work, which showed that non-spatial features (such as color or orientation) are not maintained when they are irrelevant. The findings suggests that spatial position has a special status in visual working memory.
[20*] Bae and Luck (2018)
Bae and Luck sought to decode non-spatial information maintained in working memory from EEG signals. The authors were able to decode the orientation of a stimulus maintained in working memory from the scalp distribution of slow wave potentials. In contrast, the scalp distribution of alpha-band oscillations did not carry information about stimulus orientation, but did track the spatial position of the stimulus, providing further evidence for the role of alpha-band oscillations in spatial representation.
[36*] Sadaghiani and Kleinschmidt (2016)
A recent review that examines the role of alpha-band power in top-down control processes.
[46*] Janssens et al. (2018)
In a flanker task, Janssens et al. found that alpha-band power rapidly lateralized when the target letter was flanked on one side by incongruent letters, showing that alpha-band activity is quickly modulated by control processes in response to distracting input. Although the authors interpret this result as reflecting suppression of the incongruent distractors, we note that this finding could also reflect orienting of attention away from the incongruent flankers. Indeed, letters that matched the target were presented opposite the incongruent flankers.
References and recommended reading
- [1].Carrasco M, Visual attention: The past 25 years, Vision Res 51 (2011) 1484–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Foxe JJ, Snyder AC, The role of alpha-band brain oscillations as a sensory suppression mechanism during selective attention., Front. Psychol 2 (2011) 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jensen O, Mazaheri A, Shaping functional architecture by oscillatory alpha activity: Gating by inhibition., Front. Hum. Neurosci 4 (2010) 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Worden MS, Foxe JJ, Wang N, V Simpson G, Anticipatory biasing of visuospatial attention indexed by retinotopically specific α-band electroencephalography increases over occipital cortex., J. Neurosci 20 (2000) 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Thut G, Nietzel A, Brandt SA, Pascual-Leone A, α-band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection., J. Neurosci 26 (2006) 9494–9502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kelly SP, Lalor EC, Reilly RB, Foxe JJ, Increases in alpha oscillatory power reflect an active retinotopic mechanism for distracter suppression during sustained visuospatial attention., J. Neurophysiol 95 (2006) 3844–3851. [DOI] [PubMed] [Google Scholar]
- [7].Rihs TA, Michel CM, Thut G, Mechanisms of selective inhibition in visual spatial attention are indexed by α-band EEG synchronization, Eur. J. Neurosci 25 (2007) 603– 10. [DOI] [PubMed] [Google Scholar]
- [8].Brouwer GJ, Heeger DJ, Decoding and reconstructing color from responses in human visual cortex., J. Neurosci 29 (2009) 13992–14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sprague TC, Saproo S, Serences JT, Visual attention mitigates information loss in small- and large-scale neural codes, Trends Cogn. Sci 19 (2015) 215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Serences JT, Saproo S, Computational advances towards linking BOLD and behavior, Neuropsychologia 50 (2012) 435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Foster JJ, Sutterer DW, Serences JT, Vogel EK, Awh E, Alpha-band oscillations enable spatially and temporally resolved tracking of covert spatial attention, Psychol. Sci 28 (2017) 929–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Samaha J, Sprague TC, Postle BR, Decoding and reconstructing the focus of spatial attention from the topography of alpha-band oscillations, J. Cogn. Neurosci 28 (2016) 1090–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lopes da Silva F, EEG and M G: Relevance to neuroscience, Neuron 80 (2013) 1112–1128. [DOI] [PubMed] [Google Scholar]
- [14].Awh E, Jonides J, Overlapping mechanisms of attention and spatial working memory, Trends Cogn. Sci 5 (2001) 119–126. [DOI] [PubMed] [Google Scholar]
- [15].Gazzaley A, Nobre AC, Top-down modulation: Bridging selective attention and working memory, Trends Cogn. Sci 16 (2012) 129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Foster JJ, Sutterer DW, Serences JT, Vogel EK, Awh E, The topography of alpha-band activity tracks the content of spatial working memory, J. Neurophysiol 115 (2016) 168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Foster JJ, Bsales EM, Jaffe RJ, Awh E, Alpha-band activity reveals spontaneous representations of spatial position in visual working memory, Curr. Biol 27 (2017) 3216–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].van Moorselaar D, Foster JJ, Sutterer DW, Theeuwes J, Olivers CNL, Awh E, Spatially selective alpha oscillations reveal moment-by-moment trade-offs between working memory and attention, J. Cogn. Neurosci 30 (2018) 256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ester E, Nouri A, Rodriguez L, Retrospective cues mitigate information loss in human cortex during working memory storage, J. Neurosci (2018). [DOI] [PMC free article] [PubMed]
- [20].Bae G-Y, Luck SJ, Dissociable decoding of spatial attention and working memory from EEG oscillations and sustained potentials, J. Neurosci 38 (2018) 409–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Payne L, Sekuler R, The importance of ignoring: alpha oscillations protect selectivity, Curr. Dir. Psychol. Sci 23 (2014) 171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Clayton MS, Yeung N, Cohen Kadosh R, The many characters of visual alpha oscillations, Eur. J. Neurosci (2017) 1–11. [DOI] [PubMed]
- [23].Clayton MS, Yeung N, Cohen Kadosh R, The roles of cortical oscillations in sustained attention, Trends Cogn. Sci 19 (2015) 188–195. [DOI] [PubMed] [Google Scholar]
- [24].Desimone R, Duncan J, Neural mechanisms of selective visual attention, Annu. Rev. Neurosci 18 (1995) 193–222. [DOI] [PubMed] [Google Scholar]
- [25].Hillyard SA, Vogel EK, Luck SJ, Sensory gain control (amplification) as a mechanism of selective attention: electrophysiological and neuroimaging evidence, Philos. Trans. R. Soc. Lond. B. Biol. Sci 353 (1998) 1257–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kelly SP, Foxe JJ, Newman G, Edelman JA, Prepare for conflict: EEG correlates of the anticipation of target competition during overt and covert shifts of visual attention, Eur. J. Neurosci 31 (2010) 1690–1700. [DOI] [PubMed] [Google Scholar]
- [27].Noonan MP, Adamian N, Pike XA, Printzlau F, Crittenden BM, Stokes MG, Distinct mechanisms for distractor suppression and target facilitation, J. Neurosci 36 (2016) 1797–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ergenoglu T, Demiralp T, Bayraktaroglu Z, Ergen M, Beydagi H, Uresin Y, Alpha rhythm of the EEG modulates visual detection performance in humans, Cogn. Brain Res 20 (2004) 376–383. [DOI] [PubMed] [Google Scholar]
- [29].Mathewson KE, Gratton G, Fabiani M, Beck D, Ro T, To see or not to see:prestimulus alpha phase predicts visual awareness., J. Neurosci 29 (2009) 2725–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mathewson KE, Lleras A,. Beck M, Fabiani M, Ro T, Gratton G, Pulsed out of awareness: EEG alpha oscillations represent a pulsed inhibition of ongoing cortical processing, Front. Psychol 2 (2011) 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Limbach K, Corballis PM, Pre-stimulus alpha power influences response criterion in a detection task, Psychophysiology 43 (2016) 1154–1164. [DOI] [PubMed] [Google Scholar]
- [32].Iemi L, Chaumon M, Crouzet SM, Busch NA, Spontaneous neural oscillations bias perception by modulating baseline excitability, J. Neurosci 37 (2017) 807–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Samaha J, Iemi L, Postle BR, Prestimulus alpha-band power biases visual discrimination confidence, but not accuracy, Conscious. Cogn 54 (2017) 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Iemi L, Busch NA, Moment-to-moment fluctuations in neuronal excitability bias subjective perception rather than strategic decision-making, ENeuro 5 (2018) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Benwell SY, Tagliabue CF, Veniero D, Cecere R, Savazzi S, Thut G, Pre-stimulus EEG power predicts conscious awareness but not objective visual performance, ENeuro 4 (2017) 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sadaghiani S, Kleinschmidt A, Brain networks and α-oscillations: structural and functional foundations of cognitive control, Trends Cogn. Sci 20 (2016) 805–817. [DOI] [PubMed] [Google Scholar]
- [37].Haegens S, Nacher V, Luna R, Romo R, Jensen O, α-oscillations in the monkey sensorimotor network influence discrimination performance by rhythmical inhibition of neuronal spiking, Proc. Natl. Acad. Sci 108 (2011) 19377–19382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Spaak E, Bonnefond M, Maier A, Leopold DA, Jensen O, Layer-specific entrainment of gamma-band neural activity by the alpha rhythm in monkey visual cortex, Curr. Biol 22 (2012) 2313–2318. doi: 10.1016/j.cub.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Postle BR, The cognitive neuroscience of visual short-term memory, Curr. Opin. Behav. Sci 1 (2015) 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zumer JM, Scheeringa R, Schoffelen JM, Norris DG, Jensen O, Occipital alpha activity during stimulus processing gates the information flow to object-selective cortex, PLoS Biol 12 (2014) e1001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Serences JT, Yantis S, Culberson A, Awh E, Preparatory activity in visual cortex indexes distractor suppression during covert spatial orienting, J. Neurophysiol 92 (2004) 3538–3545. [DOI] [PubMed] [Google Scholar]
- [42].Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG, Increased activity in human visual cortex during directed attention in the absence of visual stimulation, Neuron 22 (1999) 751–761. [DOI] [PubMed] [Google Scholar]
- [43].Tootell RB, Hadjikhani N, Hall EK, Marrett S, Vanduffel W, Vaughan JT, Dale AM, The retinotopy of visual spatial attention, Neuron 21 (1998) 1409–1422. [DOI] [PubMed] [Google Scholar]
- [44].Awh E, Matsukura M, Serences JT, Top-down control over biased competition during covert spatial orienting, J. Exp. Psychol. Hum. Percept. Perform 29 (2003) 52–63. doi: 10.1167/2.7.15. [DOI] [PubMed] [Google Scholar]
- [45].Haegens S, Luther L, Jensen O, Somatosensory anticipatory alpha activity increases to suppress distracting input, J. Cogn. Neurosci 24 (2012) 677–685. [DOI] [PubMed] [Google Scholar]
- [46].Janssens C, De Loof E, Boehler CN, Pourtois G, Verguts T, Occipital alpha power reveals fast attentional inhibition of incongruent distractors, Psychophysiology 55 (2018) 1–11. [DOI] [PubMed] [Google Scholar]
- [47].Händel BF, Haarmeier T, Jensen O, Alpha oscillations correlate with the successful inhibition of unattended stimuli, J. Cogn. Neurosci 23 (2011) 2494–2502. [DOI] [PubMed] [Google Scholar]
- [48].Hermes D, Nguyen M, Winawer J, Neuronal synchrony and the relation between the BOLD response and the local field potential, PLoS Biol 15 (2017) e2001461. [DOI] [PMC free article] [PubMed] [Google Scholar]



