Abstract
Objectives:
We hypothesized an association between chronic stress (as indexed by resting amygdalar activity [AmygA]), hematopoietic system activity (HMPA) and subclinical CV indices (aortic vascular inflammation [VI] and non-calcified coronary plaque burden [NCB]) in PSO. Moreover, we hypothesized that treatment of PSO would improve these parameters.
Background:
Psoriasis (PSO) is a stress-related, chronic inflammatory condition, which associates with increased prevalence of subclinical cardiovascular disease (CVD). In individuals without PSO, stress links to CVD through a serial biological pathway that involves the amygdala, hematopoietic tissues, and atherosclerotic plaques.
Methods:
164 consecutive PSO patients and 47 healthy volunteers (HV) underwent 18FDG-PET/CT scans for assessment of AmygA, HMPA, and VI, as well as CCTA scans for quantifying NCB. Furthermore, a consecutive subset of 30 severe PSO patients [Psoriasis area severity index score (PASI)>10] were followed at one-year to assess the relationship between skin disease improvement and AmygA, HMPA, VI, and NCB.
Results:
The PSO cohort was middle-aged (mean age=50), low CV risk (FRS median=3) and mild-moderate PSO activity (median PASI score=5.6). AmygA was higher in PSO compared to HV participants. AmygA associated with HMPA (bone marrow activity β=0.20, p=0.01) and subclinical CVD (VI β=0.31, p<0.001; NCB β=0.27, p<0.001) The AmygA-CVD association was in part mediated by HMPA (VI=20.9%, NCB=36.7%). Following one-year of psoriasis treatment in those with severe disease, improvement in skin disease was accompanied with reduction in AmygA, bone marrow activity, and VI, with no progression of NCB.
Conclusions:
In PSO, a chronic inflammatory disease state, AmygA, a manifestation of chronic stress, substantially contributes to the risk of subclinical CVD. Additional studies employing psychometric measures of stress are indicated.
Keywords: Stress, amygdala, atherosclerosis, inflammation, psoriasis
INTRODUCTION
In recent years, both psychological stress and depression have been increasingly recognized as independent risk factors for cardiovascular disease (1). Moreover, psychological stress is associated with multiple traditional cardiovascular risk factors, including hypertension(2) and smoking(3), as well with as clinical cardiovascular disease such as myocardial infarction(4) and stroke(5). Additionally, depression has been shown to associate with the development of coronary artery disease longitudinally in otherwise healthy populations(6). Psoriasis, a chronic inflammatory skin disease that affects 2-3% of the US adults, has been shown to associate with increased risk for myocardial infarction, major adverse cardiovascular events, and higher rates of depression (7-9). Finally, a recent study demonstrated that patients with self-reported depression and anxiety in psoriasis had higher rates of subclinical cardiovascular disease (10). However, the biological basis of the relationship between psychological stress and cardiovascular disease in humans remains poorly characterized.
The amygdala is a key component of the salience network (11) that regulates physiologic and behavioral changes in response to stress and fear (12). Resting metabolic activity in the amygdala can be reproducibly assessed using 18-fluorodeoxyglucose positron emission tomography computed tomographic (18FDG PET/CT) imaging (13). 18FDG PET/CT measures of stress-related neural activity associate with anxious temperament and are upregulated in chronic stress(14,15), anxiety disorders(16), and depression (17,18). Recently, amygdalar activity (AmygA) has been shown to strongly associate with both aortic vascular inflammation and future risk of major cardiovascular events (13). This association was in part mediated by up-regulated hematopoietic system activity, which can also be assessed by 18FDG PET/CT (13). It remains unknown whether stress-related neural activity or increased hematopoietic system activity associate with coronary plaque structural features, such as non-calcified plaque burden (NCB).
Coronary computed tomography angiography (CCTA) has been used as a reliable non-invasive imaging tool to assess coronary artery disease by quantifying coronary plaque burden. Plaque characteristics by CCTA, notably NCB, associate with the risk of cardiovascular events (19). Thus, to characterize whether coronary plaque burden relates to resting amygdalar activity, hematopoietic system activity, and vascular inflammation, all assessed by FDG uptake, we performed an observational, cross-sectional study with a one-year follow-up in patients with psoriasis, along with a healthy comparator group without psoriasis. Additionally, we followed a subset of patients with coronary phenotyping longitudinally over one-year study period. We hypothesized that AmygA would be higher in psoriasis than in normal subjects, and that it would be associated with not only aortic vascular inflammation, but also NCB beyond traditional risk factors for cardiovascular disease, in part mediated by hematopoietic system activity. Finally, we sought to explore the effect of reducing inflammation, as indexed by improvement in psoriasis severity, on AmygA, hematopoietic system activity, aortic vascular inflammation and NCB.
METHODS
Study design and population
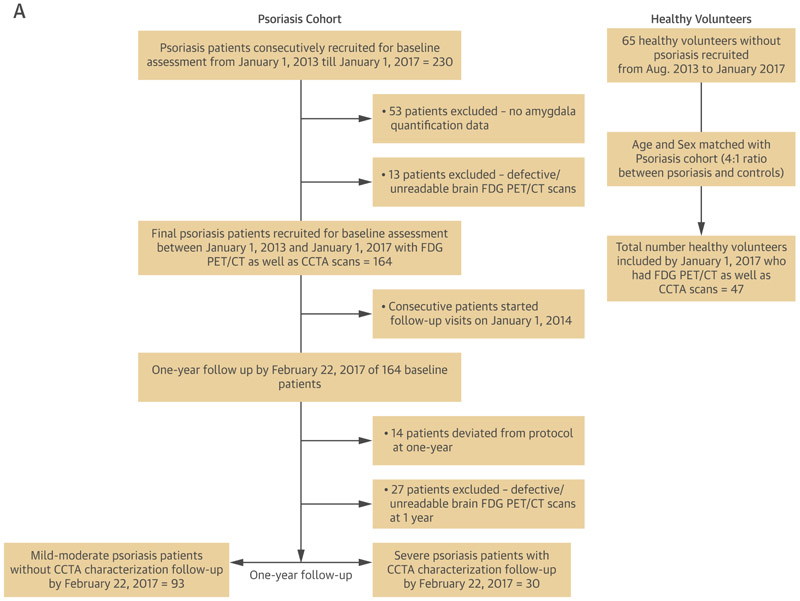
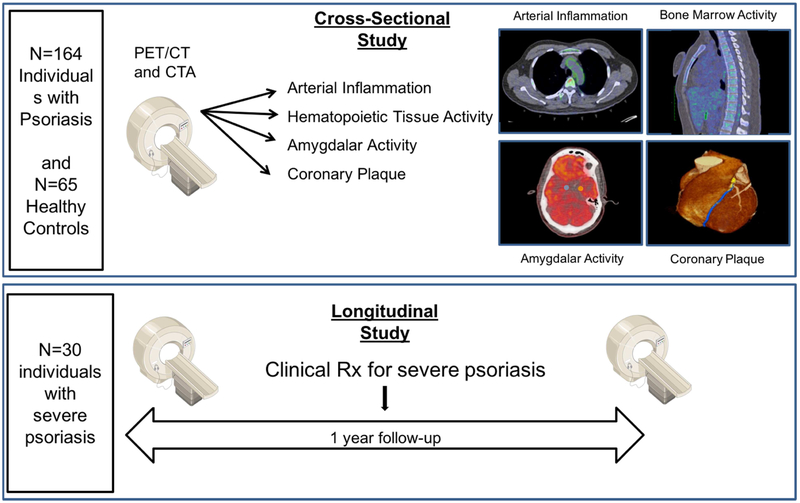
A total of 164 consecutive psoriasis patients as well as 47 healthy volunteers were recruited as part of an ongoing case-cohort study between January 1, 2013 and January 1, 2017 at the National Institutes of Health (NIH) Clinical Center: Psoriasis, Atherosclerosis and Cardiometabolic Disease Initiative (PACI: NCT01778569). In addition, a subset of severe psoriasis patients (Psoriasis area severity index score [PASI] >10) (N=30) was followed-up after 1 year of treatment to examine the effect of treatment on skin disease, AmygA, hematopoietic system activity, aortic vascular inflammation and NCB (Figure 1). The remainder of the cohort was also followed up, although without NCB characterization at one-year. Study protocols were approved by the institutional review board at the NIH. Research was conducted in accordance with the Declaration of Helsinki. For detailed inclusion/exclusion criteria as well as clinical and laboratory measurement methods, refer to Supplemental Appendix.
Figure 1. CONSORT and Flow Diagram showing recruitment scheme.
A) Recruitment scheme for patients at the National Institutes of Health. 18FDG PET/CT: 18Fluorodeoxyglucose positron emission-computed tomography. B) Study design outlining the imaging modalities used to measure outcomes of interest at cross-section and over time.
Outcomes
The primary outcome was aortic vascular inflammation measured as target-to-background ratio using 18FDG PET/CT quantification (20,21). Secondary outcomes in the study were hematopoietic system activity in the bone marrow and the spleen measured as standardized uptake values using 18FDG PET/CT quantification (13) and non-calcified coronary plaque burden quantified using CCTA (22,23). Strengthening the reporting of observational studies in epidemiology (STROBE) guidelines were followed for reporting the findings (24).
18-Fluorodeoxyglucose Positron Emission Tomography Computed Tomographic Imaging
Acquisition
Following overnight fast for at least 8 hours, PET/CT images were acquired approximately 60 minutes (mean 62minutes ± 1) (20,21) after administration of 10mCi 18-FDG.PET imaging occurred using a Siemens Biograph mCT PET/CT 64-slice scanner (Siemens Medical Solutions USA, Malvern, PA, USA), acquiring 1.5 mm axial slices of the aorta. Standard bed positions of three minutes each, scanning cranially to caudally were obtained for each patient from the vertex of the skull to the toes.
Measurement of amygdalar activity
The amygdalae are a part of the limbic system located bilaterally, dorso-medially in the temporal lobe, forming the ventral, superior and medial walls of the inferior horn of the lateral ventricle (13). After identification of amygdalar anatomical location using the above landmarks, a single reader placed right and left 3D-volume regions of interest (ROIs) with a fixed volume (0.5 cm3) in the desired area and measured 18FDG uptake as standardized uptake value (SUV) using a dedicated software (OsiriX MD, Geneva, Switzerland) by previously described methods (13). Amygdalar activity (AmygA) was calculated by dividing the maximum SUVs in each amygdala by the mean SUVs in ipsilateral temporal lobes for correction of amygdala SUV values. The highest AmygA between the two amygdalae was taken as the primary measure of amygdalar activity (13).
Measurement of aortic vascular inflammation
Aortic vascular inflammation was quantified using previously published methods (20,21). Regions of interest were placed on 1.5mm thick axial slices of the aorta from the aortic root through the bifurcation into iliac arteries. Regions of interest were also placed on 10 continuous slices of the superior vena cava to calculate and correct for background venous activity. Mean and maximum SUVs were generated using a dedicated PET/CT image analysis program Extended Brilliance Workspace (Philips electronics, Andover, MA, USA). Target-to-background ratio (TBR) was calculated by dividing the maximum SUV from each slice by the average of mean SUVs in the lumen of superior vena cava. A single aortic TBR value per patient was generated by averaging all individual TBR values.
Measurement of hematopoietic system activity
3D-volume ROIs were placed within individual vertebrae (T1 to L5) and the spleen to measure bone marrow activity and spleen activity. Total bone marrow activity was reported as the average of maximum SUVs of the individual vertebrae. Spleen activity was reported as the maximum SUV of the ROI in spleen for each individual.
Coronary Computed Tomography Angiography
Acquisition
All participants underwent CCTA using an Aquilion ONE ViSION 320-detector row CT scanner (Toshiba, Japan). Scans were performed with retrospective gating at 120 kV, tube current of 750-850 mA and a gantry rotation time of ≤420ms in accordance with NIH Radiation Exposure Committee. Image acquisition characteristics included slice thickness of 0.5mm and pitch of 0.2-0.4 (23).
Measurement of coronary plaque burden
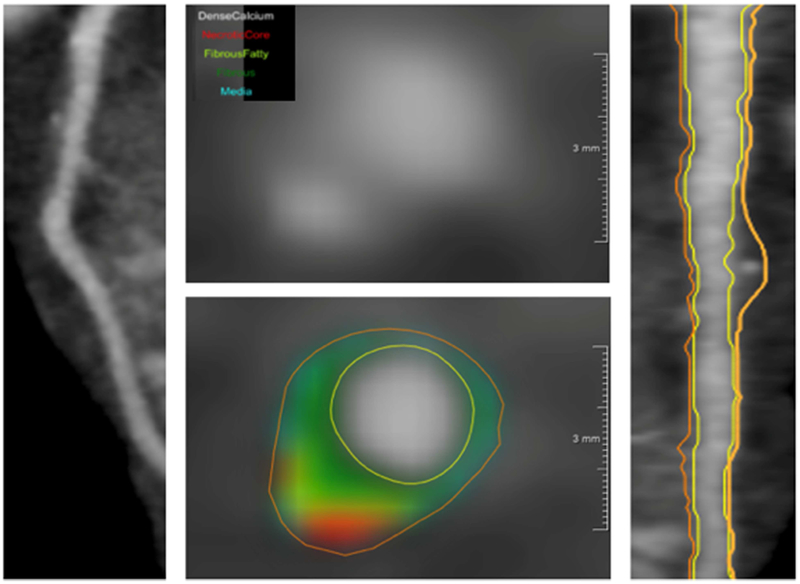
Coronary plaque characteristics were quantified across all three main coronary arteries using dedicated software QAngio CT (Medis, The Netherlands) (22,23). Automated longitudinal contouring of the inner lumen and outer wall was performed, and results were manually adjusted when clear deviations were present (Figure 2). Total burden and non-calcified burden were calculated by dividing total vessel plaque volume by total vessel length (22,23,25). Lumen attenuation was adaptively corrected on an individual-scan basis using gradient filters and intensity values within the coronary artery.
Figure 2. Multiplanar coronary CT angiography derived reconstructed views of left anterior descending coronary artery in a patient with severe psoriasis.
CCTA reconstruction from severe psoriasis patient showing the left anterior descending coronary artery (left), planar reconstruction (right) depicting non-calcified plaque burden of the coronary artery, and transverse section (middle) revealing low-attenuation lipid-rich high-risk plaque (green and red).
Statistical analysis
Data were reported as mean with standard deviation for parametric variables, median with interquartile range for non-parametric variables and percentages for categorical variables. In baseline analyses, parametric and non-parametric variables were compared between the two groups using student t-test and Mann-Whitney U test respectively. In longitudinal analyses, parametric variables were analyzed using paired-t test and non-parametric variables using Wilcoxon signed-rank test. Dichotomous variables were analyzed using Pearson’s chi-square test at baseline and follow-up. All variables undergo testing for normality using skewness, kurtosis, and histogram plots. We performed Spearman correlations for non-parametric variables including waist-to-hip ratio, C-reactive protein, Framingham risk score, homeostasis model assessment of insulin resistance and psoriasis area severity index score. Otherwise, we utilized Pearson correlation testing. In multivariable regression analyses, non-normal variables were included as covariates but if used as an outcome were transformed for normal distribution. Furthermore, the residuals were found to be normally distributed, and homoscedasticity analyses of residuals showed constant residual variance. Additionally, we performed mediation analyses using structural equation modeling and likelihood ratio tests in our nested adjusted model to calculate the association and incremental value of hematopoietic system activity on the total effect of AmygA on aortic vascular inflammation and NCB (13). Two-tailed p-values were reported throughout all analyses and p ≤0.05 was deemed significant. All statistical analyses were performed using STATA 12 (Stata Corp., College Station, TX, USA).
RESULTS
Baseline characteristics
The baseline demographics and clinical characteristics of the psoriasis study cohort (N=164) are summarized in Table 1. Overall, patients were middle aged (Mean ± SD, 50.0 ± 12.9), predominantly male (55%), low cardiovascular risk by Framingham risk score [Median (IQR), 3.0 (1.0 – 6.0)] and mild to moderate skin disease severity [PASI score 5.6 (3.0 – 9.6)]. About one-third of the participants were on systemic or biologic treatment for psoriasis. Moreover, the cohort was overweight to obese (body mass index 29.6 ± 6.1), with a high prevalence of hyperlipidemia (47%).
Table 1:
Baseline demographics of entire psoriasis cohort.
| Parameters | Psoriasis (N = 164) |
|---|---|
| Demographics and medical history | |
| Age, years | 50.0 ± 12.9 |
| Males | 91 (55%) |
| Ethnicity, Caucasians | 133 (81%) |
| Hypertension | 45 (27%) |
| Hyperlipidemia | 77 (47%) |
| Type 2 diabetes mellitus | 16 (10%) |
| Current tobacco use | 15 (9%) |
| Lipid treatment | 53 (32%) |
| Body mass index | 29.6 ± 6.1 |
| Waist-to-hip ratio | 0.95 (0.88 - 1.00) |
| Clinical and laboratory values | |
| Systolic blood pressure, mmHg | 123.6 ± 15.4 |
| Diastolic blood pressure, mmHg | 72.4 ± 10.2 |
| Total cholesterol, mg/dL | 181.5 ± 36.7 |
| HDL cholesterol, mg/dL | 55.7 ± 17.5 |
| LDL cholesterol, mg/dL | 101.2 ± 30.6 |
| Triglycerides, mg/dL | 121.8 ± 75.3 |
| Framingham risk score | 3.0 (1.0 – 6.0) |
| C-reactive protein, mg/L | 2.0 (0.8 - 4.4) |
| HOMA-IR* | 2.8 (1.7 – 4.7) |
| Cholesterol efflux capacity | 0.95 ± 0.15 |
| Psoriasis characterization | |
| Psoriasis area severity index score | 5.6 (3.0 – 9.6) |
| Total body surface area index | 4.1 (2.4 – 13.5) |
| Systemic or biologic treatment | 60 (37%) |
| Amygdalar activity (18FDG PET/CT) | |
| Amygdalar FDG uptake | 1.10 ± 0.11 |
| Aortic vascular inflammation (18FDG PET/CT) | |
| Target-to-background ratio | 1.71 ± 0.26 |
| Coronary plaque burdens (x100) | |
| Total burden, mm2 | 1.14 ± 0.45 |
| Non-calcified burden, mm2 | 1.10 ± 0.44 |
| Hematopoietic system activity (18FDG PET/CT) | |
| Bone marrow (SUV† max) | 3.93 ± 0.93 |
| Spleen (SUV† max) | 3.47 ± 0.75 |
Values reported in the table as Mean ± SD or Median (IQR) for continuous data and N (%) for categorical data.
HOMA-IR: Homeostasis model assessment of insulin resistance,
SUV: Standardized uptake value.
Psoriatic skin disease severity assessed as PASI score showed a linear relationship with AmygA after adjustment for traditional risk factors [β=0.19, p=0.05]. Additionally, since psoriatic skin disease severity has been shown to associate positively with subclinical cardiovascular disease, independent of cardiovascular risk factors (20), we sought to compare severe psoriasis patients (N=30) with 47 consecutively recruited healthy volunteers at baseline (Table 2). Furthermore, we also followed this consecutive subset of participants with severe psoriasis (N=30) at one year following intensive psoriasis treatment.
Table 2:
Baseline demographics of severe psoriasis cohort compared to healthy volunteers.
| Parameter | Severe Psoriasis (N = 30) |
Healthy Volunteers (N = 47) |
p- value |
|---|---|---|---|
| Demographics and medical history | |||
| Age, years | 51.1 ± 12.0 | 40.3 ± 12.2 | <0.001 |
| Males | 23 (77%) | 37 (79%) | 0.83 |
| Hypertension | 11 (37%) | 7 (15%) | 0.03 |
| Hyperlipidemia | 15 (50%) | 14 (30%) | 0.07 |
| Type 2 diabetes mellitus | 4 (13%) | 3 (6%) | 0.30 |
| Current tobacco use | 3 (10%) | 0 (0%) | 0.03 |
| Lipid treatment | 11 (37%) | 7 (15%) | 0.06 |
| Body mass index | 30.3 ± 7.2 | 27.1 ± 4.8 | 0.02 |
| Waist-to-hip ratio | 0.99 (0.95 - 1.05) | 0.95 (0.89 - 1.00) | 0.02 |
| Clinical and laboratory values | |||
| Systolic blood pressure, mm Hg | 122.9 ± 15.6 | 114.8 ± 12.2 | 0.01 |
| Diastolic blood pressure, mm Hg | 73.8 ± 10.5 | 70.0 ± 8.3 | 0.08 |
| Total cholesterol, mg/dL | 172.8 ± 36.0 | 183.4 ± 38.1 | 0.23 |
| HDL cholesterol, mg/dL | 51.9 ± 15.2 | 55.6 ± 18.1 | 0.36 |
| LDL cholesterol, mg/dL | 100.5 ± 32.4 | 102.7 ± 32.8 | 0. 78 |
| Triglycerides, mg/dL | 119.8 ± 75.3 | 126.2 ± 99.0 | 0.77 |
| Framingham risk score | 3.5 (1.0 – 6.0) | 1.3 (0.4 – 3. 9) | 0.004 |
| C-reactive protein, mg/L | 3.0 (1.6 – 8.7) | 1.1 (0.7 - 2.6) | 0.001 |
| HOMA-IR* | 3.5 (1.6 – 5.3) | 2.3 (1.6 – 3.4) | 0.03 |
| Cholesterol efflux capacity | 0.89 ± 0.14 | 1.00 ± 0.16 | 0.006 |
| Psoriasis characterization | |||
| Psoriasis area severity index score | 15.5 (12.5 – 23.2) | - | - |
| Total body surface area index | 28.1 (20.2 – 43.5) | - | - |
| Systemic or biologic treatment | 9 (30%) | - | - |
| Amygdalar activity(18FDGPET/CT) | |||
| Amygdalar FDG uptake | 1.12 ± 0.11 | 1.06 ± 0.12 | 0.02 |
| Aortic vascular inflammation(18FDG PET/CT) | |||
| Target-to-background ratio | 1.78 ± 0.32 | 1.62 ± 0.20 | 0.02 |
| Coronary plaque burdens (x100) | |||
| Total burden, mm2 | 1.37 ± 0.73 | 1.05 ± 0.33 | <0.001 |
| Non-calcified burden, mm2 | 1.29 ± 0.69 | 1.04 ± 0.34 | <0.001 |
| Hematopoietic system activity(18FDG PET/CT) | |||
| Bone marrow (SUV† max) | 4.05 ± 1.15 | 3.52 ± 0.96 | 0.03 |
| Spleen (SUV† max) | 3.55 ± 1.03 | 3.32 ± 0.83 | 0.29 |
Values reported in the table as Mean ± SD or Median (IQR) for continuous data and N (%) for categorical data. P-value ≤ 0.05 deemed significant. P-values were calculated by using student’s t-test or Mann-Whitney U test for continuous variables and Pearson’s chi-squared test for categorical variables.
HOMA-IR: Homeostasis model assessment of insulin resistance.
SUV: Standardized uptake value.
When compared with healthy volunteers, participants with severe skin disease had a higher Framingham risk score [3.5 (1.0 – 6.0) vs 1.3 (0.4 – 3.9), p=0.004], more insulin resistance by homeostasis model assessment of insulin resistance (HOMA-IR) [3.5 (1.6 – 5.3) vs 2.3 (1.6 – 3.4), p=0.03] and higher C-reactive protein levels [3.0 (1.6 – 8.7) vs 1.1 (0.7 – 2.6), p=0.001]. Moreover, AmygA was higher in severe psoriasis patients (1.12 ± 0.11 vs 1.06 ± 0.12, p=0.02), as was hematopoietic system activity as measured by SUVs in bone marrow (4.05 ± 1.15 vs 3.52 ± 0.96, p=0.03). Furthermore, there was evidence of subclinical cardiovascular disease assessed by 18FDG-PET/CT derived aortic vascular inflammation (1.78 ± 0.32 vs 1.62 ± 0.20, p=0.02) and CCTA derived coronary artery characteristics [total coronary plaque burden (1.37 ± 0.73 vs 1.05 ± 0.33, p<0.001) and non-calcified coronary plaque burden (1.29 ± 0.69 vs 1.04 ± 0.34, p<0.001)].
Finally, to demonstrate further validity of amygdalar FDG uptake, we characterized AmygA in psoriasis patients who report a personal history of depression and/or anxiety on survey (N=41) and then matched these patients by age and gender to randomly selected patients with psoriasis who reported no history of depression or anxiety (N=41). In this analysis, patients with depression and/or anxiety had significantly higher amygdala activity than those reporting no history of depression or anxiety (1.11 ± 0.09 vs 1.05 ± 0.01, p=0.01).
Amygdalar activity is associated with cardiometabolic disease parameters
Resting AmygA was found to be significantly associated with traditional cardiovascular risk factors including age [β=0.19, p=0.02], male gender [β=0.15, p=0.05], hyperlipidemia [β=0.16, p=0.05] and type 2 diabetes mellitus [β=0.15, p=0.05] (Table 3) in the entire psoriasis cohort (N=164). Furthermore, AmygA showed a significant association with C-reactive protein [β=0.18, p=0.02] as well as with known cardiometabolic disease biomarkers including HOMA-IR [β=0.30, p<0.001], body mass index [β=0.23, p=0.003] and waist-to-hip ratio [β=0.24, p=0.003]. Finally, PASI score was positively associated with AmygA [β=0.19, p=0.05].
Table 3:
Baseline amygdalar activity association with cardiometabolic disease parameters in psoriasis cohort.
| Variables | (N=164) Beta (p-value) |
|---|---|
| Age | 0.19 (0.02) |
| Sex | 0.15 (0.05) |
| Hypertension | 0.21 (0.008) |
| Hyperlipidemia | 0.16 (0.05) |
| Type 2 diabetes mellitus | 0.15 (0.05) |
| Current tobacco use | −0.06 (0.48) |
| Lipid treatment | 0.08 (0.34) |
| Body mass index | 0.23 (0.003) |
| Waist-to-hip ratio | 0.24 (0.003) |
| Systolic blood pressure, mm Hg | 0.23 (0.003) |
| Diastolic blood pressure, mm Hg | 0.20 (0.01) |
| Total cholesterol | −0.01 (0.86) |
| HDL cholesterol | −0.17 (0.03) |
| LDL cholesterol | −0.04 (0.60) |
| Triglycerides | 0.15 (0.05) |
| Framingham risk score | 0.30 (<0.001) |
| C-reactive protein | 0.18 (0.02) |
| HOMA-IR* | 0.30 (<0.001) |
| Cholesterol efflux capacity | −0.15 (0.06) |
| Psoriasis area severity index score | 0.19 (0.05) |
Data represented as standardized beta coefficient and p-value. Univariable linear regressions done for parametric data and Spearman’s correlations done for non-parametric data. P value ≤ 0.05 deemed significant.
HOMA-IR: Homeostasis model assessment of insulin resistance.
Amygdalar activity is associated with hematopoietic system activity
On 18FDG PET/CT, we found an association between AmygA and hematopoietic system activity in both spleen [β=0.17, p=0.03] and bone marrow [β=0.20, p=0.01], the latter of which remained significant after adjustment for cardiovascular disease risk factors [β=0.17, p=0.03] in the entire psoriasis cohort (N=164) (Table 4).
Table 4:
Adjusted associations between baseline amygdalar activity, hematopoietic system activity and vascular disease in psoriasis cohort.
| Variables (N=164) | Model-1 | Model-2 | Model-3 |
| Hematopoietic system activity &amygdalar activity | |||
| Spleen activity | 0.17 (0.03) | 0.12 (0.14) | 0.11 (0.16) |
| Bone marrow activity | 0.20 (0.01) | 0.19 (0.02) | 0.17 (0.03) |
| Aortic vascular inflammation, NCB & amygdalar activity | |||
| Aortic vascular inflammation | 0.31 (<0.001) | 0.23 (0.002) | 0.23 (0.003) |
| Non-calcified coronary plaque burden | 0.27 (<0.001) | 0.23 (<0.001) | 0.24 (<0.001) |
Values reported in Beta (p-value)
Model-1: Unadjusted
Model-2: Adjusted for Framingham risk score
Model-3: Adjusted for Framingham risk score, waist-to-hip ratio, lipid treatment (including statin use), high sensitivity c-reactive protein
Amygdalar activity is associated with aortic vascular inflammation and non-calcified coronary plaque burden
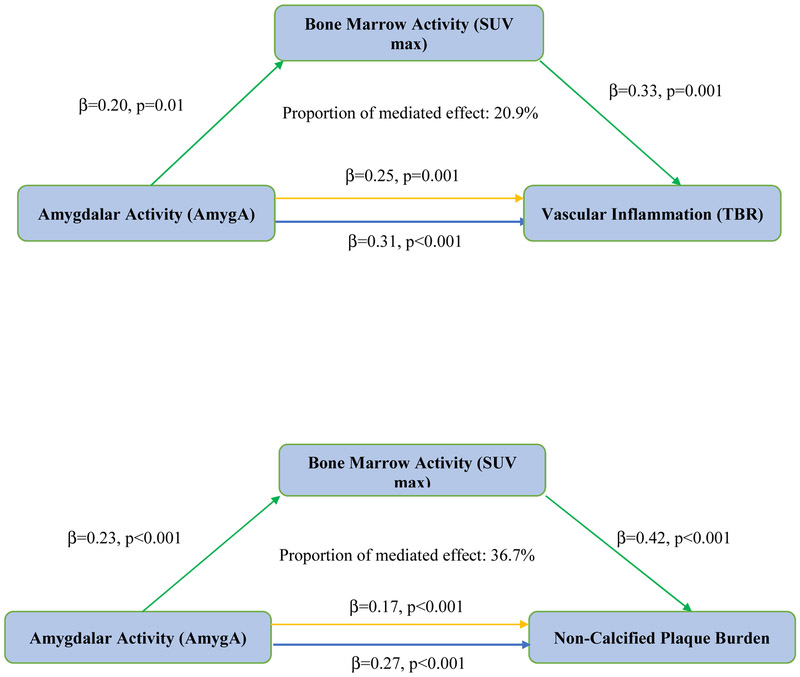
Baseline resting AmygA associated with two separate measures of atherosclerotic disease: aortic vascular inflammation [β=0.31, p<0.001] and NCB [β=0.27, p<0.001] (Online Figure 1) (Table 4). We found that bone marrow activity mediated the associations between AmygA and each atherosclerotic measure, accounting for 20.9% (p<0.001) of the relationship with aortic vascular inflammation and 36.7% (p<0.001) of the relationship with NCB (Figure 3, 4). Similar associations were noted when splenic activity was used in lieu of bone marrow activity (Online Figure 2). Finally, likelihood ratio testing confirmed the potential incremental value of bone marrow activity on the net effect of AmygA on aortic vascular inflammation and NCB (VI: χ2=18.33, p<0.001, NCB: χ2=87.50, p<0.001). Similar associations were noted when splenic activity was used in lieu of bone marrow activity.
Figure 3. Mediation analysis - amygdalar, bone marrow, and vascular disease in psoriasis (N=164).
A) Mediation analyses showing the indirect effect of bone marrow activity on the relationship between resting amygdalar and aortic vascular inflammatory activity.
B) Mediation analyses showing the added effect of bone marrow activity on the relationship between resting amygdalar activity and non-calcified coronary plaque burden.
*TBR: Target-to-background ratio; SUV: Standardized uptake value. Blue arrow: Total effect, Yellow arrow: Direct effect, Green arrow: Indirect effect
**Likelihood ratio test: A) χ2=18.33, p<0.001, B) χ2=87.50, p<0.001
***Model adjusted for Framingham risk score, waist-to-hip ratio, lipid treatment and C-reactive protein.
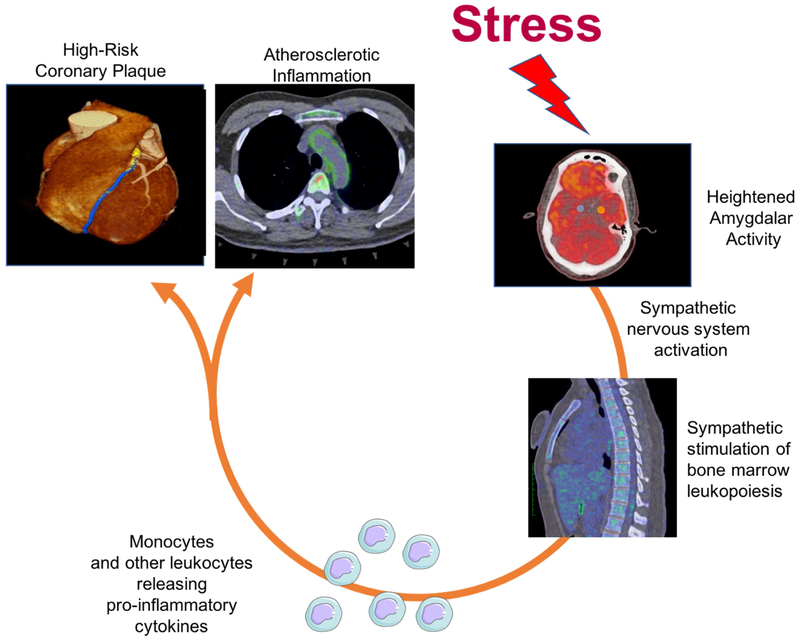
Figure 4. A psoriasis model showing stress leading to subclinical cardiovascular disease.
Amygdalar activity has been linked to cardiovascular disease through sympathetic nervous system pathways. We investigated an alternative pathway in an inflammatory disease state involving hematopoietic system activation, which in part mediates aortic vascular inflammation and non-calcified coronary plaque.
Treatment of severe psoriasis reduces skin disease severity, amygdalar activity and vascular inflammation
As mentioned earlier, a consecutive subset of participants with severe psoriasis (N=30) underwent repeat imaging and clinical evaluations over one year following intensive treatment (Table 5). At follow up, the number of patients on topical therapy (67% vs 63%), phototherapy (23% vs 30%) and systemic therapy (13% vs 17%) largely remained unchanged, however, patients on anti-inflammatory biologic therapy doubled (30% vs 60%, P<0.001). Moreover, psoriatic skin disease severity improved significantly [from 15.5 (12.5 – 23.2) to 5.9 (3.4 – 10.4), p<0.001] with concurrent decrease in AmygA (1.12 ± 0.11 to 1.05 ± 0.09, p=0.006) (Figure 5), bone-marrow activity (4.05 ± 1.15 to 3.70 ± 1.06, p<0.001) (Figure 6), and aortic vascular inflammation (1.78 ± 0.32 to 1.67 ± 0.26, p=0.01). We also observed a non-significant decrease in total coronary plaque burden (1.37 ± 0.73 to 1.31 ± 0.63, p=0.21) and NCB (1.29 ± 0.69 to 1.24 ± 0.60, p=0.23). Finally, in the remainder of the cohort (without NCB characterization), we found beneficial effects of skin disease clearance on AmygA, hematopoietic system activity and aortic vascular inflammation at one-year (Online Table 1).
Table 5:
Characteristics of severe psoriasis patients at baseline and at one-year following therapy for psoriasis.
| Parameter | Baseline (N=30) |
Follow-up (N=30) |
p- value |
|---|---|---|---|
| Demographics and medical history | |||
| Age, years | 51.1 ± 12.0 | 52.3 ± 12.3 | - |
| Males | 23 (77%) | 23 (77%) | - |
| Hypertension | 11 (37%) | 9 (30%) | 0.56 |
| Hyperlipidemia | 15 (50%) | 16 (53%) | 0.65 |
| Type 2 diabetes mellitus | 4 (13%) | 3 (10%) | 0.32 |
| Current tobacco use | 3 (10%) | 3 (10%) | 1.00 |
| Lipid treatment | 10 (33%) | 10 (33%) | 1.00 |
| Body mass index | 30.3 ± 7.2 | 29.4 ± 6.7 | 0.26 |
| Waist-to-hip ratio | 0.99 (0.95 - 1.05) | 0.98 (0.92 - 1.01) | 0.54 |
| Clinical and laboratory values | |||
| Systolic blood pressure, mmHg | 122.9 ± 15.6 | 120.2 ± 16.3 | 0.12 |
| Diastolic blood pressure, mmHg | 73.8 ± 10.5 | 70.5 ± 11.4 | 0.07 |
| Total cholesterol, mg/dL | 172.8 ± 36.0 | 172.5 ± 39.0 | 0.99 |
| HDL cholesterol, mg/dL | 51.9 ± 15.2 | 55.0 ± 17.0 | 0.25 |
| LDL cholesterol, mg/dL | 100.5 ± 32.4 | 95.4 ± 36.0 | 0.44 |
| Triglycerides, mg/dL | 119.8 ± 75.3 | 112.0 ± 52.7 | 0.67 |
| Framingham risk score | 3.5 (1.0 – 6.0) | 4.0 (1.0 – 7.0) | 0.04 |
| C-reactive protein, mg/L | 3.0 (1.6 – 8.7) | 1.5 (0.8 – 4.1) | 0.01 |
| HOMA-IR* | 3.5 (1.6 – 5.3) | 3.9 (2.1 – 7.3) | 0.16 |
| Cholesterol efflux capacity | 0.89 ± 0.14 | 0.96 ± 0.13 | 0.01 |
| Psoriasis characterization | |||
| Psoriasis area severity index score | 15.5 (12.5 – 23.2) | 5.9 (3.4 – 10.4) | <0.001 |
| Total body surface area index | 28.1 (20.2 – 43.5) | 6.6 (1.9 – 14.0) | <0.001 |
| Psoriasis treatment breakdown | |||
| Topical | 20 (67%) | 19 (63%) | - |
| Phototherapy | 7 (23%) | 9 (30%) | - |
| Systemic | 4 (13%) | 5 (17%) | - |
| Biologic | 9 (30%) | 18 (60%) | - |
| Types of biologic treatment | |||
| Anti-TNF therapy | 3 | 11 | - |
| Anti IL12/23 therapy | 3 | 3 | - |
| Anti IL17 therapy | 3 | 4 | - |
| Amygdalar activity (18FDG PET/CT) | |||
| Amygdalar FDG uptake | 1.12 ± 0.11 | 1.05 ± 0.09 | 0.006 |
| Aortic vascular inflammation (18FDG PET/CT) | |||
| Target-to-background ratio | 1.78 ± 0.32 | 1.67 ± 0.26 | 0.01 |
| Coronary plaque burden (x100) | |||
| Total burden, mm2 | 1.37 ± 0.73 | 1.31 ± 0.63 | 0.21 |
| Non-calcified burden, mm2 | 1.29 ± 0.69 | 1.24 ± 0.60 | 0.23 |
| Hematopoietic system activity (18FDG PET/CT) | |||
| Bone marrow (SUV† max) | 4.05 ± 1.15 | 3.70 ± 1.06 | <0.001 |
| Spleen (SUV† max) | 3.55 ± 1.03 | 3.74 ± 1.19 | 0.10 |
Values reported in the table as Mean ± SD or Median (IQR) for continuous data and N (%) for categorical data. P value ≤ 0.05 deemed significant. P values were calculated by using student’s t-test or Mann-Whitney U test for continuous variables and Pearson’s chi-squared test for categorical variables.
HOMA-IR: Homeostasis model assessment of insulin resistance,
SUV: Standardized uptake value.
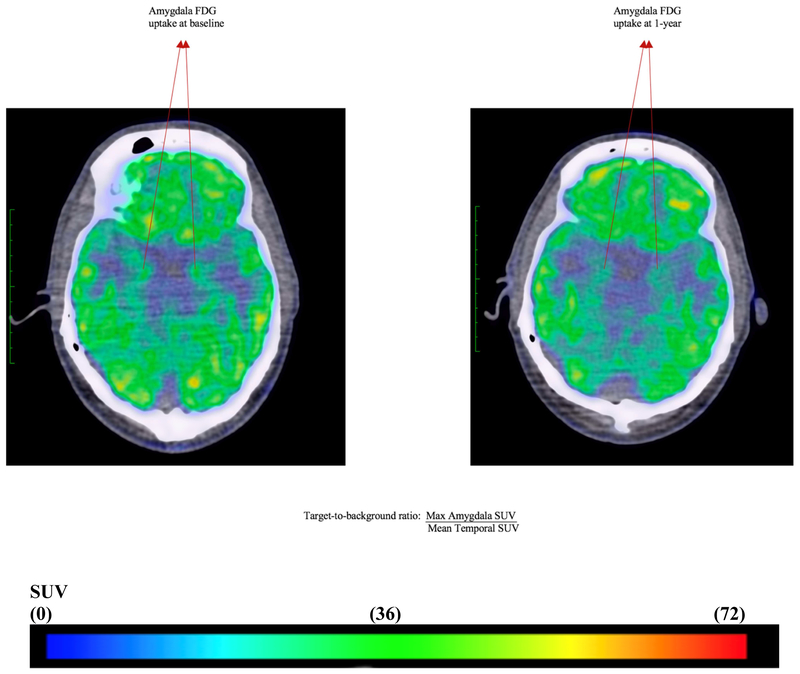
Figure 5. Amygdalar 18FDG uptake at baseline and one-year.
Representative fused 18Fluorodeoxyglucose positron emission-computed tomographic images# (axial view) from a patient who had reduction in psoriatic skin disease severity, showing 18FDG uptake in amygdala at baseline (left) and one year (right).
SUV: standardized uptake value
Baseline: Target-to-background ratio (1.14) = Max Amygdala SUV (9.71)/Mean Temporal SUV (8.50)
One-year: Target-to-background ratio (1.05) = Max Amygdala SUV (7.52)/Mean Temporal SUV (7.18)
# Blue represent mild to moderate FDG uptake, yellow and green represent moderate FDG uptake and red represents highest FDG uptake.
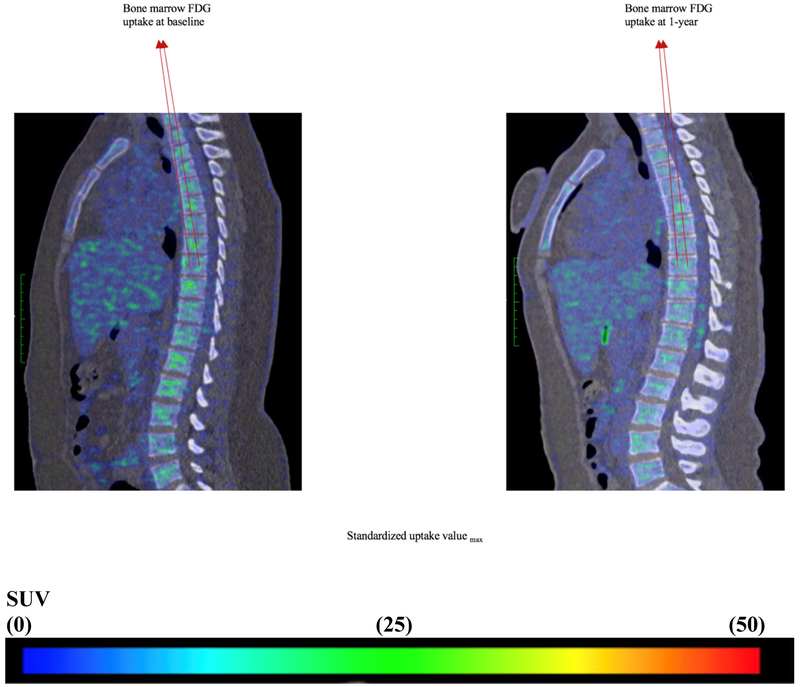
Figure 6. Bone marrow 18FDG uptake at baseline and one year.
Representative fused 18Fluorodeoxyglucose positron emission-computed tomographic images# (sagittal view) from a patient who had reduction in psoriatic skin disease severity, showing 18FDG uptake in T1 to L5 vertebrae at baseline (left) and one year (right).
SUV: standardized uptake value
Baseline: Max Amygdala SUV = 4.02
One-year: Max Amygdala SUV = 3.55
# Blue represent mild to moderate FDG uptake, yellow and green represent moderate FDG uptake and red represents highest FDG uptake.
DISCUSSION
This study yielded several important findings: 1) Chronic stress-related neural activity as measured by AmygA was higher in severe psoriasis patients than in healthy volunteers, 2) increased AmygA was related to the severity of psoriatic skin disease, 3) increased AmygA associated with subclinical cardiovascular disease including, aortic vascular inflammation and NCB, beyond traditional risk factors, 4) hematopoietic system activity potentially affected the association between AmygA and vascular disease in psoriasis and 5) improvement in psoriasis with therapy was accompanied by reductions in AmygA, hematopoietic system activity, aortic vascular inflammation and stabilization of NCB. Taken together, these findings demonstrate that in psoriasis, chronic stress-related neural activity may associate with increased burden of subclinical cardiovascular disease, at least in part through activation of an amygdala-hematopoietic-arterial wall pathway. Moreover, they also suggest that anti-inflammatory therapies targeting the skin manifestations of psoriasis may have salutary impacts on the brain-heart connection.
The effects of psychological stress on behavior and systemic processes have been well studied. Stress affects the endocrine system leading to upregulation of circulating glucocorticoids, catecholamines and inflammatory cytokines, subsequently leading to cardiovascular disease (26-28). Several epidemiological studies have highlighted the significance of psychological stress as an independent risk factor for cardiovascular disease and its associated mortality (1,29). Studies have shown poor prognosis in patients who develop depression following myocardial infarction and heart failure (30,31). Murine studies have emphasized the role of the hematopoietic system, primarily bone marrow, leading to release of activated monocytes that adhere to vessel endothelium and commence the atherosclerotic cascade in states of increased stress (32-34). Recently, Tawakol et al reported that psychological stress was associated with major cardiovascular events using 18FDG PET/CT in an observational prospective cohort (13). Whether this increase in prospective cardiovascular events was due to changes in vessel wall or plaque characteristics was not studied. Nevertheless, it prompted the question whether this stress-bone marrow-cardiovascular disease pathway exists in a chronic inflammatory disease state.
Psoriasis is a chronic inflammatory skin disease that is associated with increased prevalence of depression and psychological stress (7). Additionally, psoriasis is associated with increased aortic vascular inflammation (20) and coronary plaque burden (23) along with an elevated risk of cardiovascular mortality (8,9). CCTA, a non-invasive imaging modality used for characterization of coronary plaque burden, has been validated against intravascular ultrasound, the gold standard for coronary plaque assessment (35). Our work on CCTA-derived NCB to predict future cardiovascular risk and the effect of psoriasis treatment on NCB (23) suggests the usefulness of CCTA as a framework to study subclinical cardiovascular disease in inflammatory disease states. Studies have also shown an increase in non-calcified plaque volumes in acute coronary syndrome patients (36), which undergo modulation in response to statin therapy (37). Furthermore, aortic vascular inflammation as assessed by 18FDG PET/CT is a reliable surrogate marker for prospective cardiovascular disease as epidemiological studies have shown promising results wherein aortic vascular inflammation improved secondary to cardiovascular risk reduction by use of statins and lifestyle changes (38,39). Whether these features modulate with skin disease treatment has been the topic of investigation. We recently observed that when a sample of psoriasis patients was followed up at one year after any treatment, there was a 6% decrease in aortic vascular inflammation; however, the coronary arteries were not evaluated (21). Following that, in a small study assessing 50 psoriasis patients, we also showed that improvement in skin disease severity is associated with improvement in NCB by CCTA (23). Despite so, none of these studies investigated the role of amygdalar metabolic activity.
Herein we confirm the potential association between AmygA and aortic vascular inflammation as highlighted by a recent seminal study (13). We also provide further evidence for the role of a neuro-hematopoietic-vascular axis in psoriasis. Building upon existing literature, we present novel evidence of the association between AmygA and subclinical cardiovascular disease comprising aortic vascular inflammation and lipid-rich NCB in a chronic inflammatory disease state namely psoriasis. We show that bone marrow and spleen activity potentially mediate the pathway between AmygA and aortic vascular inflammation, suggesting that elevated hematopoietic system activity might lead to the downstream development of cardiovascular disease in a stress-associated inflammatory milieu. Lastly, we demonstrate the systemic response to psoriatic skin disease clearance, wherein AmygA, hematopoietic system activity and aortic vascular inflammation improved over one year with treatment. Total coronary plaque burden and NCB were also reduced, but not significantly. It is known that chronically inflamed psoriasis patients on no systemic treatment experience progression of total coronary plaque burden driven by an increase in NCB over time (23). Therefore, these results are reassuring considering we observed stabilization of NCB with treatment. Future studies should focus on plaque features including positive remodeling, assessment of necrotic core and other high-risk coronary features.
This study provides several novel insights. First, we present novel evidence of an association between AmygA and structural coronary atherosclerotic disease (lipid-rich coronary plaque burden) and demonstrate that elevated hematopoietic system activity may potentially mediate that association. Second, we demonstrate that one year of anti-inflammatory treatment results in reductions in AmygA, hematopoietic system activity and aortic vascular inflammation along with stabilization of non-calcified coronary plaque burden in individuals with psoriasis. Third, we associate AmygA with patient-reported depression and/or anxiety in psoriasis. Accordingly, this study provides key confirmation of findings of Tawakol et. al. (13), and furthermore provides several important extensions.
Our study indeed has limitations. Given the cross-sectional study design, our findings do not establish causality or directionality of the relationship. Though our proposed pathway of stress induced inflammation leading to increased vascular diseases through hematopoietic tissue activation might be relevant, mechanistic data are needed to establish inflammation from psoriasis as a central cause for this pathway. Despite our attempts at controlling for confounding covariates by adjusting for them in our regression models, residual confounding remains a concern. Furthermore, we could not incorporate hard cardiovascular outcomes, owing to the young age of our sample. Nevertheless, our concurrent use of 18FDG PET/CT and CCTA to shed light on cardiovascular disease risk is novel, and our outcome measures are reasonably reliable markers of future cardiovascular events (39,40). Another limitation is that our study does not incorporate validated questionnaires or interviews in the form of a perceived stress scale to quantify psychological stress or stressful events. However, increased amygdalar FDG uptake, as a manifestation of stress, has the virtue of avoiding subjective recall bias. We also acknowledge that our control group was younger than our psoriasis group, which is also a limitation and thus our results should be interpreted with caution. Although we followed up patients after psoriasis treatment, we had a relatively small sample size (N=30) of participants who met criteria for severe psoriasis. We acknowledge that the one-year post treatment reduction in amygdalar uptake is small (6.2%). However, without follow up data in the control group, we are unable to provide context for this reduction in amygdalar uptake. Finally, we have not looked at markers of hypothalamic-pituitary-adrenal axis (HPA) and sympathetic nervous system activity. Future studies should assess the amygdalar activation in patients across broader disease spectra and with wider risk profiles to provide the context to amygdalar activation as it relates to cardiovascular disease.
CONCLUSIONS
In conclusion, we demonstrate that chronic stress-related neural activity is greater in psoriasis patients. Furthermore, such neural activity may be associated with vascular inflammation and non-calcified coronary plaque burden, mediated in part by hematopoietic activity in bone marrow and spleen. Assessment of psychological stress should be undertaken in evaluating cardiovascular disease risk, especially in inflammatory disease states.
PERSPECTIVES
Competency in Medical Knowledge
Psychological stress is known to be an independent risk factor for cardiovascular disease. 18FDG PET/CT measurement of activity in the amygdala, a neural manifestation of stress, has been shown to associate with bone marrow activity, aortic vascular inflammation, and major cardiovascular events. The relationship of 18FDG PET/CT-derived assessment of amygdalar activity with CCTA-derived coronary plaque characteristics, such as non-calcified coronary plaque burden, in a chronic inflammatory disease state such as psoriasis demonstrates that chronic stress might modulate coronary plaque.
Translational Outlook One
Stress-related neural activity is greater in psoriasis and is associated with subclinical cardiovascular disease, partly mediated by hematopoietic system activity. In chronic inflammatory states, this stress-vascular pathway may be ameliorated by anti-inflammatory treatment.
Translational Outlook Two
18FDG uptake in amygdala is associated with sub-clinical cardiovascular indices in part mediated by hematopoietic system activity. Concurrent psychometric characterization of stress, as well inclusion of markers of HPA axis and sympathetic activity, and inflammatory cell subsets in blood, should be incorporated into future studies to elucidate further mechanisms by which stress associates with cardiovascular disease.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to acknowledge and thank NIH Clinical Center outpatient clinic-7 nurses for their invaluable contribution to the process of patient recruitment.
FUNDING: This study was supported by the National Heart, Lung and Blood Institute (NHLBI), National Institutes of Health, Bethesda, MD, Intramural Research Program (HL006193-01) and NIAMS K24-AR064310.
ABBREVIATIONS:
- FDG PET/CT
Flourodeoxyglucose Positron emission tomography/Computed tomography
- CCTA
Coronary computed tomography angiography
- AmygA
Resting amygdalar activity
- NCB
Non-calcified coronary plaque burden
- PASI
Psoriasis area severity index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
- Dr. Mehta has received funding from the National Institutes of Health Intramural Research Program (Z01 HL-06193); is a full-time U.S. Government employee; and has received research grants from Abbvie, Janssen, Novartis Corp, and Celgene.
- Dr. Gelfand was supported by an NIAMS grant (K24-AR-064310); Dr. Gelfand served as a consultant for BMS, Boehringer Ingelheim, GSK, Janssen Biologics, Menlo Therapeutics, Novartis Corp, Regeneron, Dr. Reddy’s labs, UCB (DSMB), Sanofi and Pfizer Inc., receiving honoraria; and receives research grants (to the Trustees of the University of Pennsylvania) from Abbvie, Janssen, Novartis Corp, Sanofi, Celgene, Ortho Dermatologics, and Pfizer Inc.; and received payment for continuing medical education work related to psoriasis that was supported indirectly by Lilly and Ortho Dermatologics.
- Dr. Tawakol reports grants from Genentech and Actelion for research outside the submitted work.
- Dr. Powell-Wiley has received funding from the National Institutes of Health Intramural Research Program (ZIA HL006168).
- All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Contributor Information
Aditya Goyal, Email: goyal.aditya90@gmail.com.
Amit K. Dey, Email: amit.dey@nih.gov.
Abhishek Chaturvedi, Email: abhishek.chaturvedi2391@gmail.com.
Youssef A. Elnabawi, Email: youssef.elnabawi@nih.gov.
Tsion M. Aberra, Email: tsion.aberra@gmail.com.
Jonathan H. Chung, Email: jonathan.chung.91@gmail.com.
Agastya D. Belur, Email: agastyabelur@gmail.com.
Jacob W. Groenendyk, Email: jake.groenendyk@nih.gov.
Joseph B. Lerman, Email: joe.lerman@gmail.com.
Joshua P. Rivers, Email: joshuaprivers@gmail.com.
Justin A. Rodante, Email: justin.rodante@nih.gov.
Charlotte L. Harrington, Email: charlotte.l.harrington@gmail.com.
Nevin J. Varghese, Email: nevinv465@gmail.com.
Gregory E. Sanda, Email: gregory.sanda@nih.gov.
Yvonne Baumer, Email: yvonne.baumer@nih.gov.
Alexander V. Sorokin, Email: alexander.sorokin2@nih.gov.
Heather L. Teague, Email: heather.teague@nih.gov.
Leonard D. Genovese, Email: leonard.genovese@nih.gov.
Balaji Natarajan, Email: drnatb.89@gmail.com.
Aditya A. Joshi, Email: adi0601@gmail.com.
Martin P. Playford, Email: playfordmp@nhlbi.nih.gov.
David A. Bluemke, Email: dbluemke@rsna.org.
Marcus Y. Chen, Email: chenmy@nhlbi.nih.gov.
Abass Alavi, Email: abass.alavi@uphs.upenn.edu.
Roger K. Pitman, Email: roger.pitman@mgh.harvard.edu.
Tiffany M. Powell-Wiley, Email: tiffany.powell-wiley@nih.gov.
Ahmed Tawakol, Email: atawakol@mgh.harvard.edu.
Joel M. Gelfand, Email: joel.gelfand@uphs.upenn.edu.
Nehal N. Mehta, Email: nehal.mehta@nih.gov.
REFERENCES
- 1.Brotman DJ, Golden SH, Wittstein IS. The cardiovascular toll of stress. Lancet 2007;370:1089–100. [DOI] [PubMed] [Google Scholar]
- 2.Davidson K, Jonas BS, Dixon KE, Markovitz JH. Do depression symptoms predict early hypertension incidence in young adults in the CARDIA study? Coronary Artery Risk Development in Young Adults. Archives of internal medicine 2000;160:1495–500. [DOI] [PubMed] [Google Scholar]
- 3.Covey LS, Glassman AH, Stetner F. Cigarette smoking and major depression. Journal of addictive diseases 1998;17:35–46. [DOI] [PubMed] [Google Scholar]
- 4.Alcantara C, Muntner P, Edmondson D et al. Perfect storm: concurrent stress and depressive symptoms increase risk of myocardial infarction or death. Circulation Cardiovascular quality and outcomes 2015;8:146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Egeberg A, Khalid U, Gislason GH, Mallbris L, Skov L, Hansen PR. Impact of Depression on Risk of Myocardial Infarction, Stroke and Cardiovascular Death in Patients with Psoriasis: A Danish Nationwide Study. Acta Derm Venereol 2016;96:218–21. [DOI] [PubMed] [Google Scholar]
- 6.Rugulies R Depression as a predictor for coronary heart disease. a review and metaanalysis. American journal of preventive medicine 2002;23:51–61. [DOI] [PubMed] [Google Scholar]
- 7.Kurd SK, Troxel AB, Crits-Christoph P, Gelfand JM. The risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol 2010;146:891–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. European heart journal 2010;31:1000–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. Jama 2006;296:1735–41. [DOI] [PubMed] [Google Scholar]
- 10.Aberra TM, Joshi AA, Lerman JB et al. Self-reported depression in psoriasis is associated with subclinical vascular diseases. Atherosclerosis 2016;251:219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang SS, Yan XB, Hofman MA, Swaab DF, Zhou JN. Increased expression level of corticotropin-releasing hormone in the amygdala and in the hypothalamus in rats exposed to chronic unpredictable mild stress. Neuroscience bulletin 2010;26:297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lagraauw HM, Kuiper J, Bot I. Acute and chronic psychological stress as risk factors for cardiovascular disease: Insights gained from epidemiological, clinical and experimental studies. Brain, behavior, and immunity 2015;50:18–30. [DOI] [PubMed] [Google Scholar]
- 13.Tawakol A, Ishai A, Takx RA et al. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet 2017;389:834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark US, Miller ER, Hegde RR. Experiences of Discrimination Are Associated With Greater Resting Amygdala Activity and Functional Connectivity. Biol Psychiatry Cogn Neurosci Neuroimaging 2018;3:367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lederbogen F, Kirsch P, Haddad L et al. City living and urban upbringing affect neural social stress processing in humans. Nature 2011;474:498–501. [DOI] [PubMed] [Google Scholar]
- 16.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 2007;164:1476–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aberra TM, Chaturvedi A, Lerman JB et al. Abstract 12605: Brain Metabolic Activity Within Regions Involved in Stress Perception Associates Directly With Arterial Inflammation and Inversely With Aortic Distensibility in Psoriasis. Circulation 2016;134:A12605–A12605. [Google Scholar]
- 18.Drevets WC, Price JL, Bardgett ME, Reich T, Todd RD, Raichle ME. Glucose metabolism in the amygdala in depression: relationship to diagnostic subtype and plasma cortisol levels. Pharmacol Biochem Behav 2002;71:431–47. [DOI] [PubMed] [Google Scholar]
- 19.Versteylen MO, Kietselaer BL, Dagnelie PC et al. Additive value of semiautomated quantification of coronary artery disease using cardiac computed tomographic angiography to predict future acute coronary syndrome. J Am Coll Cardiol 2013;61:2296–305. [DOI] [PubMed] [Google Scholar]
- 20.Naik HB, Natarajan B, Stansky E et al. Severity of Psoriasis Associates With Aortic Vascular Inflammation Detected by FDG PET/CT and Neutrophil Activation in a Prospective Observational Study. Arterioscler Thromb Vasc Biol 2015;35:2667–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dey AK, Joshi AA, Chaturvedi A et al. Association Between Skin and Aortic Vascular Inflammation in Patients With Psoriasis: A Case-Cohort Study Using Positron Emission Tomography/Computed Tomography. JAMA Cardiol 2017;2:1013–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salahuddin T, Natarajan B, Playford MP et al. Cholesterol efflux capacity in humans with psoriasis is inversely related to non-calcified burden of coronary atherosclerosis. European heart journal 2015;36:2662–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lerman JB, Joshi AA, Chaturvedi A et al. Coronary Plaque Characterization in Psoriasis Reveals High-Risk Features That Improve After Treatment in a Prospective Observational Study. Circulation 2017;136:263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Elm E, Altman DG, Egger M et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwan AC, May HT, Cater G et al. Coronary artery plaque volume and obesity in patients with diabetes: the factor-64 study. Radiology 2014;272:690–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Bosscher K, Van Craenenbroeck K, Meijer OC, Haegeman G. Selective transrepression versus transactivation mechanisms by glucocorticoid receptor modulators in stress and immune systems. Eur J Pharmacol 2008;583:290–302. [DOI] [PubMed] [Google Scholar]
- 27.Nikkheslat N, Zunszain PA, Horowitz MA et al. Insufficient glucocorticoid signaling and elevated inflammation in coronary heart disease patients with comorbid depression. Brain, behavior, and immunity 2015;48:8–18. [DOI] [PubMed] [Google Scholar]
- 28.Miller AH, Pariante CM, Pearce BD. Effects of cytokines on glucocorticoid receptor expression and function. Glucocorticoid resistance and relevance to depression. Adv Exp Med Biol 1999;461:107–16. [DOI] [PubMed] [Google Scholar]
- 29.Steptoe A, Kivimaki M. Stress and cardiovascular disease. Nat Rev Cardiol 2012;9:360–70. [DOI] [PubMed] [Google Scholar]
- 30.Frasure-Smith N, Lesperance F, Talajic M. Depression following myocardial infarction. Impact on 6-month survival. Jama 1993;270:1819–25. [PubMed] [Google Scholar]
- 31.Sokoreli I, Pauws SC, Steyerberg EW et al. Prognostic value of psychosocial factors for first and recurrent hospitalizations and mortality in heart failure patients: insights from the OPERA-HF study. Eur J Heart Fail 2018. [DOI] [PubMed] [Google Scholar]
- 32.Dutta P, Courties G, Wei Y et al. Myocardial infarction accelerates atherosclerosis. Nature 2012;487:325–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heidt T, Sager HB, Courties G et al. Chronic variable stress activates hematopoietic stem cells. Nat Med 2014;20:754–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 2013;339:161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fischer C, Hulten E, Belur P, Smith R, Voros S, Villines TC. Coronary CT angiography versus intravascular ultrasound for estimation of coronary stenosis and atherosclerotic plaque burden: a meta-analysis. J Cardiovasc Comput Tomogr 2013;7:256–66. [DOI] [PubMed] [Google Scholar]
- 36.Hoffmann U, Moselewski F, Nieman K et al. Noninvasive assessment of plaque morphology and composition in culprit and stable lesions in acute coronary syndrome and stable lesions in stable angina by multidetector computed tomography. J Am Coll Cardiol 2006;47:1655–62. [DOI] [PubMed] [Google Scholar]
- 37.Otagiri K, Tsutsui H, Kumazaki S et al. Early intervention with rosuvastatin decreases the lipid components of the plaque in acute coronary syndrome: analysis using integrated backscatter IVUS (ELAN study). Circ J 2011;75:633–41. [DOI] [PubMed] [Google Scholar]
- 38.Lee SJ, On YK, Lee EJ, Choi JY, Kim BT, Lee KH. Reversal of vascular 18F-FDG uptake with plasma high-density lipoprotein elevation by atherogenic risk reduction. J Nucl Med 2008;49:1277–82. [DOI] [PubMed] [Google Scholar]
- 39.Figueroa AL, Abdelbaky A, Truong QA et al. Measurement of arterial activity on routine FDG PET/CT images improves prediction of risk of future CV events. JACC Cardiovascular imaging 2013;6:1250–9. [DOI] [PubMed] [Google Scholar]
- 40.Motoyama S, Ito H, Sarai M et al. Plaque Characterization by Coronary Computed Tomography Angiography and the Likelihood of Acute Coronary Events in Mid-Term Follow-Up. J Am Coll Cardiol 2015;66:337–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.