Abstract
PURPOSE
A large-panel gene expression analysis was conducted to identify biomarkers associated with the effectiveness of adding palbociclib to fulvestrant.
METHODS
The PALOMA-3 (ClinicalTrials.gov identifier: NCT01942135) trial randomly assigned 521 endocrine-pretreated patients with metastatic breast cancer to receive palbociclib plus fulvestrant or placebo plus fulvestrant. Primary analysis was first conducted on 10 genes on the basis of pathway biology and evidence from previous studies followed by a systematic panel-wide search among 2,534 cancer-related genes. The association of gene expression with the effect of palbociclib on progression-free survival (PFS) was evaluated using Cox proportional hazards regression analysis, with gene expression as a continuous variable or dichotomized by median. An independent breast cancer cohort from the Preoperative Palbociclib (POP) Clinical Trial (ClinicalTrials.gov identifier: NCT02008734) was used for validation, in 61 patients with primary breast cancer treated with 2 weeks of palbociclib.
RESULTS
In the PALOMA-3 trial, 302 patients had tumor tissue analyzed (palbociclib arm, 194 patients; placebo arm, 108 patients). Palbociclib efficacy was lower in patients with high versus low cyclin E1 (CCNE1) mRNA expression (median PFS: palbociclib arm, 7.6 v 14.1 months; placebo arm, 4.0 v 4.8 months, respectively; interaction P unadjusted = .00238; false discovery rate–adjusted P = .0238). CCNE1 mRNA was more predictive in metastatic than in archival primary biopsy tissue samples. No significant interaction was found between treatment and expression levels of CDK4, CDK6, cyclin D1, and RB1. Palbociclib was efficacious in both luminal A and luminal B tumors. High CCNE1 mRNA expression was associated with poor antiproliferative activity of palbociclib in the POP trial (P = .005).
CONCLUSION
Addition of palbociclib to fulvestrant demonstrated efficacy in all biomarker groups, although high CCNE1 mRNA expression was associated with relative resistance to palbociclib.
INTRODUCTION
Palbociclib is an oral cyclin-dependent kinase (CDK) 4/6 inhibitor that decreases retinoblastoma protein (RB) phosphorylation, which blocks cell cycle progression from the G1 to the S phase and reduces proliferation of breast cancer cells.1-3 Large, randomized, prospective clinical studies have demonstrated the efficacy and safety of palbociclib in combination with letrozole or fulvestrant,4-7 which supports palbociclib plus an aromatase inhibitor or fulvestrant as a standard of care for treating hormone receptor–positive, human epidermal growth factor receptor 2 (HER2)–negative metastatic breast cancer (MBC) in premenopausal or postmenopausal women.2,3,8 Extensive analyses have shown that clinical subgroups derive similar benefit from palbociclib combination treatment.9-11 Identification of biomarkers would assist in distinguishing patient subgroups that would derive the greatest benefit from palbociclib as well as in elucidating resistance mechanisms that could lead to rational selection of patients with CDK4/6 combination therapy.
Preclinical research has suggested potential mechanisms of resistance to CDK4/6 inhibitors, including bypass activation of CDK2,12 with high cyclin E1 (CCNE1) expression correlated with palbociclib resistance in cell line models of breast and ovarian cancer.13,14 Other studies have shown that CDK6 amplification is associated with acquired resistance to CDK4/6 inhibitors15 and that luminal subtype breast cancer cell lines are more responsive to CDK4/6 inhibitors than nonluminal subtypes.16 In the small nonrandomized study, Neoadjuvant Palbociclib, a Cyclin-Dependent Kinase 4/6 Inhibitor, and Anastrozole for Clinical Stage 2 or 3 Estrogen Receptor–Positive Breast Cancer (NeoPalAna), exploratory analysis showed that high levels of CCNE1 and CDKN2D mRNA may predict palbociclib resistance.17
No predictive biomarkers have been identified in randomized trials of CDK4/6 inhibitors. In PALOMA-1, neither CCND1 amplification nor p16 loss was predictive for palbociclib efficacy.5 In PALOMA-2, CDK4 and CDK6 expression were not predictive of efficacy for palbociclib plus letrozole.18 In PALOMA-3, neither estrogen receptor 1 (ESR1) nor phosphatidylinositol-4,5-biphosphate 3-kinase (PIK3CA) mutations predicted palbociclib plus fulvestrant efficacy.4,19 In addition, data from the Preoperative Palbociclib (POP) Randomized Clinical Trial showed that PIK3CA mutations and CCND1 amplification are not predictive for palbociclib efficacy.20
We describe herein an analysis of baseline tumor tissue from PALOMA-3. We used a large gene expression panel to identify predictive biomarkers for the relative efficacy of adding palbociclib to fulvestrant.
METHODS
Samples
PALOMA-3 randomly assigned 521 patients with endocrine-pretreated MBC to receive palbociclib plus fulvestrant or placebo plus fulvestrant.4 This study was approved by an institutional review board or independent ethics committee at each site; all patients provided informed consent before enrollment. Patients consented to the assessment of biomarkers associated with sensitivity or resistance to palbociclib combination treatment per study protocol. Except for patients with bone-only disease or relapse while on adjuvant therapy and who had surgery within 3 years who could provide an archival primary sample, all patients provided formalin-fixed paraffin-embedded (FFPE) tissue taken from metastatic disease. One FFPE tissue sample (two slides per patient) was stained with hematoxylin and eosin, and a board-certified pathologist assessed tumor content and tissue necrosis (additional details provided in the Data Supplement).
To independently validate the association between CCNE1 mRNA expression and efficacy of palbociclib, we analyzed gene expression data from 61 patients in the POP trial (Data Supplement).21 This trial allocated women with untreated early-stage breast cancer three to one to receive oral palbociclib for 14 days until the day before surgery or to no treatment.
Gene Expression Analysis
The EdgeSeq Oncology BM Panel (HTG Molecular Diagnostics, Tucson, AZ) was used for mRNA profiling, which assessed 2,534 cancer-related genes. Gene expression analysis was performed while blinded to the clinical information. The EdgeSeq system used targeted capture sequencing to quantitate RNA expression levels of gene targets in FFPE tissues and was extensively validated (Data Supplement). Sample preparation was conducted by following the laboratory process and manufacturer protocols. Sequencing was performed on a NextSeq 500 sequencer (Illumina, San Diego, CA). Raw data are deposited in the Gene Expression Omnibus. In the POP trial, CCNE1 mRNA expression data were obtained from gene expression analysis on the GeneChip Human Gene Array ST2.1 (Affymetrix, Santa Clara, CA).
Gene Expression Hypothesis-Driven Statistical Analysis
Gene expression data were quantile normalized and log2 transformed (HTG Molecular Diagnostics). Hypothesis-driven analysis was conducted on 10 genes on the basis of pathway biology and evidence from preclinical and the NeoPalAna studies.17 Cox proportional hazards regression analysis was performed to investigate potential interactions between biomarker levels, as a continuous variable or dichotomized by median level, and treatment effects in terms of progression-free survival (PFS). Interaction P values were adjusted using the Benjamini-Hochberg false discovery rate (FDR) to account for multiplicity. Varying levels of CCNE1 mRNA expression by treatment interaction was evaluated further using the nonparametric subpopulation treatment effect pattern plot (STEPP) method22 (Data Supplement). Data analyses were performed using R (www.r-project.org) and MATLAB (MathWorks, Natick, MA) statistical software. All tests were two-sided, unless otherwise noted.
To investigate whether higher levels of CCNE1 mRNA were associated with lower absolute antiproliferative response (ln protein encoded by the MKI67 gene [Ki-67] < 1% at day 15) in the POP trial, we performed a Cochran-Armitage test for trend using the three tertiles of CCNE1 mRNA expression. We also performed an analysis of covariance of the change from baseline in ln Ki-67 in the palbociclib arm across the three CCNE1 mRNA tertiles. Analyses were carried out on the basis of a two-sided significance level of .05.
Molecular Subtype Classification
Only hormone receptor–positive patients comprised the PALOMA-3 cohort, with no large diverse reference tumor sets profiled with the EdgeSeq Oncology platform, which limited classification with PAM50 (Prosigna; NanoString Technologies, Seattle, WA) where the subtype is determined relative to a baseline of heterogeneous tumors.23 The absolute intrinsic molecular subtyping single-sample predictor algorithm thus was applied to assign subtypes through a set of binary rules that compare expression measurements for pairs of genes from a single patient24 (Data Supplement).
Exploratory Unbiased Discovery Statistical Analysis
A data-driven exploratory unbiased discovery analysis was performed for gene expression biomarkers suggestive of greater efficacy from adding palbociclib to fulvestrant. Using Cox proportional hazards regression analysis, the search was initially narrowed to genes whose expression as a continuous variable was significantly associated with treatment effect within the palbociclib arm (P < .01); a cross-arm interaction analysis was then performed on these genes. To investigate further the underlying biological processes that mediate palbociclib plus fulvestrant response, all genes were sorted by the coefficient values of the expression-treatment interaction from continuous analysis followed by gene set enrichment analyses (Data Supplement).
RESULTS
Gene Expression Analysis of PALOMA-3 Tumor Tissue
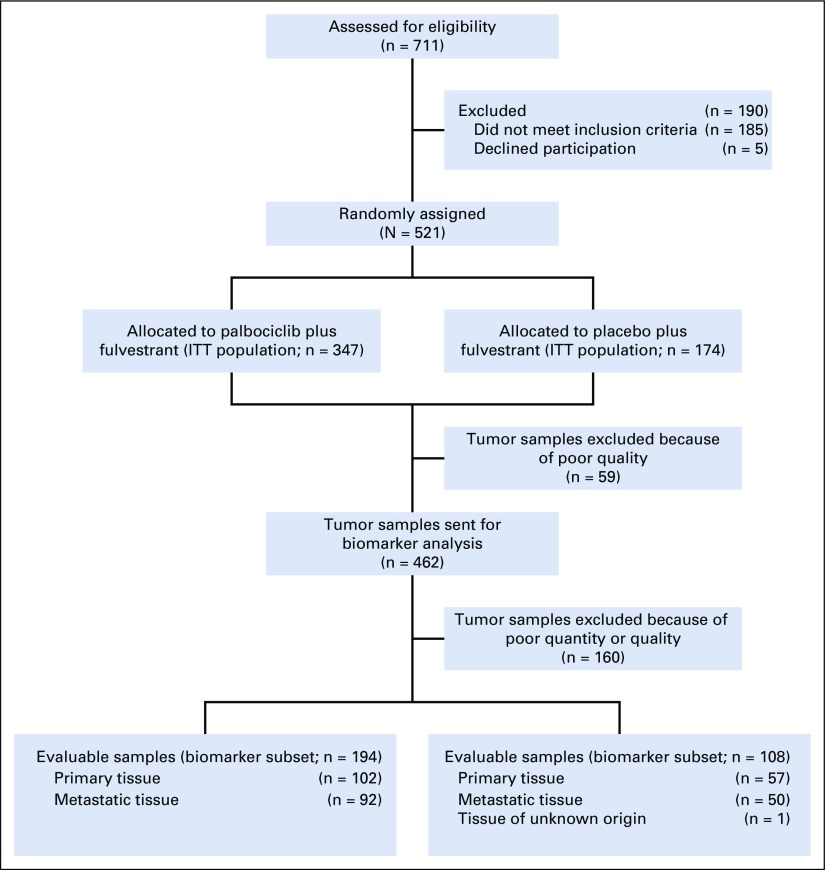
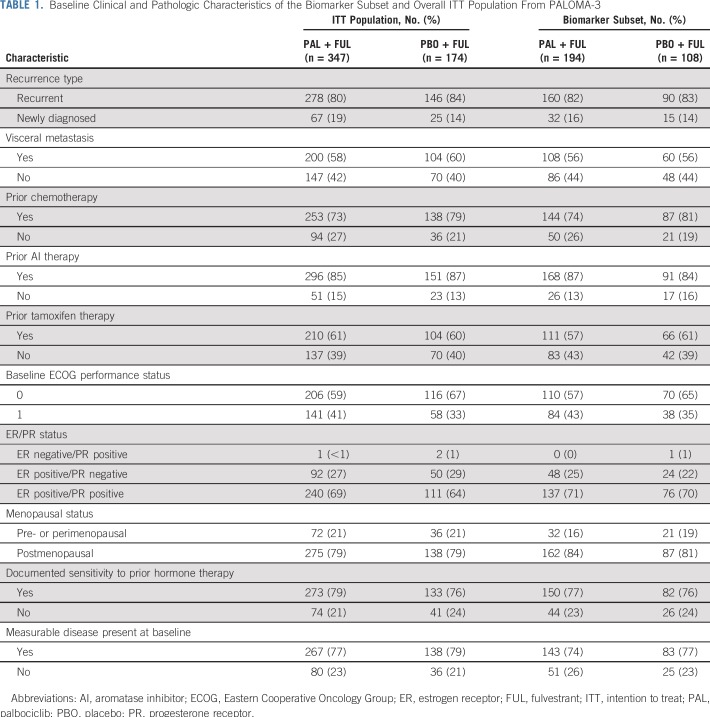
In total, 462 tumor samples from 521 patients were analyzed by the HTG EdgeSeq Oncology panel for gene expression (Fig 1), with 302 tumor samples evaluable for analysis (159 archival primary samples [53%] and 142 metastatic biopsy samples [47%]). Of the evaluable samples, 194 (64%) were from the palbociclib arm (102 primary samples and 92 metastatic samples), and 108 (36%) were from the placebo arm (57 primary samples and 50 metastatic samples). Baseline clinical and pathologic characteristics (Table 1) and PFS (Data Supplement) were similar between the biomarker and overall PALOMA-3 populations. Gene expression of ESR1 mRNA and progesterone receptor (PR) mRNA showed high correlation with protein expression of the estrogen receptor (ER; Spearman r = 0.54; P < .001) and PR (Spearman r = 0.77; P < .001) as assessed centrally by immunohistochemistry H-score at the same time point (Data Supplement). ER and PR H-scores were most correlated with their own transcripts across all genes in the EdgeSeq Oncology panel.
FIG 1.
CONSORT diagram of breast cancer tissues analyzed for gene expression. ITT, intention to treat.
TABLE 1.
Baseline Clinical and Pathologic Characteristics of the Biomarker Subset and Overall ITT Population From PALOMA-3
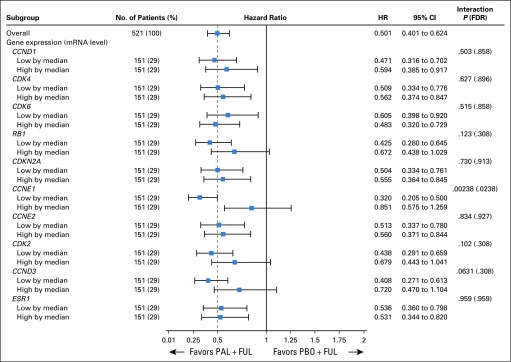
Our primary hypothesis was that the expression of CDK4/6-RB1 axis genes would have an impact on the addition of palbociclib to fulvestrant. Expression of CDK4, CDK6, and CCND1 mRNA were not predictive of palbociclib efficacy (Fig 2; Data Supplement). Similarly, although ESR1 mRNA expression was prognostic with low expression associated with shorter PFS in both treatment arms, the efficacy of palbociclib did not differ significantly by ESR1 mRNA expression level (Data Supplement).
FIG 2.
Association of cell cycle pathway gene expression and the efficacy of palbociclib (PAL) in combination with fulvestrant (FUL). Expression of cell cycle pathway genes dichotomized by median expression, with hazard ratios (HRs) for progression-free survival of PAL plus FUL versus placebo (PBO) plus FUL. HRs were derived from a Cox proportional hazards regression model. Interaction P value for statistical interaction between gene expression and treatment. CCND1, cyclin D1; CCND3, cyclin D3; CCNE1, cyclin E1; CCNE2, cyclin E2; CDK2, cyclin-dependent kinase 2; CDK4, cyclin-dependent kinase 4; CDK6, cyclin-dependent kinase 6; CDKN2A, cyclin-dependent kinase inhibitor 2A; ESR1, estrogen receptor 1; FDR, false discovery rate; RB1, retinoblastoma 1.
CCNE1 mRNA Expression Is Predictive of Palbociclib Efficacy When Assessed in Metastatic Tissues
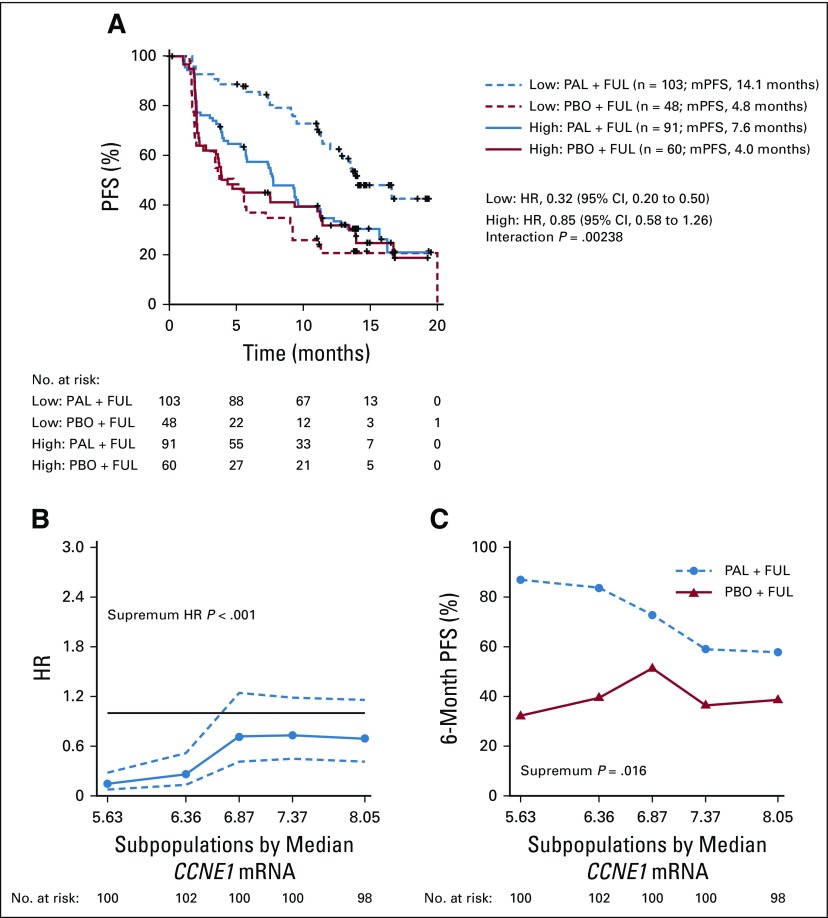
In line with prior preclinical evidence, lower CCNE1 mRNA expression was associated with improved efficacy from palbociclib (Fig 3A). Division of samples by median CCNE1 mRNA expression value showed that the median PFS of patients with high CCNE1 mRNA levels was 7.6 months with palbociclib plus fulvestrant and 4.0 months with placebo plus fulvestrant (hazard ratio [HR], 0.85; 95% CI, 0.58 to 1.26), whereas the median PFS of those with lower CCNE1 mRNA levels was 14.1 months with palbociclib plus fulvestrant and 4.8 months with placebo plus fulvestrant (HR, 0.32; 95% CI, 0.20 to 0.50), with a significant interaction between treatment effect and CCNE1 mRNA expression (unadjusted P = .00238; FDR P = .0238; Figs 2 and 3A). The interaction with CCNE1 mRNA remained significant after accounting for baseline clinicopathologic characteristics, including recurrence type, tumor tissue collection site, baseline Eastern Cooperative Oncology Group performance status, visceral metastases, prior chemotherapy, prior aromatase inhibitor therapy, and prior tamoxifen therapy (P = .00167).
FIG 3.
Association between cyclin E1 (CCNE1) mRNA expression and palbociclib (PAL) efficacy. (A) Progression-free survival (PFS) in tumors with low or high CCNE1 mRNA expression by median. Hazard ratios (HRs) were derived from a Cox proportional hazards regression model. P value from the interaction test between gene expression and treatment. (B) Subpopulation treatment effect pattern plot analysis of CCNE1 mRNA expression as measured by HR (PAL plus fulvestrant [FUL] v placebo [PBO] plus FUL). The x-axis represents the median CCNE1 mRNA expression for patients in each overlapping subpopulation. The dashed lines represent the corresponding 95% pointwise CIs. The solid black line indicates a reference HR of 1, with HR less than 1 favoring the PAL plus FUL combination. (C) Subpopulation treatment effect pattern plot analysis of CCNE1 mRNA expression as measured by 6-month PFS rates. mPFS, median progression-free survival.
The STEPP analysis also supported a significant interaction between CCNE1 mRNA expression and the relative treatment effect on the basis of the HRs across CCNE1 mRNA expression levels (supremum HR P < .001; Fig 3B). STEPP analysis of absolute treatment effect on the basis of 6-month PFS across CCNE1 mRNA expression levels consistently provided evidence of heterogeneous treatment effects related to CCNE1 mRNA expression (P = .016; Fig 3C).
The source of tumor biopsy had an impact on the association between CCNE1 mRNA expression and palbociclib efficacy. CCNE1 mRNA was highly predictive in metastatic biopsies (n = 142; interaction P < .001) but marginal in primary biopsy samples (n = 159; interaction P = .09). Of note, primary and metastatic biopsy samples expressed similar levels of CCNE1 mRNA at baseline (P = .57; Data Supplement), which suggests that a more contemporaneous assessment of gene expression may explain the improved prediction power of assessment in metastatic biopsy samples. Tumors with documented sensitivity to prior hormone therapy tended to have lower CCNE1 mRNA expression levels (P = .0032; Data Supplement). Overall, 54% of primary and 52% of metastatic tumor samples were supplied by slides, whereas 45% of primary and 47% of metastatic samples were supplied by blocks. As anticipated, both ER and PR immunohistochemistry levels were significantly higher in blocks than in slides (P < .001 and P = .004, respectively). CCNE1 mRNA levels were not affected by block versus slide tissue type analyzed (slide v block P = .085).
Independent Validation of High CCNE1 mRNA as a Marker of Palbociclib Resistance in the POP Trial
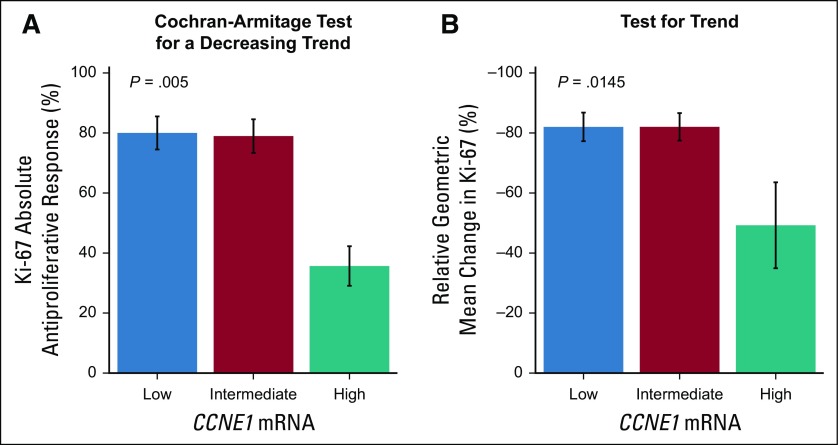
In the POP trial, high CCNE1 mRNA expression was associated with lower absolute antiproliferative response to palbociclib (high CCNE1 mRNA, 36%; intermediate CCNE1 mRNA, 79%; low CCNE1 mRNA, 80%; P = .005; Fig 4A). High CCNE1 mRNA expression also was associated with a reduced geometric mean change in Ki-67 with palbociclib treatment (high CCNE1 mRNA, –49%; intermediate CCNE1 mRNA, –82%; low CCNE1 mRNA, –82%; P = .015; Fig 4B).
FIG 4.
Independent validation of high cyclin E1 (CCNE1) mRNA as a marker of palbociclib resistance in the Modulation of Rb Phosphorylation and Antiproliferative Response to Palbociclib: The Preoperative-Palbociclib (POP) Randomized Clinical Trial. (A) Antiproliferative response by CCNE1 expression tertile. (B) Geometric mean change in protein encoded by the MKI67 gene (Ki-67) expression with palbociclib treatment by CCNE1 expression tertile.
Intrinsic Subtypes and Efficacy of Palbociclib
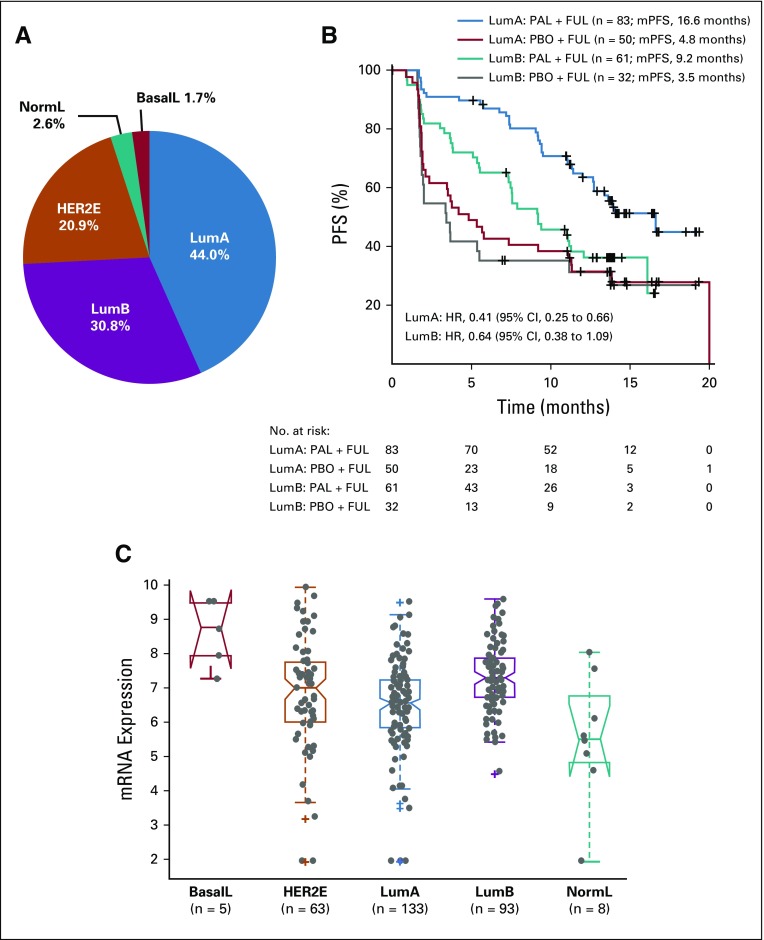
Of tumors with gene expression data, 133 (44%) were luminal A, 93 (31%) were luminal B, and 76 (25%) were nonluminal (five basal-like, 63 HER2-enriched, eight normal-like; Fig 5A). In patients with luminal A tumors, median PFS was 16.6 months with palbociclib plus fulvestrant and 4.8 months with placebo plus fulvestrant (HR, 0.41; 95% CI, 0.25 to 0.66), whereas in patients with luminal B tumors, median PFS was 9.2 months with palbociclib plus fulvestrant and 3.5 months with placebo plus fulvestrant (HR, 0.64; 95% CI, 0.38 to 1.09; Fig 5B). No significant interaction was found between luminal A versus luminal B and treatment effect of palbociclib (P = .20). Patients with nonluminal hormone receptor–positive tumors had a median PFS of 9.5 months with palbociclib plus fulvestrant and 5.5 months with placebo plus fulvestrant (HR, 0.58; 95% CI, 0.34 to 0.99; Data Supplement).
FIG 5.
Intrinsic molecular subtype and efficacy of palbociclib (PAL). (A) Intrinsic subtype distribution of tumors in the PALOMA-3 trial. (B) Progression-free survival (PFS) in luminal A (LumA) and B (LumB) tumors. (C) Cyclin E1 (CCNE1) mRNA expression by intrinsic molecular subtype. BasalL, basal-like; FUL, fulvestrant; HER2E, human epidermal growth factor receptor 2–enriched; HR, hazard ratio; mPFS, median progression-free survival; NormL, normal-like; PBO, placebo.
CCNE1 mRNA expression seemed highest in the few basal-like subtype tumors, followed by luminal B (across all subtypes, P < .001; Fig 5C; Data Supplement). The CCNE1 mRNA expression level of luminal A tumors was significantly lower than that in luminal B tumors (P < .001, Mann-Whitney U test). In an exploratory subtype-specific analysis, the effect of CCNE1 mRNA as a continuous variable on improvement in PFS from adding palbociclib to fulvestrant was observed in both luminal B and nonluminal subtypes (interaction P = .03 and .007, respectively) but not in the luminal A subtype (interaction P = .49).
Discovery Analysis of Genes and Pathways Associated With Efficacy of Palbociclib
After correcting for multiple hypothesis testing, 20 candidate genes were identified with an FDR P < .1, including 11 relative resistance markers and nine relative sensitivity markers (Data Supplement). The unbiased search independently identified high CCNE1 mRNA expression as the second most significant gene panel–wide linked to lack of efficacy from the addition of palbociclib to fulvestrant. The only more significant gene was neuromedin U, which previously had been implicated in drug-resistant HER2-positive breast cancer by driving increased levels of transforming growth factor-β125 and expanding the cancer stem-cell phenotype.26 We noted that higher CDKN2D mRNA (p19) expression level also was associated with reduced efficacy with palbociclib combination as well as with possibly CDKN2C mRNA (p18) expression (Data Supplement). Both genes belong to the INK4 family, which regulates kinase activities of CDK4/6.
Among the 50 hallmark gene sets from the Molecular Signatures Database,27 E2F targets (regulon) demonstrated the most significant association with lack of improvement in PFS from palbociclib combination (normalized enrichment score, −2.36; FDR P < .001) followed by other cell cycle–related pathways, including Myc regulon, mechanistic target of rapamycin complex 1 signaling, G2/M checkpoint, DNA repair, and mitotic spindle (Data Supplement). These results from the unbiased search support and confirm the critical role of E2F transcriptional activity, and CCNE1 mRNA in particular, in determining the relative clinical efficacy from the addition of palbociclib to fulvestrant.
DISCUSSION
We present a gene expression analysis of breast cancer tissues in the PALOMA-3 trial and identify the first, to our knowledge, predictive marker of efficacy from CDK4/6 inhibition, with low expression of CCNE1 mRNA associated with greater efficacy of palbociclib. Low E2F transcriptional activity was associated with relative improved efficacy, with CCNE1 mRNA seeming to be the most significant predictive biomarker gene within the regulon. In contrast, we found no evidence that either ER expression or luminal subtype was associated with efficacy of palbociclib.
Previous studies have shown discordance between primary and metastatic biopsy samples in genetic profiles28 and HER2 29 and ER and PR status.30 PALOMA-3 was one of the first phase III trials to mandate the provision of recurrent disease biopsy samples, unless patients had bone-only disease or experienced a relapse in the first 3 years of adjuvant endocrine therapy and could provide tissue from the primary tumor. The current results demonstrate that the collection of tissue temporally closer to the time of trial entry greatly facilitates the identification of predictive markers and could be considered a norm in all phase III advanced breast cancer trials.
CCNE1 canonically activates CDK2,31 and our findings build on a wealth of preclinical and early clinical evidence that CCNE1 expression is a marker of resistance to CDK4/6 inhibition.13,14,17,32 High CCNE1 mRNA expression correlates with resistance to therapy in cell line models of breast and ovarian cancer.13,14 In triple-negative cancer cell lines with resistance to CDK4/6 inhibition, CCNE1 mRNA expression remains high directly after mitosis, which bypasses the restriction point at which CDK4/6 traditionally has been viewed as being required for G1 transition by activating CDK2.33 High expression of low-molecular-weight CCNE1 assessed by immunohistochemistry has been associated with poor outcomes, including in a cohort of patients treated with palbociclib in routine clinical practice.34,35 Of interest for future research is an exploration of the relative importance of assessing CCNE1 mRNA versus CCNE1 protein and the post-translational modification of CCNE1.
We independently validated high CCNE1 mRNA as a resistance biomarker in the POP trial. The POP trial assessed gene expression with Affymetrix arrays, demonstrating the potential of CCNE1 mRNA expression to be predictive across various platforms. Limited biomarker work from other preoperative palbociclib trials also supports high CCNE1 mRNA as a biomarker that identifies ER-positive and HER2-negative cancers resistant to CDK4/6 inhibition.17 These data suggest that CCNE1 mRNA expression may be associated with the benefit from palbociclib in early-stage breast cancer. Future research will be required to assess the impact of endocrine therapy resistance on CCNE1 mRNA expression and to identify the cellular processes that allow CCNE1 mRNA expression to become decoupled from the requirement for prior CDK4/6 activation.
Overall, both luminal A and B breast cancer subtypes derived benefit from adding palbociclib to fulvestrant. The current data add to recent data that the small subset of nonluminal ER-positive breast cancers may be a distinct and separate entity characterized by disparate treatment responsiveness.16,36 Nonluminal ER-positive breast cancers may derive less benefit from endocrine therapy than luminal breast cancers.37 The current data suggest that CDK4/6 inhibition combination therapy may improve PFS in nonluminal cancers, which are dominated by the HER2-enriched phenotype (Data Supplement). In unsupervised exploratory analysis of genes and pathways associated with palbociclib efficacy, we identified a potential association between high expression levels of p19-CDKN2D mRNA, as well as p18-CDKN2C mRNA, and reduced efficacy from palbociclib. This observation is exploratory and requires validation but possibly identifies that high levels of CCNE1 may predict lower response to palbociclib. Mechanistic target of rapamycin complex 1 signaling was associated with reduced response to palbociclib addition, which is consistent with previous preclinical studies.38
The current study has important limitations. The PALOMA-3 backbone endocrine therapy was fulvestrant,4,7 and whether the biomarkers identified in this study are relevant to aromatase inhibitor-CDK4/6 combinations is unknown. Our analysis was not conducted with a clinical assay and should not be used to select patients for therapy without additional validation of the results and of clinical grade diagnostics. In addition, whether measurement of CCNE1 mRNA would be useful to make decisions for individual patients is not clear from this analysis because the subgroup with high CCNE1 mRNA expression potentially derived some PFS improvement with the addition of palbociclib to fulvestrant, albeit to a substantially lesser degree than cancers of low expression.
In this correlative analysis of the PALOMA-3 trial, we have identified the first potential biomarker to our knowledge that is predictive of the efficacy of palbociclib. The findings reinforce CDK2 as a key bypass kinase of CDK4/6 inhibition and identify potential therapeutic approaches to prevent early progression on CDK4/6 inhibitors. The effect of CCNE1 mRNA expression was most evident in metastatic biopsy samples, which are more contemporaneous to treatment than archival primary biopsy samples, and demonstrates the importance of collecting metastatic/recurrent tissue biopsies in clinical studies. Additional methodologic and clinical validations are warranted to elucidate the role of CCNE1 mRNA expression as a biomarker of CDK4/6 inhibitor therapy.
Footnotes
Presented at the American Association for Cancer Research Annual Meeting 2018, Chicago, IL, April 14-18, 2018.
Supported by Pfizer. The Preoperative Palbociclib trial was sponsored by and analyzed at Institut Gustave Roussy. Editorial support was provided by Anny Wu, PharmD, of Complete Healthcare Communications (North Wales, PA), a CHC Group company, and was funded by Pfizer.
Clinical trial information: NCT01942135.
See accompanying Editorial on page 1148
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Provision of study material or patients: All authors
Collection and assembly of data: Yuan Liu, Zhou Zhu, Zhe Zhang
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Cyclin E1 Expression and Palbociclib Efficacy in Previously Treated Hormone Receptor–Positive Metastatic Breast Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Nicholas C. Turner
Consulting or Advisory Role: Roche, Novartis, AstraZeneca, Pfizer, Tesaro, Bicycle Therapeutics
Research Funding: Pfizer (Inst), Roche (Inst), AstraZeneca (Inst), Inivata (Inst), Clovis Oncology (Inst), Bio-Rad (Inst)
Yuan Liu
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Zhou Zhu
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Sherene Loi
Consulting or Advisory Role: AstraZeneca (Inst), MedImmune (Inst), Seattle Genetics (Inst), Bristol-Myers Squibb (Inst), Pfizer (Inst), Novartis (Inst), Roche (Inst), Genentech (Inst), Merck Sharp & Dohme (Inst)
Research Funding: Roche (Inst), Genentech (Inst), Pfizer (Inst), Novartis (Inst), Merck (Inst), Puma Biotechnology (Inst), Bristol-Myers Squibb (Inst)
Marco Colleoni
Honoraria: Novartis
Consulting or Advisory Role: Pierre Fabre, Pfizer, OBI Pharma, Puma Biotechnology, Celldex Therapeutics, AstraZeneca
Sibylle Loibl
Consulting or Advisory Role: Pfizer (Inst), Roche (Inst), Novartis (Inst), Seattle Genetics (Inst), Celgene (Inst), Eli Lilly (Inst), AstraZeneca (Inst), MedImmune (Inst)
Research Funding: AbbVie (Inst), AstraZeneca (Inst), Seattle Genetics (Inst), TEVA Pharmaceuticals Industries (Inst), Vifor Pharma (Inst), Amgen (Inst), Celgene (Inst), Novartis (Inst), Pfizer (Inst), Roche (Inst)
Angela DeMichele
Honoraria: Pfizer
Consulting or Advisory Role: Calithera Biosciences, Novartis, Context Therapeutics, Pfizer (I)
Research Funding: Pfizer (Inst), Genentech (Inst), Incyte (Inst), Millennium Pharmaceuticals (Inst), Bayer AG (Inst), Veridex (Inst), Calithera Biosciences (Inst), GlaxoSmithKline (Inst), Wyeth (Inst)
Travel, Accommodations, Expenses: Pfizer, Calithera Biosciences, Novartis, Pfizer
Nadia Harbeck
Honoraria: Roche, Novartis, Celgene, Amgen, Pfizer, Genomic Health, NanoString Technologies
Consulting or Advisory Role: Roche, Genentech, Novartis, Celgene, Pfizer, Eli Lilly, Sandoz, Daiichi Sankyo, Agendia, AstraZeneca, Merck Sharp & Dohme, Odonate Therapeutics, Seattle Genetics
Research Funding: Roche (Inst), Genentech (Inst), Novartis (Inst), Pfizer (Inst), Eli Lilly (Inst), MSD (Inst), Merck (Inst)
Fabrice André
Research Funding: AstraZeneca (Inst), Novartis (Inst), Pfizer (Inst), Eli Lilly (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Novartis, Roche, GlaxoSmithKline, AstraZeneca
Stefan Michiels
Consulting or Advisory Role: IDDI, Hexal AG, Johnson & Johnson, Genticel, mAbxience, Roche, QuintilesIMS
Patents, Royalties, Other Intellectual Property: Patent pending on a prognostic gene score in early breast cancer: WO2017EP66533
Zhe Zhang
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Carla Giorgetti
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Monica Arnedos
Honoraria: Novartis, AstraZeneca
Consulting or Advisory Role: Seattle Genetics
Research Funding: Pfizer, Eli Lilly
Travel, Accommodations, Expenses: Pfizer, AstraZeneca
Cynthia Huang Bartlett
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Massimo Cristofanilli
Honoraria: Pfizer, Novartis, Merus, CytoDyn
No other potential conflicts of interest were reported.
REFERENCES
- 1.Asghar U, Witkiewicz AK, Turner NC, et al. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov. 2015;14:130–146. doi: 10.1038/nrd4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ibrance (Palbociclib). Summary of Product Characteristics. Sandwich, Kent, UK, Pfizer, 2018. [Google Scholar]
- 3. Ibrance (Palbociclib). Full Prescribing Information. New York, NY, Pfizer, 2018. [Google Scholar]
- 4.Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–439. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 5.Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 6.Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 7.Turner NC, Ro J, André F, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373:209–219. doi: 10.1056/NEJMoa1505270. [DOI] [PubMed] [Google Scholar]
- 8. https://www.tri-kobe.org/nccn/guideline/breast/english/breast.pdf National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Breast Cancer. Version 3.2015.
- 9.Turner NC, Finn RS, Martin M, et al. Clinical considerations of the role of palbociclib in the management of advanced breast cancer patients with and without visceral metastases. Ann Oncol. 2018;29:669–680. doi: 10.1093/annonc/mdx797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loibl S, Turner NC, Ro J, et al. Palbociclib combined with fulvestrant in premenopausal women with advanced breast cancer and prior progression on endocrine therapy: PALOMA-3 results. Oncologist. 2017;22:1028–1038. doi: 10.1634/theoncologist.2017-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwata H, Im SA, Masuda N, et al. PALOMA-3: Phase III trial of fulvestrant with or without palbociclib in premenopausal and postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer that progressed on prior endocrine therapy-safety and efficacy in Asian patients. J Glob Oncol. 2017;3:289–303. doi: 10.1200/JGO.2016.008318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dean JL, Thangavel C, McClendon AK, et al. Therapeutic CDK4/6 inhibition in breast cancer: Key mechanisms of response and failure. Oncogene. 2010;29:4018–4032. doi: 10.1038/onc.2010.154. [DOI] [PubMed] [Google Scholar]
- 13.Taylor-Harding B, Aspuria PJ, Agadjanian H, et al. Cyclin E1 and RTK/RAS signaling drive CDK inhibitor resistance via activation of E2F and ETS. Oncotarget. 2015;6:696–714. doi: 10.18632/oncotarget.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrera-Abreu MT, Palafox M, Asghar U, et al. Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor-positive breast cancer. Cancer Res. 2016;76:2301–2313. doi: 10.1158/0008-5472.CAN-15-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang C, Li Z, Bhatt T, et al. Acquired CDK6 amplification promotes breast cancer resistance to CDK4/6 inhibitors and loss of ER signaling and dependence. Oncogene. 2017;36:2255–2264. doi: 10.1038/onc.2016.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finn RS, Dering J, Conklin D, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11:R77. doi: 10.1186/bcr2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma CX, Gao F, Luo J, et al. NeoPalAna: Neoadjuvant palbociclib, a cyclin-dependent kinase 4/6 inhibitor, and anastrozole for clinical stage 2 or 3 estrogen receptor-positive breast cancer. Clin Cancer Res. 2017;23:4055–4065. doi: 10.1158/1078-0432.CCR-16-3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Finn R, Jiang Y, Rugo H, et al: Biomarker analyses from the phase 3 PALOMA-2 trial of palbociclib (P) with letrozole (L) compared with placebo (PLB) plus L in postmenopausal women with ER+/HER2‒ advanced breast cancer (ABC). Ann Oncol 27, 2016 (suppl 6; abstr LBA15) [Google Scholar]
- 19.Fribbens C, O’Leary B, Kilburn L, et al. Plasma ESR1 mutations and the treatment of estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2016;34:2961–2968. doi: 10.1200/JCO.2016.67.3061. [DOI] [PubMed] [Google Scholar]
- 20. Arnedos M, Cheaib B, Bayer MA, et al: Anti-proliferative response and predictive biomarkers to palbociclib in early breast cancer: The Preoperative Palbociclib (POP) randomized trial. Cancer Res 76, 2016 (suppl 14; abstr CT041) [Google Scholar]
- 21.Arnedos M, Bayar MA, Cheaib B, et al. Modulation of Rb phosphorylation and antiproliferative response to palbociclib: The Preoperative-Palbociclib (POP) randomized clinical trial. Ann Oncol. 2018;29:1755–1762. doi: 10.1093/annonc/mdy202. [DOI] [PubMed] [Google Scholar]
- 22.Lazar AA, Cole BF, Bonetti M, et al. Evaluation of treatment-effect heterogeneity using biomarkers measured on a continuous scale: Subpopulation treatment effect pattern plot. J Clin Oncol. 2010;28:4539–4544. doi: 10.1200/JCO.2009.27.9182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parker JS, Mullins M, Cheang MC, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160–1167. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paquet ER, Hallett MT. Absolute assignment of breast cancer intrinsic molecular subtype. J Natl Cancer Inst. 2014;107:357. doi: 10.1093/jnci/dju357. [DOI] [PubMed] [Google Scholar]
- 25. Martinez VG, O’Driscoll L: Neuromedin U drives increased expression of TGFβ1 in HER2-positive breast cancer cells and their extracellular vesicles: a novel biomarker of response to HER-targeted drugs. Cancer Res 76, 2016 (suppl 14; abstr LB-116) [Google Scholar]
- 26.Martinez VG, Crown J, Porter RK, et al. Neuromedin U alters bioenergetics and expands the cancer stem cell phenotype in HER2-positive breast cancer. Int J Cancer. 2017;140:2771–2784. doi: 10.1002/ijc.30705. [DOI] [PubMed] [Google Scholar]
- 27.Liberzon A, Birger C, Thorvaldsdóttir H, et al. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015;1:417–425. doi: 10.1016/j.cels.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stoecklein NH, Klein CA. Genetic disparity between primary tumours, disseminated tumour cells, and manifest metastasis. Int J Cancer. 2010;126:589–598. doi: 10.1002/ijc.24916. [DOI] [PubMed] [Google Scholar]
- 29.Tapia C, Savic S, Wagner U, et al. HER2 gene status in primary breast cancers and matched distant metastases. Breast Cancer Res. 2007;9:R31. doi: 10.1186/bcr1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liedtke C, Broglio K, Moulder S, et al. Prognostic impact of discordance between triple-receptor measurements in primary and recurrent breast cancer. Ann Oncol. 2009;20:1953–1958. doi: 10.1093/annonc/mdp263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caldon CE, Musgrove EA. Distinct and redundant functions of cyclin E1 and cyclin E2 in development and cancer. Cell Div. 2010;5:2. doi: 10.1186/1747-1028-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garrido-Castro AC, Goel S. CDK4/6 inhibition in breast cancer: Mechanisms of response and treatment failure. Curr Breast Cancer Rep. 2017;9:26–33. doi: 10.1007/s12609-017-0232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asghar US, Barr AR, Cutts R, et al. Single-cell dynamics determines response to CDK4/6 inhibition in triple-negative breast cancer. Clin Cancer Res. 2017;23:5561–5572. doi: 10.1158/1078-0432.CCR-17-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vijayaraghavan S, Karakas C, Doostan I, et al. CDK4/6 and autophagy inhibitors synergistically induce senescence in Rb positive cytoplasmic cyclin E negative cancers. Nat Commun. 2017;8:15916. doi: 10.1038/ncomms15916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keyomarsi K, Tucker SL, Buchholz TA, et al. Cyclin E and survival in patients with breast cancer. N Engl J Med. 2002;347:1566–1575. doi: 10.1056/NEJMoa021153. [DOI] [PubMed] [Google Scholar]
- 36.Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies--improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol. 2015;26:1533–1546. doi: 10.1093/annonc/mdv221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prat A, Cheang MC, Galván P, et al. Prognostic value of intrinsic subtypes in hormone receptor-positive metastatic breast cancer treated with letrozole with or without lapatinib. JAMA Oncol. 2016;2:1287–1294. doi: 10.1001/jamaoncol.2016.0922. [DOI] [PubMed] [Google Scholar]
- 38.Michaloglou C, Crafter C, Siersbaek R, et al. Combined inhibition of mTOR and CDK4/6 is required for optimal blockade of E2F function and long-term growth inhibition in estrogen receptor-positive breast cancer. Mol Cancer Ther. 2018;17:908–920. doi: 10.1158/1535-7163.MCT-17-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]