Highlights
-
•
Monochromatic light source affected growth of gamma irradiated in vitro cultures of Dendrobium
-
•
Yellow and red monochromatic lights significantly improved survival rate and growth of irradiated cultures.
-
•
Intra-somatic competition among mutated and non-mutated cultures can be managed by monochromatic light through better growth in vitro.
Keywords: Orchids, Gamma irradiation, Monochromatic light, In vitro mutagenesis, In vitro growth
Abstract
The effects of gamma radiation and monochromatic lights on growth of in vitro shoot cultures of orchid, Dendrobium sonia, were investigated. The gamma irradiated shoot cultures grown under white, blue, yellow and red monochromatic lights exhibited differential growth pattern. Shoot cultures gamma irradiated at 15–45 Gy showed reduced shoot length, fresh weight and leaf area. The monochromatic light significantly influenced survival rate and growth of irradiated shoots. The yellow and red light treatments positively influenced survival of gamma irradiated shoots with significant increase in fresh weight, shoot length and chlorophyll content. Yellow light was found to be most effective as leaf area was increased across the radiation dose range (15–100 Gy) compared to red light. The results demonstrated that the method of post-irradiation exposure could be useful to improve growth of gamma irradiated in vitro shoots, and help to recover orchid mutants with novel modifications.
1. Introduction
Radiation mutagenesis has become an established tool in plant breeding with significant contribution to crop improvement by improving existing cultivars [1,2]. There is a great interest in developing mutants of ornamental plants for flower shapes and colors [3] as the species are amenable to mutation induction and selection based on flower color and plant type mutations is relatively easy [4]. Of the >3200 officially released, improved mutant plant varieties, more than 625 belong to ornamental plant category, improved for flower type, leaf size, pigmentation, photoperiodic response and early flowering [5,6].
Orchids are the popular ornamental plants among the members of the Orchidaceae that constitute the largest botanical family of higher plants [7]. These ornamental plants are known for their long lasting fragrance and quality of flowers. In India, orchids are mainly grown in the north-eastern region in the dense evergreen forests under conditions of low temperature and high humidity [8]. The genus Dendrobium is the largest genus belonging to Orchidaceae with most members being epiphytic. Dendrobium has approximately 1500 species and almost one fourth of them are used for their high ornamental value [9]. Because of their commercial value in the horticulture sector, development of new and novel orchid varieties having exotic color, size and shape of flowers under diverse agro-climatic conditions has become an attractive option [10]. One of the breeding strategies for orchids is through induced mutation in which orchid plants or cells are exposed to mutagens such as gamma rays, x-rays, electrons or ion beams or chemical agents [10]. Among the targeted breeding traits for orchid mutants are flower color, sizes, morphology and shelf life as well as plant architecture and vigor. Although several new mutant varieties have been developed in ornamental plants using gamma radiation and are propagated for new traits [[11], [12], [13]], only few mutant varieties of D. sonia have been successfully generated through mutagenesis namely ‘Keena Oval’, ‘Ahmad Sobri’, ‘Keena radiant’ and ‘Hieng Ding’ [14].
In vitro organogenesis is the process where de novo organs can be induced from cultured tissues (explants), under the influence of certain physical and chemical conditions such as the type of explants, basal medium, growth regulators, carbohydrate source, light, and temperature [15]. In vitro cell and tissue cultures provide several advantages for mutation breeding, as sufficient, high amount of in vitro material can be generated for mutagenesis and ease in post-mutagenesis handling [16]. However, the method is often challenged by lower mutation induction frequencies and lower regeneration rate because there is always discrimination of mutagenized cells against normal, non-mutagenized cells resulting in loss of mutant cell progenies [17]. This is referred to as intrasomatic competition and can be manipulated by in vitro conditions such as medium ingredients or physical environment to stimulate better growth of mutant cells [17]. In this regard, partial desiccation has been shown to favour regeneration response of high dose gamma irradiated in vitro cultures of sugarcane [18]. Since monochromatic light treatment has shown stimulatory effect on in vitro morphogenesis in different plant species [19] including orchids [20,21], we have applied this method to augment in vitro regeneration of mutagenized orchid tissue cultures.
Plant growth and development is strongly influenced by light, depending on wavelength (quality), intensity (quantity) and duration [22,23]. Red and blue light spectrums have the maximum impact on plant growth because they are the major energy sources for photosynthetic CO2 assimilation in plants [24,25]. Spectral light changes can induce different morphogenetic and photosynthetic responses among different plant species [26]. Such photo-responses are of practical importance in plant cultivation practices since the possibility of specific light spectra could facilitate the control of growth, development and nutritional features [27]. Terrestrial orchids need stronger light than epiphytic orchids [28]. Reports on the effect of artificial light spectral intensities on plant growth, particularly in orchids are limited [21]. In other plants, blue light treatment improved the efficiency of favourable adventitious rooting [29]. However, such studies in relation to radiation mutagenesis to see if light exposure can be used to stimulate growth and regeneration of irradiated cultures are scarce, especially in an important commercial orchid, Dendrobium sp. In this study, we report the in vitro growth responses of Dendrobium orchid to gamma radiation and effect of different monochromatic lights on growth of gamma irradiated in vitro shoot cultures.
2. Materials and methods
Dendrobium sonia orchid plants were maintained at the greenhouse facility (Department of Life Science, Maharaja Ranjit Singh College of Professional Sciences, Indore, India) at 22–25 °C temperature and >60% relative humidity. Rhizome buds from the ex vitro plants were used as explants to initiate in vitro shoot cultures. These explants were thoroughly washed in running tap water for 10 min, surface sterilized with 0.1% mercuric chloride for 3 min followed by 70% alcohol for 30 s and finally rinsed thrice with sterilized distilled water [21]. These surface sterilized explants were then cultured on to Murashige and Skoog’s (MS) [30] basal medium supplemented with sucrose (3% w/v), 6-benzylaminopurine (BAP 2 mg/l) and gelling agent agar-agar (0.6% w/v). The cultures were incubated under white fluorescent Light Emitting Diodes (LEDs) light (17.7 μmol/m2/s) with 16 h illumination and 8 h dark photoperiod at 22–25 °C and >60% humidity.
2.1. Gamma irradiation of in vitro shoot cultures
One month old in vitro regenerated shoots of approximately 3 cm in length were exposed to gamma radiation using 60Co gamma irradiator – Gamma Cell 220 (5.7 Gy/min dose rate) at the Bhabha Atomic Research Centre (BARC), Trombay, Mumbai, India. The shoots were exposed to gamma radiation at different doses (0, 15, 30, 45, 60, 80 and 100 Gy) by varying duration of exposure based on the dose rate. After irradiation, the shoots were immediately transferred to fresh shoot multiplication medium comprising of MS basal medium fortified with BAP (2.5 mg/l), indole-3 acetic acid (IAA 2 mg/l), sucrose (3% w/v) and gelling agent agar-agar (0.6% w/v) for further studies.
2.2. Post irradiation exposure of shoots with monochromatic light regimes
Subsequent to gamma irradiation, cultures were incubated separately in four chambers illuminated with different monochromatic lights. For this purpose, four separate wooden chambers painted with white colored reflective paint were fabricated and the inside-top was fitted with monochromatic light sources of four different wavelengths. These monochromatic lights were provided using Light Emitting Diodes (LEDs) of blue (450 nm), yellow (570 nm) and red (680 nm) lights with intensity range between 15–25 μmol/m2/s (Table 1). For comparison, a separate set of cultures maintained under white LED light (400–700 nm wide range). Light illumination of respective wooden chamber was kept under digital timer control that was set for 16 h illumination and 8 h dark to maintain the photoperiod.
Table 1.
Experimental setup with monochromatic lights of varying wavelength and intensity.
| Light | Wavelength (nm) | Intensity (μmol/m2/s) |
|---|---|---|
| White | 400-700 | 17.7 |
| Blue | 450 | 22.5 |
| Yellow | 570 | 24.6 |
| Red | 680 | 15.6 |
The gamma irradiated shoots were passed through M1V1 up to M1V4 subculture cycles (with 30 days of subculture interval). Post irradiation, survival rate (%) was recorded at M1V1 and M1V4 stage and observations were recorded on different growth parameters like total fresh weight (gm), leaf area (mm²), shoot length (cm), and chlorophyll content (μg g−1 FW).
2.3. Estimation of total chlorophyll content
The total chlorophyll content of the newly emerged leaf was determined by extracting the pigment with 80% (v/v) acetone [31]. Briefly, leaves of each treatment were excised and washed with sterile distilled water, and excess moisture was removed with blotting paper. Known quantity (0.1 g) of freshly chopped leaves was ground in 5 ml of 80% acetone and centrifuged at 6000 rpm for 12 min. Absorbance of the supernatant was measured at 645 nm, and 663 nm wavelengths with the help of spectrophotometer (Systronics 117, UV-VIS Spectrophotometer) by keeping 80% (v/v) acetone as blank.
Total Chlorophyll (μg g−1 FW) = (20.2*A645 + 8.02*A663) * (V/1000 * W)
Where, A is the absorbance at given wavelength, V is the total volume of acetone extract (ml) and W is the fresh weight (g) of the sample.
2.4. Plant acclimatization
The individual shoots measuring 1–2 cm long were transferred onto MS medium containing 3% sucrose fortified with 3 mg/L IAA for root induction. The plants with 3–4 roots in number and approximately 1 inch in length were selected for hardening and acclimatization [21]. The well established plantlets were transplanted into small pots containing coco-peat for two weeks and kept under 70–80% relative humidity at 24–28℃, followed by transfer to large sized pots containing coco-peat under greenhouse conditions. The plants were sprayed with water, twice every day in the morning and evening.
2.5. Statistical analysis
All the experiments were repeated twice with ten shoots for each treatment and results were expressed as mean ± standard error (SE). The data were subjected to one-way analysis of variance (ANOVA) and the differences between means were tested using Duncan’s multiple range test (DMRT) (P ≤ 0.05) using SPSS 16.0.
3. Results and discussion
3.1. Effect of gamma radiation on in vitro shoot cultures
In vitro shoot cultures of Dendrobium sonia were exposed to gamma rays at different doses (0, 15, 30, 45, 60, 80 and 100 Gy) and maintained under white LED-light. Gamma irradiation significantly affected growth of the in vitro shoots (Fig. 1). Survival rate of irradiated shoots was found to decrease with increase in gamma radiation dose up to 45 Gy and beyond this dose; cultures did not survive (Table 2). The LD50 (lethal dose 50) appeared to be around 30 Gy, based on the survival rate of the cultures. Shoot cultures irradiated at 15–45 Gy gamma ray doses showed reduced shoot length (Table 3), leaf area (Table 4) and fresh weight (Table 5).
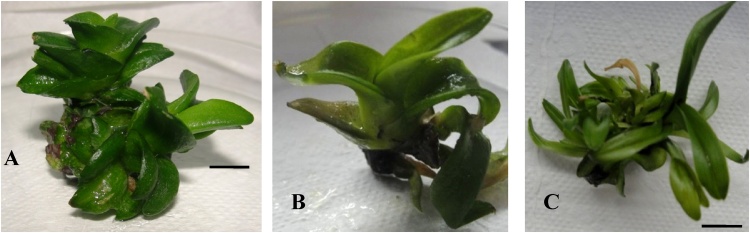
Fig. 1.
Morphology of 15 Gy gamma irradiated shoots of Dendrobium under the influence of different monochromatic lights; (A) White light, (B) Blue light, (C) Yellow light and (D) Red light treatments. Bar = 10 mm.
Table 2.
Effect of different monochromatic light treatments on survival rate of gamma irradiated in vitro shoots of Dendrobium sonia in M1V1 generation.
| Treatments | Survival rate (%) |
|||
|---|---|---|---|---|
| White | Blue | Yellow | Red | |
| Control | 100 | 100 | 100 | 100 |
| 15 Gy | 100 | 20 | 100 | 60 |
| 30 Gy | 40 | 60 | 100 | 20 |
| 45 Gy | 20 | 40 | 100 | 40 |
| 60 Gy | 0 | 20 | 100 | 40 |
| 80 Gy | 0 | 0 | 100 | 20 |
| 100 Gy | 0 | 0 | 100 | 20 |
Table 3.
Effect of different monochromatic lights on shoot length of gamma irradiated in vitro shoots of Dendrobium sonia in M1V4 generation.
| Treatment | Shoot length (cm) |
|||
|---|---|---|---|---|
| White | Blue | Yellow | Red | |
| Control | 4.70 (±0.46)a | 5.43 (±0.39)a | 3.60 (±0.40)a | 4.00 (±0.49)ab |
| 15 Gy | 4.37 (±0.44)a | 4.37 (±0.29)b | 3.50 (±0.42)a | 5.07 (±0.98)a |
| 30 Gy | 4.13 (±0.15)a | 3.77 (±0.35)b | 3.83 (±0.29)a | 2.90 (±0.31)bc |
| 45 Gy | 3.93 (±0.49)a | 4.13 (±0.26)b | 4.03 (±0.38)a | 3.40 (±0.31)bc |
| 60 Gy | – | – | 2.33 (±0.15)b | 1.83 (±0.47)c |
| 80 Gy | – | – | 1.57 (±0.18)b | 3.13 (±0.15)bc |
| 100 Gy | – | – | 2.17 (±0.41)b | 3.07 (±0.09)bc |
Values in the parenthesis represent ± standard error of mean, Different letters in superscript indicate significance of differences using DMRT at p ≤ 0.05.
Table 4.
Effect of different monochromatic lights on leaf area of gamma irradiated in vitro shoots of Dendrobium sonia in M1V4 generation.
| Treatment | Leaf area (mm2) |
|||
|---|---|---|---|---|
| White | Blue | Yellow | Red | |
| Control | 19.77 (±0.98)b | 15.83 (±0.58)b | 32.00 (±1.24)abc | 15.93 (±1.19)c |
| 15 Gy | 27.37 (±0.91)a | 22.73 (±1.65)a | 25.60 (±0.64)cde | 22.70 (±1.67)b |
| 30 Gy | 17.60 (±0.89)c | 20.60 (±1.15)a | 28.67 (±1.37)bcd | 20.47 (±1.10)b |
| 45 Gy | 21.63 (±0.98)b | 21.73 (±1.65)a | 33.37 (±2.02)ab | 31.17 (±2.68)a |
| 60 Gy | – | – | 37.00 (±2.11)a | 31.70 (±1.04)a |
| 80 Gy | – | – | 19.33 (±3.85)e | 9.87 (±0.41)d |
| 100 Gy | – | – | 22.83 (±1.70)de | 10.07 (±1.01)d |
Values in the parenthesis represent ± standard error of mean, Different letters in superscript indicate significance of differences using DMRT at p ≤ 0.05.
Table 5.
Effect of different monochromatic lights on fresh weight of gamma irradiated in vitro shoots of Dendrobium sonia in M1V4 generation.
| Treatment | Fresh weight (gm) |
|||
|---|---|---|---|---|
| White | Blue | Yellow | Red | |
| Control | 3.90 (±0.58)a | 5.20 (±0.23)a | 5.50 (±0.40)a | 5.17 (±0.23)a |
| 15 Gy | 3.00 (±0.40)a | 3.00 (±0.12)b | 4.80 (±0.52)ab | 5.30 (±0.47)a |
| 30 Gy | 3.57 (±0.38)a | 3.43 (±0.38)b | 3.60 (±0.29)bc | 3.80 (±0.52)bc |
| 45 Gy | 3.47 (±0.23)a | 4.57 (±0.41)a | 3.47 (±0.58)c | 4.37 (±0.43)ab |
| 60 Gy | – | – | 2.90 (±0.46)c | 2.93 (±0.49)c |
| 80 Gy | – | – | 2.50 (±0.35)c | 3.27 (±0.12)bc |
| 100 Gy | – | – | 2.37 (±0.18)c | 3.17 (±0.41)bc |
Values in the parenthesis represent ± standard error of mean, Different letters in superscript indicate significance of differences using DMRT at p ≤ 0.05.
3.2. Effect of monochromatic light treatments on gamma irradiated in vitro shoot cultures
The effects of monochromatic light (Blue, Yellow and Red) on the morphology of in vitro shoot cultures of Dendrobium sonia are presented in Fig. 2, Fig. 3. In vitro shoot cultures irradiated at higher gamma ray doses above 45 Gy and exposed to blue LED light did not survive, whereas yellow and red LED light exposure favored survival of irradiated cultures even at higher gamma ray doses (Table 2, Fig. 3). In blue light treatment, cultures irradiated with 15, 30, and 45 Gy gamma doses had shown low (20–60%) survival rate (Table 2), while no shoots survived at 60, 80 and 100 Gy gamma doses. Shoot cultures irradiated and maintained under blue light showed reduced shoot length (Table 3), leaf area (Table 4) and fresh weight (Table 5).
Fig. 2.
Morphology of gamma irradiated shoots (30 Gy) of Dendrobium under the influence of different monochromatic lights.
(A) White light, (B) Yellow light, (C) Red light treatments. Bar = 10 mm.
Fig. 3.
Morphology of in vitro shoots of Dendrobium gamma irradiated at higher doses (A) 60 Gy (B) 80 Gy and (C) 100 Gy and grown under the influence of Yellow light treatment; Bar = 10 mm.
In the present study, yellow light significantly enhanced fresh weight and leaf area as compared to all other light sources (Figs. 2B, 3). The yellow light treatment was found favorable for the survival of gamma irradiated shoots with maximum survival percentage (100%) at all the radiation doses (Table 2). Yellow light treatment resulted in significant increase in fresh weight (Table 5) and shoot length to (Table 3) at 15, 30 and 45 Gy. Higher doses 60, 80 and 100 Gy showed decrease in fresh weight (Table 5) and shoot length (Table 3). Similar trend was observed in case of exposure of cultures to red light. Exposure of irradiated shoot cultures to yellow and red LED lights did not differ much in terms of shoot length, fresh weight and leaf area at 10 to 45 Gy but at higher gamma doses (beyond 45 Gy) better shoot length and fresh weight were recorded under red light treatment (Table 3, Table 5). Yellow light was also found to be very effective as leaf area was increased (ranging from 22.8-37 mm2) at all the radiation doses (15, 30, 45, 60, 80 and 100 Gy) compared with red light (Table 4).
The leaf total chlorophyll content of all the gamma irradiated and monochromatic light treated shoot cultures was determined except for the shoots irradiated at higher doses (beyond 45 Gy) as these shoot cultures failed to survive and/or produce leaves. The shoots irradiated at 15 and 30 Gy followed by white and blue light treatment exhibited higher chlorophyll content than respective control (non-irradiated) shoots (Table 6). Similar trend was also noticed for 15 and 30 Gy irradiated cultures under yellow and red light (Fig. 1, Fig. 2). In case of yellow and red light treatments for shoot cultures exposed to higher doses (45 Gy–100 Gy), there was no definite trend for changes seen in chlorophyll content. The gamma irradiated cultures under white light showed higher chlorophyll content followed by yellow light treatment (Table 6). Plantlets transplanted into small pots containing coco-peat (Fig. 4) were acclimatized with good survival (100%).
Table 6.
Effect of different monochromatic lights on leaf chlorophyll content of gamma irradiated in vitro shoots of Dendrobium sonia in M1V4 generation.
| Treatment | Total chlorophyll content (μg g−1 FW) |
|||
|---|---|---|---|---|
| White | Blue | Yellow | Red | |
| Control | 7.50 (±0.19)c | 1.58 (±0.00)c | 6.03 (±0.00)c | 2.94 (±0.01)f |
| 15 Gy | 9.26 (±0.06)a | 4.71 (±0.02)a | 4.02 (±0.02)e | 4.73 (±0.03)c |
| 30 Gy | 8.78 (±0.01)b | 4.71 (±0.02)a | 7.11 (±0.03)a | 4.05 (±0.01)e |
| 45 Gy | 7.21 (±0.30)c | 3.34 (±0.04)b | 2.90 (±0.04)g | 5.30 (±0.02)a |
| 60 Gy | – | – | 6.79 (±0.04)b | 2.80 (±0.00)g |
| 80 Gy | – | – | 5.21 (±0.01)d | 5.20 (±0.00)b |
| 100 Gy | – | – | 3.42 (±0.02)f | 4.33 (±0.02)d |
Values in the parenthesis represent ± standard error of mean, Different letters in superscript indicate significance of differences using DMRT at p ≤ 0.05.
Fig. 4.
Hardened plants from 30 Gy gamma-ray irradiated shoot cultures of Dendrobium sonia. (A) Rooted plantlets (B and C) Acclimatization of plants in pots containing cocopeat. Bar = 10 mm.
Gamma radiation mutagenesis is one of the viable options for the generation of new and novel genetic variability in ornamental plant species [[32], [33], [34]]. Gamma rays, in combination with in vitro cultures, have been used in ornamental plants such as orchid [13], chrysanthemum [35], and rose [36]. Dendrobium is the most popular commercial orchid and new varieties with improved plant and floral characteristics are in constant demand. Mutations could generate phenotypic variations in both vegetative and reproductive characteristics and the approach is particularly useful for Dendrobium improvement because it is highly heterozygous and is genetically diverse. In our earlier study, we reported effects of monochromatic lights on growth and morphogenesis of D. sonia [21]. It is essential to study the radiosensitivity in each case to estimate lethal dose (LD50) to focus on the effective doses at which higher frequency of the mutants can be isolated [1,2]. In this study, protocorm-like bodies (PLBs) were exposed to each irradiation dose and growth parameters were assessed. The results showed that LD50 was 30 Gy. In contrast, previous report on Dendrobium sonia-28 the LD50 for the PLBs was approximately 43 Gy [37]. In Cymbidium orchid, Kozlowska-kalisz [38] reported that a dose of 20 Gy inhibited growth of PLBs and 70 Gy was the lethal dose. In a recent study, Lee et al. [39] assessed the relative growth rate of PLBs of Cymbidium hybrid, RB001 and found LD50 γ-ray dose was approximately 40 Gy. In our study, lower dose of 15 Gy did not show much inhibitory effect on survival but stimulated shoot length, leaf area and chlorophyll content. Low dose irradiation induced stimulatory effects have been shown in other plants and this is attributed to change in hormonal balance or increase the antioxidant capacity of cells [40,41]. Higher doses beyond 45 Gy did not result in survival and growth of cultures which is possibly due to disturbed protein synthesis, hormone imbalance and altered leaf gas exchange [42]. In Oncidium lanceanum orchids, ion beam irradiation induced highest average fresh weight of PLBs up to 6.0 Gy which decreased at higher doses (12 Gy) but, the PLBs didn’t regenerate into complete shoots [43]. Such growth inhibition at higher doses could be attributed to the cell cycle arrest during somatic cell division and (or) varying damage to the entire plant genome [44].
Radiation mutagenesis in combination with in vitro culture has become useful for the generation of new genetic variability, selection and multiplication of the mutant clones [3]. For undertaking mutagenesis, meristematic cells or tissues are propagated in vitro to generate adequate plant material for mutagenic treatment and handling post-mutagenesis. Often, mutagen affected cells are discriminated against the large population of normal growing cells and this causes potential loss of mutant cell progenies. This process referred to as intrasomatic competition can be handled by amending in vitro conditions (medium composition or some other factors) resulting in stimulating growth of mutant cells [17]. In sugarcane, partial desiccation has been shown to be effective for augmenting plant regeneration response of higher gamma ray dose irradiated in vitro cultures [18].
The post-irradiation treatment of monochromatic light regimes demonstrated significant positive effects on survival, growth and development of gamma irradiated shoots of Dendrobium (Table 2, Table 3, Table 4, Table 5). Further, our results showed that yellow and red monochromatic light spectra ameliorated the impact of radiation damage and survival of the in vitro shoots. The LEDs have been successfully employed to stimulate in vitro morphogenesis [[45], [46], [47]]. In our previous study, we have reported differential morphogenic responses in PLBs of Dendrobium sonia to different monochromatic lights and it was found that yellow and red light positively influences in vitro shoot multiplication response [21]. The PLBs of Oncidium and Dendrobium officinale cultured under red LED showed poor productivity, while the application of blue LED resulted in the enhanced productivity [45,48]. Red light significantly improved the adventitious bud development and stem elongation in different plants including Petunia and Gerbera [[49], [50], [51], [52]]. There is also evidence that such attributes might be due to the presence of some photosensors and spectral overlaps that switch between green, orange and red spectral regions that can be selectively toggled to control plant growth, development, physiology and morphogenesis [27]. The wavelengths ranging between 500–600 nm also represent the photosynthetically active radiation (PAR) which could be useful to manipulate plant stature, color, nutrients and other attributes [46].
Chlorophyll is fundamental to photosynthesis for absorbing light energy. In this study, we have shown that shoots grown under yellow and red LED lights had higher chlorophyll content compared to those under white and blue LED lights. In Houttuynia cordata seedlings, yellow and blue LED resulted in the higher soluble sugar contents [53]. Although, blue LED is known to have a major absorption spectrum for chlorophyll, in our study, we observed that yellow LED exhibited positive effects on growth of gamma-irradiated shoots in terms of leaf area and chlorophyll content. It is interesting to investigate further the precise mechanism underlying such stimulatory effect. Wang et al. [54] found that carbohydrates contents were consistent with the transcript levels of Calvin cycle genes of Cucumis sativus plants exposed to different LEDs and suggested that yellow and blue LED light could up-regulate the carbohydrates biosynthesis related genes.
4. Conclusion
Our results show that the post-gamma irradiation exposure of in vitro shoots of Dendrobium to monochromatic light regimes has a positive effect on survival, growth and leaf area. Although the plant regeneration response from in vitro shoot cultures of PLBs of Dendrobium sonia as achieved in this study is not high, the findings can be useful especially in view of low or no regeneration occurring in high gamma ray dose-irradiated (mutagenized) cultures. Further fine-tuning in the treatment methodology can improve regeneration response in high dose irradiated in vitro cultures.
Author contribution statement
VB, MJ and PS conceived the study, designed the experiments; VB performed experiments, analyzed data; PS and SJM wrote the manuscript. PS and MJ coordinated the entire study. All authors read and approved the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethical standards
The present research did not involve experiments with Human Participants and/or Animals.
Contributor Information
Penna Suprasanna, Email: penna888@yahoo.com, science2002@rediffmail.com.
Monica Jain, Email: science2002@rediffmail.com.
References
- 1.Jain S.M. Major mutation-assisted plant breeding programs supported by FAO/IAEA. Plant Cell Tissue Organ Cult. 2005;82(1):113–123. [Google Scholar]
- 2.Suprasanna P., Mirajkar S.J., Bhagwat S.G. Induced mutations and crop improvement. In: Bahadur Bir, Rajam M.V., Sahijram Leela, Krishnamurthy K.V., editors. Plant Biology and Biotechnology. Springer; 2015. pp. 593–618. [Google Scholar]
- 3.Ahloowalia B., Maluszynski M. Induced mutations- A new paradigm in plant breeding. Euphytica. 2001;118:167–173. [Google Scholar]
- 4.Broertjes C., van Harten A.M. Elsevier Scientific Publishing Company; Amsterdam: 1978. Application of Mutation Breeding Methods in the Improvement of Vegetatively Propagated Crops; p. 316. [Google Scholar]
- 5.Kharkwal M.C., Shu Q.Y. The role of induced mutation in world food security. In: Shu Q.Y., editor. Induced Plant Mutations in the Genomics Era. Food and Agriculture Organization of the United Nations; Rome: 2009. pp. 33–38. [Google Scholar]
- 6.Jain S.M., Suprasanna P. Induced mutation for enhancing nutrition and food production. Gene Conserv. 2011;40:201–215. [Google Scholar]
- 7.Arditti J. John Wiley and Sons; New York: 1992. Fundamentals of Orchid Biology. [Google Scholar]
- 8.De L.C., Rao A.N., Singh D.R. Endangered orchids and their conservation in north east India. In: Purkayastha J., editor. Bioprospecting of Indigenous Bioresources of North-East India. Springer; Singapore: 2016. pp. 61–75. [Google Scholar]
- 9.De L.C. New India Publishing Agency; Pitampur, New Delhi: 2011. Value Addition in Flowers and Orchids; p. 294. [Google Scholar]
- 10.Dutta S., Chowdhury A., Bhattacharjee B., Nath P.K., Dutt B.K. In vitro multiplication and protocorm development of Dendrobium aphyllum (Roxb.) Assam Univ. J. Sci. Technol. J. Biol. Environ. Sci. 2011;7(1):57–62. [Google Scholar]
- 11.Affrida A.H., Sakinah A., Zaiton A., Mohd Nazir B., Oono Y., Hase Y., Shikazono N., Tanaka A. Flower morphology of ion beam irradiated Dendrobium crumenatum. 17th Scientific Meeting of the Malaysian Society for Molecular Biology and Biotechnology. 2008 [Google Scholar]
- 12.Luan L.Q., Nguyen H.P.U., Vo T.T.H. In vitro mutation breeding of Paphiopedilum by ionization radiation. Sci. Hortic. 2012;144:1–9. [Google Scholar]
- 13.Ahmad Z., Ariffin S., Hassan A.A., Ahmad Ramli R.A., Basiran M.N., Ibrahim R. Induction of insect resistance in Dendrobium mirbelianum using ion beam irradiation. Acta Hortic. 2015;1087:499–505. [Google Scholar]
- 14.Sakinah A., Azhar M., Affrida A.H., Zaiton A., Mohd Nazir B. Flower morphology of Dendrobium sonia mutants. Research and Development Seminar. 2010 [Google Scholar]
- 15.Hesami M., Daneshvar M.H.J. Indirect organogenesis through seedling-derived leaf segments of Ficus religiosa - a multipurpose woody medicinal plant. J. Crop Sci. Biotechnol. 2018;21:129–136. [Google Scholar]
- 16.Suprasanna P., Jain S.M., Ochatt S.J., Kulkarni V.M., Predieri S. Applications of in vitro techniques in mutation breeding of vegetatively propagated crops. In: Shu Q.Y., Forster B.P., Nakagawa H., editors. Plant Mutation Breeding and Biotechnology. CAB International; Wallingford: 2012. pp. 369–383. [Google Scholar]
- 17.van Harten A.M. Cambridge Univ Press; Cambridge: 1998. Mutation Breeding: Theory and Practical Applications; p. 353. [Google Scholar]
- 18.Suprasanna P., Rupali C., Desai N.S., Bapat V.A. Partial desiccation augments plant regeneration from irradiated embryogenic cultures of sugarcane. Plant Cell Tissue Organ Cult. 2008;92(1):101–105. [Google Scholar]
- 19.Gupta S.D., Jatothu B. Fundamentals and applications of light-emitting diodes LEDs in in vitro plant growth and morphogenesis. Plant Biotechnol. Rep. 2013;7:211–220. [Google Scholar]
- 20.Cybularz-Urban T., Hanus-Fajerska E., Świderski A. Effect of light wavelength on in vitro organogenesis of a Cattleya hybrid. Acta Biol. Crac. Ser. Bot. 2007;49(1):113–118. [Google Scholar]
- 21.Billore V., Jain M., Suprasanna P. Monochromic radiation through light-emitting diode (LED) positively augments in vitro shoot regeneration in Orchid (Dendrobium sonia) Can. J. Biotech. 2017;1(2):50–58. [Google Scholar]
- 22.Arditti J., Ernst R. John Wiley & Sons; New York: 1993. Micropropagation of Orchids; p. 682. [Google Scholar]
- 23.Zoratti L., Karppinen K., LuengoEscobar A., Haggman H., Jaakola L. Light-controlled flavonoid biosynthesis in fruits. Front. Plant Sci. 2014;5:534. doi: 10.3389/fpls.2014.00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olle M., Virsile A. The effects of light-emitting diode lighting on greenhouse plant growth and quality. Agric. Food Sci. 2013;22(2):223–234. [Google Scholar]
- 25.Shengxin C., Chunxia L., Xuyang Y., Song C., Xuelei J., Xiaoying L., Zhigang X., Rongzhan G. Morphological, photosynthetic, and physiological responses of rapeseed leaf to different combinations of red and blue lights at the rosette stage. Front. Plant Sci. 2016;7:1144. doi: 10.3389/fpls.2016.01144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu W., Liu Y., Song L., Jacobs D.F., Du X., Ying Y., Wu J. Effect of differential light quality on morphology, photosynthesis, and antioxidant enzyme activity in Camptotheca acuminata seedlings. J. Plant Growth Regul. 2017;36(1):148–160. [Google Scholar]
- 27.Folta K.M., Carvalho S.D. Photoreceptors and control of horticultural plant traits. HortScience. 2015;50:1274–1280. [Google Scholar]
- 28.Soontornchainaksaeng P., Chaicharoen S., Sirijuntarut M., Kruatrachue M. In vitro studies on the effect of light intensity on plant growth of Phaius tankervilliae(Banks ex L’ Herit.) Bl. andVanda coerulea Griff. Sci. Asia. 2001;27:233–237. [Google Scholar]
- 29.Pinker I., Zoglauer K., Göring H. Influence of light on adventitious root formation in birch shoot cultures in vitro. J. Plant Biol. 1989;31(4):254–260. [Google Scholar]
- 30.Murashige T., Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962;15:473–497. [Google Scholar]
- 31.Arnon D.I. Copper enzymes in isolated chloroplasts, polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahloowalia B.S. In vitro techniques and mutagenesis for the improvement of vegetatively propagated plants. In: Jain S.M., Brar D.S., Ahloowalia B.S., editors. Somaclonal Variation and Induced Mutations in Crop Improvement. Kluwer Acad. Publishers; London: 1998. pp. 293–309. [Google Scholar]
- 33.Jain S.M. Mutation-assisted breeding for improving ornamental plants. Acta Hortic. 2006;714:85–98. [Google Scholar]
- 34.Sheela V.L., Sheena A. Novel trends and achievements in breeding of tropical ornamental crops especially orchids and anthuriums: the mutation breeding approach. In: Tomlekova N.B., Kozgar M.I., Wani M.R., editors. Mutagenesis: Exploring Genetic Diversity of Crops. Wageningen Academic Publ; The Netherlands: 2014. pp. 141–158. [Google Scholar]
- 35.Yamaguchi H., Shimizu A., Degi K., Morishita T. Effects of dose and dose rate of gamma ray irradiation on mutation induction and nuclear DNA content in chrysanthemum. Breed. Sci. 2008;58:331–335. [Google Scholar]
- 36.Kahrizi Z.A., Jafarkhani Kermani M., Amiri M., Vedadi S., Hosseini Z. In vitro radiosensitivity of different genotypes and explants of rose (Rosa hybrida) J. Hortic. Sci. Biotechnol. 2013;88(1):47–52. [Google Scholar]
- 37.Dehgahi R., Joniyasa A. Gamma irradiation-induced variation in Dendrobium sonia-28 Orchid protocorm-like bodies (PLBs) Fungal Genom. Biol. 2017;7:2. [Google Scholar]
- 38.Kozlowska-Kalisz J. The influence of ionizing radiation on biological activity of endogenous growth regulators in orchids Cymbidium in tissue culture. Acta Hortic. 1979;91:261–286. [Google Scholar]
- 39.Lee Y.M., Lee H.J., Kim Y.S., Kang S.Y., Kim D.S., Kim J.B., Ahn J.W., Ha B.K., Kim S.H. Evaluation of the sensitivity to ionising γ-radiation of a Cymbidium hybrid. J. Hortic. Sci. Biotechnol. 2016;91(2):109–116. [Google Scholar]
- 40.Kim J.H., Beak M.H., Chung B.Y., Wi S.G., Kim J.S. Alterations in the photosynthetic pigments and antioxidant machineries of red pepper (Capsicum annuum L.) plants from control and gamma-irradiated seeds. J. Plant Biol. 2004;47:314–321. [Google Scholar]
- 41.Wi S.G., Chung B.Y., Kim J.S., Kim J.H., Baek M.H., Lee J.W., Kim Y.S. Effects of gamma irradiation on morphological changes and biological responses in plants. Micron. 2007;38(6):553–564. doi: 10.1016/j.micron.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 42.Marcu D., Damian G., Cosma C., Cristea V. Gamma radiation effects on seed germination, growth and pigment content, and ESR study of induced free radicals in maize (Zea mays) J. Biol. Phys. 2013;39(4):625–634. doi: 10.1007/s10867-013-9322-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahmad Z., Hassan A.A., Idris N.A., Basiran M.N., Tanaka A., Shikazono N., Oono Y., Hase N. Effects of ion beam irradiation on Oncidium lanceanum orchids. J. Nucl. Rel. Technol. 2006;3:1–8. [Google Scholar]
- 44.Preuss S.B., Britt A.B. A DNA-damage-induced cell cycle checkpoint in Arabidopsis. Genetics. 2003;164:323–334. doi: 10.1093/genetics/164.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin Y., Li J., Li B., He T., Chun Z. Effects of light quality on growth and development of protocorm-like bodies of Dendrobium officinale in vitro. Plant Cell Tissue Organ Cult. 2011;105:329–335. [Google Scholar]
- 46.Wang Y., Folta K.M. Contribution of green light to plant growth and development. Am. J. Bot. 2013;100:70–78. doi: 10.3732/ajb.1200354. [DOI] [PubMed] [Google Scholar]
- 47.Cioć M., Szewczyk A., Żupnik M., Kalisz A., Pawłowska B. LED lighting affects plant growth, morphogenesis and phytochemical contents of Myrtus communis L. in vitro. Plant Cell Tiss. Organ Cult. 2018;132:433–438. [Google Scholar]
- 48.Xu Z.G., Cui J., Di X.R. Effect of different spectral energy distribution on tissue culture of Oncidium in vitro. J. Beijing Forest Univ. 2009;31:45–50. [Google Scholar]
- 49.Gabryszewska E., Rudnicki R. The influence of light quality on the shoot proliferation and rooting of Gerbera jamesonii in vitro. Acta Agrobot. 1995;48(2):105–111. [Google Scholar]
- 50.Bach A., Świderski A. The effect of light quality on organogenesis of Hyacinthus orientalis L. in vitro. Acta Biol. Crac. Ser. Bot. 2000;42(1):115–120. [Google Scholar]
- 51.Bach A., Malik M., Ptak A., Kędra M. Effect of light quality on morphogenesis of bulbous ornamental microplant. Acta Hortic. 2000;53:173–180. [Google Scholar]
- 52.Sivakumar G., Heo J.W., Kozai T., Paek K.Y. Effect of continuous or intermittent radiation on sweet potato plantlets in vitro. J. Hortic. Sci. Biotechnol. 2006;81:546–548. [Google Scholar]
- 53.Wang Z., Tian J., Yu B., Yang L., Sun Y. LED light spectrum affects the photosynthetic performance of Houttuynia cordata seedlings. Am. J. Opt. Photonics. 2015;3(3):38–42. [Google Scholar]
- 54.Wang H., Gu M., Cui J., Shi K., Zhou Y., Yu J. Effects of light quality on CO2 assimilation, chlorophyll-fluorescence quenching, expression of Calvin cycle genes and carbohydrate accumulation in Cucumis sativus. J. Photochem. Photobiol. B, Biol. 2009;96:30–37. doi: 10.1016/j.jphotobiol.2009.03.010. [DOI] [PubMed] [Google Scholar]