Abstract
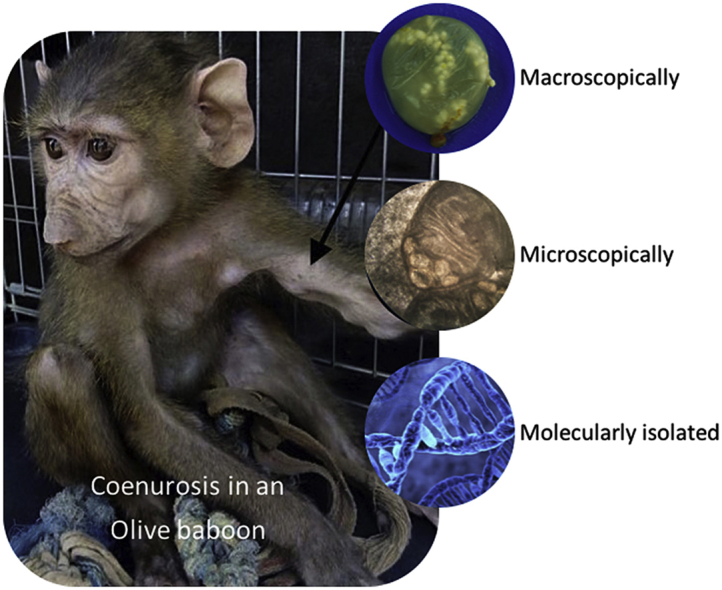
In March 2017, a captive male juvenile (ca. 6 months old) olive baboon (Papio anubis) was brought to a primate rescue center in Benin with multiple subcutaneous swellings of unknown aetiology. At the general inspection of the body, around 15 partially mobile masses of variable sizes were found in different locations across the body. Following two surgical procedures, several cyst-like structures were removed and placed either in 10% formalin or in absolute ethanol. The cysts had a typical coenurus-like morphology. Genomic DNA was extracted from one cyst using a commercially available kit. The molecular characterization was performed by PCR amplification and sequencing of a region of the nuclear ITS-2 rDNA and a fragment of the mitochondrial 12S rDNA gene, revealing its identity as T. serialis, with 88%–98% similarity to T. serialis isolates from USA, Japan and Germany This study reports a new host for the larval form of T. serialis and confirms the role of primates as intermediate host, highlighting also the risk for human infection. Further genetic studies from multiple geographic isolates are needed to clarify the taxonomic status of this group.
Keywords: Olive baboon, Intermediate host, Taenia serialis, Coenurus
Graphical abstract
Highlights
-
•
The present study reports a the olive baboon as new host for the larval form of Taenia serialis.
-
•
This is the first report of T. serialis in Benin.
-
•
The relevance and risk for the health and welfare of primates in rescues centers is discussed.
1. Introduction
Taeniidae is among the most studied family of cestodes due to its worldwide distribution, common occurrence in domestic animals, economic impact and immense public health importance. All taeniids use mammals both as definitive and intermediate hosts, having a complex life cycle which exploits the predator-prey relationships (Bush et al., 2001). The life cycle and host spectrum was studied in detail for some species of Taeniidae, mainly those with zoonotic potential (e.g. Echinococcus granulosus, E. multilocularis, Taenia solium). However, some species remain poorly known, and their real host spectrum, including the zoonotic potential, is largely unexplored. Humans are dead-end hosts for larval cestodes due to ecological factors and their position in the food chain. However, non-human primates may serve as true intermediate hosts, as large predators often prey on them (Jolly, 2013). Understanding the role of primates in the life cycle of various species of Taenia can shed light on their ecology and contribute to the understanding on their zoonotic and public health importance.
Several species of the family Taeniidae are known to have monocystic polycephalic larval stages, known as coenurus type larvae and causing the disease “coenurosis”. The most commonly reported are the zoonotic Taenia multiceps (circulating between Canidae and Hyaenidae and ruminants) and T. serialis (Canidae, Hyaenidae and lagomorphs, rodents, primates) (Verster, 1969; Loos-Frank, 2000). However, a reliable morphological differentiation between T. serialis and T. multiceps is possible only for adult stages collected from the intestines of definitive hosts. Although rostellar hook length has been suggested for larval differentiation, such criteria are not currently considered reliable to differentiate T. serialis from T. multiceps (Schneider-Crease et al., 2017b). Many authors assigned coenurus larvae to species based on the host and/or anatomic location, however, these are not consistent differentiation criteria (Schneider-Crease et al., 2017b). Hence, most of the reports of coenurus type larvae in various hosts cannot be reliably assigned to the species only if molecular tools are employed (Schneider-Crease et al., 2017b). Therefore, previous records were species were identified using only morphological criteria should be treated with caution.
With this view, our aim was to identify to species level larval tapeworms found in an olive baboon, Papio anubis, characterize it molecularly to confirm its identity and discuss the importance of our finding in the light of the parasite's ecology and conservation medicine.
2. Materials and methods
In March 2017, a juvenile (ca. 6 months old) male olive baboon (Papio anubis) was brought to the only primate rescue center in Benin (ATO – Monkey Conservation Center - Veronique Tessier, Manigri village, Bassila commune, Donga Department, Benin) with multiple subcutaneous swellings of unknown aetiology. Swellings were visible and palpable through the skin and located in various regions of the body. The swellings had developed while the baboon was held captive in a private yard with stray dogs permanently present in the same enclosure.
Following two surgical procedures performed eight months (December 2017) and ten months (February 2018) after the arrival in the center, several cyst-like structures were removed from the subcutaneous and intermuscular tissues. Some were analyzed under microscope directly after the surgery and the remaining were placed either in 10% formalin (for further morphological analysis), or in absolute ethanol (for DNA extraction).
Genomic DNA was extracted from one cyst using a commercially available kit (Isolate II Genomic DNA Kit, Bioline, UK). The molecular characterization was performed by PCR amplification and sequencing (Macrogen Europe) of a region of the nuclear ITS-2 rDNA and a fragment of the mitochondrial 12S rDNA gene, using previously published primers and protocols (Gasser and Chilton, 1995; von Nickisch-Rosenegk et al., 1999). The sequences were compared to other sequences from the GenBank database by Basic Local Alignment Search Tool (BLAST) analysis.
Sequences of the 12S s rDNA gene of cestodes with coenurus type larvae were downloaded from GenBank and aligned using Mega X software (Kumar et al., 2016). For the phylogenetic analyses, 21 sequences were included (320 bp) and the tree was constructed using the Maximum Likelihood method and General Time Reversible model (Tamura and Kumar, 2000). The support for clades was assessed by bootstrap resampling (1000 replicates). The tree was rooted by using Echinococcus granulosus as outgroup.
3. Results
3.1. Case presentation
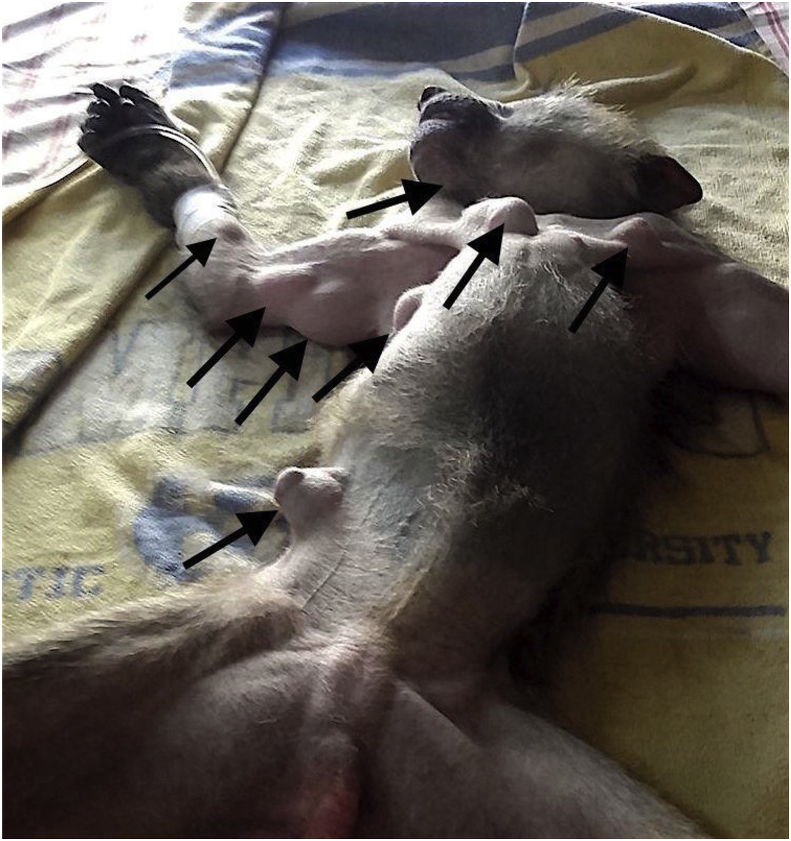
Fifteen partially mobile masses of variable size (from 1 cm to 6 cm) were observed across the juvenile baboon's body upon its arrival to the rescue center in March 2017. Swellings were located mainly in the ventral abdominal and thoracic regions (8 cysts), in the dorsal region close to the thoracic vertebrae (4 cysts), on the inner part of the forelegs (2 cysts) and in the intermandibular region (1 cyst) (Fig. 1). The general state of the animal was otherwise normal, with no other clinical symptoms. The baboon was maintained under observation in the rescue center, firstly in quarantine, isolated from the other baboons, and then with other juveniles as it seemed not directly contagious. Although the general state remained unchanged, the size and number of palpable swellings increased and, as a consequence, 8 months after the arrival of the baboon to the center, the decision to perform an exploratory surgery was taken by the vet. Following an anaesthetic protocol (Acepromazine 0.2 mg/kg IM + Ketamine 10 mg/kg IM + Buprenorphine 0.02 mg/kg IM), surgery was performed in December 2017, and five cyst-like structures were removed. Most of the cysts were located in the intermuscular connective tissue. Only one cyst was located directly in the subcutaneous tissue. The cysts appeared as bladder-like structures, with a transparent or semi-transparent membrane, with clear fluid and multiple small white structures. As the remaining cysts continued to increase in size, the baboon underwent a second surgery in February 2018, when seven more cysts, with similar location and morphology were removed. Three remaining cysts that could be palpated were not removed because of their location and complicated surgical procedure required. These three cysts did not continue to grow or to resorb through the following months. No other baboons in the center presented similar clinical findings.
Fig. 1.
Clinical presentation of the baboon with the clear presence of swellings in various areas of the body: ventral abdominal and thoracic parts, inner part of forearms, intermandibular region (arrow heads) and dorsal region also.
3.2. Parasite identification
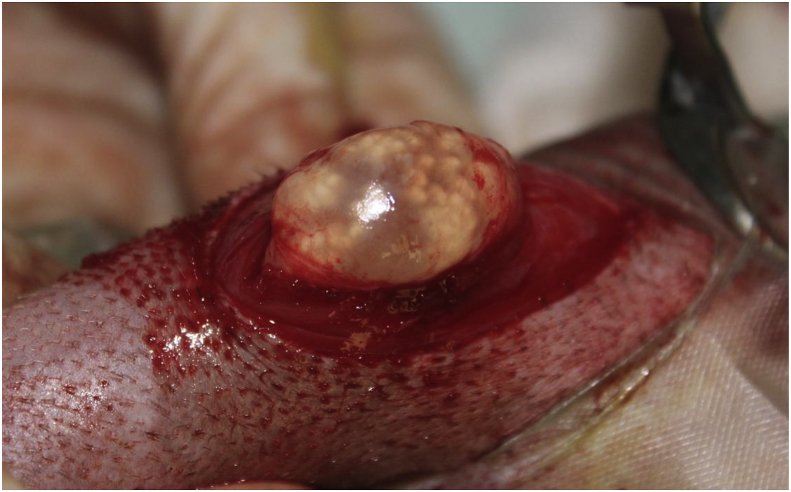
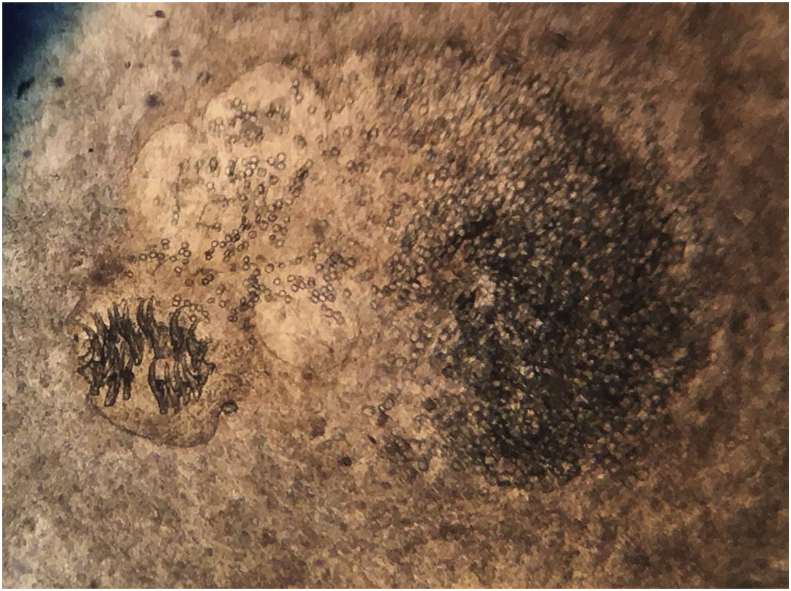
Macroscopically, the cysts had a typical coenurus-like morphology (presence of multiple protoscolices) (Fig. 2, Fig. 3).
Fig. 2.
Macroscopic appearance of a Taenia serialis cyst during surgery, located in intermuscular tissues. Multiple protoscolices are visible as white spots on the inner surface of the cyst.
Fig. 3.
Detail of a protoscolex extracted from a cyst.
The ITS-2 sequence from the cyst removed from this baboon was 96% similar to an isolate of T. serialis from a rabbit in Canada (DQ099571), 88%–98% similar to other T. serialis isolates from coyotes in California, USA (DQ099572, DQ099573, DQ099575, DQ099576) and 99% similar to a T. serialis isolate from a gelada in Ethiopia (KF414738) (Table 1). Its similarity with various T. multiceps isolates ranged between 83% (e.g. KX377745) and 84% (e.g. KX377752), while the similarity with T. saginata isolates ranged between 82% (e.g. AY791899) and 85% (e.g. KU041644). The 12S rDNA sequence revealed a similarity of 99% a T. serialis isolate from a gelada from Ethiopia (KF414738), of 98% to a T. serialis isolate from a domestic dog from Japan (LC085644) and of 96% to a T. serialis isolate (environmental sample) from Germany (EU219546). The similarity to a variety of T. multiceps isolates was of 95% (e.g. LC271556, KX377744), while a similarity of 94% with isolates of T. saginata (AM902708) and T. asiatica (AF445798, AP017670, EF420719) was noted.
Table 1.
Sequence similarity between our sample and other T. serialis sequences available in GenBank.
| Gene | Query cover (%) | Similarity (%) | Acc. no. | Stage | Host | Origin |
|---|---|---|---|---|---|---|
| ITS2 | 100 | 88 | DQ099573 | adult | Canis latrans | California, USA |
| 90 | DQ099576 | adult | Canis latrans | California, USA | ||
| 96 | DQ099571 | larva | Oryctolagus cuniculus | Canada | ||
| 97 | DQ099572 | adult | Canis latrans | California, USA | ||
| 98 | DQ099575 | adult | Canis latrans | California, USA | ||
| 80 | 99 | KF414738 | larva | Theropithecus gelada | Ethiopia | |
| 12S | 100 | 96 | EU219546 | eggs | Canis familiaris | Germany |
| 98 | LC085644 | not specified | Canis familiaris | Japan | ||
| 98 | 98 | LC085837 | not specified | Canis familiaris | Japan | |
| 99 | AB731674 | adult | Canis familiaris | Australia | ||
| 95 | 99 | KF414739 | larva | Theropithecus gelada | Ethiopia |
Our sequences were deposited in the GenBank database, under the Accession numbers MH231230 and MH236068.
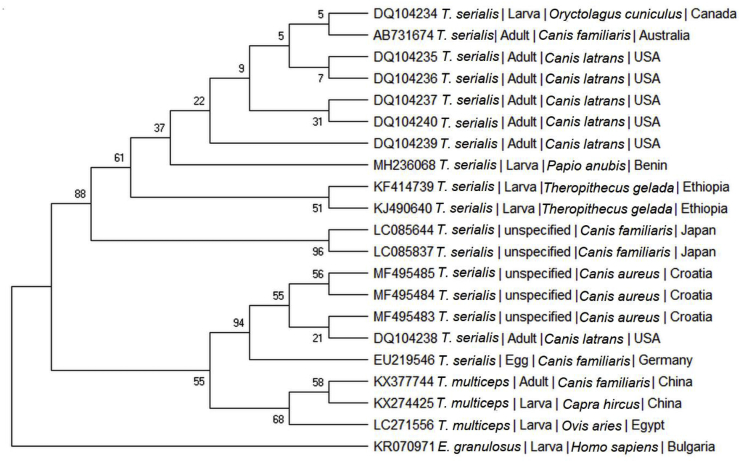
The 12S rDNA gene analysis placed our sequence in a clade including various T. serialis isolates from several host species originating in North America, Australia, Asia and Africa (Fig. 4). A second clade included T. serialis sequences from North America and Europe and all of the T. multiceps isolates. The mean distance within the two clades was of 0.01. The mean distance between the two clades was of 0.0430. The mean distance between the outgroup and the clade was of 2.7487 for the first one and 2.7471 for the second one.
Fig. 4.
Bootstrap consensus tree inferred from 1000 replicates, using the Maximum Likelihood method. The analysis involved 20 sequences of 12S rDNA gene of cestodes having coenurus type larvae (Taenia serialis and T. multiceps) and one sequence of Echinococcus granulosus, as outgroup. For each sequence, the GenBank Accession number, species, developmental stage, host and geographic origin are provided. A total of 320 positions were included in the dataset. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches.
4. Discussion
Lagomorphs (Oryctolagus cuniculus, Lepus europaeus, L. townsendii, L. americanus, L. californicus, L. timidus, L. callotis) are traditionally regarded as the typical intermediate hosts for T. serialis. However, larval forms were reported in a greater variety of hosts, including rodents (Sciurus vulgaris, S. niger, Cricetomys gambianus, Chinchilla lanigera, Myocastor coypus, Dipus sagitta, Microtus sp.), marsupials (Pseudocheirus peregrinus, Macropus giganteus), ruminants (Sylvicapra grimmia), domestic cats (Felis catus) and primates (Macaca sinica, Theropithecus gelada, Cercopithecus nictitans, Homo sapiens) (Gough, 1910; Hall, 1910; Railliet and Marullaz, 1919; Brglez and Bidovec, 1980; Shults and Rickard, 1985; Kennedy, 1986; Schneider-Crease et al., 2017b). Most of these records assigned the species identification based on the assumed intermediate host specificity, the anatomical location in the intermediate host or the hook size, but according to Schneider-Crease et al. (2017b) such criteria should be reconsidered. Molecular approaches to characterize the larval forms of Taenia serialis have been performed to date on cysts collected from rabbits (Padgett et al., 2005), geladas (Schneider-Crease et al., 2013; Nguyen et al., 2015) and humans (Tappe et al., 2016). In one case, the cyst was experimentally fed to a domestic dog and T. serialis was identified based on the morphology of the adult tapeworms (Schwartz, 1927). In the present study, the species was confirmed by sequencing of two genes. In both cases the highest obtained similarity was with isolates of T. serialis, both in adult and larval stage (Table 1). The phylogenetic analysis (Fig. 4) of 12S rDNA gene shows the presence of two clades of T. serialis. The first, where also our sequence clusters, includes isolates from Africa, North America and Japan. Other T. serialis isolates from Europe and North America cluster in the same branch with T. multiceps from Africa and Asia. However, the number of 12S rDNA sequences labelled as T. serialis available at this time in GenBank is too limited for further conclusions.
The first report of a coenurus in a non-human primate seems to be the one of Cobbold in 1859 (cited by Hall, 1910) in the black lemur (Eulemur macaco) from Madagascar, as “Multiceps lemuris”, later synonymized as T. serialis (Loos-Frank, 2000). Subsequently, a coenurus cyst was found in the perineum of a captive toque macaque (Macaca sinica) (Railliet and Marullaz, 1919). Geladas (Theropithecus gelada) in Africa are commonly affected by coenurus larvae. Dunbar and Dunbar (1975) and Ohsawa (1978, 1979) reported coenurus of T. serialis in free-ranging geladas from Ethiopia, but at that time, the specific assignation to Taenia serialis was not molecularly confirmed. The molecular confirmation of the assignation as T. serialis was done in the same gelada population by Schneider-Crease et al. (2013) and by Nguyen et al. (2015). Moreover, there are surprisingly many earlier reports of coenurus type cysts in wild-caught geladas found in captivity in various zoos or private collections (Schwartz, 1926, 1927; Scott, 1926; Urbain and Bullier, 1935; Elek and Finkelstein, 1939; Rodhain and Wanson, 1954; Bertolino, 1957; Clark, 1969; Kaufmann and Whittaker, 1972; Leith and Satterfield, 1974). Additionally, coenurus cysts were reported in the brain of a captive spotted-nose monkey (Cercopithecus nictitans), wild-caught from an unspecified location in West Africa (Sandground, 1937). To our knowledge, this is the first report of coenurus in olive baboons and the first report of this parasite species in Benin.
As in our case the baboon spent most of his life captive in the village, we assume the infection occurred there, as otherwise, the contact with feces of domestic dogs in the typical habitat of baboons is less probable. However, this highlights the importance of assessing the detailed history of the animal prior to their transfer to rescue centers. Moreover, as the dogs seem to be the primary source of infection for intermediate hosts, we highlight the importance of controlling the access of dogs in the recuse centers, as larval T. serials can seriously impact the health and welfare of primates. Dunbar (1980) and Schneider-Crease et al. (2017a) have shown that the parasitism with larval T. serialis is an important cause of adult and infant mortality in wild geladas. Nguyen et al. (2015) demonstrated also a decreased reproductive success in wild geladas infected with larvae of T. serialis. The prevalence in a gelada population from Ethiopia varied according to the age group from 3.2 to 24.1%, with adults being more commonly affected (Dunbar, 1980). In another study, the overall prevalence was 14%, with adults and females more commonly affected than young and males, respectively (Schneider-Crease et al., 2017a). The overall average prevalence (evaluated as “visible swellings”) in the gelada population was 9.8% and 10.6%, in two studies performed in two different seasons (Dunbar, 1980). Coenurosis was reported also as a case of mortality (Scott, 1926; Urbain and Bullier, 1935; Elek and Finkelstein, 1939) or as a reason for euthanasia (Schwartz, 1927; Clark, 1969; Leith and Satterfield, 1974) in geladas from zoos. The presence of multiple large cysts can affect the animals’ ability to move, even in free-ranging individuals (Ohsawa, 1979) or can induce limb paralysis (Elek and Finkelstein, 1939). Some cysts readily ulcerate, become infected and suppurate, causing evident pain and discomfort to the animals, with risk of septicaemia (Schwartz, 1927; Urbain and Bullier, 1935; Elek and Finkelstein, 1939; Clark, 1969; Ohsawa, 1979). As geladas are phylogenetically related to baboons (Zinner et al., 2013), we may expect similar pathology and clinical signs of coenurosis in these two primate speices.
Given the relatively common occurrence in African primates, the apparently low intermediate host specificity and the ubiquity of free-ranging dogs across Africa, the zoonotic risk associated with T. serialis and its real impact should be further investigated. Coenurus type larvae have been reported on various occasions in humans in Europe, Africa, Middle East and the Americas (list of records in Schneider-Crease et al., 2017b). However, only one study in humans was able to confirm molecularly the larvae as belonging to T. serialis (Tappe et al., 2016).
5. Conclusion
The present study reports a new host new geographical record for the larval form of T. serialis. As an addition to the previously known presence of T. serialis larvae in wild primate populations, our study brings further evidence that this parasite can circulate also in captive wildlife around human settlements. Given the apparent flexibility in host selection, we recommend a more in-depth parasitological diagnosis for larval cestodes found in humans. Human coenurosis is probably more common in Africa than currently estimated, as coenurus larvae could be easily misidentified by medical personnel with other larval cestodes (e.g. cysticercus type), or, in the majority of cases, the post-surgical identification may not be attempted. Further genetic studies from multiple geographic isolates are needed to clarify the taxonomic status of this group.
Conflicts of interest
The authors declare that they have no conflict of interest.
Acknowledgments
We are grateful to Veronique Tessier, the director of the ATO center, for allowing the data from this orphan baboon to be shared, and for her collaboration. We thank Julie Brunet from Parasitology at Strasbourg University and the laboratory team from USAMV Cluj-Napoca for the identification on the molecular part. This research did not receive any specific grant from funding agencies in the public, commercial, or non-profit-sectors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2019.04.008.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Bertolino P. Studio clinico ed anatomo-patologico su due casi di cenurosi in Theropithecus gelada. Profil. 1957;30:3–10. [Google Scholar]
- Brglez J., Bidovec A. Taenia cervi larvae in roe deer and Multiceps serialis larvae in hare and shrew in Slovenia (Yugoslavia) Acta Parasitol. Iug. 1980;11:91–93. [Google Scholar]
- Bush A.O., Fernández J.C., Esch G.W., Seed J.R. Cambridge University Press; 2001. Parasitism: the Diversity and Ecology of Animal Parasites. [Google Scholar]
- Clark J.D. Coenurosis in a gelada baboon (Theropithecus gelada) J. Am. Vet. Med. Assoc. 1969;155:1258–1263. [PubMed] [Google Scholar]
- Dunbar R., Dunbar P. vol. 6. Karger; Basel: 1975. (Social Dynamics of Gelada Baboons. Contrib. Primatol). [PubMed] [Google Scholar]
- Dunbar R.I.M. Demographic and life history variables of a population of gelada baboons (Theropithecus gelada) J. Anim. Ecol. 1980;49:485–506. [Google Scholar]
- Elek S.R., Finkelstein L.E. Multiceps serialis infestation in a baboon. Report of a case exhibiting connective tissue cystic masses. Zoologica. 1939;24:323–328. [Google Scholar]
- Gasser R.B., Chilton N.B. Characterization of taeniid cestode species by PCR-RFLP of ITS2 ribosomal DNA. Acta Trop. 1995;59:31–40. doi: 10.1016/0001-706x(94)00085-f. [DOI] [PubMed] [Google Scholar]
- Gough L.H. Note on a coenurus of the duikerbok. Trans. Roy. Soc. S. Afr. 1910;1:343–345. [Google Scholar]
- Hall M.C. The gid parasite and allied species of the cestode genus Multiceps. I. Historical review. US Dep. Agric. Bur. Anim. Ind. Bull. 1910;125 Part 1. [Google Scholar]
- Jolly C.J. Genus Papio baboons. In: Mutynski M., Kingdon J., Kalina J., editors. Mammals of Africa. Volume II. Primates. Bloomsbury; London: 2013. pp. 217–238. [Google Scholar]
- Kaufmann P.H., Whittaker F.H. Coenurosis in a zoo collection of gelada baboons (Theropithecus gelada) Vet. Med. Small Anim. Clin. 1972;67:1100–1104. [PubMed] [Google Scholar]
- Kennedy M.J. Alberta Agriculture, Animal Health Division; Edmonton Alta: 1986. Synopsis of the Parasites of the Vertebrates of Canada: Helminths and Protozoa of Terrestrial Mammals. [Google Scholar]
- Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2016;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leith J.D., Satterfield W.C. Coenurus cysts of Taenia multiceps in a baboon, Theropithecus gelada. J. Zoo Anim. Med. 1974;5:32–34. [Google Scholar]
- Loos-Frank B. An up-date of Verster's (1969) ‘Taxonomic revision of the genus Taenia Linnaeus’ (Cestoda) in table format. Syst. Parasitol. 2000;45:155–183. doi: 10.1023/a:1006219625792. [DOI] [PubMed] [Google Scholar]
- Nguyen N., Fashing P.J., Boyd D.A., Barry T.S., Burke R.J., Goodale C.B., Jones S.C., Kerby J.T., Kellogg B.S., Lee L.M., Miller C.M. Fitness impacts of tapeworm parasitism on wild gelada monkeys at Guassa, Ethiopia. Am. J. Primatol. 2015;77:579–594. doi: 10.1002/ajp.22379. [DOI] [PubMed] [Google Scholar]
- Ohsawa H. The local gelada population and environment of the Gich area. Contrib. Primatol. 1978;16:3–45. [PubMed] [Google Scholar]
- Ohsawa H. The local gelada population and environment of theGish area. In: Kawai M., editor. Ecological and Sociological Studies of Gelada Baboons. Karger Publishers; Tokyo: 1979. pp. 4–45. [Google Scholar]
- Padgett K.A., Nadler S.A., Munson L., Sacks B., Boyce W.M. Systematics of Mesocestoides (Cestoda: mesocestoididae): evaluation of molecular and morphological variation among isolates. J. Parasitol. 2005;91:1435–1443. doi: 10.1645/GE-3461.1. [DOI] [PubMed] [Google Scholar]
- Railliet A., Marullaz M. Sur un cenure nouveau du Bonnet chinois (Macacus sinicus) Bull. Soc. Path. Exot. 1919;12:223. [Google Scholar]
- Rodhain J., Wanson M. Un nouveau cas de coenurose chez le babouin, Theropithecus gelada Ruppell. Riv. Parasitol. 1954;15:613–620. [Google Scholar]
- Sandground J.H. On a coenurus from the brain of a monkey. J. Parasitol. 1937;23:482–490. [Google Scholar]
- Schneider Crease I., Griffin R.H., Gomery M.A., Bergman T.J., Beehner J.C. High mortality associated with tapeworm parasitism in geladas (Theropithecus gelada) in the Simien Mountains National Park, Ethiopia. Am. J. Primatol. 2017;79 doi: 10.1002/ajp.22684. [DOI] [PubMed] [Google Scholar]
- Schneider Crease I., Griffin R.H., Gomery M.A., Dorny P., Noh J.C., Handali S., Chastain H.M., Wilkins P.P., Nunn C.L., Snyder-Mackler N., Beehner J.C., Bergman T.J. Identifying wildlife reservoirs of neglected taeniid tapeworms: non-invasive diagnosis of endemic Taenia serialis infection in a wild primate population. PLoS Neglected Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Crease I.A., Snyder-Mackler N., Jarvey J.C., Bergman T.J. Molecular identification of Taenia serialis coenurosis in a wild Ethiopian gelada (Theropithecus gelada) Vet. Parasitol. 2013;198:240–243. doi: 10.1016/j.vetpar.2013.08.015. [DOI] [PubMed] [Google Scholar]
- Schwartz B. A subcutaneous tumor in a baboon due to Multiceps larvae. J. Parasitol. 1926;12:159–160. [Google Scholar]
- Schwartz B. A subcutaneous tumor in a primate caused by tapeworm larvae experimentally reared to maturity in dogs. J. Agric. Res. 1927;35 471-448. [Google Scholar]
- Scott H.H. Report on deaths occurring in the Society's Gardens during the year 1925. Proc. Zool. Soc. Lond. 1926;1:231–244. [Google Scholar]
- Shults L.M., Rickard L.G. Helminth parasites of the white-tailed jackrabbit, Lepus townsendi, from Northwestern Colorado and southern Wyoming. Gt. Basin Nat. 1985;45:604–606. [Google Scholar]
- Tamura K., Kumar S. Oxford University Press; New York: 2000. Molecular Evolution and Phylogenetics. [Google Scholar]
- Tappe D., Berkholz J., Mahlke U., Lobeck H., Nagel T., Haeupler A., Muntau B., Racz P., Poppert S. Molecular identification of zoonotic tissue-invasive tapeworm larvae other than Taenia solium in suspected human cysticercosis cases. J. Clin. Microbiol. 2016;54:172–174. doi: 10.1128/JCM.02171-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbain A., Bullier P. Un cas de cenurose conjonctive chez un Gelada. Bull. Acad. Vét. de France. 1935;8:322–324. [Google Scholar]
- Verster A. A taxonomic revision of the genus Taenia Linnaeus, 1758 s. str. Onderstepoort J. Vet. Res. 1969;36:3–58. [PubMed] [Google Scholar]
- von Nickisch-Rosenegk M., Lucius R., Loos-Frank B. Contributions to the phylogeny of the Cyclophyllidea (Cestoda) inferred from mitochondrial 12S rDNA. J. Mol. Evol. 1999;48:586–596. doi: 10.1007/pl00006501. [DOI] [PubMed] [Google Scholar]
- Zinner D., Wertheimer J., Liedigk R., Groeneveld L.F., Roos C. Baboon phylogeny as inferred from complete mitochondrial genome. Am. J. Phys. Anthropol. 2013;150:133–140. doi: 10.1002/ajpa.22185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.