The treatment of estrogen receptor (ER)–positive metastatic breast cancer has been dramatically transformed through the introduction of ATP-competitive inhibitors of the cyclin-dependent kinases 4 and 6 (CDK4/6i) in combination with antiestrogen therapies. Seven phase III studies demonstrated clear improvements in progression-free survival through the addition of these agents in either the first- or later-line setting, with new studies underway to evaluate the benefit of these agents as adjuvant therapy.1-7 A core scientific premise backing these agents is that in a large subset of tumors, ER inhibition is only partially effective at blocking the G1 checkpoint kinases CDK4/6, either because of ineffective ER inhibition (eg, ESR1 mutations)8-10 or hormone-independent inputs into CDK4/6 activation (eg, NF1 loss).11 The addition of highly selective CDK4/6 kinase inhibitors enables more potent and durable blockade of the cell cycle, which may accrue additional benefits such as inducing tumor cell senescence or augmenting antitumor immunity.
Despite the clear benefit of this combination, a subset of cancers (10% to 20%) remain insensitive, whereas a much larger group of cancers (70% to 80%) become resistant after 12 to 36 months of therapy.1-7 Even with such great clinical heterogeneity, there remain virtually no biomarkers to separate these subgroups of patients. This is particularly poignant given the potential efficacy of alternative forms of therapy in ER-positive metastatic breast cancer, including mammalian target of rapamycin inhibition (everolimus)12 as well as chemotherapy. In the companion article, Turner et al13 use pretreatment tumor samples from the PALOMA-3 (Palbociclib [PD-0332991] Combined With Fulvestrant In Hormone Receptor+ HER2-Negative Metastatic Breast Cancer After Endocrine Failure) trial of fulvestrant versus fulvestrant plus palbociclib to investigate biomarkers that may identify tumors that are de novo resistant to the fulvestrant plus palbociclib combination and, specifically, find high levels of cyclin E1 (CCNE1) associated with an attenuated benefit.
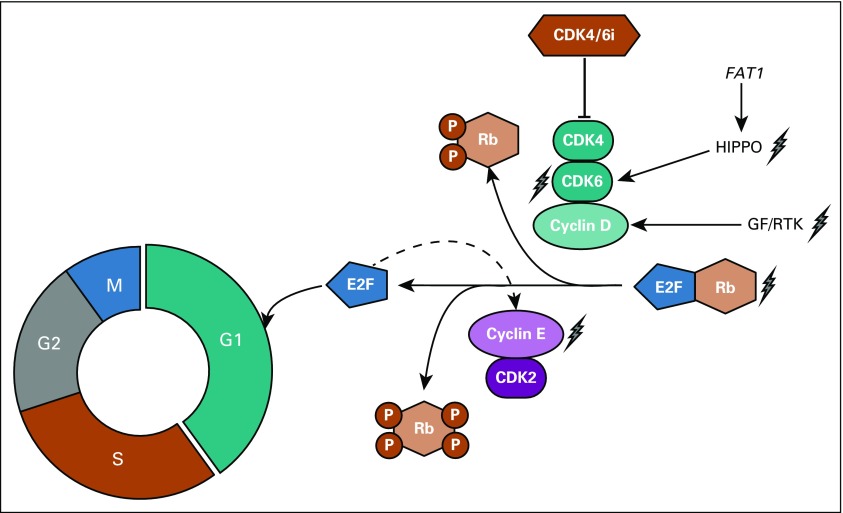
As a backdrop to this investigation, several groups have used preclinical models to help nominate potential genomic alterations that may promote resistance to CDK4/6 inhibitors in the clinic. The work, depicted in Fig 1, has identified: (1) mechanisms that bypass the requirement for G1 checkpoint kinases to phosphorylate Rb and release E2F, and (2) mechanisms that hyperactivate the G1 checkpoint kinases and thereby render the drugs insufficiently potent. With respect to the former, loss of RB1 expression has been noted in subsets of models and patient samples and ably causes drug resistance.14-17 However, this event is rare enough in ER-positive breast cancer11,18-21 that preemptive exclusion of patients with RB1 loss was largely abandoned in the clinical trials in ER-positive breast cancer, to no apparent detriment.
FIG 1.
Putative mechanisms of resistance to CDK4/6i. Cartoon shows G1 checkpoint kinases CDK4/6 coupled to D-cyclins promoting cell cycle progression through the G1-S transition by phosphorylating Rb and thereby inducing release of E2F. E2F further supports Cyclin E (CCNE1) expression by increasing Rb phosphorylation and thereby accelerating progression. Potential mechanisms of drug resistance are denoted by lightning symbol with hyperactivation of CDK4/6 activity, loss of Rb, or hyperactivation of CCNE1-CDK2 representing potential mechanisms. CDK, cyclin-dependent kinase; GF, growth factor; P, phosphorylation; RTK, receptor tyrosine kinase.
Apart from RB1 loss, a number of different mechanisms (Fig 1) have been identified that result in restored Rb phosphorylation, such as amplification of CDK6,22 hyperactivation of growth factor signaling,23 and aberrant activation of CCNE1-CDK2 downstream of CDK4/6.23-25 Among these, it is notable that CCNE1 is not only a potential mediator of resistance. In addition, CCNE1 overexpression may prove to be a sensor of other mechanisms of resistance. This results in a constitutively bypassed G1 checkpoint because CCNE1 transcription is induced by E2F itself. In their study, Turner et al13 attempted to identify transcripts that may reflect these different states of resistance and highlight the finding of high levels of CCNE1 being associated with reduced response to palbociclib.
To identify potential biomarkers that predicted intrinsic resistance to the combination of palbociclib and fulvestrant in the PALOMA-3 clinical trial,1 Turner et al13 made use of 194 samples (92 of 194 metastatic samples) from the combination arm and 108 samples (50 of 108 metastatic samples) from the fulvestrant-only arm. These samples were subjected to a well-validated and relatively small-panel mRNA profiling platform (EdgeSeq Oncology platform; 2,534 genes; HTG Molecular Diagnostics, Inc., Tucson, AZ). The effect of 10 genes relevant to the G1 checkpoint were primarily examined. In this analysis, CCNE1 mRNA levels correlated with the degree of benefit. Tumors with low levels of CCNE1 showed marked improvement with the addition of palbociclib (median progression-free survival, 14.1 months in the palbociclib arm v 4.8 months in the placebo arm). Tumors with high levels of CCNE1 had an attenuated benefit (median progression-free survival, 7.6 months in the palbociclib arm v 4.0 months in the placebo arm). Curiously, these effects were mainly evident when metastatic tumor samples were used, suggesting that tumor samples collected temporally closer to the start of therapy may more accurately represent the genomic landscape of the disease than archival samples. The other nine target genes examined, including CDK2 and RB1, did not show a significant difference by expression. Importantly, the authors attempted to validate the finding of CCNE1 mRNA and response in an independent cohort of patients with breast cancer from the Preoperative Palbociclib (POP) adjuvant trial. They found that high CCNE1 levels were associated with impaired reduction in tumor proliferation (Ki67) by palbociclib treatment. Finally, the authors performed an unbiased screening analysis across the 2,534 genes included in the assay. Interestingly, they found that high levels of p18 (CDKN2C) and p19 (CDKN2D; both of which are endogenous inhibitors of CDK4/6) were also associated with reduced response to palbociclib.
The work is commendable on multiple levels. First, the trial successfully collected metastatic tissues on a large proportion of patients enrolled in a multicenter phase III trial and then collaborated to validate their key finding in another trial. Second, the search for biomarkers of response used both candidate and unbiased methods, enabling a robust finding of CCNE1. Third, the validation of CCNE1 ties together the biology of CCNE1-CDK2 as an alternative means to Rb phosphorylation and the dynamic activation of CCNE1 transcription in response to E2F activity. Finally, the work helps bring to the fore a biomarker for a candidate set of tumors for CDK2-selective inhibitors currently in development.
There are a few important cautions and questions for future work, which are naturally raised by the study. First and critically, a top-level statement must be made that even patients with high levels of CCNE1 derived benefit from the addition of palbociclib to fulvestrant. This may be as the result of many reasons. These include heterogeneity and time required for selection of resistant subclones under the pressure of drug therapy, spatial tumor heterogeneity resulting in incomplete representation of disease status in the biopsied tumor tissue, intrinsic deficiencies of the assay and cut points used, among others. For instance, CCNE1 mRNA may only partially reflect the true state of CCNE1-CDK2 activity such that other factors (eg, CCNE1 isoforms, localization, and even proteostasis) may be of significance.26-29 Irrespective of the intriguing results reported by Turner et al,13 physicians should continue their current practice of adding CDK4/6i to endocrine therapy without reference to any biomarkers.
A second point raised is the lack of accounting for what caused reduced response in patients who derived little benefit but did not have high levels of CCNE1. To this point, a single, steady state look at mRNA may prove an inadequate reflection of the underlying biology of the tumor and potential resilience. Perhaps an augmentation to these data will come through the use of provocative biomarkers such as the response of transcripts, circulating tumor DNA, or proteins to a challenge of CDK4/6i exposure. Moreover, integration with less dynamic measures such as DNA mutations (eg, RB1) may further help to provide a composite marker of nonresponders.30 Finally, although studying a panel of 2,534 genes at a single time point is far from trivial, there are a number of other potential inputs into cancer cell growth control. These include unexpected players such as the FAT1/Hippo pathway, which was recently identified as a mediator of CDK4/6i resistance.30 Therefore, ongoing efforts to survey broadly are needed.
We laud Turner et al13 for conducting this outstanding study of biomarkers of response to CDK4/6i in metastatic breast cancer. The work strongly validates the investigators’ prespecified collection of tissues for understanding biology and developing future biomarkers. It nominates CCNE1 as a specific transcript that, when elevated, seems to predict a reduced response to CDK4/6i.
Footnotes
See accompanying article on page 1169
AUTHOR CONTRIBUTIONS
Manuscript writing: Sarat Chandarlapaty, Pedram Razavi
Final approval of manuscript: Sarat Chandarlapaty, Pedram Razavi
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Cyclin E mRNA: Assessing Cyclin-Dependent Kinase (CDK) Activation State to Elucidate Breast Cancer Resistance to CDK4/6 Inhibitors
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Sarat Chandarlapaty
Consulting or Advisory Role: Sermonix Pharmaceuticals, Novartis, Revolution Medicines, Context Therapeutics
Research Funding: Novartis (Inst)
Travel, Accommodations, Expenses: Novartis, Sun Pharma
Pedram Razavi
Consulting or Advisory Role: Novartis
Research Funding: GRAIL (Inst), Illumina (Inst)
REFERENCES
- 1.Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–439. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 2.Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 3.Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375:1738–1748. doi: 10.1056/NEJMoa1609709. [DOI] [PubMed] [Google Scholar]
- 4.Sledge GW, Jr, Toi M, Neven P, et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35:2875–2884. doi: 10.1200/JCO.2017.73.7585. [DOI] [PubMed] [Google Scholar]
- 5.Goetz MP, Toi M, Campone M, et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35:3638–3646. doi: 10.1200/JCO.2017.75.6155. [DOI] [PubMed] [Google Scholar]
- 6.Slamon DJ, Neven P, Chia S, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018;36:2465–2472. doi: 10.1200/JCO.2018.78.9909. [DOI] [PubMed] [Google Scholar]
- 7.Tripathy D, Im SA, Colleoni M, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): A randomised phase 3 trial. Lancet Oncol. 2018;19:904–915. doi: 10.1016/S1470-2045(18)30292-4. [DOI] [PubMed] [Google Scholar]
- 8.Toy W, Shen Y, Won H, et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat Genet. 2013;45:1439–1445. doi: 10.1038/ng.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson DR, Wu YM, Vats P, et al. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat Genet. 2013;45:1446–1451. doi: 10.1038/ng.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeselsohn R, Yelensky R, Buchwalter G, et al. Emergence of constitutively active estrogen receptor-α mutations in pretreated advanced estrogen receptor-positive breast cancer. Clin Cancer Res. 2014;20:1757–1767. doi: 10.1158/1078-0432.CCR-13-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Razavi P, Chang MT, Xu G, et al. The genomic landscape of endocrine-resistant advanced breast cancers. Cancer Cell. 2018;34:427–438.e6. doi: 10.1016/j.ccell.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turner NC, Liu Y, Zhu Z, et al. Cyclin E1 expression and palbociclib efficacy in previously treated hormone receptor–positive metastatic breast cancer. J Clin Oncol. 2019;37:1169–1178. doi: 10.1200/JCO.18.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsushime H, Ewen ME, Strom DK, et al. Identification and properties of an atypical catalytic subunit (p34PSK-J3/cdk4) for mammalian D type G1 cyclins. Cell. 1992;71:323–334. doi: 10.1016/0092-8674(92)90360-o. [DOI] [PubMed] [Google Scholar]
- 15.DeCaprio JA, Ludlow JW, Lynch D, et al. The product of the retinoblastoma susceptibility gene has properties of a cell cycle regulatory element. Cell. 1989;58:1085–1095. doi: 10.1016/0092-8674(89)90507-2. [DOI] [PubMed] [Google Scholar]
- 16.Fry DW, Harvey PJ, Keller PR, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004;3:1427–1438. [PubMed] [Google Scholar]
- 17.Dean JL, Thangavel C, McClendon AK, et al. Therapeutic CDK4/6 inhibition in breast cancer: Key mechanisms of response and failure. Oncogene. 2010;29:4018–4032. doi: 10.1038/onc.2010.154. [DOI] [PubMed] [Google Scholar]
- 18.Stephens PJ, Tarpey PS, Davies H, et al. The landscape of cancer genes and mutational processes in breast cancer. Nature. 2012;486:400–404. doi: 10.1038/nature11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ciriello G, Gatza ML, Beck AH, et al. Comprehensive molecular portraits of invasive lobular breast cancer. Cell. 2015;163:506–519. doi: 10.1016/j.cell.2015.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nik-Zainal S, Davies H, Staaf J, et al. Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature. 2016;534:47–54. doi: 10.1038/nature17676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang C, Li Z, Bhatt T, et al. Acquired CDK6 amplification promotes breast cancer resistance to CDK4/6 inhibitors and loss of ER signaling and dependence. Oncogene. 2017;36:2255–2264. doi: 10.1038/onc.2016.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor-Harding B, Aspuria PJ, Agadjanian H, et al. Cyclin E1 and RTK/RAS signaling drive CDK inhibitor resistance via activation of E2F and ETS. Oncotarget. 2015;6:696–714. doi: 10.18632/oncotarget.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrera-Abreu MT, Palafox M, Asghar U, et al. Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor-positive breast cancer. Cancer Res. 2016;76:2301–2313. doi: 10.1158/0008-5472.CAN-15-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma CX, Gao F, Luo J, et al. NeoPalAna: Neoadjuvant palbociclib, a cyclin-dependent kinase 4/6 inhibitor, and anastrozole for clinical stage 2 or 3 estrogen receptor-positive breast cancer. Clin Cancer Res. 2017;23:4055–4065. doi: 10.1158/1078-0432.CCR-16-3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porter DC, Zhang N, Danes C, et al. Tumor-specific proteolytic processing of cyclin E generates hyperactive lower-molecular-weight forms. Mol Cell Biol. 2001;21:6254–6269. doi: 10.1128/MCB.21.18.6254-6269.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delk NA, Hunt KK, Keyomarsi K. Altered subcellular localization of tumor-specific cyclin E isoforms affects cyclin-dependent kinase 2 complex formation and proteasomal regulation. Cancer Res. 2009;69:2817–2825. doi: 10.1158/0008-5472.CAN-08-4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nanos-Webb A, Jabbour NA, Multani AS, et al. Targeting low molecular weight cyclin E (LMW-E) in breast cancer. Breast Cancer Res Treat. 2012;132:575–588. doi: 10.1007/s10549-011-1638-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunt KK, Karakas C, Ha MJ, et al. Cytoplasmic cyclin E predicts recurrence in patients with breast cancer. Clin Cancer Res. 2017;23:2991–3002. doi: 10.1158/1078-0432.CCR-16-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Z, Razavi P, Li Q, et al. Loss of the FAT1 tumor suppressor promotes resistance to CDK4/6 inhibitors via the Hippo pathway. Cancer Cell. 2018;34:893–905.e8. doi: 10.1016/j.ccell.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]