Abstract
Introduction In recent years, cold snare polypectomy (CSP) has increasingly been used over hot snare polypectomy (HSP) for the removal of colorectal polyps (4 – 10 mm in size). However, the optimal technique (CSP vs. HSP), in terms of complete polyp resection and complications, is uncertain. Our aim was to compare incomplete resection rate (IRR) of polyps and complications using CSP vs. HSP.
Methods Randomized controlled studies (RCTs) comparing CSP and HSP for removal of 4 – 10 mm colorectal polyps were considered. Studies were included in the analysis if they obtained biopsy specimens from the resection margin to confirm the absence of residual tissue and reported complications. IRR and complication rate were the outcome measures. Pooled rates were reported as Odds Ratios (OR) or risk difference with 95 % Confidence Interval (CI).
Results In total, three RCTs were included in the final analysis. A total of 1051 patients with 1485 polyps were randomized to either HSP group (n = 741 polyps) or CSP group (n = 744 polyps). The overall IRR did not differ between the two groups (HSP vs. CSP: 2.4 % vs. 4.7 %; OR 0.51, 95 %CI 0.13 – 1.99, P = 0.33, I 2 = 73 %). The HSP group had a lower rate of overall complications compared to the CSP group (3.7 % vs. 6.6 %; OR 0.53, 95 % CI 0.3 – 0.94, P = 0.03, I 2 = 0 %). Polyp retrieval rates were not different between the two groups (99 % vs. 98.1 %).
Conclusion Our results suggest that HSP and CSP techniques can be effectively used for the complete removal of 4 – 10 mm colorectal polyps; however, HSP has a lower incidence of overall complications.
Introduction
Colorectal cancer (CRC) is the third most common cancer in the US with 135 – 420 new cases per year 1 . Screening colonoscopy with polypectomy substantially reduces CRC incidence and mortality 2 3 . However, an unexpected high rate of incomplete resection of colon polyps has been reported in some recent studies, and this has in turn been related to a higher risk of post-colonoscopy interval CRC 4 .
Diminutive (< 5 mm) and small (6 – 9 mm) polyps represent the vast majority of polyps removed at screening colonoscopy 5 . In theory, two major techniques are available for these lesions, namely biopsy-forceps polypectomy and snare polypectomy. The former, however, has been associated with a much higher rate of incomplete resection, especially for small polyps, and its use is not generally recommended 6 . The latter may be further classified into hot (HSP) and cold (CSP) snare polypectomy. The basic difference is the use of a high frequency generator for HSP. Such use may, on the one hand, minimize immediate post-polypectomy bleeding by coagulation, but, on the other, it may also damage deeper vessels with increased risk of delayed bleeding 7 8 or even perforation. For this reason, CSP is usually considered safer, while resulting in equivalent rates of complete resection, and its use has dramatically increased in recent years 9 10 11 12 , due also to the development of specific CSP-snares. CSP- and HSP-incomplete resection rates (IRR) for ≤ 10 mm polyps range widely, being 0.5 – 6.4 % and 1.2 – 7.4 %, respectively 12 13 14 15 , and these estimates are much lower compared with forceps-polypectomy 6 16 . However, any advantage of one technique (HSP vs. CSP) over the other remains unclear 17 .
The current European Society of Gastrointestinal Endoscopy (ESGE) guidelines recommend CSP as the preferred technique for removal of diminutive polyps (size ≤ 5 mm) due to high rates of complete resection, adequate tissue sampling for histology, and low complication rates 18 . They also suggest CSP for sessile polyps 6 – 9 mm in size because of its superior safety profile, although evidence comparing efficacy with HSP is lacking 18 .
Recently, randomized controlled studies (RCTs) have compared the efficacy of HSP with CSP for colorectal polyps ranging between 4 and 10 mm with a disparity in results 19 20 21 . Given this discrepancy and the lack of supporting evidence for current guidelines for polypectomy, we performed a systemic review and meta-analysis of available evidence with the objective to compare the IRR and adverse events between CSP and HSP when removing polyps between 4 and 10 mm in size.
Materials and methods
A comprehensive search was performed using PubMed, Embase, and abstracts of conferences presented at Digestive Diseases Week and the United European Gastroenterology Week. These sources were searched using the keywords “polypectomy”, “cold polypectomy”, “cold snare polypectomy”, “hot snare polypectomy”, “remnant adenoma”, and “endoscopic mucosal resection” to extract studies up to December 2017. Only English language and human studies were searched. Related data suggested by PubMed were also searched. Two authors (RJ and MA) individually searched the databases for article screening. The abstracts were not blinded for authors, institutions or journals during review. Only data from randomized controlled trials comparing the efficacy of and adverse events related to the use of CSP with those of HSP (with or without submucosal injection) for removal of 4 – 10 mm colorectal polyps were included in our study. Such ‘4 – 10 mm’ definition was preferred over the generally reported ‘6 – 9 mm’ for small polyps, in order not to exclude relevant studies, and also include 4-, 5- or 10-mm polyps. In addition, we limited our inclusion to only those studies where biopsy specimens were obtained from the post-polypectomy resection margin to confirm the absence of any residual polypoid tissue (i. e. endoscopic and histologic radical resection). If multiple articles were found from the same institution, then the most recent article was used for analysis. Letters to editors, case reports, case series, case-control, and cohort studies were excluded.
Data collection and bias assessment
Baseline demographic data (age, gender), number of study participants, number of polyps examined, morphology of polyps, histology of polyps, location of polyps, IRR, type of snare used, procedure time, and complication rates were extracted from each study. Paris classification 22 and Vienna classification 23 systems were used to assess the morphology and histology of polyps, respectively. Polyps were classified as either right-sided (if found in the cecum, ascending colon or transverse colon) or left-sided (if found in the descending colon, sigmoid colon, or rectum). IRR was defined as the presence of any residual polypoid tissue in biopsied specimen post-polypectomy with either technique. Procedural time was defined as the time required from identification of polyp to complete resection of the polyp using either technique 19 21 .
Complications included immediate bleeding during the procedure, delayed bleeding after the procedure, and perforations of the intestinal wall. Immediate bleeding was defined as continuous hemorrhage usually for ≥ 30 seconds immediately after polypectomy 19 . Delayed bleeding was defined as hemorrhage after colonoscopy requiring endoscopic hemostasis. All three studies have excluded patients who were treated with antithrombotic agents.
Outcome measures
The primary outcome of our study was IRR and secondary outcomes were complication rates, polyp retrieval rates, and procedure time.
Risk of bias assessment
The risk of bias was evaluated based on guidelines from the Cochrane Handbook for Systematic Reviews of Interventions 24 using the following: adequacy of random sequence generation, allocation concealment, blinding of the participant, blind outcome assessment, incomplete outcome data, and selective outcome reporting.
Statistical analysis
Pooled odds ratio (OR) and 95 % confidence interval (CI) were calculated for primary and secondary outcomes. For pooled analysis of rare events, risk difference was used as an estimate to compare any possible detectable difference, when applicable. Heterogeneity of the study was assessed using the I 2 statistic 25 . Percentages of 25 % ( I 2 = 25), 50 % ( I 2 = 50), and 75 % ( I 2 = 75) were considered to be a low, moderate, and high degree of heterogeneity, respectively. In the presence of substantial heterogeneity ( I 2 > 50 %), a random effect model was used as the pooling method; otherwise, a fixed effect model was adopted as the pooling method. Statistical software used was Review Manager (RevMan) v.5.3 (The Cochrane Collaboration, Oxford, Oxfordshire, UK).
Results
A total of 244 studies were retrieved using the databases ( Fig. 1 ). After screening through the title, abstracts, and full texts, a total of three RCTs were selected 19 20 21 . All three studies were published between April 2017 and August 2017 ( Table 1 ). A total of 1051 patients were found to have 1485 small polyps (4 – 10 mm in size) which were randomized to either the HSP group (n = 741 polyps) or the CSP group (n = 744 polyps). Of 1485 colorectal polyps, 1266 polyps were included in the final analysis with 630 polyps in the HSP group and 636 polyps in the CSP group. Two RCTs 19 21 included only neoplastic polyps for the final analysis while one RCT 20 included all colorectal polyps which were excised. The mean age of patients randomized to the CSP group was 64.1 years old, while those randomized to the HSP group was 65.3 years old, as described in two studies 20 21 . The mean percentages of males were 55.5 % in the CSP group and 56.8 % in the HSP group.
Fig. 1.
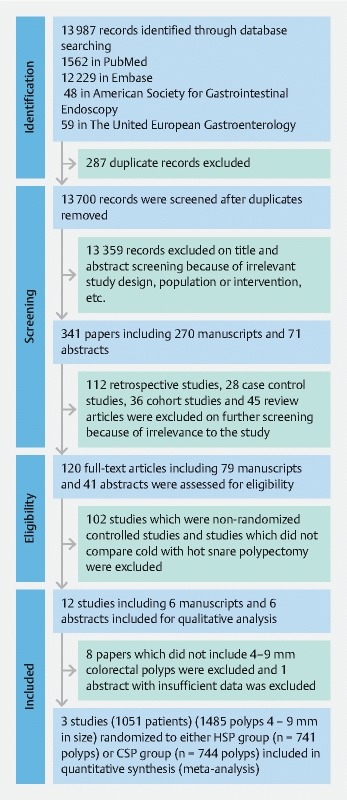
PRISMA flow diagram for selection of studies.
Table 1. Details of the three studies included.
| Reference | Patient age, mean ± SD, years | Gender male, % | No. of polyps, n | Polyp size, mean ± SD, mm | Macroscopic appearance, n | Histological findings, n | Location of the polyp, n | No. of all adverse events, n | Incomplete resection rate, % | Complete retrieval rate, % | ||||||
| 0 – I | 0 – II | TA | AA | HP | SSP | OP | Right | Left | ||||||||
| Cold snare polypectomy | ||||||||||||||||
| Kawamura et al., 2017 19 | NA | NA | 341 | NA | 234 | 107 | 333 | 10 | 21 | 11 | 12 | 207 | 134 | 34 | 1.8 | 98.2 |
| Zhang et al., 2017 21 | 64.5 ± 7.7 | 96 (53.6 %) | 267 | 7.4 ± 1.4 | 164 | 81 | 189 | 45 | 46 | 25 | 7 | 141 | 126 | 5 | 8.5 | 100 |
| Papastergiou et al., 2018 20 | 63.1 ± 10.3 | 46 (59.7 %) | 83 | 8.2 ± 1.6 | 38 | 45 | 59 | 0 | 7 | 17 | 0 | 40 | 43 | 3 | 7.2 | 92.8 |
| Hot snare polypectomy | ||||||||||||||||
| Kawamura et al., 2017 19 | NA | NA | 346 | NA | 234 | 112 | 336 | 15 | 32 | 9 | 7 | 193 | 153 | 16 | 2.6 | 99.3 |
| Zhang et al., 2017 21 | 65.8 ± 9.4 | 101 (56.4 %) | 258 | 7.7 ± 1.5 | 164 | 74 | 175 | 41 | 49 | 26 | 15 | 140 | 118 | 3 | 1.5 | 100 |
| Papastergiou et al., 2018 20 | 64.1 ± 10.9 | 45 (57.7 %) | 81 | 8.3 ± 1.4 | 34 | 47 | 60 | 0 | 8 | 13 | 0 | 36 | 45 | 1 | 3.7 | 95.1 |
NA, not available; TA, tubular adenoma; AA, advanced adenoma; HP, hyperplastic polyps; SSP, sessile serrated polyps; OP, other polyps.
The risks of bias assessment are reported in Fig. 2 .
Fig. 2.
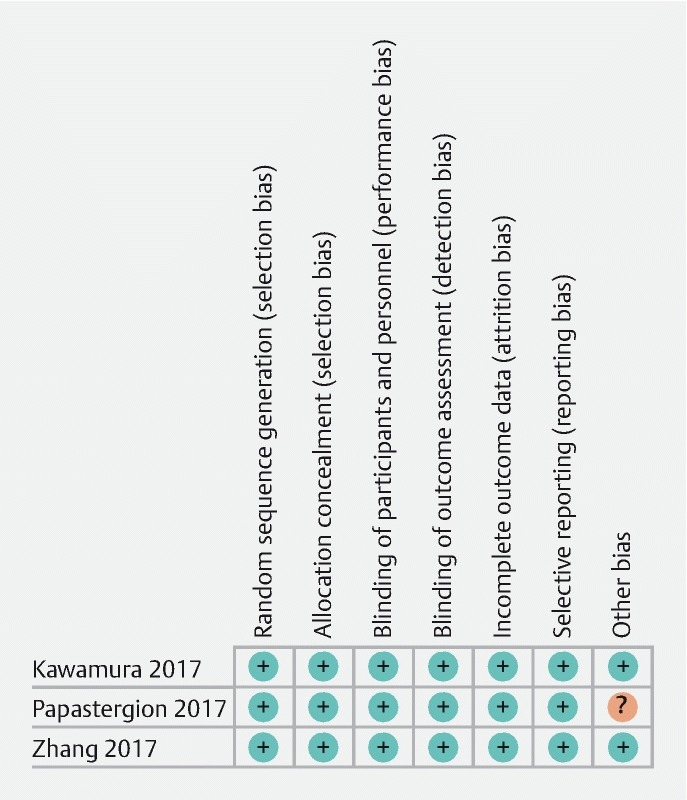
Risk of bias per Cochrane risk of bias assessment among studies included.
Type of snare
The snares used in these studies were: Captivator II 10 mm (Boston Scientific, Natick, MA, United States) 19 20 , the Acusnare (Cook Medical, Bloomington, IN, USA) 20 , Snare-Master 10 mm (Olympus Medical, Tokyo, Japan) 20 21 and Exacto/9 mm (US Endoscopy, Mentor, OH, United States) 19 . For HSP, electro-cautery was performed with the ERBE ICC 200 electrosurgical generator (ERBE Electromedizin, Tübingen, Germany) 20 21 . Only one study mentioned the setting for cautery as fractionated cutting mode ENDO CUT Q (effect 3; cut duration 1; cut interval 3) 20 . Sub-mucosal injection before polypectomy was performed for both groups in one study 19 , however, it was only performed for the HSP group in the other two studies 20 21 .
Polyp characteristics
Based on the morphology of polyps, 436 out of 691 (63.1 %) and 432 out of 685 (63.1 %) were classified as type 0 – I polyps, while 255 out of 691 (36.9 %) and 263 out 685 (38.4 %) were classified as type 0 – II in the CSP and HSP group, respectively. On a histological basis, the following polyps were removed: 1152 (78.1 %) tubular adenomas, 111 (7.5 %) advanced adenomas (prominent villous component and high grade dysplasia), 86 (5.8 %) sessile serrated polyps/adenoma, 83 (5.6 %) hyperplastic polyps and 43 (2.9 %) polyps without histological diagnosis. The locations of polyps were reported in all studies with a total of 757 out of 1485 (51 %) found to be right-sided. All three studies reported the average polyp sizes which were not significantly different on comparison. The size averaged between 5.4 and 8.3 mm as provided in Table 1 .
Endoscopic mucosal resection
Submucosal injection before polypectomy for endoscopic mucosal resection (EMR) was performed for both groups in one study 19 ; however, it was only performed for selected polyps in the HSP group in the other two studies based on endoscopist preference 20 21 . The injected submucosal solution included 1:10 000 epinephrine in one of the studies that allowed submucosal injection for HSP only 21 while the other two studies did not use any epinephrine in the injected submucosal solution.
Incomplete resection rate
IRR was assessed via four-quadrant biopsy along with biopsy of the base of the post-polypectomy site in two studies 19 20 and biopsy of the left and right lateral margins in one study 21 . IRR was collectively found to be 2.4 % (15/630) and 4.7 % (30/636) for HSP and CSP group, respectively. The study heterogeneity was substantial ( I 2 = 73 %), hence a random effects model was used to assess the difference in incomplete resection. The pooled odds ratio was 0.51 (95 % CI 0.13 – 1.99, P = 0.33). The difference in IRR between HSP and CSP was not statistically significant ( Fig. 3 ).
Fig. 3.
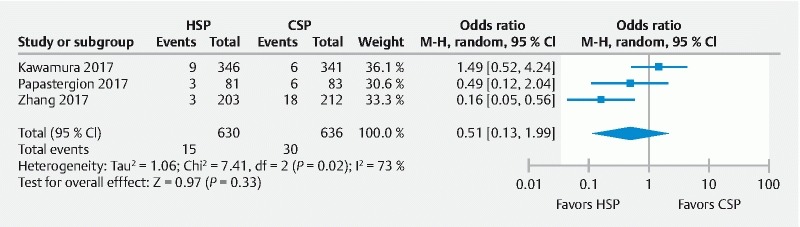
Forest plot of all RCTs assessing incomplete resection rate (IRR) between HSP/EMR and CSP group.
Adverse events
All three studies reported adverse events (immediate procedural bleeding, delayed bleeding, and perforations). A total of 741 polyps were removed using HSP and 744 polyps were removed employing CSP. Clinically and statistically, a lower rate of overall adverse events was observed with HSP compared to CSP (20/546 [3.7 %] vs. 36/545 [6.6 %], pooled OR 0.53, 95 % CI 0.3 – 0.94, P = 0.03; I 2 = 0 %) ( Fig. 4a ).
Fig. 4.
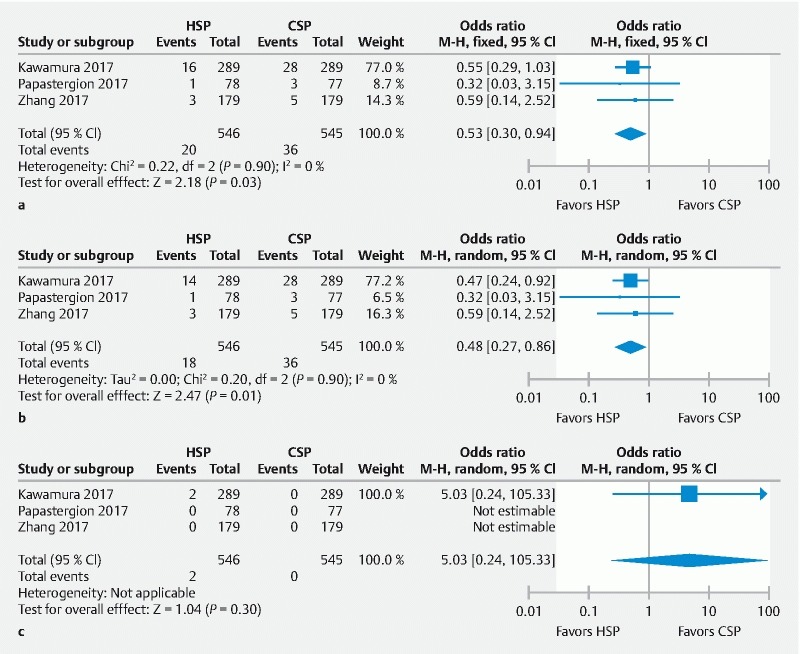
Forest plot of all RCTs assessing: a total complication rate; b immediate bleeding rate; c delayed bleeding rate between HSP/EMR and CSP group.
When assessing adverse events separately, immediate bleeding rate was lower in the HSP group (18/546 [3.3 %]) compared to the CSP group (36/545 [6.6 %]), yielding a pooled OR 0.48 (95 % CI 0.27 – 0.86, P = 0.01; I 2 = 0 %) ( Fig. 4b ). All patients with immediate post-polypectomy bleeding required endoscopic hemostasis to control bleeding and was clinically successful in all three studies. Two studies used hemostatic clip placement for endoscopic hemostasis 20 21 while one study did not mention the technique used for endoscopic hemostasis 19 .
The delayed bleeding rates were not different between the two groups (2/546 [0.4 %] vs. 0/545 [0 %]) ( Fig. 4c ). Delayed bleeding occurred in only two patients who underwent HSP in one of the studies 19 while the other two studies did not have any occurrence of delayed bleeding in either group. Since delayed bleeding was considered a “rare event” among the studies included, we used pooled risk difference as pooled estimate to detect if any difference existed between the groups. Pooled risk difference was zero and results were not statistically significant ( P = 0.45).
No case of perforation was reported in either group in any of the three studies. Preventive hemostasis, defined as prophylactic coagulation of vessels or red spots in the ulcer or clipping of a non-bleeding post-polypectomy mucosal defect, was allowed in the HSP group, but not in the CSP group in one study 19 .
Polyp retrieval rate
The polyp retrieval rate was not clinically or statistically significantly different between the two groups. Collective retrieval rates were 99 % for the HSP group and 98.1 % for the CSP group yielding a pooled OR of 1.9 (95 % CI 0.74 – 4.83, P = 0.18; I 2 = 0 %) as shown in Fig. 5 .
Fig. 5.
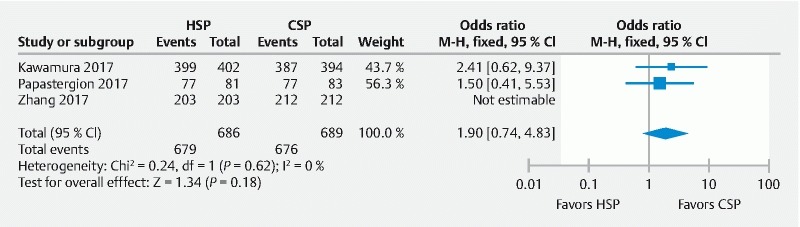
Forest plot of all RCTs assessing polyp retrieval rate between HSP/EMR and CSP group.
Procedure time
Procedural time was defined as the time required from identification of polyp to complete resection of the polyp using either technique 19 21 . Only two studies reported procedural time for polypectomies. Kawamura et al. reported median times of 83 seconds and 60 seconds for the HSP and CSP group, respectively 19 . Zhang et al. had comparatively longer times for their polypectomies with mean procedural times of 330 seconds and 282 seconds, respectively for the HSP and CSP group 21 .
Discussion
The results of this meta-analysis suggest that there is no significant difference between cold and hot snare in achieving a complete resection of 4 – 10 mm colorectal polyps, but there is a higher rate of adverse events with cold snare, albeit this is limited to immediate post-polypectomy bleeding that was amenable to endoscopic treatment in all of the cases.
The main result of our analysis is the equivalence between the two techniques in achieving a complete endoscopic resection. This is clinically relevant as completeness of resection represents by far the dominant end point when approaching the risk/benefit ratio of endoscopic resection for sub-centimetric lesions. Our meta-analysis, however, showed that there is residual uncertainty on the equivalence between the two techniques, as shown by the very high heterogeneity level in this estimate. In detail, in two of the three studies, the rate of incomplete resection was 2- to 6-fold higher with cold snare than hot snare 20 21 , while in the remaining study a very low rate of incomplete resection was shown in both of the arms 19 . The risk of incomplete resection with cold snare, was however < 10 % in all three trials, marginalizing the clinical impact of a possible superiority of the hot snare technique for such an end point. For instance, the overall 4.7 % rate of incomplete resection with cold-snaring is much lower than the nearly 20 % estimated for forceps biopsy when removing diminutive polyps 16 26 .
Adverse events related to both CSP and HSP include risk of bleeding (immediate bleeding during the procedure or delayed bleeding after colonoscopy) and intestinal perforation. In a large, prospective, non-randomized study, Repici et al. reported the rate of immediate bleeding for CSP to be 1.8 % 10 27 . The rate for immediate bleeding has been reported to be 0 – 1.4 % for HSP 13 15 . The immediate bleeding rates in our meta-analysis were 6.6 % and 3.3 % for CSP and HSP groups, respectively. There were two major limitations in the included studies which could have affected immediate bleeding rate between the two techniques. One of the three studies included allowed submucosal injection which also included epinephrine in the HSP group only 21 , and which could have decreased immediate post-polypectomy bleeding in the HSP group. Also, only one study 19 defined immediate bleeding to be continuous bleeding for ≥ 30 seconds while the other two studies did not provide any specific definition. Immediate oozing is destined to occur after cold snare polypectomy due to lack of coagulation current. In most cases, it is a slow, capillary bleeding that spontaneously stops and does not need intervention 13 15 .
The incidences of delayed post-polypectomy bleeding observed in previous prospective studies were 0.6 – 2 % and 0 % for the HSP and CSP groups, respectively 10 15 28 29 . The delayed bleeding rates in our study were 0.3 % and 0 % for the HSP and CSP groups, respectively, which are consistent with previous studies. As this was a rare event in our included studies, we used risk difference as pooled estimate; pooled risk difference was zero and results were not statistically significant ( P = 0.45) suggesting there was no difference between either method. Colonic perforation has been reported to be below 1 % using HSP and 0 % with CSP 30 31 . The overall adverse events for our meta-analysis were lower for the HSP group compared to CSP, with sub-analysis revealing immediate post-procedural bleeding to be lower for the HSP group. Clinically, this does not have any major significance as immediate bleeding is usually self-limiting and managed with observation on the majority of occasions or with post-procedural clipping immediately after polyp removal 10 32 . Since all three studies excluded patients treated with antithrombotic agents, the use of antithrombotic agents did not have any influence on post-polypectomy bleeding.
Several factors pose limitations to our meta-analysis. Firstly, despite a wide literature search on the main electronic databases, only three RCTs were available in the literature which were evaluated and compared in this systematic review and meta-analysis. Secondly, there was no follow-up colonoscopy for these patients to confirm “true” complete resection as polyps may recur in previously biopsied negative margin sites. Further studies are needed that not only biopsy the resection margins to confirm complete removal but also assess the patients at a follow-up surveillance colonoscopy and biopsy the scar area to determine the IRR. The post-polypectomy sites can also be evaluated by performing endoscopic mucosal resection of the 1 – 3 mm margin around the resection site to determine IRR as suggested by Matsuura et al. 33 . Third, the rates of incidence of colon cancer and mortality from colon cancer were not assessed in these studies which undermines the true efficacy of removing small colorectal polyps. Large, multicenter long-term studies are needed to assess the incidence of colon cancer when comparing the two techniques in removing small colorectal polyps. Moreover, two studies reported four-quadrant biopsy along with biopsy of base of polypectomy site compared to one study that biopsied only left and right resected margins to display any residual polypoid tissue. This can imply potential bias and possibly false IRR. There is potential bias involved with different physicians as each endoscopist has a different skill set with different procedural times. There was also substantial heterogeneity noted between the three included studies which could be partially explained by different types of snare used between studies for polypectomy and the difference in the utility of submucosal injection between CSP and HSP groups in the included studies. Also, immediate bleeding was only defined in one study, while the other studies used the term “intra-procedural bleeding” that was summed together as “immediate bleeding” for our systematic review. Finally, we only searched articles published in the English language and may have missed relevant studies published in other languages.
Despite the above limitations, the main strength of this meta-analysis is the fact that all three studies being compared were randomized controlled trials (RCTs) with a low risk of bias based on the Cochrane risk of bias assessment scale that demonstrated incomplete resection rate (IRR) using post-polypectomy biopsy to confirm residual tissue. Furthermore, all RCTs assessed polyp size between 4 and 10 mm which is in accordance with our study objective and further decreased the bias among polyp sizes.
In conclusion, our study findings show that there is no statistically significant difference between the two techniques, although the rate of overall complications is higher with CSP; however, this is self-limiting. Based on currently available RCTs, we suggest that, clinically, either CSP or HSP can be safely used as one of the standard techniques for the resection of 4 – 10 mm colorectal polyps. More studies are needed in future to assess recurrence of polyps and incidence of cancer to further gauge the efficacy of these two techniques.
Footnotes
Competing interests None
References
- 1.American Cancer Society Cancer Facts & Figures 2017Available from:https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf[cited 21 December 2017]
- 2.Winawer S, Zauber A, Ho M et al. Prevention of colorectal cancer by colonoscopic polypectomy. NEJM. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 3.Zauber A, Winawer S, O’Brien M et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. NEJM. 2012;366:687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pohl H, Srivastava A, Bensen S et al. Incomplete polyp resection during colonoscopy – results of the Complete Adenoma Resection (CARE) study. Gastroenterology. 2013;144:74–800. doi: 10.1053/j.gastro.2012.09.043. [DOI] [PubMed] [Google Scholar]
- 5.Hassan C, Pickhardt P, Kim D et al. Systematic review: distribution of advanced neoplasia according to polyp size at screening colonoscopy. Aliment Pharmacol Ther. 2010;31:210–217. doi: 10.1111/j.1365-2036.2009.04160.x. [DOI] [PubMed] [Google Scholar]
- 6.Gómez V, Badillo R, Crook J et al. Diminutive colorectal polyp resection comparing hot and cold snare and cold biopsy forceps polypectomy. Results of a pilot randomized, single-center study (with videos) Endosc Int Open. 2014;03:E76–E80. doi: 10.1055/s-0034-1390789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Metz A, Moss A, Mcleod D et al. A blinded comparison of the safety and efficacy of hot biopsy forceps electrocauterization and conventional snare polypectomy for diminutive colonic polypectomy in a porcine model. Gastrointest Endosc. 2013;77:484–490. doi: 10.1016/j.gie.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Yamashina T, Fukuhara M, Maruo T et al. Cold snare polypectomy reduced delayed postpolypectomy bleeding compared with conventional hot polypectomy: a propensity score-matching analysis. Endosc Int Open. 2017;05:E587–E594. doi: 10.1055/s-0043-105578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piraka C, Saeed A, Waljee A et al. Cold snare polypectomy for non-pedunculated colon polyps greater than 1 cm. Endosc Int Open. 2017;05:E184–E189. doi: 10.1055/s-0043-101696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Repici A, Hassan C, Vitetta E et al. Safety of cold polypectomy for < 10 mm polyps at colonoscopy: a prospective multicenter study. Endoscopy. 2011;44:27–31. doi: 10.1055/s-0031-1291387. [DOI] [PubMed] [Google Scholar]
- 11.Chukmaitov A, Bradley C, Dahman B et al. Polypectomy techniques, endoscopist characteristics, and serious gastrointestinal adverse events. J Surg Oncol. 2014;110:207–213. doi: 10.1002/jso.23615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aslan F, Camci M, Alper E et al. Cold snare polypectomy versus hot snare polypectomy in endoscopic treatment of small polyps. Turk J Gastroenterol. 2014;25:279–283. doi: 10.5152/tjg.2014.5085. [DOI] [PubMed] [Google Scholar]
- 13.Ichise Y, Horiuchi A, Nakayama Y et al. Prospective randomized comparison of cold snare polypectomy and conventional polypectomy for small colorectal polyps. Digestion. 2011;84:78–81. doi: 10.1159/000323959. [DOI] [PubMed] [Google Scholar]
- 14.Horiuchi A, Nakayama Y. 587g: Prospective randomized comparison of cold snare polypectomy and conventional polypectomy. Gastrointest Endosc. 2010;71:AB127. [Google Scholar]
- 15.Paspatis G, Tribonias G, Konstantinidis K et al. A prospective randomized comparison of cold vs hot snare polypectomy in the occurrence of postpolypectomy bleeding in small colonic polyps. Colorectal Dis. 2011;13:e345–e348. doi: 10.1111/j.1463-1318.2011.02696.x. [DOI] [PubMed] [Google Scholar]
- 16.Efthymiou M, Taylor A, Desmond P et al. Biopsy forceps is inadequate for the resection of diminutive polyps. Endoscopy. 2011;43:312–316. doi: 10.1055/s-0030-1256086. [DOI] [PubMed] [Google Scholar]
- 17.Fujiya M, Sato H, Ueno N et al. Efficacy and adverse events of cold vs hot polypectomy: A meta-analysis. World J Gastroenterol. 2016;22:5436. doi: 10.3748/wjg.v22.i23.5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.European Society of Gastrointestinal Endoscopy (ESGE) Colorectal polypectomy and endoscopic mucosal resection (EMR) 2018. Available from:http://www.esge.com/colorectal-polypectomy-and-endoscopic-mucosal-resection-emr-esge.html[cited 5 January 2018] [DOI] [PubMed]
- 19.Kawamura T, Takeuchi Y, Asai S et al. A comparison of the resection rate for cold and hot snare polypectomy for 4–9 mm colorectal polyps: a multicentre randomised controlled trial (CRESCENT study) Gut. 2018;67:1950–1957. doi: 10.1136/gutjnl-2017-314215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papastergiou V, Paraskeva K, Fragaki M et al. Cold versus hot endoscopic mucosal resection for nonpedunculated colorectal polyps sized 6–10 mm: a randomized trial. Endoscopy. 2018;50:403–411. doi: 10.1055/s-0043-118594. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Q, Gao P, Han B et al. Polypectomy for complete endoscopic resection of small colorectal polyps. Gastrointest Endosc. 2018;87:733–740. doi: 10.1016/j.gie.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 22.Endoscopic Classification Review Group . Update on the Paris Classification of superficial neoplastic lesions in the digestive tract. Endoscopy. 2005;37:570–578. doi: 10.1055/s-2005-861352. [DOI] [PubMed] [Google Scholar]
- 23.Schlemper R. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251–255. doi: 10.1136/gut.47.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shuster J. Review: Cochrane Handbook for Systematic Reviews for Interventions, Version 5.1.0, published 3/2011. Julian P.T. Higgins and Sally Green, Editors. Res Synth Methods. 2011;2:126–130. [Google Scholar]
- 25.Higgins J, Thompson S G, Deeks J J et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu S, Ho S, Krinsky M. Quality of polyp resection during colonoscopy: are we achieving polyp clearance? Dig Dis Sci. 2012;57:1786–1791. doi: 10.1007/s10620-012-2115-6. [DOI] [PubMed] [Google Scholar]
- 27.Levin T, Zhao W, Conell C et al. Complications of colonoscopy in an integrated health care delivery system. Ann Intern Med. 2006;145:880. doi: 10.7326/0003-4819-145-12-200612190-00004. [DOI] [PubMed] [Google Scholar]
- 28.Sorbi D, Norton I, Conio M et al. Postpolypectomy lower GI bleeding: Descriptive analysis. Gastrointest Endosc. 2000;51:690–696. doi: 10.1067/mge.2000.105773. [DOI] [PubMed] [Google Scholar]
- 29.Park S, Lee M, Jeong S et al. Prospective analysis of minor adverse events after colon polypectomy. Dig Dis Sci. 2017;62:2113–2119. doi: 10.1007/s10620-017-4586-y. [DOI] [PubMed] [Google Scholar]
- 30.Luigiano C, Consolo P, Scaffidi M et al. Endoscopic mucosal resection for large and giant sessile and flat colorectal polyps: a single-center experience with long-term follow-up. Endoscopy. 2009;41:829–835. doi: 10.1055/s-0029-1215091. [DOI] [PubMed] [Google Scholar]
- 31.Doniec M, Löhnert M, Schniewind B et al. Endoscopic removal of large colorectal polyps. Dis Colon Rectum. 2003;46:340–348. doi: 10.1007/s10350-004-6553-x. [DOI] [PubMed] [Google Scholar]
- 32.Heldwein W, Dollhopf M, Rösch T et al. The Munich Polypectomy Study (MUPS): Prospective analysis of complications and risk factors in 4000 colonic snare polypectomies. Endoscopy. 2005;37:1116–1122. doi: 10.1055/s-2005-870512. [DOI] [PubMed] [Google Scholar]
- 33.Matsuura N, Takeuchi Y, Yamashina T et al. Incomplete resection rate of cold snare polypectomy: a prospective single-arm observational study. Endoscopy. 2017;49:251–257. doi: 10.1055/s-0043-100215. [DOI] [PubMed] [Google Scholar]


