Abstract
In Northern Italy, a hastening of hare population decline was noticeable from 2008. In the same year hunters reported a sudden increase of hares infected by Taenia sp. larvae, whose morphology was consistent with T. pisiformis cysticerci. The aim of the survey was: i)to identify the parasites through morphological features and molecular techniques; ii)to quantify the prevalence and abundance of cysticerci in hunted hares; iii)to describe pathological aspects of parasite-induced lesions; iv)to evaluate the short-term trend of the infection comparing two different hunting seasons; v)to highlight possible relationship between T. pisiformis infection and hare-related variables.
In 2013, 2015 the viscera of 54 and 61 hares legally hunted in agro-ecosystems of the Po Plain were collected.
Peritoneum, liver and lungs were examined for cysticercosis; abundance was estimated counting superficial parasites in liver; parasites were microscopically identified by shape and measure of both large and small hooks. One cysticercus from each hare was analized by a PCR targeting Taeniid species and then sequenced. Frozen liver, lungs and gastrointestinal peritoneum were macroscopically observed and, after thawing, representative samples from the available organs were collected for histologic examination to verify parasitic cysts and the subsequent damage of the involved organs. Sex, weight and age class of the animals were recorded. Generalized linear models were used for statistical analysis.
T. pisiformis was isolated in 8 hares in 2013 (prevalence 14.8%; abundance range: 0–400; mean abundance 17.8) and in 2 hares in 2015 (prevalence 3.28%; abundance range: 0–180; mean abundance 3.22). Identification was confirmed morphologically and by PCR. The DNA sequencing confirmed T. pisiformis in all samples. The sequences were all identical each-other. Infection was significantly related with adult age class, sampling year and low full-weight.
Epidemiological and pathological pattern suggest both a possible role on host population health and a tendency toward host-parasite equilibrium.
Keywords: Lepus europaeus, Taenia pisiformis, Cysticercosis, Pathological findings, Epidemiology
Highlights
-
•
Hastening of hare population decline was noticeable in Northern Italy.
-
•
Hunters reported a sudden increase of hares infected by Taenia sp. larvae.
-
•
T. pisiformis was isolated.
-
•
Observed pattern suggest both a possible role on host population health and a tendency toward host-parasite equilibrium.
1. Introduction
Since the Seventies, a reduction of the European Brown Hare population (Lepus europaeus) has been observed in most European Countries (Edwards et al., 2000; Schmidt et al., 2004; Smith et al., 2005).
In Italy, an increase of the rate of hare population decline was notable since 2008; in particular, in the Emilia Romagna region the hares captured for restocking from the protected areas dropped from about 7000 in 2007-’08 to 1891 in 2014-‘15 (Zanin, 2017).
In the same year, 2008, some hunters reported a sudden increase of hares infected by Taenia sp. larvae, morphologically consistent with T. pisiformis cysticerci.
T. pisiformis is a heteroxenous parasite belonging to the Phylum Plathelminthes (Eucestoda, Cyclophillidea, Taeniidae). Adults occur in the small intestine of canids and, rarely, felids, while the larval stage (called cysticercus) is found in the serosa of the body cavity and viscera of lagomorphs and rodents (Jones and Pybus, 2001) causing an infection called cysticercosis. Generally, cysticercosis does not give rise to clinically relevant signs in lagomorphs, although a loss of prolificacy was observed in rabbit (Hallal-Calleros et al., 2016) and a negative relation to kidney fat index was observed in Iberian hare (Lepus granatensis) by Alzaga et al. (2008).
The aim of the survey was: i)to correctly identify the parasites through morphological features and molecular techniques; ii)to quantify the prevalence and abundance of cysticerci in hunted hares; iii)to describe pathological aspects of parasite-induced lesions; iv)to evaluate the short-term trend of the infection comparing two different hunting seasons; v)to highlight possible relationship between T. pisiformis infection and hare-related variables.
2. Material and methods
The examined hares come from a hunting area (ATC BO2) located in the eastern part of the Bologna Province (Emilia-Romagna Region), an agro-ecosystems of the Po Plain (Italy). The hares were all legally shut during hunting seasons.
Anterior leg, gastrointestinal tract, abdominal and toracic organs of hares were collected during two hunting seasons, namely in 2013 and 2015. All specimens were frozen within few hours, delivered frozen to our facilities and therefore preserved at −20 °C until examination. All sampled hares were hunted between September 15th and October 2nd, at the beginning of each hunting season.
The sex and the weight of the animals were recorded by hunters in a relevant form provided by the authors, while age class was assigned observing foreleg Stroh's tubercle according to Bujalska et al. (1965).
Macroscopic examination was carried out to verify the presence of parasitic cysts morphologically consistent with cysticerci of T. pisiformis in liver, lungs and peritoneum.
Representative samples from the available organs were collected, fixed in 10% buffered formalin at pH 7–7.6 for 24 h, embedded in paraffin wax, and five micron sections were stained with haematoxylin and eosin (H&E) for histological examination. Histological sections of tissues with the most interesting findings were photographed.
In order to obtain a comparable index of parasite abundance, the cyst number was estimated in each infected hare counting the parasites on liver surface and used as parasite abundance for statistical analysis. However, if present, also the number of cysts in the liver parenchima was recordered.
For each parasitized hare, at least 3 parasites were microscopically identified by shape and measure of both large and small hooks and compared with the measure and description provided by Abuladze (1964) and Loos-Frank (2000).
One cysticercus from each hare was therefore analized by a PCR targeting Taeniid species and then sequenced.
DNA was extracted from cysts using a DNeasy Blood & Tissue kit (Qiagen, Valencia, CA, USA), according to the manufacturer's instructions.
Each sample was analysed by conventional PCR using primers TaeSf-UnivSr (12S rRNA gene) and protocols described elsewhere (Barabàsi et al., 2010), in particular the used primers were:
UnivSr: 5′-GCGGTGTGTACMTGAGCTAAAC-3′
TaeSf: 5′-YGAYTCTTTTTAGGGGAAGGTGTG-3’.
Negative (sterile water) and positive controls (DNA of Taenia pisiformis) were included in each run.
The PCR products were sequenced in both directions using the Big Dye Terminator v3.1 chemistry and ABI PRISM 3130xl Genetic Analyzer (Applied Biosystem, Foster City, CA, USA). Sequence data were assembled and edited with SeqScape software v2.5 (Applied Biosystem, Foster City, CA, USA) and compared with those available in GenBank database by Basic Local Alignment Search Tool (BLAST -http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Statistical analysis was performed using STATA 11. Negative binomial regression was used to evaluate factors possibly influencing parasite abundance while parasite prevalence was analysed using logistic regression and/or Fisher exact test. The possible influence of parasite prevalence and abundance on hare weight was analysed using linear regression.
3. Results
A total of 114 hare were examined: 54 during the first hunting season (2013) and 60 during the second one (2015), two years later.
The sex and age of examined hares in each sampling season are reported in Table 1.
Table 1.
Hares examined in each hunting season. Y:young; A: adult; M: male; F: female.
| Young | Adult | Y/A | Male | Female | M/F | |
|---|---|---|---|---|---|---|
| 2013 | 33 | 21 | 1.48 | 23 | 31 | 0.74 |
| 2015 | 37 | 23 | 1.6 | 27 | 34 | 0.79 |
Cysicerci were isolated in adult hares only. Identification as T. pisiformis was confirmed by both morphology and PCR. All the DNA sequences were identical each-other and showed the highest similarity (99%) with a sequence from Japan (Accession number AB329716).
In particular, T. pisiformis was isolated in 8 hares in 2013 (prevalence: 14.8%; mean liver surface abundance 17.8, range: 0–202) and in 2 hares in 2015 (prevalence 3.28%; mean liver surface abundance 3.22, range: 0–180).
The difference in parasite prevalence between juveniles and adults was significant (Fisher exact test, p < 0.001).
Since no juvenile was infected, the following analyses were performed for adult hares only.
Logistic regression highlighted a significant reduction of prevalence from 2013 to 2015 and higher prevalence in animals with low full weight, while no relation was observed with hare sex (Table 2).
Table 2.
Logistic regression model performed on prevalence data in adult hare. A significant reduction in prevalence in 2015 and a significant negative relation with hare weight were highlighted.
| T. pisiformis prevalence | coefficient | p-value | 95% C.I. | ||
|---|---|---|---|---|---|
| Year | |||||
| 2013 | – | ||||
| 2015 | −1.914 | 0.049 | −3.820 | −0.006 | |
| sex | |||||
| male | – | ||||
| female | 0.646 | 0.545 | −1.449 | 2.741 | |
| full weight | −3.201 | 0.033 | −6.147 | −0.255 | |
| constant | 10.53 | 0.042 | 0.399 | 20.676 | |
Parasite abundance had no significant relationship with year and hare sex, while a significant negative relationship was observed with hare full weight as highlighted with negative binomial regression (Table 3).
Table 3.
Negative binomial regression model performed on liver surface abundance data in adult hare. A significant negative relation with hare weight was highlighted.
| T. pisiformis abundance | coefficient | p-value | 95% C.I. | ||
|---|---|---|---|---|---|
| year | |||||
| 2013 | – | ||||
| 2015 | 0.847 | 0.581 | −2.163 | 3.856 | |
| sex | |||||
| male | – | ||||
| female | 3.176 | 0.165 | −1.311 | 7.662 | |
| full weight | −9.337 | 0.022 | −17.301 | −1.372 | |
| constant | 33.651 | 0.013 | 7.161 | 60.142 | |
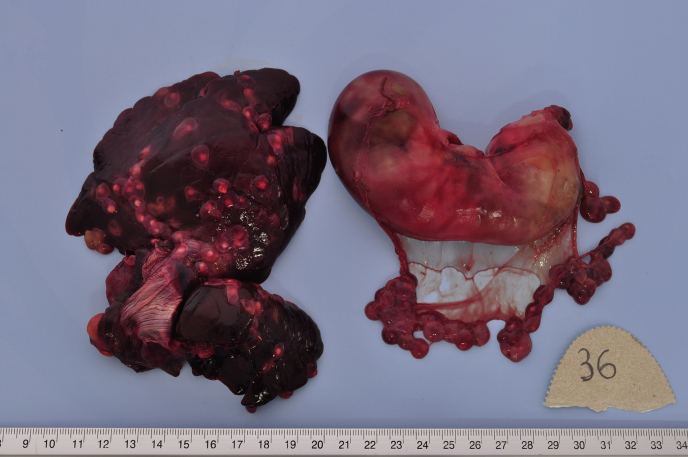
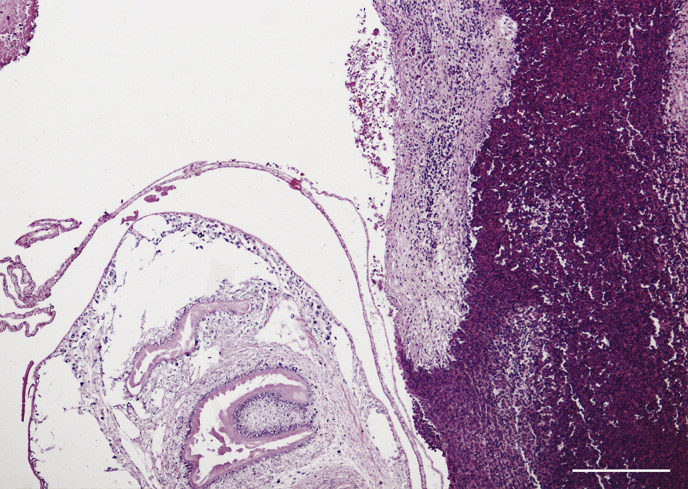
The most important results of pathological investigations related to cysticercosis of the hares are reported in Table 4. Fig. 1, Fig. 2 show exemples of macroscopic and microscopic aspect of cysticercosis.
Table 4.
Macroscopic examination results for individual positive hare and related microscopic features of hepatic lesions.
| Hare-id/year | Macroscopic examination | Microscopic examination | Number of cysticerci on liver surface |
|---|---|---|---|
| 2/2013 | Parasitic cysts in liver surface and diaphragm serosa. |
Description: rare inflammatory lymphocytic foci within the liver parenchyma. Diagnosis: subacute focal interstitial hepatitis associated with parasitic infection. |
16 |
| 9/2013 | Parasitic cysts in small intestine visceral serosa. Liver with massive cysticercosis, some of them (10) in the parenchima. Some cysts were also visible on the renal capsula. |
Description: inflammatory lympho-plasmacytic foci within the liver parenchyma around protoscolex sections of T. pisiformis cysts, surrounded by a fibrous reaction. Diagnosis: focal subacute/chronic interstitial hepatitis with cysts of T. pisiformis |
150 |
| 13/2013 | Parasitic cysts in intestinal visceral serosa. Moderate to severe hepatomegaly with several peritoneal cysts, some of them (10) inside the hepatic parenchyma. |
Description: multifocal lymphocytic lymphocytic foci within the liver parenchyma. Diagnosis: multifocal to disseminated interstitial chronic hepatitis associated with cysts of T. pisiformis with biliary stasis. |
202 |
| 35/2013 | Parasitic cysts in intestinal surface. Hepatomegaly with several (about 400) yellowish-white cysts, of 3 mm diameter, even within the hepatic parenchyma. |
Description: multifocal lymphocytic lymphocytic foci within the liver parenchyma. Diagnosis: multifocal to disseminated interstitial chronic hepatitis associated with cysts of T. pisiformis, with diffuse biliary stasis. |
100 |
| 36/2013 | Peritoneal cysts on of the great gastric curvature and on liver surface (Fig. 1). | Slight focal interstitial hepatitis. | 140 |
| 41/2013 | Few parasitic cysts in intestinal surface and one on the liver surface. | No microscopic analysis performed | 1 |
| 42/2013 | Parasitic cysts in intestinal surface. Moderate hepatomegaly with multifocal peritoneal (hepatic and diaphragmatic) cysts. |
Description: multifocal lymphocytic lymphocytic foci within the liver parenchyma. Diagnosis: multifocal interstitial chronic hepatitis associated with cysts of T. pisiformis visible also in the peritoneum of the diaphragmatic sections. |
30 |
| 47/2013 | Only 3 cysts on the diaphragmatic liver surface. | No evidence of parasite related alterations. | 3 |
| 7/2015 | Several parasitic cysts in hepatic surface. |
Description: scattered inflammatory lympho-plasmacytic foci within the liver parenchyma. Diagnosis: focal interstitial lymphocytic hepatitis with severe parasitic infection associated with peritoneal cysts of T. pisiformis. |
180 |
| 19/2015 | Some parasitic cysts in hepatic surface |
Description: small scattered lymphocytic foci with parasitic cysts, surrounded by a granulomatous inflammation with multinucleated giant cells, macrophages, plasma cells and many eosinophils. Diagnosis: severe chronic portal hepatitis (Fig. 2). |
17 |
Fig. 1.
Hare 36/2013, liver and stomach. Cysticercosis (T. pisiformis) in liver surface and gastric peritoneum.
Fig. 2.
Hare 19/2015. Histological image of a hepatic cyst (T. pisiformis), visible on the left. Focal infiltrate of eosinophil granulocytes with few lymphocytes, macrophages and plasma cells on the right. H&E, Bar = 200 μm.
4. Discussion
No published study regarding specifically T. pisiformis epidemiology and pathology infection in hare or in definitive hosts (domestic or wild canids) is available nor from Italy or from other european Countries, becoming the present paper the first one dealing specifically with hare cysticercosis epidemiology and pathology.
Studies about hare parasites reporting T. pisiformis cysticercosis usually base the identification of this parasite only on macroscopic morphological characters of the cysticercus and on host specificity. For the present study, macroscopic morphology was confirmed both by microscopic examination of the parasite hooks and by genetic analyses, allowing to exclude possible confounding infection due to other Taenia species infecting wildlife such as T. crassiceps (Jones and Pybus, 2001).
Taenia pisiformis cysticercosis is known to be a typical parasitosis of lagomorphs and in particular of rabbit and hare.
From 2008, informations about this infection came firstly by newspapers and other media claming the appearance of a dangerous zoonotic parasite in hares followed by communications, during scientific congresses and conventions, aimed, overall, to specify that hare cysticercosis is not a zoonotic infection and to focus on possible causes of the emergence of this parasite (Spaggiari et al., 2009; Maioli et al., 2010; Stancampiano et al., 2016).
Previous papers dealing with hare parasites in Italy seldom reported T. pisiformis therefore suggesting the absence or a very low burden of T. pisiformis in Northern Italy brown hares until 2008. In particular, no infection due to T. pisiformis were reported in several paper dealing with hare parasites (Canestri-Trotti et al., 1988; Poglayen et al., 1994; Zanni et al., 1995) while in our best knowledge the only italian report was in 2 out of 52 hares hunted in the Liguria Region (Poglayen et al., 2002).
A pattern typical of epidemic infections, characterized by unexpected and sudden increase of cases, was therefore observed in northern italian areas such as the area of the present study. The infection is still not reported in hare in central Italy (Sergi et al., 2018). The significant lower prevalence of cysticercosis in the second year of the present survey is consistent with the typical decrease of the case number after an epidemic peak.
The source of the cysticercosis outbreak observed is still undefined. We studied intermediate hosts only, and very few data are available about possible definitive hosts. A recent survey on helminth parasites in wild canids in Emilia-Romagna Region -the same italian Region of the present survey-reported only low prevalence in fox (Fiocchi et al., 2016), consistently with Jones and Pybus (2001) statement that foxes are refractory to experimental infection, unlike dogs. It is therefore reasonable that the epidemiology included, at least in the early period, also domestic hosts.
Pathological features show a very low host reaction to the parasite, with moderate interstitial lymphocytic hepatitis in most cases. Only in one case in the second sampling year (hare number 19/2015) a parasitic granulomatous hepatitis suggesting host immuno response, possibly caused by the rupture of some cysts, was observed (Marcato et al., 2015). It is noteworthy that this kind of immuno reaction was observed in a hare with a relatively low parasite burden (Table 4 and Fig. 2).
Although the very low number of infected hares in 2015 do not allows clear conclusions, the lack of signs of specific immune response in 2013 and its appearance in 1 hare out of 2 hares in 2015 suggest that cysticercosis decline should be related to acquired resistance of host population to the parasite. A certain degree of protective immune response to Taenia spp. has long been recognized in intermediate hosts (Flisser et al., 1979); moreover vaccines have recently been developed both for T. pisiformis (Chen et al., 2014) and for other Taenia species (Gauci et al., 2008). We believe that the lymphocytic foci observed are an expression of an inflammatory response to the parasite, but no investigation was conducted to verify the presence of other or concomitant infections, viruses or other pathogens.
The massive presence of parasitic cysts and the histologically detected lesions were sufficient for a reliable anatomopathological diagnosis (Marcato and Rosmini, 1986).
The relationship with host age and the lack of differences related to host sex observed in this study are not perfectly consistent with data collected in different lagomorph hosts: some authors found highest intensities in juveniles Lepus americanus and Sylvilagus floridanus in USA (Jones and Pybus, 2001); others authors, likewise in the present paper, observed highest intensities in adult in L. granatensis (Alzaga et al., 2008); Berg and Beck (1968) found almost double prevalence in male than female naturally infected S. floridanus but the examined sample included more juvenile females than males and most juveniles were uninfected. Consistently with our results, Alzaga et al. (2008) found negative relationship between cysticerci abundance and body condition in L. granatensis, supporting the potential role of this parasite as a contributory factor of hare population decline.
In conclusion, we were able to identify the parasites infecting L. europaeus in Northern Italy as T. pisiformis cysticerci, whose epidemiological and pathological pattern suggest both a possible role on host population health and a tendency toward host-parasite equilibrium.
Acknowledgments
Thanks to Irene Cicognani and Rossella Ciarlo who collaborated to organ examination; to Valter Trocchi and Cristian Geminiani who supported sample collection and age-class determination. Thanks to Elisa Armaroli for her useful comments and discussion and to Vittorio Guberti for his enduring conceptual support. The authors thank also Silvia Sabattini for the photographic work and Enrica Bellinello for her comments on the paper draft.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2019.04.004.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Abuladze K.I. Taeniata of animals and man and diseases caused by them. In: Skrjabin K.I., editor. vol. IV. Israel Program for Scientific Translations; Jerusalem: 1964. (Essentials of Cestodology). 1970, 530 pp. [Google Scholar]
- Alzaga V., Vicente J., Villanua D., Acevedo P., Casas F., Gortazar C. Body condition and parasite intensity correlates with escape capacity in Iberian hares (Lepus granatensis) Behav. Ecol. Sociobiol. 2008;62:769–775. [Google Scholar]
- Barabàsi S.S., Deplazes P., Cozma V., Pop S., Tivadar C., Bogolin I., Popescu R. Echinococcus multilocularis confirmed in Romania. Sci. Parasitol. 2010;11(2):89–96. [Google Scholar]
- Berg E., Beck R.D. Possible role of a sex factor in rabbit hosts naturally infected with Taenia pisiformis cysticerci. J. Parasitol. 1968;54(6):1252–1253. [Google Scholar]
- Bujalska G., Cabon-Raczynska K., Raczynski J. Studies on the european hare. VI. Comparison of different criteria of age. Acta Theriol. 1965;10(1):1–10. [Google Scholar]
- Canestri-Trotti G., Corradini L., Bassi S. Osservazioni sulle elmintiasi gastrointestinali di lepri delle province di Ferrara e Modena e lepri di importazione. Suppl. Ric. Biol. Selvaggina. 1988;14(1):317–321. [Google Scholar]
- Chen L., Yang D.Y., Xie Y., Nong X., Huang X., Fu Y., Gu X.B., Wang S.X., Peng X.R., Yang G.Y. Protection against Taenia pisiformis larval infection induced by recombinant oncosphere antigen vaccine. Genet. Mol. Res. 2014;13(3):6148–6159. doi: 10.4238/2014.February.13.14. [DOI] [PubMed] [Google Scholar]
- Edwards P.J., Fletcher M.R., Berny P. Review of the factors affecting the decline of the European brown hare, Lepus europaeus (Pallas, 1778) and the use of wildlife incident data to evaluate the significance of paraquat. Agric. Ecosyst. Environ. 2000;79:95–103. [Google Scholar]
- Fiocchi A., Gustinelli A., Gelmini L., Rugna G., Renzi M., Fontana M.C., Poglayen G. Helminth parasites of the red fox Vulpes vulpes (l.1758) and the wolf Canis lupus italicus Altobello, 1921 in Emilia-Romagna. Italy. Ital. J.Zool. 2016;83(4):503–513. [Google Scholar]
- Flisser A., Pérez-Montfort R., Larralde C. The immunology of human and animal cysticercosis: a review. Bull. WHO. 1979;57(5):839–856. [PMC free article] [PubMed] [Google Scholar]
- Gauci C., Vural G., Öncel T., Varcasia A., Damian V., Kyngdon C.T., Craig P.S., Anderson G.A., Lightowlers M.W. Vaccination with recombinant oncosphere antigens reduces the susceptibility of sheep to infection with Taenia multiceps. Int. J. Parasitol. 2008;38:1041–1050. doi: 10.1016/j.ijpara.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallal-Calleros C., Morales-Montor J., Orihuela-Trujillo A., Togno-Peirce C., Murcia-Mejía C., Bielli A., Hoffman K.L., Flores-Pérez F.A. Taenia pisiformis cysticercosis induces decreased prolificacy and increased progesterone levels in rabbits. Vet. Parasitol. 2016;229:50–53. doi: 10.1016/j.vetpar.2016.09.015. [DOI] [PubMed] [Google Scholar]
- Jones A., Pybus M.J. Teniasis and echinococcosis. In: Samuel W.M., Pybus M.J., Kocan A.A., editors. Parasitic Diseases of Wild Mammals. Iowa State University Press; 2001. pp. 150–192. [Google Scholar]
- Loos-Frank B. An up-date of Verster's (1969) ‘Taxonomic revision of the genus Taenia Linnaeus’ (Cestoda) in table format. Syst. Parasitol. 2000;45:155–183. doi: 10.1023/a:1006219625792. [DOI] [PubMed] [Google Scholar]
- Maioli G., Fontana M.C., Zanin D., Rugna G., Renzi M., Merialdi G. Cluster of cysticercosis (Taenia pisiformis) in european brown hares in Bologna province. XXVI congresso Nazionale della Societa' Italiana di Parassitologia, 22-25 Giugno 2010, Perugia. Parassitologia. 2010;52(1–2):279. [Google Scholar]
- Marcato P.S., Bettini G., Sarli G., Perillo A. Fegato e Pancreas. In: Marcato P.S., editor. Patologia Sistematica Veterinaria. Seconda Edizione, Capitolo 6, Vol II. Edagricole - Edizioni agricole di New Business Media srl; Milano: 2015. pp. 791–886. [Google Scholar]
- Marcato P.S., Rosmini R. Società Editrice Esculapio; Bologna: 1986. Pathology of the Rabbit and Hare. A Color Atlas and Compendium. [Google Scholar]
- Poglayen G., Gaglio G., Brianti E., Capelli G., Agretti D. Control of the health status of brown hare (Lepus europaeus). The parasitic fauna. Atti della Società Italiana delle Scienze Veterinarie. 2002;56:199–200. [Google Scholar]
- Poglayen G., Roda R., Zanni M.L., Amendola B., Pepa M. Parassiti dell’apparato digerente della lepre (Lepus europaeus) nelle province di Bologna e Bolzano. Selezione Veterinaria. 1994;35:193–199. [Google Scholar]
- Schmidt N.M., Asferg T., Forchhammer M.C. Long-term patterns in European brown hare population dynamics in Denmark: effects of agriculture, predation and climate. BMC Ecol. 2004:4–15. doi: 10.1186/1472-6785-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergi V., Romeo G., Serafini M., Torretta E., Macchioni F. Endoparasites of the european hare (Lepus europaeus) (Pallas, 1778) in central Italy. Helminthol. 2018;55(2) doi: 10.2478/helm-2018-0011. 157-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R.K., Jennings N.V., Harris S. A quantitative analysis of the abundance and demography of European hares Lepus europaeus in relation to habitat type, intensity of agriculture and climate. Mamm Rev. 2005;35:1–24. [Google Scholar]
- Spaggiari B., Gelmini L., Fontana M.C., Lavazza A., Merialdi G. vol. 12. 2009. Diagnostic investigation on found dead brown hares (Lepus europaeus) in three Emilia Romagna Provinces during 2008; p. 34. (III Convegno di Ecopatologia della Fauna Selvatica, 15-17 ottobre 2009, Torino. Poster). [Google Scholar]
- Stancampiano L., Militerno G., Cicognani I., Cazzin S., Capelli G. XXIX Congress SoIPa-Società Italiana di Parassitologia & European Veterinary Parasitology College. 21-24 June 2016, Bari. 106. 2016. Cysticercosis in Lepus europaeus hunted in plain areas of Bologna province (Emilia Romagna region, Italy) [Google Scholar]
- Zanin D. 2017. Programma annuale di gestione A.T.C. BO2 Imolese e Bologna Orientale – 2017/2018. Centro servizi e Coordinamento degli Ambiti territoriali di Caccia di Bologna.www.atcbologna.org [Google Scholar]
- Zanni M.L., Poglayen G., Marzadori F., Benassi M.C., Capucci L., Carpenè E., Tasselli A. Monitoraggio sanitario nella lepre (Lepus europaeus) in provincia di Ravenna. Selezione Veterinaria. 1995;36:1–25. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.