SUMMARY
Chronic inflammation predisposes to aging-associated disease, but it is unknown whether immunity regulation might be important for extending healthy lifespan. Here we show that in C. elegans, dietary restriction (DR) extends lifespan by modulating a conserved innate immunity pathway that is regulated by p38 signaling, and the transcription factor ATF-7. Longevity from DR depends upon p38–ATF-7 immunity being intact, but downregulated to a basal level. p38–ATF-7 immunity accelerates aging when hyperactive, influences lifespan independently of pathogen exposure, and is activated by nutrients independently of mTORC1, a major DR mediator. Longevity from reduced insulin/IGF-1 signaling (rIIS) also involves p38–ATF-7 downregulation, with signals from DAF-16/FOXO reducing food intake. We conclude that p38–ATF-7 is an immunometabolic pathway that senses bacterial and nutrient signals, that immunity modulation is critical for DR, and that DAF-16/FOXO couples appetite to growth regulation. These conserved mechanisms may influence aging in more complex organisms.
In Brief
Wu et al. report that longevity from dietary restriction and reduced insulin/IGF-1 signaling involves modulation of a conserved p38–ATF-7 innate immunity pathway. This immunometabolic pathway is activated by nutrients independently of mTORC1. DAF-16/FOXO inhibits p38–ATF-7 immunity by reducing food consumption, thereby linking growth, appetite, immunity, and lifespan regulation.
Graphical Abstract:
INTRODUCTION
Eukaryotic lifespans range from days to centuries. Across this spectrum, the progression of aging can be slowed by dietary restriction (DR), the reduction of food intake without malnutrition (Fontana and Partridge, 2015; Kapahi et al., 2017; Kenyon, 2010). When food is freely available, lifespan can be extended by decreasing the activity of mechanisms that promote growth. Reduced insulin/IGF-1 signaling (rIIS) is associated with longer life in species ranging from C. elegans to mice, and possibly humans (Kenyon, 2010; Shore and Ruvkun, 2013). From yeast to mammals, lifespan can be extended through inhibition of the mTORC1 (mechanistic target of rapamycin complex 1) kinase, a driver of growth that integrates amino acid and growth factor signals (Fontana and Partridge, 2015; Greer and Brunet, 2009; Johnson et al., 2013; Kapahi et al., 2017; Kenyon, 2010). An understanding of how reductions in anabolic signals promote longevity holds promise for enhancing healthy aging in humans.
Genetic analyses of model organisms have been invaluable for identifying mechanisms that extend lifespan. It is generally agreed that DR lifespan extension is mediated in part through reduced mTORC1 activity, and under some conditions rIIS as well (Fontana and Partridge, 2015; Hou et al., 2016; Johnson et al., 2013; Kapahi et al., 2017). Analyses in C. elegans have provided extensive insight into rIIS lifespan extension (Kenyon, 2010; Shore and Ruvkun, 2013). An evolutionarily conserved IIS pathway initiates from the receptor DAF-2 to inhibit the transcription factor DAF-16 (FOXO), which is required for rIIS longevity. By relieving repression of DAF-16 and other transcription factors, rIIS increases the activity of cell- and tissue-protective mechanisms that promote proteostasis, stress resistance, neuronal function, germ cell fitness, and extracellular matrix remodelling (Ewald et al., 2018; Kenyon, 2010; Shore and Ruvkun, 2013). The paradigm of enhanced cytoprotection has also been linked to DR, mTORC1 inhibition, and other lifespan extension scenarios (Fontana and Partridge, 2015; Greer and Brunet, 2009; Hou et al., 2016; Kapahi et al., 2017; Kenyon, 2010; Robida-Stubbs et al., 2012; Shore and Ruvkun, 2013).
An important hallmark of human aging is immune dysregulation (Shaw et al., 2013). Aging increases chronic innate immune activity and inflammation, a major risk factor for age-related pathologies that include diabetes, cancer, and neurodegenerative and cardiovascular disease (Kotas and Medzhitov, 2015). Obesity or an unhealthy diet can reprogram the innate immune system to a long-term inflammatory state, further predisposing to age-related disease (Christ et al., 2018; Dandona et al., 2004; Newton and Dixit, 2012). By contrast, in mammals DR seems to reduce chronic immunity and inflammation (Meydani et al., 2016; Shaw et al., 2013). It is unknown whether this DR benefit stems from a slower rate of tissue aging and damage, or immune regulation. The emerging idea that innate immunity is influenced by metabolic activity is of great interest, because of the potential importance in metabolic disease (Hotamisligil, 2017; Kotas and Medzhitov, 2015).
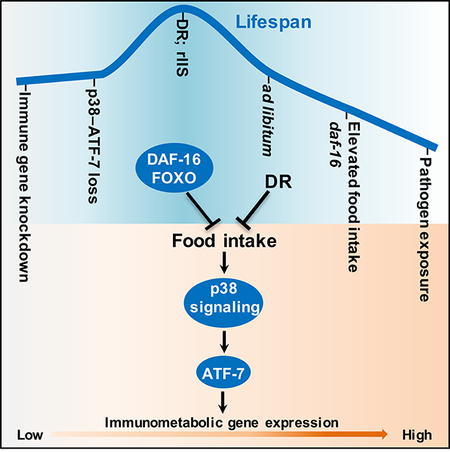
Here we have investigated relationships between nutrient intake, innate immunity, and aging in the nematode C. elegans. We find that DR longevity depends upon modulation of a conserved innate immunity pathway that is controlled by p38 signaling and the transcription factor ATF-7 (Irazoqui et al., 2010; Kim et al., 2002; Shivers et al., 2010). In contrast to other protective mechanisms implicated in longevity, for lifespan extension this pathway must be downregulated to a more favorable level. This immunometabolic pathway is activated by nutrients independently of mTORC1, and influences lifespan independently of pathogen defense. Modulation of the p38–ATF-7 pathway is also important for rIIS longevity. Unexpectedly, this is mediated through a reduction in food intake that is imposed by DAF-16, in part through non-autonomous signaling from the gut to the nervous system. Our findings show that metabolic regulation of innate immunity plays a major role in aging and DR lifespan extension. They also implicate p38 signaling in nutrient responses, and DAF-16/FOXO as a suppressor of feeding behavior.
RESULTS
DR Lifespan Extension Requires a Conserved Innate Immunity Pathway
To investigate the role of immunity in DR we examined the importance of p38 MAPK (mitogen-activated protein kinase) signalling, which regulates innate immune functions from C. elegans to humans (Arthur and Ley, 2013; Irazoqui et al., 2010). In humans the p38 cascade is activated by pathogen exposure and inflammatory cytokines (Arthur and Ley, 2013; Newton and Dixit, 2012). In C. elegans, a conserved p38 cassette consisting of NSY-1(ASK1 MAPKKK), SEK-1(MKK3/MKK6 MAPKK), and PMK-1(p38 MAPK) activates an innate immunity program that is induced by diverse pathogens (Figure S1A) (Irazoqui et al., 2010; Kim et al., 2002). This response includes activation of various antimicrobial genes, and processes that protect the host and clear damaged structures (Chikka et al., 2016; Troemel et al., 2006).
Both liquid- and plate-culture DR regimens failed to extend lifespan when p38 pathway components were ablated by mutation (Figures 1A–1D, and S1B–S1E; Data S1). This indicates that lifespan extension from DR requires the p38 pathway independently of any differences among these conditions, such as the swimming that occurs in liquid culture. Besides immune defense, in C. elegans p38 signaling is involved in the response to reactive oxygen species (ROS), and neuronal specification and regeneration (Andrusiak and Jin, 2016; Inoue et al., 2005). DR lifespan extension largely required the Toll/interleukin-1 receptor (TIR) protein TIR-1 (Figures 1E, 1F, and S1F), which functions upstream of the p38 cascade specifically in response to pathogens (Andrusiak and Jin, 2016; Inoue et al., 2005), suggesting a requirement for p38-regulated immunity.
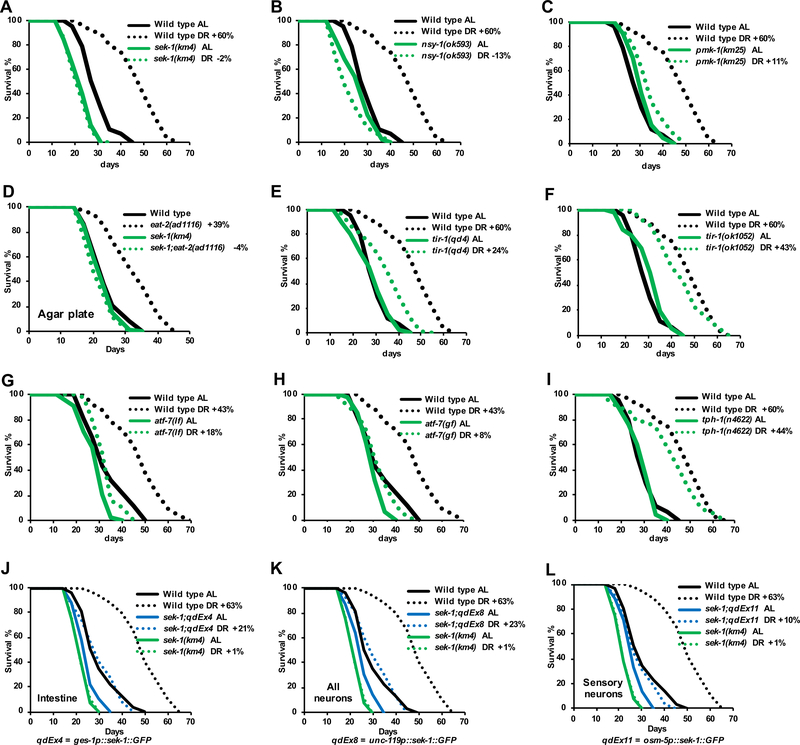
Figure 1. The p38–ATF-7 Innate Immunity Pathway is Required for DR Longevity.
(A–C) DR lifespan extension is blocked by the p38 pathway loss-of-function mutations sek-1(km4) (A), nsy-1(ok593) (B), and pmk-1(km25) (C).
(D) Lack of SEK-1 abolished lifespan extension by the feeding-impairment mutation eat-2, a putative DR model (Greer and Brunet, 2009).
(E, F) The upstream immunity regulator TIR-1 is largely required in DR. Lifespan extension was reduced considerably by a pathogen-sensitive tir-1 mutant (qd4), but only modestly by a tir-1 allele (ok1052) that does not impair pathogen resistance (Shivers et al., 2009).
(G, H) Impairment of DR by the atf-7 loss-of-function (lf) allele atf-7(qd22 qd130lf) (G), and the gain-of-function constitutive repressor mutation atf-7(qd22gf) (H).
(I). Lack of the serotonin biosynthesis enzyme TPH-1, which is required in the pathogen avoidance response (Shivers et al., 2009), had only a modest effect on DR. tph-1(n4622) is a presumed null deletion mutant.
(J–L) DR lifespan extension was limited in extrachromosomal transgenic strains in which sek-1 expression was rescued specifically in the intestine (J), all neurons (K) or ciliated sensory neurons (L) (Shivers et al., 2009).
Ad libitum (AL) food concentration is OD600 at 3 and DR is OD600 at 0.1. Unless otherwise specified, all AL/DR experiments were performed in liquid culture by dilution of growth-arrested bacterial food, and lifespans were measured at 20°C from hatching. The percent to which DR Increased lifespan (%, DR vs. AL) is indicated for individual strains in each figure. Complete data and statistics for all lifespan experiments, including repeat trials, are presented in Data S1. See also Figure S1 and Data S1.
We tested this idea further by examining the role of the transcription factor ATF-7 (Figure S1A), which also appears to function specifically in p38-regulated immunity (Shivers et al., 2010). Mammalian ATF-7 orthologs (ATF2/ATF7/CREB5) are regulated by p38, and are involved in innate immune responses (Newton and Dixit, 2012; Shivers et al., 2010), suggesting conservation of this pathway. In C. elegans, ATF-7 has a dual role in immunity regulation (Figure S1G) (Shivers et al., 2010). ATF-7 functions as a repressor that prevents immunity genes from being transcribed constitutively, but when it is phosphorylated by p38 (PMK-1) this repression is lifted, and ATF-7 acts as an activator that promotes full immune gene induction. This phosphorylation is blocked by the atf-7 gain-of-function (gf) mutation qd22, so that ATF-7 then constitutively represses immune gene expression (Shivers et al., 2010). An intragenic double mutation in atf-7 (qd22 qd130) creates a loss-of-function (lf), because it impairs both the activator and repressor functions of ATF-7. Because the repressor function is lacking, atf-7(lf) allows weak immune gene expression and partially suppresses the stronger sek-1 and pmk-1 defects, indicating that atf-7 functions downstream of sek-1 and pmk-1 in immunity (Shivers et al., 2010). Similarly to their effects on pathogen resistance, atf-7 mutations largely prevented DR longevity (Figures 1G, 1H, and S1H), and atf-7(lf) partially rescued the complete ablation of DR longevity resulting from SEK-1 loss (Figures S1I and S1J). Our data indicate that the p38–ATF-7-regulated immunity pathway is required for DR lifespan extension, with p38 signaling functioning upstream of ATF-7, although they do not rule out other p38-regulated mechanisms also being important. These DR experiments involved growth-arrested bacterial food (Moroz et al., 2014), suggesting that this requirement for p38–ATF-7 immunity reflects an effect on longevity and not infection resistance.
p38 signaling through the MAPKK SEK-1 acts in multiple tissues to resist pathogens (Shivers et al., 2009). An avoidance behavioral response is mediated by neuronal SEK-1, and pathogen defense functions involve SEK-1 expression in the intestine, the digestive system counterpart (Shivers et al., 2009). DR longevity was fully rescued by expression of SEK-1 from its own promoter (Figure S1K), and was rescued by intestinal SEK-1 partially (Figure 1J) but less robustly than pathogen resistance (Shivers et al., 2009). DR longevity was also partially rescued by neuronal SEK-1 (Figures 1K and 1L), although the pathogen avoidance response itself was not essential (Figure 1I). During DR, p38 signaling therefore acts in both the gut and nervous system but apparently does not function identically to its role in acute pathogen responses.
Modulation of Innate Immunity by DR and rIIS
To investigate why p38 signaling is required for DR longevity, we performed expression profiling of wild type (WT) and sek-1 animals under AL (ad libitum) and DR conditions (Figures 2A–2C, and S2A–S2C; Data S2). In WT animals, DR induced gene expression changes seen in other long-lived scenarios, including upregulation of cytoprotective genes (i.e. Small Heat Shock, Glutathione-S-Transferase, Cytochrome P450, UDP-Glucuronosyl Transferase) and downregulation of vitellogenins (Figures 2D, S2D, and S2E) (Fontana and Partridge, 2015; Hsu et al., 2003; Kenyon, 2010; Seah et al., 2016; Shore and Ruvkun, 2013). A previous comprehensive analysis of DR-induced gene expression also observed upregulation of many cytoprotective genes (Hou et al., 2016). A simple model to explain why the p38–ATF-7 immune response is required in DR is that many p38-dependent genes might activated by food deprivation as a protective mechanism. However, our DR-upregulated and SEK-1-dependent gene sets shared only 46 genes, a small set without obvious functions in common (Figures 2B, and S2A; Data S2). In addition, many DR-upregulated genes were expressed at elevated levels in sek-1 mutants (SEK-1-downregulated genes, Figure 2B), and expression of many heat shock and other cytoprotective genes was increased by either DR or sek-1 mutation (Figures 2B, 2D, S2A, S2D, and S3K). Thus, when p38 signaling was blocked DR nevertheless induced many gene regulation events that are associated with longevity, but these were insufficient for lifespan extension.
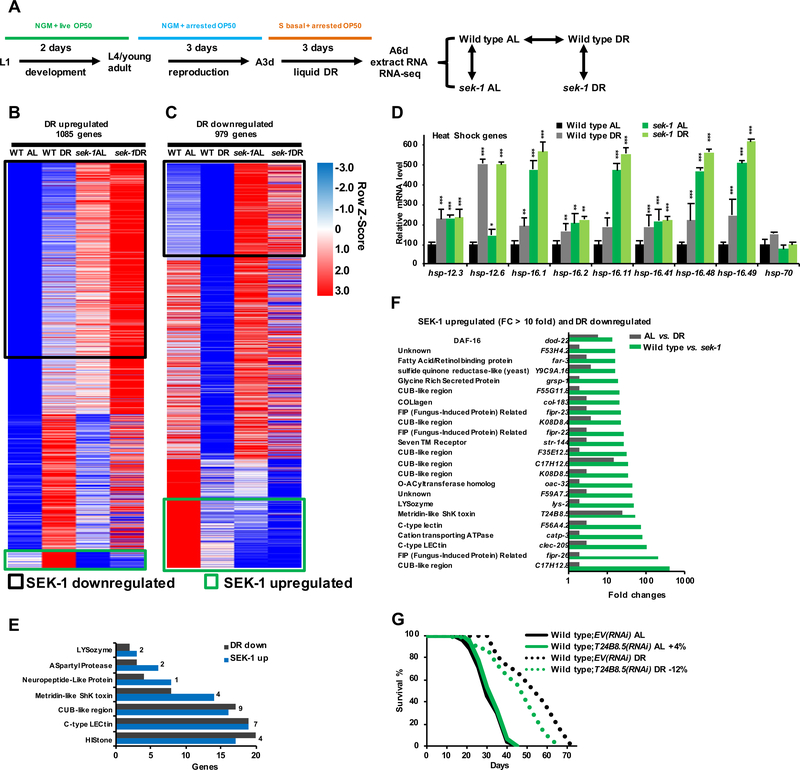
Figure 2. DR Increases Cytoprotection but Inhibits p38-regulated Immunity Genes.
(A) Plan for RNA-seq experiments. Analysis of sek-1 overcame possible redundancy among the three PMK kinases.
(B, C) Heatmap of DR-upregulated (B) and -downregulated (C) genes. DR-upregulated genes are defined as those genes having a higher expression under DR than AL conditions in WT animals. Throughout the manuscript, SEK-1 up- or down-regulated refers to genes that are expressed differentially between WT and sek-1(−) animals under both DR and AL conditions. SEK-1-upregulated genes that are upregulated (46 genes) or downregulated (162 genes) by DR are indicated with green boxes. A threshold of P < 0.05 is set to define differentially expressed targets.
(D) Expression change for small heat shock protein genes in response to DR and sek-1 deletion, derived from RNA-Seq data.
(E) DR-downregulated and SEK-1-upregulated genes (numbers indicate overlaps) are involved in proposed immune response gene classes (Troemel et al., 2006).
(F) Immunity genes that are strongly dependent upon sek-1, but suppressed by DR, as indicated by RNAseq. For each fold change shown, P < 0.0001.
(G) Effect of RNAi knockdown of a DR-downregulated immune response gene on DR longevity.
All the RNA-seq data sets generated in this study are summarized in Data S2. Mean ± SEM, two-tailed t-test. * P < 0.05, ** P < 0.01, *** P < 0.0001. See also Figure S2, Data S1 and S2.
Although DR longevity depended upon p38 signaling, more SEK-1-dependent genes were downregulated by DR (162 genes) than were upregulated (46 genes) (Figures 2B, 2C, S2A, and S2B). Many immunity genes were present in our SEK-1-dependent set, consistent with previous findings (Figures 2E, 2F, and S2C) (Troemel et al., 2006), and immunity genes were prominent among the sek-1-dependent genes we identified as downregulated by DR (Figures 2E and 2F; Data S2). Several of these DR-downregulated immunity genes are controlled directly by ATF-7 (Figures S2F and S2G), as predicted (Shivers et al., 2010). We also determined that many of these genes had been downregulated by DR and intermittent fasting in a previous expression profiling analysis (Data S2) (Hou et al., 2016). DR reduced expression of many p38–ATF-7-regulated immune response genes much more modestly than did sek-1 mutation (Figure 2F), indicating that their expression was not completely eliminated by reduced food intake. p38-dependent immunity genes function largely redundantly in pathogen resistance (Schulenburg et al., 2008; Troemel et al., 2006). However, RNAi (RNA interference) against several of them modestly impaired DR longevity (Figures 2G and S2H), consistent with the requirement for sek-1 and the p38–ATF-7 pathway (Figure 1). Thus, while DR lifespan extension depends upon p38–ATF-7-regulated innate immunity being functionally intact, DR reduces expression of these genes to a basal level.
Previous work showed that lifespan extension from rIIS is largely suppressed by mutation of sek-1 (Troemel et al., 2006) or pmk-1 (Zarse et al., 2012). This suppression has been proposed to reflect a requirement for immunity (Troemel et al., 2006) or the p38 response to ROS (Zarse et al., 2012). To assess the importance of p38–ATF-7 immunity specifically, we investigated whether lifespan extension from daf-2 RNAi requires the immunity regulator ATF-7 along with upstream p38 pathway components, comparing under AL and DR conditions in liquid culture (Figures 3A–3C and S3A–S3D). The atf-7(gf) double mutation (constitutive repressor) reduced AL lifespan only minimally compared to WT, but almost completely suppressed lifespan extension from both DR and daf-2 RNAi. Also supporting a role for this immunity pathway, RNAi against several p38–ATF-7-regulated immunity genes modestly decreased rIIS lifespan extension (Figure S3F). Many genes that are upregulated by rIIS were expressed at considerably higher background levels in sek-1 mutants (Figures 3D and S3K), arguing against the idea that the p38–ATF-7 signaling might be generally required for rIIS-induced genes to be expressed. We conclude that the p38–ATF-7-regulated immunity pathway is largely required for rIIS lifespan extension.
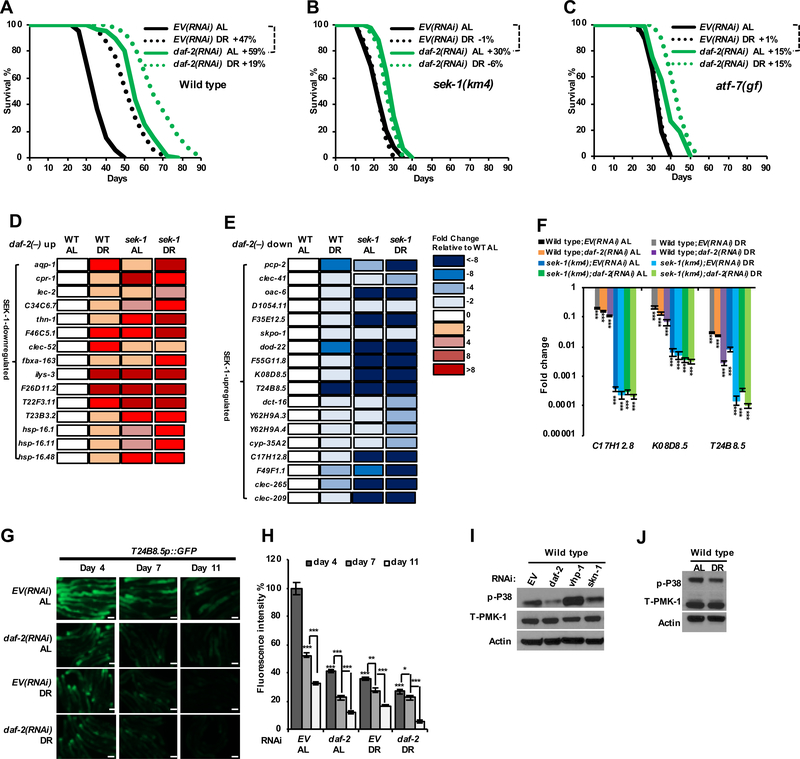
Figure 3. Similar modulation of p38–ATF-7-mediated immunity by DR and rIIS.
(A–C) Comparison of DR and daf-2 RNAi effects on the survival of wild type (A), sek-1(km4) (B) and atf-7(qd22gf) (C) animals.
(D, E) Effects of DR and sek-1 mutation on genes that are upregulated (D) or downregulated (E) in daf-2(−) animals. All expression data presented are from RNA-Seq; see also Data S2. These daf-2(−)-upregulated and -downregulated genes are from (Murphy et al., 2003).
(F) Effects of DR and daf-2 RNAi on expression of p38–ATF-7-regulated immunity genes (C17H12.8, K08D8.5 and T24B8.5) in wild type and sek-1(km4) animals (Shivers et al., 2010), examined by qRT-PCR. DR was performed for three days.
(G, H) Effects of DR and daf-2 RNAi on expression of the immune response gene T24B8.5, as indicated by a transcriptional GFP (green fluorescent protein) fusion reporter (Shivers et al., 2009). Fluorescence microscopy images (G) and quantification (H) indicate GFP levels at the indicated number of days after DR was initiated, or in parallel AL-fed controls.
(I, J) DR and daf-2 RNAi suppress PMK-1 (p38) activity. Immunoblot analyses of total and activated (phosphorylated) PMK-1 from three-day RNAi-treated day-one-adult (I) and three-day DR treated worms (J) are shown.
Scale bar, 100 μm. Mean ± SEM, two-tailed t-test. * P < 0.05, ** P < 0.01, *** P < 0.0001. See also Figure S3, Data S1 and S2.
rIIS increases the activity of many cytoprotective mechanisms (Introduction), but the example of DR raises the question of whether p38–ATF-7 immunity might be broadly suppressed when IIS is reduced. When we compared our DR data to DAF-2-regulated gene sets (Figures S3G–S3J; Data S2) (Murphy et al., 2003; Tepper et al., 2013), we found that many sek-1-dependent immunity genes that are downregulated by DR are also downregulated by rIIS (Figure 3E), consistent with earlier reports (Murphy et al., 2003; Troemel et al., 2006). Expression of a transcriptional reporter for the p38–ATF-7-regulated immunity gene T24B8.5 is detectable most prominently in the intestine, the digestive system counterpart, and declines with age (Youngman et al., 2011) (Figures 3G and 3H). DR and rIIS comparably decreased expression of T24B8.5 and other immune response genes, by reducing the p38 signal that drives their expression (Figures 3E–3H and S3K). By contrast, neither DR nor rIIS decreased the levels of total PMK-1 protein (Figures 3I and 3J), and DR did not inhibit expression of the nsy-1, sek-1, or pmk-1 mRNAs (Data S2), suggesting that DR and rIIS inhibit p38–ATF-7 immunity by reducing signaling through the p38 pathway.
In wild-type animals, the age-related decline in p38–ATF-7 immunity is associated with increased pathogen susceptibility (Youngman et al., 2011). By contrast, rIIS reduces p38–ATF-7 immune activity but increases pathogen resistance through largely uncharacterized immunoprotective effects of DAF-16 (Garsin et al., 2003; Murphy et al., 2003; Troemel et al., 2006). DR also enhanced pathogen resistance (Figure S3E), suggesting that DR compensates for reduced p38–ATF-7 immunity by enhancing other protective activities.
Activation of p38-ATF-7 Immunity by Nutrients
How might DR and rIIS decrease p38–ATF-7 immune activity? p38 signaling and immunity gene expression are higher in AL-fed compared to DR animals (Figures 3E–3J), suggesting that food intake activates the p38–ATF-7 pathway. Consistent with this idea, many of the DR-downregulated immunity genes we identified here were found to cycle up and down in their expression in response to feeding during intermittent fasting (Data S2) (Hou et al., 2016). Importantly, our AL conditions involved growth-arrested bacterial food, and the p38–ATF-7-regulated immunity reporter was activated by this food in a dose-dependent manner and could be upregulated by feeding of UV-killed bacteria (Figures 4A–4C), arguing against the idea that this p38–ATF-7 activity derives from bacterial colonization or pathogenesis.
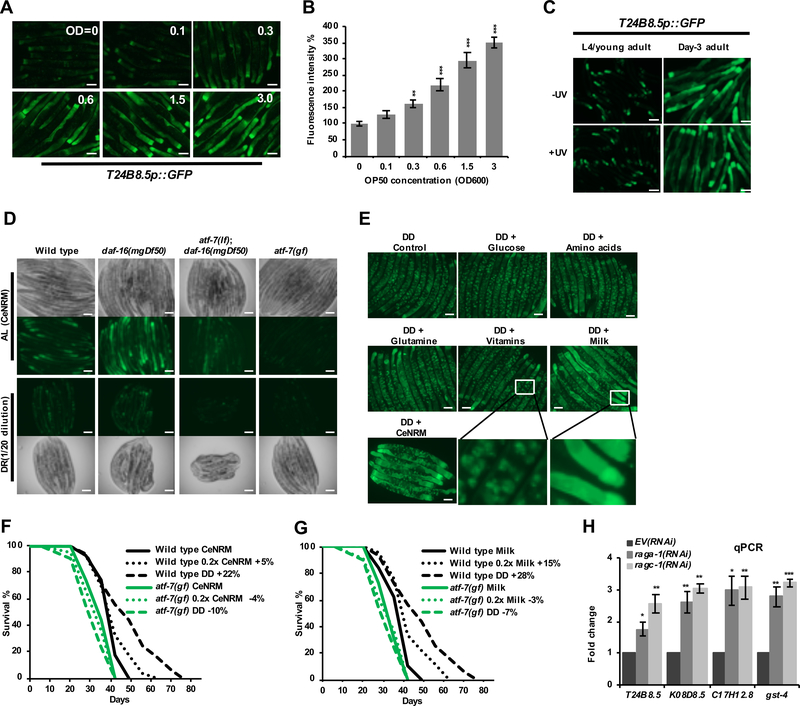
Figure 4. Regulation of p38–ATF-7 immunity and DR longevity by nutrients.
(A, B) Dose-response effect of bacterial food on T24B8.5 immunity reporter expression. Fluorescence microscopy images (A) and quantification (B) indicate GFP expression from day-six-adult WT worms that carry the agIs219 transgene, and have been fed for three-days under different concentrations of antibiotic-arrested OP50 bacteria in liquid medium.
(C) UV treatment of cold- and antibiotic-treated OP50 did not affect T24B8.5 reporter expression.
(D) Expression of T24B8.5 in C. elegans nutrient rich medium (CeNRM), a liquid axenic medium. Worms were grown in CeNRM until the second day of adulthood (day 7 from the L1 stage), then transferred into fresh CeNRM (AL, 1X) or diluted medium (DR, 0.05X) for four days.
(E) Effect of nutrients on T24B8.5 expression. Day-three-adults were transferred to DD (dietary deprivation) conditions that included the indicated CeNRM components. Fluorescence microscopy images show day-seven adults. Only milk powder was sufficient to drive immune gene expression. The presence of FUdR in the DD medium resulted in embryonic growth arrest and reporter expression in embryos (apparent as speckles).
(F, G) Survival of wild type N2 and atf-7(gf) animals that had been fed with CeNRM (F) or milk powder (G). Lifespan extension from A6d-initiated DD (F) was suppressed by continued CeNRM feeding, and reduced to a lesser extent by feeding with 0.2X CeNRM. Similar effects were seen upon feeding with milk powder (G).
(H) Effects of mTORC1 inhibition on p38–ATF-7-regulated immunity gene expression (C17H12.8, K08D8.5, T24B8.5). raga-1 and ragc-1 transduce amino acid signals to mTORC1 (Saxton and Sabatini, 2017), and the cytoprotective gene gst-4 is activated by mTORC1 inhibition (Robida-Stubbs et al., 2012). Day 3 adults were examined by qRT-PCR after initiation of RNAi at the L4 stage.
Scale bar, 100 μm. Mean ± SEM, two-tailed t-test. * P < 0.05, ** P < 0.01, *** P < 0.0001. See also Figure S4 and Data S1.
We investigated whether p38–ATF-7 immunity might be activated by nutrients independently of microbial exposure. The chemically-defined media developed to date support C. elegans growth and development very poorly, but C. elegans can readily develop into fertile adults when fed an axenic medium in which a defined set of nutrients is supplemented with sterilized milk powder (Rao et al., 2005; Samuel et al., 2014; Scanlan et al., 2018). Our version of the latter feeding regimen (C. elegans nutrient rich medium; CeNRM) activated the T24B8.5 immunity reporter in a dose-dependent manner (Figure 4D), enabled robust growth and development (see Methods), and in a dose- and ATF-7-dependent manner suppressed lifespan extension from complete dietary deprivation (DD) (Figure 4F), a form of DR (Steinkraus et al., 2008). The capability of CeNRM components to activate the immunity reporter correlated with their effects on growth and lifespan: milk powder was essential for CeNRM to support C. elegans growth or activate the reporter (Figure 4E) (Rao et al., 2005; Samuel et al., 2014; Scanlan et al., 2018), and feeding with milk powder but not other CeNRM components suppressed DD longevity and upregulated immunity (Figures 4G and S4E–S4G). CeNRM activated theT24B8.5 immunity reporter comparably to feeding of E. coli OP50 at OD 0.6 (approximately 5 × 108 E. coli/ml.) (Figure 4A and 4E), a bacterial concentration that is far greater than would be possible for any contaminant of a CeNRM component. This reporter was also not induced detectably by either an E. coli culture supernatant or a bacterial lipopolysaccharide preparation (Figures S4A and S4B). Together, the data indicate that CeNRM activated immunity gene expression by means of its nutritional content, not any bacterial derivatives that might be present at trace levels. Inhibition of the nutrient sensor mTORC1 did not attenuate immunity analogously to DR and instead increased immune gene expression (Figure 4H), and did not affect immunity gene suppression by DR (Figures S4C and S4D). We conclude that the p38–ATF-7-regulated immune response is an immunometabolic pathway that can be activated by nutrients independently of bacterial signals, and mTORC1.
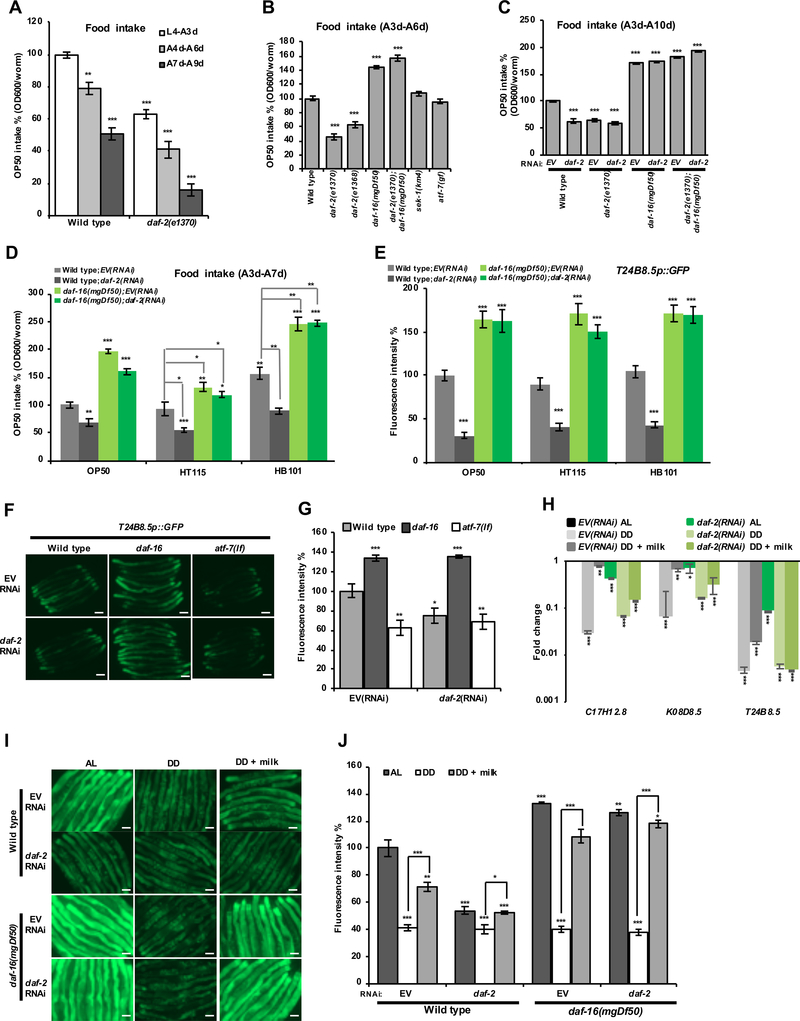
rIIS and DAF-16 Suppress Immunity by Decreasing Food Consumption
rIIS might conceivably suppress the p38–ATF-7 pathway through a signal that is regulated by IIS, or by reducing food intake. Analyses of C. elegans feeding have typically measured pharyngeal pumping rates, but not the amount of bacterial food that is consumed. When we measured the relative levels of food consumption directly, we were surprised to find that food intake was decreased to 50–60% of WT by rIIS, and was increased significantly in daf-16 mutants (Figures 5A–5C). A similar assay that has been corroborated by mass spectroscopy (Gomez-Amaro et al., 2015) yielded essentially identical results (M. Petracheck, pers. comm.). In analyses of three E. coli food strains, rIIS reduced food intake and immunity gene expression similarly, and in a daf-16-dependent manner (Figures 5D–5E and S5A). rIIS and DAF-16 decreased the levels of bacteria present in the intestine, presumably reflecting enhanced bacterial clearance but also consistent with decreased feeding (Figures S5D and S5E). In contrast to rIIS, food deprivation decreased expression of the T24B8.5 immunity reporter independently of daf-16 (Figures 5I, 5J, S5B, and S5C), indicating that DAF-16 modulates immunity by regulating feeding. These effects of rIIS and DAF-16 on immunity gene expression were recapitulated when animals were fed on agar plates instead of in liquid medium, and with milk powder instead of bacterial food (Figures 5F–5J, S5B, and S5C). In contrast to rIIS, mTORC1 inhibition did not reduce either feeding or p38–ATF-7 immunity (Figures 4H and 6A). We conclude that rIIS reduces p38–ATF-7 immune activity through a DAF-16-mediated decrease in feeding and nutrient consumption.
Figure 5. rIIS and DAF-16 inhibit food consumption.
(A) The long-lived mutant daf-2(e1370) consumes reduced amounts of food. The relative consumption of food (antibiotic-arrested OP50 in liquid medium) was determined by the change in OD600 absorbance over time.
(B, C) Effects of rIIS and DAF-16 on food intake. DAF-16 is required for rIIS to reduce food intake, and daf-16 mutants consume more food than WT animals.
(D, E) Effects of rIIS and DAF-16 on food intake (D) and immunity gene expression (E) when animals were fed with the three E. coli strains most commonly used in the laboratory as C. elegans food sources. Here HT115 is carrying the L4440 plasmid.
(F, G) Effect of daf-2 RNAi on expression of the p38–ATF-7 target gene T24B8.5 in daf-16 and atf-7(lf) mutants. Fluorescence microscopy images (F) and quantification (G) indicate GFP levels of day-one-adults in solid agar plates.
(H) Three immunity genes (C17H12.8, K08D8.5, T24B8.5) are downregulated by DD or the decreased feeding associated with rIIS, with this partially reversed by milk feeding. Expression was examined by qRT-PCR.
(I, J) Effects of IIS, DD, and autoclaved milk powder on expression of the p38–ATF-7-regulated gene T24B8.5. Day-three-adults were transferred to DD medium, and fluorescence microscopy images (I) and quantification (J) indicate GFP levels after four days of DD treatment (day-seven adult).
Scale bar, 100 μm. Mean ± SEM, two-tailed t-test. * P < 0.05, ** P < 0.01, *** P < 0.0001. See also Figure S5.
Figure 6. Effects of mTORC1 and tissue-specific DAF-16 on food consumption.
(A) Effects of mTOR signaling on bacterial food intake. Inhibition of mTORC1-specific genes did not affect feeding, and the small increase seen with let-363 RNAi might reflect inhibition of the related but distinct complex mTORC2 (Saxton and Sabatini, 2017), as suggested by the effect of rict-1 (Rictor) RNAi.
(B) Tissue-specific activities of DAF-16 in the regulation of food intake. DAF-16 is expressed from transgenes in the intestine (ges-1) or neurons (unc-119), or from its own promoter (Pdaf-16) (Libina et al., 2003).
Mean ± SEM, two-tailed t-test. * P < 0.05, ** P < 0.01, *** P < 0.0001.
The amount of food C. elegans consumes is determined by the rate and efficiency of pharyngeal pumping, and the proportion of time spent pumping (Avery and You, 2012; Gomez-Amaro et al., 2015; Lemieux and Ashrafi, 2016). Consistent with some earlier observations, rIIS modestly reduced the frequency of both pumping and defecation (Figures S5F and S5G). Importantly, C. elegans regularly enters and exits a quiescent state in which pumping and movement cease (McCloskey et al., 2017; Skora et al., 2018; You et al., 2008). The frequency of quiescence is increased in liquid culture, and elevated by food deprivation in either liquid or plate culture, with rIIS enhancing the propensity of fasting to promote quiescence (McCloskey et al., 2017; Skora et al., 2018). We determined that rIIS substantially increased the proportion of time spent in quiescence under typical plate culture AL feeding conditions (Figure S5H; Movie S1–S4). Together, our data suggest that rIIS and DAF-16 decrease both the pumping rate and time spent feeding, effects that would each reduce food intake.
We investigated whether DAF-16 might inhibit feeding by acting autonomously within the nervous system, which controls activity and pharyngeal pumping (Avery and You, 2012). The reduction in food consumption induced by rIIS was rescued slightly by neuronal DAF-16 expression, but intestinal DAF-16 provided a more robust rescue (Figure 6B). We conclude that DAF-16 inhibits feeding by acting in multiple tissues, in part by directing the gut to send signals that modulate neuronal control of feeding.
Importance of p38–ATF-7 Immunity Modulation for Lifespan Extension
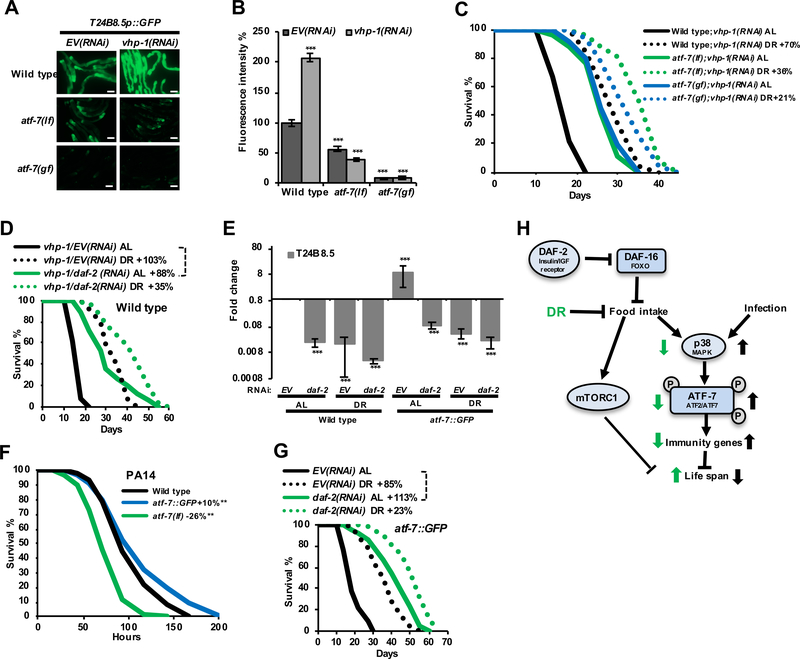
The association of reduced p38–ATF-7 activity with longevity from both DR and rIIS suggests that high-level activation of this pathway might be deleterious. Accordingly, lifespan was decreased by RNAi knockdown of the phosphatase VHP-1 (Figure S6A) which inhibits PMK-1 (p38) (Irazoqui et al., 2010; Kim et al., 2004) and downstream ATF-7-regulated gene expression (Figures 7A, 7B, S6A, and S6B). This lifespan reduction was largely rescued by atf-7 mutations, indicating that it derived to a great extent from excessive immune activity (Figure 7C). Moreover, the relative extent to which DR or rIIS extended lifespan was greater in the vhp-1(RNAi) background, presumably because DR and rIIS reverse the elevated p38–ATF-7 activity (Figures 7C and 7D, compare to 3A). Transgenic ATF-7 overexpression similarly increased immune gene expression and pathogen resistance, but decreased lifespan (Figures 7E, 7F, and S6C). Notably, ATF-7 overexpressors also responded dramatically to DR and daf-2 RNA, so that their increased immune activity and reduced lifespan were largely overcome (Figures 7G, S6C, and S6D). Modulation of the p38–ATF-7 immunometabolic pathway to an optimal level is therefore an important part of the “package” of benefits through which DR and rIIS slow aging.
Figure 7. Modulation of p38–ATF-7 downstream gene expression determines lifespan.
(A, B) Regulation of T24B8.5 expression by ATF-7 and VHP-1. Fluorescence microscopy images (A) and quantification (B) indicate GFP expression from day-one-adult WT, atf-7(qd22 qd130lf), and atf-7(qd22gf) worms that carry the agIs219 immunity reporter transgene, and have been subjected to two-day RNAi treatment. Scale bar, 100 μm.
(C) The survival impairment induced by hyperactive p38 signaling (vhp-1 RNAi) is ameliorated by atf-7 mutations.
(D) DR and rIIS partially compensate for the lifespan reduction induced by vhp-1 RNAi.
(E) Comparison of the effects of DR and daf-2 RNAi on T24B8.5 mRNA expression in WT and atf-7::GFP integrated transgenic animals after three days of liquid DR treatment, assayed by qRT-PCR.
(F) Pathogenesis assay of day-one-adult worms exposed to P. aeruginosa PA14. Survival is enhanced or reduced by ATF-7 overexpression and loss-of-function, respectively.
(G) DR and rIIS partially compensate for the lifespan reduction induced by transgenic ATF-7 overexpression.
(H) Regulation of the p38–ATF-7 immunometabolic pathway. Effects of DR and DAF-16-mediated reduced food intake on this pathway are shown in green.
Scale bar, 100 μm. Mean ± SEM, two-tailed t-test. * P < 0.05, ** P < 0.01, *** P < 0.0001. See also Figure S6 and Data S1.
DISCUSSION
DR Longevity Depends Upon Immunity Modulation
DR appears to reduce development of aging-related chronic inflammation in mammals (see Introduction), but paradigms have not been developed in which immunity regulation has been implicated in lifespan extension. Here we have shown that C. elegans lifespan extension from DR depends upon the p38–ATF-7 immunometabolic pathway being intact, but downregulated (Figure 7H). We find that this pathway is activated not only by exposure to bacterial pathogens, but also by nutrients. A considerable body of evidence indicates that DR longevity involves downregulation of the mTORC1 pathway, the best-understood mechanism of nutrient signaling (Fontana and Partridge, 2015; Johnson et al., 2013). We find that nutrients activate p38 signaling to ATF-7 in parallel to mTORC1 (Figures 4 and 7), identifying this pathway as an independent nutrient-regulated mechanism that is inhibited by DR, and as a mediator of DR lifespan extension. Milk components are required for our axenic media to support C. elegans larval development and immune activation, suggesting that these essential nutrients might include specific metabolites or signalling molecules. Our data provide an entry point for understanding responses to nutrients, as well as unravelling how immunity is influenced by nutritional status and possibly gut microbiome signals, and how this interaction affects aging. Modulation of nutrient-regulated immune activities may be a generally essential part of the “package” of benefits through which aging is slowed by DR.
It is well-established that lifespan extension depends upon a variety of cellular and tissue protective mechanisms, the activity of which is typically enhanced in scenarios of long life (Blackwell et al., 2015; Fontana and Partridge, 2015; Kenyon, 2010; Shore and Ruvkun, 2013). By contrast, DR longevity involves p38–ATF-7 activity being reduced. Why would fine-tuning of this immunometabolic pathway be important? Activation of p38–ATF-7 immunity or its components in distant tissues has been observed either to inhibit or induce neuronal degeneration, depending upon the circumstances (Chikka et al., 2016; E et al., 2018), suggesting that some ATF-7-regulated functions may be advantageous for pathogen defense but not long life. A gf mutation in the apparent immunity protein IRG-7 requires ATF-7 to extend lifespan and interacts functionally with multiple longevity-associated transcription factors (Yunger et al., 2017), suggesting that innate immunity systems might influence maintenance of cellular and tissue homeostasis. It is also possible that even relatively simple organisms may possess “inflammatory” activities that promote age-related pathologies, as suggested by antimicrobial peptide expression being associated with intestinal pathology, aging, and death in Drosophila (Pletcher et al., 2002; Rera et al., 2012). Our results imply that a complex balance must be maintained between the positive and potentially negative effects of these innate immune activities. Perhaps the dual function of ATF-7 as a transcriptional repressor and activator evolved to maximize the range and precision of immunity gene activity, providing a tightly repressed ground state along with the capacity for rapid activation in response to a phosphorylation signal (Figure S1J).
Immune activity and pathogen responses are influenced by mitochondrial activity or stress, possibly as an early-warning mechanism to detect pathogen attack (Chikka et al., 2016; Kirienko et al., 2015; Pellegrino et al., 2014; Weinberg et al., 2015). Our finding that nutrients regulate the p38–ATF-7 pathway independently of microbial signals suggests that this pathway also has important metabolic functions that are independent of pathogen defense, and that metabolic parameters may have been an early driver of immunity evolution. One intriguing possibility is that the p38–ATF-7 pathway might have arisen initially as a transcriptional response to nutrients or other signals from food. The decline in p38–ATF-7 immune activity that is seen during aging (Figure 3H) (Youngman et al., 2011) could be explained in part by the reduction in food consumption that occurs with age (Figure 5A). Interestingly, both DR and rIIS enhance pathogen resistance despite reducing p38–ATF-7 pathway activity (Figure S3E) (Garsin et al., 2003). Parallel protective mechanisms thus might have evolved to provide pathogen defense in times of reduced food availability.
IIS Modulates Immunity and Lifespan by Regulating Food Consumption
Like DR longevity, rIIS lifespan extension depends upon modulation of the p38–ATF-7 pathway, in this case through a reduction in food intake that is imposed by DAF-16 (Figures 3, 5, and 7). This effect of IIS on feeding had not been described previously, because the amounts of food consumed had not been examined directly. Assessment of pharyngeal pumping, defecation, or movement in awake animals only partially indicates feeding activity, because daf-2 adults spend an increased proportion of time in a quiescent state from which they become aroused when stimulated by even slight movement, or light (Figure S5; mov. S4). The effects of IIS on quiescence and pumping frequency we detected would reduce the amount of food consumed, although it is also possible that IIS influences feeding by affecting the strength or efficiency of pumping.
The IGF-1 and insulin signaling pathways promote growth and energy consumption across metazoa (Taguchi and White, 2008). In C. elegans IIS inhibits entry into the larval diapause form known as dauer, in which pharyngeal pumping and movement largely cease (Ewald et al., 2018; Kenyon, 2010). rIIS and DAF-16 increase lifespan through mechanisms that are involved in or independent of the dauer program, with dauer-related mechanisms predominating at higher temperatures (Ewald et al., 2018). One of the daf-2 alleles we analyzed (e1370) exhibits mild dauer-related traits at 20°C (Ewald et al., 2018; P odshivalova et al., 2017), and is quiescent approximately half of the time at 25°C even as a da y-one adult (McCloskey et al., 2017). The rIIS-induced quiescence and reduced feeding we observed at 20°C are unlikely to be strictly dauer-related, however, because they were seen in daf-2(RNAi) animals (Figure S5), which do not show dauer-like traits (Ewald et al., 2018). DAF-16 localizes to nuclei upon food withdrawal, and is important for starvation survival (Kenyon, 2010). The reduction in feeding behavior we found to be established by DAF-16 might serve to conserve energy when food is scarce. On the other hand, when IIS activity is high DAF-16 is inhibited, and feeding can increase (Figure 5). This regulation of feeding by DAF-16 provides a paradigm through which growth factor activity might determine nutrient intake in other organisms.
In C. elegans DAF-16 controls a wide array of downstream processes, many of which are associated with longevity, and exerts its effects through direct regulation of target genes, indirect effects on gene expression, and cell-non-autonomous signals between tissues (Kenyon, 2010; Murphy et al., 2003; Tepper et al., 2013; Zhang et al., 2013). In Drosophila, dFOXO also promotes longevity by acting both directly and tissue-non-autonomously (Alic et al., 2014). We find that DAF-16 modulates p38–ATF-7 immunity indirectly, by controlling feeding (Figure 7H), and regulates feeding in part through tissue non-autonomous signals from the intestine to the nervous system (Figure 6B). Our results raise the interesting question of which biological functions of DAF-16 might be mediated through its regulation of feeding, and provide an unexpected paradigm (feeding regulation) that could account for some of its indirect or tissue-non-autonomous effects across the organism.
The extent to which reductions in IIS are involved in DR longevity is a topic of active investigation (Fontana and Partridge, 2015; Hou et al., 2016; Kenyon, 2010). We find that DR and rIIS are interwined in an unexpected way, in that the rIIS-induced reduction in feeding appears to lead to a DR-like state, as suggested by the lower levels of p38–ATF-7 activity. This DR-like state appears to contribute to rIIS lifespan extension: rIIS and DR each increase mean lifespan to a greater proportional extent when the baseline activity of p38–ATF-7 immunity is increased (Figure 7). It is clear that rIIS does not increase lifespan simply by reducing feeding, however, given that the lifespan extensions from DR and rIIS are additive (Figure 3), and that rIIS enhances many longevity-associated processes by acting through DAF-16 and other regulators (Ewald et al., 2018; Kenyon, 2010; Shore and Ruvkun, 2013). In contrast to rIIS, mTORC1 inhibition does not reduce feeding or p38–ATF-7 immune activity (Figures 4H and 6A), indicating a specific effect of IIS and DAF-16. Elucidating the importance of feeding regulation in other scenarios of C. elegans lifespan extension should further illuminate relationships between food intake and longevity.
Mammals are far complex than C. elegans with respect to regulation of growth, behavior, and appetite, and the sophistication of the innate immune system. However, it is important to note that the pathways we have studied here are largely conserved, including the regulation of innate immunity functions by p38, and of FOXO by growth factor signals. An understanding of how the p38–ATF-7 pathway is regulated by nutrients and bacterial signals, and feeding by DAF-16, may therefore reveal new possibilities for influencing human appetite, immunity, and longevity.
Limitations of Study
Our study has raised a number of interesting questions for future work. It remains to be determined how p38-ATF-7 immunity so profoundly influences longevity. Similarly, this immunometabolic pathway is regulated by nutrients in a manner that correlates with the capacity for growth and development, but the mechanisms involved are still unknown. Another important question is how DAF-16 inhibits feeding behavior and food intake, apparently by acting in multiple tissues. It will be of great interest to elucidate this unexpected DAF-16 function, and to determine whether in mammals FOXO proteins might similarly link appetite regulation to growth factor activity.
STAR+METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, T. Keith Blackwell (keith.blackwell@joslin.harvard.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animals
Worms were maintained on NGM plates seeded with Escherichia coli (OP50–1) at 15°C. Strains obtained from other sources were outcrossed to the appropriate laboratory strains as indicated in KEY RESOURCES TABLE. The sek-1::GFP transgenic strains were a gift of Nick Bishop and Leonard Guarente (MIT).
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| anti-phospho-p38 | Cell Signaling | #9211; RRID: AB_331762 |
| anti-PMK-1 | Inoue et al., 2005 | N/A |
| Anti-β-Actin | Sigma | #A5441; RRID: AB_476744 |
| Anti-GFP | Sigma | #11814460001; RRID: AB_390913 |
| Bacterial and Virus Strains | ||
| E. coli: Strain OP50–1 | Caenorhabditis Genetics Center | #OP50–1; RRID:WB-STRAIN: OP50–1 |
| E. coli: Strain HB101 | Caenorhabditis Genetics Center | #HB101; RRID:WB-STRAIN:H B101 |
| E. coli: Strain HT115 | Caenorhabditis Genetics Center | #HT115; RRID:WB-STRAIN:H T115 |
| Pseudomonas aeruginosa: Strain PA14 | Laboratory of Dennis Kim | #PA14; RRID:WB-STRAIN:P A14 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Nystatin | Sigma | N1638 |
| 5-Fluoro-2’-deoxyuridine (FUDR) | Thermo Fisher Scientific | AC227600025 |
| TRI Reagent | Sigma | T9424 |
| Protein A Sepharose | Abcam | ab193256 |
| Isopropyl β-D-1-thiogalactopyranoside (IPTG) | Fisher Scientific | BP1620–10 |
| Lipopolysaccharides from Escherichia coli O111:B4 | Sigma | L2630 |
| Skim Milk Powder | Thermo Scientific | OXLP0031B |
| RPMI 1640 amino acids solution | Sigma | R7131 |
| L-glutamine solution | Sigma | G7513 |
| RPMI 1640 vitamins solution | Sigma | R7256 |
| Trace metal mix | Sigma | 92949 |
| Adenosine | Sigma | A1752 |
| Guanosine | Sigma | G8377 |
| Hemin | Sigma | 51280 |
| Ampicillin | Sigma | A9518 |
| Kanamycin | Sigma | K1377 |
| Tetracycline | Sigma | T7660 |
| Texas Red-dextran | Thermo Fisher Scientific | D1829 |
| Critical Commercial Assays | ||
| Direct-zol™ RNA Kits | Zymo Research | R2050 |
| First-Strand Synthesis SuperMix | Thermo Fisher Scientific | 11752050 |
| SYBR Green | Thermo Fisher Scientific | 11760500 |
| Deposited Data | ||
| RNA-seq data | This paper | Gene Expression Omnibus (GEO) :GSE92902 |
| Experimental Models: Organisms/Strains | ||
| C. elegans: Strain N2: wild type | Caenorhabditis Genetics Center | N2 |
| C. elegans: Strain AU1: sek-1(ag1) | Caenorhabditis Genetics Center | AU1 |
| C. elegans: Strain AU3: nsy-1(ag3) | Caenorhabditis Genetics Center | AU3 |
| C. elegans: Strain CB1370: daf-2(e1370) | Caenorhabditis Genetics Center | CB1370 |
| C. elegans: Strain CF1038: daf-16(mu86) | Caenorhabditis Genetics Center | CF1038 |
| C. elegans: Strain CF1139: daf-16(mu86); daf-16a::GFP | Caenorhabditis Genetics Center | CF1139 |
| C. elegans: Strain CF1724: daf-16(mu86); daf-2(e1370); muIs105[daf-16p::GFP::daf-16 + rol-6(su1006)] | Caenorhabditis Genetics Center | CF1724 |
| C. elegans: Strain CF2005: daf-16(mu86); daf-2(e1370); muIs120[ges-1p::GFP::daf-16 + rol-6(su1006)] | Caenorhabditis Genetics Center | CF2005 |
| C. elegans: Strain CF2093: daf-16(mu86); daf-2(e1370); muIs131[unc-119p::GFP::daf-16 + rol-6(su1006)] | Caenorhabditis Genetics Center | CF2093 |
| C. elegans: Strain CF2570: daf-16(mu86); daf-2(e1370); muIs142[ges-1p::GFP::daf-16 + odr-1p::RFP] | Caenorhabditis Genetics Center | CF2570 |
| C. elegans: Strain CL2070: dvIs70 [hsp-16.2p::GFP + rol-6(su1006)] | Caenorhabditis Genetics Center | CL2070 |
| C. elegans: Strain DA1116: eat-2(ad1116) | Caenorhabditis Genetics Center | DA1116 |
| C. elegans: Strain DR1572: daf-2(e1368) | Caenorhabditis Genetics Center | DR1572 |
| C. elegans: Strain GR1307: daf-16(mgDf50) | Caenorhabditis Genetics Center | GR1307 |
| C. elegans: Strain HT1890: daf-16(mgDf50); daf-2(e1370) | Caenorhabditis Genetics Center | HT1890 |
| C. elegans: Strain IG685: tir-1(tm3036) | Caenorhabditis Genetics Center | IG685 |
| C. elegans: Strain LD1271: N2; Ex1060[pRF4(rol-6(su1006))] | This paper | N/A |
| C. elegans: Strain LD1757: sek-1(km4) 4X | This paper | N/A |
| C. elegans: Strain LD1758: pmk-1(km25) 5X | This paper | N/A |
| C. elegans: Strain LD1759: nsy-1(ok593) 4X | This paper | N/A |
| C. elegans: Strain LD1760: agIs219[T24B8.5p::GFP::unc-54–3’UTR + ttx-3p::GFP::unc-54–3’UTR] 5X | This paper | N/A |
| C. elegans: Strain LD1761: agIs219; atf-7(qd22 qd130) 4X | This paper | N/A |
| C. elegans: Strain LD1762: agIs219; atf-7(qd22) 4X | This paper | N/A |
| C. elegans: Strain LD1763: atf-7(gk715) 4X | This paper | N/A |
| C. elegans: Strain LD1764: atf-7(qd22 qd130) 4X | This paper | N/A |
| C. elegans: Strain LD1765: atf-7(qd22) 4X | This paper | N/A |
| C. elegans: Strain LD1766: tir-1(qd4) 4X | This paper | N/A |
| C. elegans: Strain LD1767: tph-1(n4622) 4X | This paper | N/A |
| C. elegans: Strain LD1768: sek-1(km4); eat-2(ad1116) | This paper | N/A |
| C. elegans: Strain LD1781: N2; ldIs1603[atf-7::GFP; unc-119(+)]; unc-119 (ed3) 5X | This paper | N/A |
| C. elegans: Strain LD1814: agIs219; atf-7(qd22 qd130); daf-16(mgDf50) | This paper | N/A |
| C. elegans: Strain LD1815: agIs219; daf-16(mgDf50) | This paper | N/A |
| C. elegans: Strain LD1816: sek-1(km4); sek-1::GFP | This paper | N/A |
| C. elegans: Strain LD1817: N2; ldEx1605[atf-7::GFP; rol-6(su1006)] | This paper | N/A |
| C. elegans: Strain LD1818: sek-1(km4); dvIs70 [hsp-16.2p::GFP + rol-6(su1006)] | This paper | N/A |
| C. elegans: Strain LG328: sek-1::GFP | Laboratory of Leonard Guarente | N/A |
| C. elegans: Strain JT366: vhp-1(sa366) | Caenorhabditis Genetics Center | JT366 |
| C. elegans: Strain RB1085: tir-1(ok1052) | Caenorhabditis Genetics Center | RB1085 |
| C. elegans: Strain ZD39: agIs219; pmk-1(km25) | Caenorhabditis Genetics Center | ZD39 |
| C. elegans: Strain ZD193: sek-1(km4);qdEx4[ges-1p::sek-1(cDNA)::GFP::unc-54–3’ UTR + myo-2p::mStrawberry::unc-54–3’ UTR] | Caenorhabditis Genetics Center | ZD193 |
| C. elegans: Strain ZD202: sek-1(km4);qdEx8[unc-119p::sek-1(cDNA)::GFP::unc-54 –3’ UTR + myo-2p::mStrawberry::unc-54–3’ UTR] | Caenorhabditis Genetics Center | D202 |
| C. elegans: Strain ZD260: sek-1(km4);qdEx11[osm-5p::sek-1(cDNA)::GFP::unc-54 –3’ UTR + myo-2p::mStrawberry::unc-54–3’ UTR] | Caenorhabditis Genetics Center | ZD260 |
| C. elegans: Strain ZD326: agIs219; atf-7(qd22;qd130); pmk-1(km25) | Caenorhabditis Genetics Center | ZD326 |
| C. elegans: Strain ZD340: agIs219; atf-7(qd22;qd130); sek-1(km4) | Caenorhabditis Genetics Center | ZD340 |
| C. elegans: Strain ZD395: agIs219; sek-1(km4) | Caenorhabditis Genetics Center | ZD395 |
| Oligonucleotides | ||
| Primer: C17H12.8 Forward: CAT GTT GAA CGA TTT GCG A | This paper | N/A |
| Primer: C17H12.8 Reverse: GCA ATC GAC AGT GAA ATT CTC | This paper | N/A |
| Primer: K08D8.5 Forward: TTT ATG CGA CTG CTG TTG C | This paper | N/A |
| Primer: K08D8.5 Reverse: CTC ACG TTT GCA TTG TAT GGA | This paper | N/A |
| Primer: T24B8.5 Forward: TAC ACT GCT TCA GAG TCG TG | This paper | N/A |
| Primer: T24B8.5 Reverse: CGA CAA CCA CTT CTA ACA TCT G | This paper | N/A |
| Primer: tba-1 Forward: CCT TCG TTC ACT GGT ACG TC | This paper | N/A |
| Primer: tba-1 Reverse: CAG CCA AGT CTT CAC GAG CC | This paper | N/A |
| Primer: sod-3 Forward: ATG GAC ACT ATT AAG CGC GA | This paper | N/A |
| Primer: sod-3 Reverse: GCC TTG AAC CGC AAT AGT G | This paper | N/A |
| Primer: hsp-16.1 Forward: CTC AGA TGG AAC GTC AAT TTA CTC | This paper | N/A |
| Primer: hsp-16.1 Reverse: ATC TCA GAA GAT TCA GAT GGA GAG | This paper | N/A |
| Primer: hsp-16.11 Forward: GGC TCA GAT GGA ACG TCA A | This paper | N/A |
| Primer: hsp-16.11 Reverse: GCT TGA ACT GCG AGA CAT TG | This paper | N/A |
| Primer: hsp-16.48 Forward: GAG AAA TGC TGA TCA CAA CTC | This paper | N/A |
| Primer: hsp-16.48 Reverse: GAA ACA TCG AGT TGA ACA GAG | This paper | N/A |
| Primer: dod-17 Forward: AAT TCA CAC TCA CTG TCG C | This paper | N/A |
| Primer: dod-17 Reverse: AAT CTA CTG TTG GAA GTG GC | This paper | N/A |
| Primer: dod-24 Forward: ATT GAA TTG CTC CAG AAC GA | This paper | N/A |
| Primer: dod-24 Reverse: CAT TTC TGT TGT CCG TCC C | This paper | N/A |
| Primer: clec-265 Forward: AAC TTT GCA GTT GGA GAA CC | This paper | N/A |
| Primer: clec-265 Reverse: CTG AGC ATA CCA GTA TCC GT | This paper | N/A |
| Primer: clec-209 Forward: CAA CTG GAG CTT CCG ATC TAC | This paper | N/A |
| Primer: clec-209 Reverse: GCT CCC GAA GCT GGA TAA AT | This paper | N/A |
| Primer: spp-21 Forward: CTG AAG ATA AAT TTC TCG CCG A | This paper | N/A |
| Primer: spp-21 Reverse: ATG ATT GGA CCG AAC TCC G | This paper | N/A |
| Primer: cpr-2 Forward: AAC CTT CAA ACT CCA CCG T | This paper | N/A |
| Primer: cpr-2 Reverse: CGG AGT TAC CAT AGT TCT TGT C | This paper | N/A |
| Primer: clec-60 Forward: GAA CAA ATC CTT CTG AGC CA | This paper | N/A |
| Primer: clec-60 Reverse: TAA GAT CGG CTT GAA TCG C | This paper | N/A |
| Primer: F49F1.1 Promoter Forward: GCC CTC TCA CAT CAT CAT TTC | This paper | N/A |
| Primer: F49F1.1 Promoter Reverse: AGT TCT ATA AAA AGG CAG CGT TA | This paper | N/A |
| Primer: F49F1.1 UTR Forward: ACT ATT TTA TTA CTT GCA CGT TTG G | This paper | N/A |
| Primer: F49F1.1 UTR Reverse: TGA TGT CAA TTG TTG CTC TCC A | This paper | N/A |
| Primer: C17H12.8 Promoter Forward: CGG CGT TCG TCT TCA ACA TA | This paper | N/A |
| Primer: C17H12.8 Promoter Reverse: AGA TCG GCT GAA CTT TTC TGT | This paper | N/A |
| Primer: C17H12.8 UTR Forward: TGG GTG GTT ATC AAC AGC AT | This paper | N/A |
| Primer: C17H12.8 UTR Reverse: ACA GTA GAT ACA ACG TTA GAC ACA | This paper | N/A |
| Primer: T24B8.5 Promoter Forward: AGG TAG AGC TCC ATC TTT CCA | This paper | N/A |
| Primer: T24B8.5 Promoter Reverse: CGT CAA GGG TAA AGT TTA GAA CA | This paper | N/A |
| Primer: T24B8.5 UTR Forward: GCA CAT TTG CAT TTC AGA CCA | This paper | N/A |
| Primer: T24B8.5 UTR Reverse: CTG CAA GTT TGT CGT GAA GG | This paper | N/A |
| Primer: K08D8.5 Promoter Forward: TCA TAC TTT TGC GTG TGG GG | This paper | N/A |
| Primer: K08D8.5 Promoter Reverse: GCA CGC ATA TAA TGC TCC CAT | This paper | N/A |
| Primer: K08D8.5 UTR Forward: GAT CCT TTT GAA TCG AGT TGT ATT T | This paper | N/A |
| Primer: K08D8.5 UTR Reverse: CAA TGA ATT GCT CAC CTT GTC A | This paper | N/A |
| Primer: clec-265 Promoter Forward: GAC CAA ACT CCC TAA TGA CGA | This paper | N/A |
| Primer: clec-265 Promoter Reverse: GCG ATATCA ATG CCT CCG AT | This paper | N/A |
| Primer: clec-265 UTR Forward: TAC GGT TTC AAC ATA AAT TAT CCG T | This paper | N/A |
| Primer: clec-265 UTR Reverse: TCT TGG TTC CGA ACA CAT AAA AA | This paper | N/A |
| Primer: dod-22 Promoter Forward: TAA CGA ACG TCG AGG AAA ACA | This paper | N/A |
| Primer: dod-22 Promoter Reverse: AAG CAT AGG AGA AGT GTG AGC | This paper | N/A |
| Primer: dod-22 UTR Forward: TGG CAT TTG TTA AGC CCT GT | This paper | N/A |
| Primer: dod-22 UTR Reverse: TGT TTC ATC CAG CTT ATG GCA | This paper | N/A |
| Primer: C17H12.6 Promoter Forward: GGG TGC CAA TAT CCA ACT GA | This paper | N/A |
| Primer: C17H12.6 Promoter Reverse: CAC CTG ATC TTG GAA CCT TGT | This paper | N/A |
| Primer: C17H12.6 UTR Forward: AAG GTG GAA GTA ACA AAC TGT G | This paper | N/A |
| Primer: C17H12.6 UTR Reverse: CGT TAA ATT GCG GGA ACA ACC | This paper | N/A |
| Software and Algorithms | ||
| SMALT version 0.7.4 | Wellcome Trust Sanger Institute | http://www.sanger.ac.uk/science/tools/smalt-0 |
| Subread version 1.4.6 | Liao et al., 2014 | http://subread.sourceforge.net |
| edgeR version 3.20.9 | Anders et al., 2013 | https://bioconductor.org/packages/release/bioc/html/edgeR.html |
| JMP version Pro 12.1.0 | SAS Institute Inc | https://www.jmp.com/en_us/home.html |
Microbial Strains and RNAi
E. coli (OP50–1) bacteria was cultured overnight in Luria-Bertani (LB) with 10 mg/L streptomycin at 37°C, after which 1 mL of liquid culture was see ded on standard NGM plates (100mm × 15mm petri dish) to grow for 2 days at room temperature. For pathogenesis experiments a single colony of P. aeruginosa PA14 was cultured in LB without antibiotics at 37°C (Shivers et al., 2010). Feeding RNAi was performed using HT115 bacteria carrying the pL4440 plasmid as empty vector (EV) control. RNAi cultures were grown overnight at 37°C in 50 mL conical tubes with 5 mL LB medium containing 50 μg/mL carbenicillin. Cultures were then diluted 1:5 in LB containing the carbenicillin to allow for re-entry into the logarithmic growth phase (~6 hours). Cultures were then centrifuged at 3,900 g for 10 minutes, concentrated to a volume of 3 mL LB with 0.2 g/L (1 mM) IPTG prior to seed onto NGM plates containing 50 μg/mL carbenicillin and 0.2 g/L IPTG. In all liquid culture experiments, RNAi was performed on plates for three days (L4 to A3d) prior to transfer to liquid culture. For double RNAi, clones were grown separately in parallel and after spin-down equal amounts of two clones or one clone plus L4440 control were mixed and spread on plates.
METHOD DETAILS
DR Liquid Medium Preparation
Bacterial food that maintained growth arrest under DR conditions was prepared as described previously (Moroz et al., 2014). OP50–1 bacteria were grown in 500 ml LB with 10 mg/L streptomycin in a 2 L flask overnight at 37°C, and spun down at 3,900 × g for 10 min. Cells were re-suspended in S basal (1× SB solution: 5.85 g/L NaCl, 1 g/L K2HPO4, 6 g/L KH2PO4, autoclaved) with 5 mg/L cholesterol, 50 mg/L ampicillin, 10 mg/L kanamycin, 1 mg/L tetracycline, 50 mg/L nystatin at 3 OD (optical density) at 600 nm in 50 mL conical tubes, and maintained at room temperature for ~7 hours before being placed at 4°C for one week. Im mediately prior to transferring animals to liquid cultures, the growth medium was replaced by fresh S basal containing the same antibiotic regimen, and 20 mg/L FUdR.
DR Lifespan Assays
All worms were passaged at 20°C for at least two ge nerations before lifespan assays were initiated. Synchronized populations of L1 animals were obtained by hypochlorite treatment, then allowed to develop at 20°C on standard NGM plates s eeded with OP50–1. After two days, the animals were transferred to standard RNAi plates described above, or to NGM agar plates containing 5 mg/L cholesterol, 50 mg/L ampicillin, 10 mg/L kanamycin, 1 mg/L tetracycline, 50mg/L nystatin, and 100 mg/L FUdR, and seeded with the cold- and antibiotic- treated OP50–1 described above. Three days later the animals were washed twice in S basal, and transferred to a 24 well plate containing 1 mL cold- and antibiotic- arrested OP50–1 at different concentrations in media containing the antibiotic cocktail in which the bacteria were growth-arrested. In this culture protocol the bacteria do not proliferate, but remain alive (Moroz et al., 2014). 10–20 worms were placed in each well. Unless otherwise specified, cultures were maintained without shaking (Petrascheck et al., 2007). All lifespan results shown in figure panels are representative of at least two independent experiments, with detailed information presented in Table S1.
NGM Plate Lifespan Assay
Lifespans were performed on NGM agar plates essentially as described (Robida-Stubbs et al., 2012), with feeding RNAi initiated at the L4/young adult stage for three days at 20°C on the standard RNAi plates described above. Solid DR was performed by dilution of the same food used in the liquid DR assay. Animals were washed twice in M9, and approximately 30 animals were transferred to these prepared plates, with 6 plates scored per strain. Animals that crawled off the plate, ruptured, or died from internal hatching were excluded.
RNA Sequencing (RNA-Seq)
For each RNAseq condition, three independent biological replicates that had been obtained in parallel were examined. For each sample, approximately 2,000 synchronized animals that were treated with DR or AL conditions in parallel for three-days (A6d) were collected, and frozen in liquid nitrogen. Total RNA was extracted using TRIzol (Sigma-Aldrich, St. Louis, MO), and purified with Direct-zol™ RNA Kits (Zymo Research, Irvine, CA). Purified RNA samples were DNase treated before sending for sequencing. Single-read 50 bp RNA sequencing with poly(A) enrichment was performed using a HiSeq 2000 (Illumina, San Diego, CA). FASTQ output files were aligned to C. elegans WBcel246 reference genome using SMALT (Wellcome Trust Sanger Institute). The proportion of reads successfully mapped to the genome was more than 90% for all samples. Quantification of mapped reads in the aligned SAM output files was performed using featureCounts (Liao et al., 2014). Transcripts that did not have at least 0.5 count per million reads in at least two samples were filtered out. Differential expression analysis was performed in R using the bioconductor package edgeR (Anders et al., 2013). A threshold of p < 0.05 was set to determine differentially expressed targets. Genes are induced (DR-upregulated) or suppressed (DR-downregulated) by DR when compared to AL condition in N2 animals, and induced (SEK-1-downregulated or sek-1(−) upregulated) or suppressed (SEK-1-upregulated or sek-1(−) downregulated) in sek-1(−) animals when compared to N2 animals under both AL and DR conditions. Functional annotations, biological process, and human ortholog matching were obtained from Wormbase build WS246. Protein functional classifications were based on the InterPro database and DAVID functional annotations (Dennis et al., 2003). InterPro categorizes sequences at superfamily, family and subfamily levels and predicts the occurrence of functional domains and important sites.
Quantitative RT-PCR (qRT-PCR)
For each treatment, approximately 200 synchronized animals were collected, and frozen in liquid nitrogen. Total RNA was extracted using TRIzol (Sigma-Aldrich, St. Louis, MO), and purified with Direct-zol™ RNA Kits (Zymo Research, Irvine, CA). First-strand cDNA was synthesized in duplicate from each sample (Invitogen SuperScript III). SYBR green was used to perform qRT-PCR (ABI 7900). At least two biological replicates were examined for each sample. Gene expression fold change was calculated using the ΔΔCt method.
ChIP-qPCR
Worms were maintained on standard OP50–1-seeded NGM plates, and mixed-stage animals were collected for ChIP assay as described previously (Kaletsky et al., 2016). Samples were fixed in 1.1% formaldehyde for 20 min at room temperature. For immunoprecipitation, 5 μg anti-GFP antibodies was added to 3 mg protein sample and incubated at 4°C overnight. 40 μL of Protein A Sepharose was added to each ChIP sample and incubated 4 hour at 4°C. DNA was purified using Qiagen PCR purification kit and eluted in 50 μl Qiagen Elution buffer. SYBR green was used to perform qPCR. Fold change was calculated using the ΔΔCt method.
Transgenic Strain Construction
The qdEx16[atf-7::GFP] construct was generously provided by Dennis Kim (Shivers et al., 2010). Transgenic lines were made by microparticle bombardment as described (Isik and Berezikov, 2013), using unc-119(ed3) animals that were outcrossed from the EG6699 strain. Transformants were screened for stable integration. Appropriate atf-7::GFP expression patterns were confirmed in integrants, which were backcrossed five times to N2 prior to analysis. Extrachromosomal arrays were generated by injecting transgene DNA (25 ng/mL) along with the rol-6 marker (pRF4) at 50 ng/mL into the gonads of young adult N2 animals.
Fluorescence Microscopy and Image Analysis
Animals were randomly selected and imaged with ZEN 2012 software on an Axio Imager M2 microscope with a 10X/0.25 objective (Zeiss, Jena, Germany). Fluorescence brightness was quantified blindly using NIH ImageJ.
Western Blotting
Approximately 1000 animals were sonicated in Laemmli buffer. Protein samples were boiled 95°C for 10 min. Western blot analysis was performe d under standard conditions with antibodies against phospho-p38 (Cell Signaling) or total PMK1 antibodies (Inoue et al., 2005).
Pathogenesis Assays
Pathogenesis assays with P. aeruginosa PA14 were performed as described previously (Shivers et al., 2010). In brief, single colonies of PA14 were inoculated in 3 ml cultures of LB, then cultured overnight at 37°C. Five microliters of the overnigh t culture were seeded to the center of a 35-mm NGM agar plate containing 20 mg/L FUdR. Seeded plates were incubated at 37°C overnight, then incubated at room temperature overnight. Approximately 40 animals were transferred to these prepared plates, with 5 plates scored per strain. The assays were conducted at 20°C. Animals that did not respond to gentle prodding from a platinum wire were scored as dead.
OP50 UV Killing
UV killing was performed by using a Stratagene UV Stratalinker 2400 as described previously (Steinkraus et al., 2008). In brief, OP50 were UV-killed by exposing the surface of the plates twice to the maximal energy setting of a Stratagene UV Stratalinker 2400 (La Jolla, CA, USA).
OP50 Supernatant Preparation
The cold- and antibiotic- treated OP50–1 described above were spun down at 3,900 × g for 10 min, and then the supernatant was filtered further using a sterile syringe filter with a 0.22 μm pore size hydrophilic Polyethersulfone (PES) membrane.
Axenic Liquid Medium Preparation
We developed a simplified protocol for preparing a C. elegans nutrient-rich liquid medium (CeNRM) for propagating animals without bacteria. This method is a variation on previously-published mCeHR (modified C. elegans Habitation and Reproduction media) that are composed of specific nutrients and sterilized milk powder (Rao et al., 2005; Samuel et al., 2014; Scanlan et al., 2018). Briefly, using sterile technique to prepare 100 mL CeNRM, the following components were added to 46 mL 1× SB solution: 20 mL autoclaved (121°C, 5 min) 100 g/L skim milk powder in 1× SB solution, 2 mL RPMI 1640 amino acids solution, 10 mL L-glutamine solution, 20 mL RPMI 1640 vitamins solution, 100 μL trace metal mix, 100 μL 1 M adenosine, 100 μL 1 M guanosine, 400 μL 2 mM hemin in 0.1 M NaOH, 100 μL 50 g/L ampicillin, 100 μL 10 g/L kanamycin, 100 μL 1 g/L tetracycline, 1 mL nystatin solution. To culture worms in CeNRM, 20–40 hypochlorite-bleached and synchronized L1 animals were placed in each well of a 24-well plate containing 1 mL freshly prepared media.
When fed CeNRM or the previously published mCeHR media, animals developed more slowly than on bacterial food but were all able to develop into adults (Rao et al., 2005; Samuel et al., 2014; Scanlan et al., 2018). For the strains we analyzed (wild type N2, sek-1, pmk-1, atf-7, daf-16 and daf-2 mutants), four days were typically required to reach the L4 stage, with 5 days needed to initiate egg laying. By contrast, for each strain we analyzed, if milk powder was omitted from CeNRM no animals completed larval development. When fed CeNRM from hatching, wild type N2 were all gravid and produced 54 ± 10 progeny per animal (10 worms analyzed), compared to the 341 ± 51 progeny we typically see produced with feeding on OP50 food on NGM plates. In lifespan experiments 20 mg/L FUdR was added at the L4 stage to prevent hatching.
For analyses of individual CeNRM components, these were added to S basal medium without OP50 (DD, dietary deprivation) at the concentrations indicated above. Where indicated glucose was added at 20 g/L.
Food Intake Assay
This assay measures food consumption directly, by quantifying the relative amount of growth-arrested bacteria that are present in a culture before and after incubation with C. elegans. Animals were washed twice in S basal, and transferred to a 24 well plate containing 1 ml cold- and antibiotic- arrested OP50–1 at OD 3 (600 nm), a treatment identical to the AL condition for lifespan assays, with 30–50 worms placed in each well. After the indicated number of days, bacteria were removed from the wells by washing, and diluted 10× with S basal prior to measuring the OD600. For each experimental data point at least 6 wells were measured (at least 200 worms in total), with the results shown being representative of at least three independent assays. The relative food intake was determined by the change in OD for each well, normalized to the number of worms. Under these conditions ample OP50 were available for feeding throughout the analysis, and similar results were obtained whether animals were maintained in the same wells for the entire experiment, or introduced into a new quantified bacterial sample at the start of each assay period.
Pumping
Worms were cultured at NGM agar plates with growth-arrested bacteria. Pharyngeal pumping was assessed by observing the number of pharyngeal contractions under a Zeiss Stereo Discovery.V12 microscope at 100× magnification. To reduce the effect of irregular rhythm of pumping, we recorded the pumping of each worm during a 3-minute interval. As the young adult worm pumping rate is over 300/min (Huang et al., 2004) counting was performed by tapping of a smartphone screen, which allowed faster counting than is feasible with a common laboratory counter. A total of 20 healthy worms for each strain were examined blindly.
Defecation
Defecation behavior was assessed by observing the anal depressor and the intestinal muscle contractions (Thomas, 1990) under the same conditions as the pumping assay. Under these conditions the two major motor steps of a defecation cycle, contraction of a set of posterior body muscles (pBoc) and contraction of a set of anterior body muscles (aBoc), are typically very clear and can be scored readily in every cycle. Two cycles were recorded for each worm and a total of 10 worms were examined blindly for each strain.
Quiescence
Quiescence is defined as a state of complete cessation of both pumping and moving (McCloskey et al., 2017; You et al., 2008). Prior to assay, worms were maintained under the same conditions as in the above experiments. At 20°C the majority o f wild type N2 adult worms were not found to be quiescent, but at any given time a high proportion of daf-2 mutants were in quiescence beginning around day 3 of adulthood. These animals were detected as quiescent upon initial transfer of their plate from incubator to a microscope, but within a few minutes typically ‘woke up’ and reinitiated pumping and moving (mov. S4). Worms could be stimulated to wake up by light, heat from the microscope stage, or shaking. To minimize the disruption of the animals, we turned the microscope light down to as low a setting as would allow us to observe pumping clearly, and we conducted our observations and counting within two minutes of placement on the microscope. For each mutant, four plates and more than 100 worms were examined, and scored for whether the animals were in quiescence when first placed on the microscope stage.
Intestinal Lumen Labelling
To assess intestinal lumen morphology and determine the extent to which the lumen was filled with bacteria, we firstly fed worms with live OP50-GFP E. coli on a standard NGM plate to assess the extent of bacterial accumulation in the intestine. To determine the size of the intestinal lumen, the worms were stained with a fluorescent fluid-phase marker, Texas Red-dextran (40,000 MW, Thermo Fisher Scientific) at 1 mg/L in M9 for 1 hour. The OP50-GFP E. coli was obtained from CGC and the dextran stain assay was performed as described previously (Saegusa et al., 2014).
QUANTIFICATION AND STATISTICAL ANALYSIS
All data are presented as mean ± standard error of mean (SEM). The P values (log-rank test) for lifespan comparison, mean and median lifespan were determined using Kaplan-Meier survival curves in JMP software (version JMP Pro 12). The analysis of variance for each set of biological replicates was carried out with the SAS statistical program (version SAS 9.00), and significance was determined by Duncan’s multiple range test (more than two treatments) or Student t-test procedure (two treatments) at * P < 0.05, ** P < 0.01, and *** P < 0.0001. These statistical methods were chosen because they are standard in the field and are typically used for the assays we performed. Statistical details of experiments can be found in the figure legends. No methods were used to determine whether the data met assumptions of the statistical approach.
Supplementary Material
Movie S1. A feeding wild type (N2) worm at adulthood day 9, Related to Figures 5
Movie S2. A feeding daf-16(mgDf50);daf-2(e1370) worm at adulthood day 9, Related to Figures 5
Movie S3. A feeding daf-2(e1370) worm at adulthood day 9, Related to Figures 5
Movie S4. A quiescent daf-2(e1370) worm at adulthood day 9. Note that the increased light stimulates exit from quiescence, Related to Figures 5
Highlights.
Dietary restriction longevity requires modulation of nutrient-regulated immunity
Nutrients activate the p38-ATF-7 immunometabolic pathway independently of mTORC1
Insulin/IGF-1 signaling affects immunity and aging in part by curtailing food intake
DAF-16/FOXO lowers food consumption, linking feeding and immunity to growth signals
ACKNOWLEDGEMENTS
We thank Leonard Guarente, Dennis Kim, and Kunihiro Matsumoto for reagents, Nick Bishop, Leonard Guarente, and Michael Petrascheck for sharing unpublished results, and Rachel Beltzhoover and Blackwell lab members for helpful discussions or comments on the manuscript. The work was funded by support to TKB from the NIH (R35 GM122610 and R01 AG054215) and NIDDK DRC and T32 grants to the Joslin Diabetes Center (P30 DK036836 and T32 DK007260). Some strains were provided by the CGC, which is funded by the NIH (P40 OD010440).
Footnotes
DECLARATION OF INTERESTS
We declare that no competing interests exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alic N, Tullet JM, Niccoli T, Broughton S, Hoddinott MP, Slack C, Gems D, and Partridge L (2014). Cell-nonautonomous effects of dFOXO/DAF-16 in aging. Cell Rep 6, 608–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S, McCarthy DJ, Chen Y, Okoniewski M, Smyth GK, Huber W, and Robinson MD (2013). Count-based differential expression analysis of RNA sequencing data using R and Bioconductor. Nat Protoc 8, 1765–1786. [DOI] [PubMed] [Google Scholar]
- Andrusiak MG, and Jin Y (2016). Context specificity of stress-activated mitogen-activated protein (MAP) kinase signaling: the story as told by Caenorhabditis elegans. J Biol Chem 291, 7796–7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur JS, and Ley SC (2013). Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol 13, 679–692. [DOI] [PubMed] [Google Scholar]
- Avery L, and You Y.j. (2012). C. elegans feeding In WormBook. Jorgensen EM, ed. (http://www.wormbook.org/ The C. elegans Research Community; ). [Google Scholar]
- Blackwell TK, Steinbaugh MJ, Hourihan JM, Ewald CY, and Isik M (2015). SKN-1/Nrf, stress responses, and aging in Caenorhabditis elegans. Free Radical Bio Med 88, 290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikka MR, Anbalagan C, Dvorak K, Dombeck K, and Prahlad V (2016). The Mitochondria-regulated immune pathway activated in the C. elegans intestine is neuroprotective. Cell Rep 16, 2399–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ A, Günther P, Lauterbach MA, Duewell P, Biswas D, Pelka K, Scholz CJ, Oosting M, Haendler K, and Baßler K (2018). Western diet triggers NLRP3-dependent innate immune reprogramming. Cell 172, 162–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandona P, Aljada A, and Bandyopadhyay A (2004). Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol 25, 4–7. [DOI] [PubMed] [Google Scholar]
- Dennis G Jr., Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, and Lempicki RA (2003). DAVID: database for annotation, visualization, and integrated discovery. Genome Biol 4, R60. [PubMed] [Google Scholar]
- Dillon J, Holden-Dye L, O’Connor V, and Hopper NA (2016). Context-dependent regulation of feeding behaviour by the insulin receptor, DAF-2, in Caenorhabditis elegans. Invertebr Neurosci 16, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E L, Zhou T, Koh S, Chuang M, Sharma R, Pujol N, Chisholm AD, Eroglu C, Matsunami H, and Yan D (2018). An antimicrobial peptide and its neuronal receptor regulate dendrite degeneration in aging and infection. Neuron 97, 125–138.e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans EA, Chen WC, and Tan MW (2008). The DAF‐2 insulin‐like signaling pathway independently regulates aging and immunity in C. elegans. Aging cell 7, 879–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald CY, Castillo-Quan JI, and Blackwell TK (2018). Untangling longevity, dauer, and healthspan in Caenorhabditis elegans insulin/IGF-1-signalling. Gerontology 64, 96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, and Partridge L (2015). Promoting health and longevity through diet: from model organisms to humans. Cell 161, 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garsin DA, Villanueva JM, Begun J, Kim DH, Sifri CD, Calderwood SB, Ruvkun G, and Ausubel FM (2003). Long-lived C. elegans daf-2 mutants are resistant to bacterial pathogens. Science 300, 1921. [DOI] [PubMed] [Google Scholar]
- Gems D, Sutton AJ, Sundermeyer ML, Albert PS, King KV, Edgley ML, Larsen PL, and Riddle DL (1998). Two pleiotropic classes of daf-2 mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans. Genetics 150, 129–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Amaro RL, Valentine ER, Carretero M, LeBoeuf SE, Rangaraju S, Broaddus CD, Solis GM, Williamson JR, and Petrascheck M (2015). Measuring food intake and nutrient absorption in Caenorhabditis elegans. Genetics 200, 443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, and Brunet A (2009). Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell 8, 113–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS (2017). Inflammation, metaflammation and immunometabolic disorders. Nature 542, 177–185. [DOI] [PubMed] [Google Scholar]
- Hou L, Wang D, Chen D, Liu Y, Zhang Y, Cheng H, Xu C, Sun N, McDermott J, Mair WB, and Han JD (2016). A systems approach to reverse engineer lifespan extension by dietary restriction. Cell Metab 23, 529–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu AL, Murphy CT, and Kenyon C (2003). Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science 300, 1142–1145. [DOI] [PubMed] [Google Scholar]
- Huang C, Xiong C, and Kornfeld K (2004). Measurements of age-related changes of physiological processes that predict lifespan of Caenorhabditis elegans. P Natl Acad Sci USA 101, 8084–8089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Hisamoto N, An JH, Oliveira RP, Nishida E, Blackwell TK, and Matsumoto K (2005). The C. elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN-1 in oxidative stress response. Genes Dev 19, 2278–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irazoqui JE, Urbach JM, and Ausubel FM (2010). Evolution of host innate defence: insights from Caenorhabditis elegans and primitive invertebrates. Nat Rev Immunol 10, 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isik M, and Berezikov E (2013). Biolistic transformation of Caenorhabditis elegans. Methods Mol Biol 940, 77–86. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Rabinovitch PS, and Kaeberlein M (2013). mTOR is a key modulator of ageing and age-related disease. Nature 493, 338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaletsky R, Lakhina V, Arey R, Williams A, Landis J, Ashraf J, and Murphy CT (2016). The C. elegans adult neuronal IIS/FOXO transcriptome reveals adult phenotype regulators. Nature 529, 92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapahi P, Kaeberlein M, and Hansen M (2017). Dietary restriction and lifespan: lessons from invertebrate models. Ageing Res Rev 39, 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, and Tabtiang R (1993). A C. elegans mutant that lives twice as long as wild type. Nature 366, 461–464. [DOI] [PubMed] [Google Scholar]
- Kenyon CJ (2010). The genetics of ageing. Nature 464, 504–512. [DOI] [PubMed] [Google Scholar]
- Kim DH, Feinbaum R, Alloing G, Emerson FE, Garsin DA, Inoue H, Tanaka-Hino M, Hisamoto N, Matsumoto K, Tan MW, and Ausubel FM (2002). A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science 297, 623–626. [DOI] [PubMed] [Google Scholar]
- Kim DH, Liberati NT, Mizuno T, Inoue H, Hisamoto N, Matsumoto K, and Ausubel FM (2004). Integration of Caenorhabditis elegans MAPK pathways mediating immunity and stress resistance by MEK-1 MAPK kinase and VHP-1 MAPK phosphatase. Proc Natl Acad Sci USA 101, 10990–10994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirienko NV, Ausubel FM, and Ruvkun G (2015). Mitophagy confers resistance to siderophore-mediated killing by Pseudomonas aeruginosa. Proc Natl Acad Sci USA 112, 1821–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotas ME, and Medzhitov R (2015). Homeostasis, inflammation, and disease susceptibility. Cell 160, 816–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapierre LR, Silvestrini MJ, Nuñez L, Ames K, Wong S, Le TT, Hansen M, and Meléndez A (2013). Autophagy genes are required for normal lipid levels in C. elegans. Autophagy 9, 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux GA, and Ashrafi K (2016). Investigating connections between metabolism, longevity, and behavior in Caenorhabditis elegans. Trends Endocrin Met 27, 586–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Smyth GK, and Shi W (2014). featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930. [DOI] [PubMed] [Google Scholar]
- Libina N, Berman JR, and Kenyon C (2003). Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell 115, 489–502. [DOI] [PubMed] [Google Scholar]
- McCloskey RJ, Fouad AD, Churgin MA, and Fang-Yen C (2017). Food responsiveness regulates episodic behavioral states in Caenorhabditis elegans. J Neurophysiol 117, 1911–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meydani SN, Das SK, Pieper CF, Lewis MR, Klein S, Dixit VD, Gupta AK, Villareal DT, Bhapkar M, and Huang M (2016). Long-term moderate calorie restriction inhibits inflammation without impairing cell-mediated immunity: a randomized controlled trial in non-obese humans. Aging (Albany NY) 8, 1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroz N, Carmona JJ, Anderson E, Hart AC, Sinclair DA, and Blackwell TK (2014). Dietary restriction involves NAD(+) -dependent mechanisms and a shift toward oxidative metabolism. Aging Cell 13, 1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, and Kenyon C (2003). Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 424, 277–283. [DOI] [PubMed] [Google Scholar]
- Newton K, and Dixit VM (2012). Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol 4, a006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang S, and Curran SP (2014). Adaptive capacity to bacterial diet modulates aging in C. elegans. Cell Metab 19, 221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino MW, Nargund AM, Kirienko NV, Gillis R, Fiorese CJ, and Haynes CM (2014). Mitochondrial UPR-regulated innate immunity provides resistance to pathogen infection. Nature 516, 414–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrascheck M, Ye X, and Buck LB (2007). An antidepressant that extends lifespan in adult Caenorhabditis elegans. Nature 450, 553–556. [DOI] [PubMed] [Google Scholar]
- Pletcher SD, Macdonald SJ, Marguerie R, Certa U, Stearns SC, Goldstein DB, and Partridge L (2002). Genome-wide transcript profiles in aging and calorically restricted Drosophila melanogaster. Curr Biol 12, 712–723. [DOI] [PubMed] [Google Scholar]
- Podshivalova K, Kerr RA, and Kenyon C (2017). How a mutation that slows aging can also disproportionately extend End-of-Life Decrepitude. Cell Rep 19, 441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao AU, Carta LK, Lesuisse E, and Hamza I (2005). Lack of heme synthesis in a free-living eukaryote. Proc Natl Acad Sci USA 102, 4270–4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rera M, Clark RI, and Walker DW (2012). Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc Natl Acad Sci USA 109, 21528–21533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robida-Stubbs S, Glover-Cutter K, Lamming DW, Mizunuma M, Narasimhan SD, Neumann-Haefelin E, Sabatini DM, and Blackwell TK (2012). TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab 15, 713–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saegusa K, Sato M, Sato K, Nakajima-Shimada J, Harada A, and Sato K (2014). Caenorhabditis elegans chaperonin CCT/TRiC is required for actin and tubulin biogenesis and microvillus formation in intestinal epithelial cells. Molecular biology of the cell 25, 3095–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel TK, Sinclair JW, Pinter KL, and Hamza I (2014). Culturing Caenorhabditis elegans in axenic liquid media and creation of transgenic worms by microparticle bombardment. JoVE 90, 51796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton RA, and Sabatini DM (2017). mTOR signaling in growth, metabolism, and disease. Cell 168, 960–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlan LD, Lund SP, Coskun SH, Hanna SK, Johnson ME, Sims CM, Brignoni K, Lapasset P, Petersen EJ, Elliott JT, and Nelson BC (2018). Counting Caenorhabditis elegans: protocol optimization and applications for population growth and toxicity studies in liquid medium. Sci Rep 8, 904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulenburg H, Hoeppner MP, Weiner J, and Bornberg-Bauer E (2008). Specificity of the innate immune system and diversity of C-type lectin domain (CTLD) proteins in the nematode Caenorhabditis elegans. Immunobiology 213, 237–250. [DOI] [PubMed] [Google Scholar]
- Seah NE, de Magalhaes Filho CD, Petrashen AP, Henderson HR, Laguer J, Gonzalez J, Dillin A, Hansen M, and Lapierre LR (2016). Autophagy-mediated longevity is modulated by lipoprotein biogenesis. Autophagy 12, 261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw AC, Goldstein DR, and Montgomery RR (2013). Age-dependent dysregulation of innate immunity. Nat Rev Immunol 13, 875–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivers RP, Kooistra T, Chu SW, Pagano DJ, and Kim DH (2009). Tissue-specific activities of an immune signaling module regulate physiological responses to pathogenic and nutritional bacteria in C. elegans. Cell Host Microbe 6, 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivers RP, Pagano DJ, Kooistra T, Richardson CE, Reddy KC, Whitney JK, Kamanzi O, Matsumoto K, Hisamoto N, and Kim DH (2010). Phosphorylation of the conserved transcription factor ATF-7 by PMK-1 p38 MAPK regulates innate immunity in Caenorhabditis elegans. PLoS Genet 6, e1000892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore DE, and Ruvkun G (2013). A cytoprotective perspective on longevity regulation. Trends cell biol 23, 409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skora S, Mende F, and Zimmer M (2018). Energy Scarcity Promotes a Brain-wide Sleep State Modulated by Insulin Signaling in C. elegans. Cell Rep 22, 953–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinkraus KA, Smith ED, Davis C, Carr D, Pendergrass WR, Sutphin GL, Kennedy BK, and Kaeberlein M (2008). Dietary restriction suppresses proteotoxicity and enhances longevity by an hsf-1‐dependent mechanism in Caenorhabditis elegans. Aging cell 7, 394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A, and White MF (2008). Insulin-like signaling, nutrient homeostasis, and life span. Annu Rev Physiol 70, 191–212. [DOI] [PubMed] [Google Scholar]
- Tepper RG, Ashraf J, Kaletsky R, Kleemann G, Murphy CT, and Bussemaker HJ (2013). PQM-1 complements DAF-16 as a key transcriptional regulator of DAF-2-mediated development and longevity. Cell 154, 676–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JH (1990). Genetic analysis of defecation in Caenorhabditis elegans. Genetics 124, 855–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troemel ER, Chu SW, Reinke V, Lee SS, Ausubel FM, and Kim DH (2006). p38 MAPK regulates expression of immune response genes and contributes to longevity in C. elegans. PLoS Genet 2, e183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg SE, Sena LA, and Chandel NS (2015). Mitochondria in the regulation of innate and adaptive immunity. Immunity 42, 406–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J-A, Liu X-J, Yuan J, Jiang J, and Cai S-Q (2014). Longevity manipulations differentially affect serotonin/dopamine level and behavioral deterioration in aging Caenorhabditis elegans. J Neurosci 34, 3947–3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Y. j., Kim J, Raizen DM, and Avery L (2008). Insulin, cGMP, and TGF-β signals regulate food intake and quiescence in C. elegans: a model for satiety. Cell Metab 7, 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngman MJ, Rogers ZN, and Kim DH (2011). A decline in p38 MAPK signaling underlies immunosenescence in Caenorhabditis elegans. PLoS Genet 7, e1002082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunger E, Safra M, Levi-Ferber M, Haviv-Chesner A, and Henis-Korenblit S (2017). Innate immunity mediated longevity and longevity induced by germ cell removal converge on the C-type lectin domain protein IRG-7. PLoS Genet 13, e1006577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarse K, Schmeisser S, Groth M, Priebe S, Beuster G, Kuhlow D, Guthke R, Platzer M, Kahn CR, and Ristow M (2012). Impaired insulin/IGF1 signaling extends life span by promoting mitochondrial L-proline catabolism to induce a transient ROS signal. Cell Metab 15, 451–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Judy M, Lee S-J, and Kenyon C (2013). Direct and indirect gene regulation by a life-extending FOXO protein in C. elegans: roles for GATA factors and lipid gene regulators. Cell Metab 17, 85–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie S1. A feeding wild type (N2) worm at adulthood day 9, Related to Figures 5
Movie S2. A feeding daf-16(mgDf50);daf-2(e1370) worm at adulthood day 9, Related to Figures 5
Movie S3. A feeding daf-2(e1370) worm at adulthood day 9, Related to Figures 5
Movie S4. A quiescent daf-2(e1370) worm at adulthood day 9. Note that the increased light stimulates exit from quiescence, Related to Figures 5