Abstract
Introduction
The peri-operative use of high-dose dexamethasone to reduce cerebral oedema may result in worsening glycaemic control in people with diabetes and glucocorticoid-induced diabetes in susceptible individuals. This study aims to examine the incidence of glucocorticoid-induced diabetes in a cohort of neurosurgical patients receiving high-dose dexamethasone peri-operatively.
Materials and methods
Adult non-diabetic neurosurgical patients receiving high-dose dexamethasone were prospectively studied. Exclusion criteria included pregnancy, HbA1c > 6.0%, and use of anti-diabetes therapies. The following data were collected: Family history of diabetes, body mass index, fasting glucose, insulin, C-peptide, and HbA1c (prior to surgery and 6 weeks after last dose of dexamethasone). Homeostatic model assessment values were calculated. Peri-operative glucose readings were recorded and 75 g oral glucose tolerance tests performed at the end of 6 weeks. Paired student t tests and multiple linear regressions were used.
Results
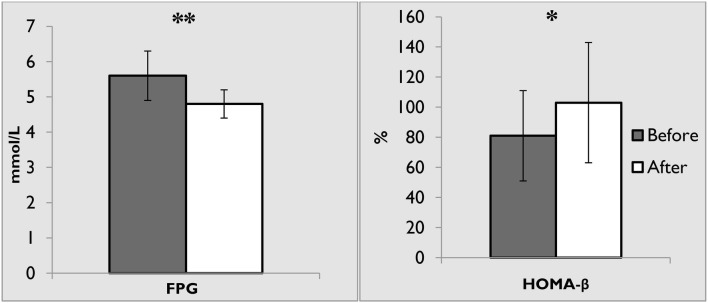
Data from 21 participants (11 women) were available. The mean total dose of dexamethasone was 96 ± 34 mg, and treatment duration was 17 ± 7 days. A total of 105 random blood glucose levels were documented peri-operatively (mean 7.0 ± 1.0 mmol/L). Six weeks following cessation of dexamethasone course, none of the participants developed diabetes, defined either by fasting glucose or by 75 g OGTT. There was a statistically significant increase in the mean HOMA-β from 81.5 to 102.1% (p = 0.01) and a significant decrease in the mean fasting glucose from 5.7 to 4.8 mmol/L (p = 0.001).
Conclusions
The use of high-dose dexamethasone in this cohort of neurosurgical patients did not result in glucocorticoid-induced diabetes. Hyperglycaemia was transient and had resolved by 6 weeks.
Keywords: Glucocorticoid, Dexamethasone, Diabetes, Neurosurgery
Introduction
Glucocorticoids (GC) are used during the peri-operative periods of several surgical operations, including neurosurgical procedures, to reduce oedema and inflammation [1]. Several mechanisms for GC-induced inflammation reduction have been proposed: inhibition of phospholipase A2, improvement in microcirculation, and stabilization of lysosomal membranes [2]. Dexamethasone is the second most potent GC after Betamethasone and it is approximately six times as potent as prednisolone [3]. Cerebral oedema is a prominent feature of brain tumours and often contributes to the neurologic dysfunction caused by the tumour. Thus, high-dose dexamethasone has been used in clinical practice to reduce cerebral oedema since the early 1960s, and has been responsible for the remarkable decline in peri-operative mortality, indicating the importance of this therapy. Furthermore, dexamethasone use may reduce the size of brain tumour by 15% [2].
It is well known that one of the adverse effects of GC is worsening of glycaemic control in people with diabetes, and hyperglycaemia or even the development of new onset diabetes in those without pre-existing diabetes [2]. GC exerts their adverse effect on glycaemic level through two main mechanisms: increasing insulin resistance (IR) (mainly at the level of skeletal muscle and liver) and causing β-cell dysfunction [1]. The effect of GC on blood glucose level (BGL) has been extensively reported in the literature. However, most of the studies were conducted on people with pre-existing diabetes. On the contrary, there is a paucity of evidence regarding incidence of GCID in people without a prior history of diabetes. Several previous studies examined the incidence of GCID. Never the less, these studies were retrospective with a relatively short follow-up period. Five studies tested the effect of administration of a single dose of dexamethasone intraoperatively [1, 4–7] and found an acute rise in BGL within 48 h of administration. None of these studies followed the patients for longer. Two open-labelled randomized controlled trials tested the effect of high-dose oral prednisolone for approximately 22 weeks and found the incidence of GCID to be around 17% [8, 9]. Ashley et al. found the incidence of hyperglycaemia higher among patients who received dexamethasone in a dose of 13.5 mg/day for an average of 5 days than those who received other types of steroids [10]. However, the follow-up period in this study was short (4.4 days). The exact effect of relatively long course of high-dose dexamethasone on the incidence of GCID needs to be further clarified. Organ transplantation and oncology patients usually receive high-dose GC to dampen the side effects of chemotherapeutic agents mainly nausea and vomiting. Several studies were conducted on those patients, but are biased by the confounding effect of some chemotherapies on BGL [11–16]. Furthermore, a meta-analysis conducted by Liu et al. found the incidence of GCID to be approximately 19% (p = 0.002) [17]. However, 12 out of the 13 included studies were retrospective. Furthermore, incidence of GCID was found to be 27–67% in all patients during induction of chemotherapy [18, 19]. A recent pilot study conducted in Korea examined the incidence of GCID in patients without diabetes receiving high-dose dexamethasone adjuvant to chemotherapy. In this study, the incidence was found to be 22% after 6 months of follow-up [16]. However, the exact incidence of GCID in people without diabetes remains undefined. Furthermore, risk factors associated with GCID were not established clearly in the literature.
This study aims to examine the incidence of GCID in a cohort of neurosurgical patients receiving high-dose dexamethasone. It also attempts to identify its risk factors and to examine the effect of high-dose dexamethasone on glucose homeostasis using HOMA. To achieve these goals, we conducted a prospective observational study on patients with brain tumour who do not have diabetes or have pre-diabetes and receiving high-dose dexamethasone peri-operatively.
Materials and methods
Study design
This was a prospective observational study. Subjects were recruited from the neurosurgery ward at Macquarie University Hospital, Sydney, Australia. Adult neurosurgical patients receiving high-dose dexamethasone peri-operatively for brain tumour resection without diabetes, but may have pre-diabetes were included. Diabetes was diagnosed according to the World Health Organization (WHO) criteria [20, 21]. Further exclusion criteria extended to pregnant women, those on palliative care or unable to give consent (Table 1). High-dose dexamethasone was defined as more than or equal to 4 mg/day for at least 10 days.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| 1. All neurosurgical patients with brain tumour receiving high-dose dexamethasone (more than 4 mg/day) for 10 days or more 2. Age range 18–90 years 3. Pre-diabetes, or without history of diabetes |
1. Known diabetes on any treatment 2. HbA1c > 6% 3. Pregnancy 4. Palliative care 5. Unable to give consent 6. Previously on high-dose steroid treatment |
The study was divided into three phases. During phase I at baseline, before starting dexamethasone treatment, an informed consent was taken followed by a detailed medical history including family history of diabetes. In addition, demographic data were recorded, body weight and body mass index (BMI) were measured. All patients fasted for more than 8 h before blood collection for measurement of fasting plasma glucose (FPG), haemoglobin A1C (HbA1C), insulin, and C-peptide. Patients showing diabetic levels in laboratory tests performed before the start of dexamethasone were excluded. Phase II included the period of dexamethasone treatment (peri-operative period) when the total dose and duration of dexamethasone were recorded. In addition, several random BGLs were documented to examine glycaemic excursion. In addition, histopathological examination of the tumour was documented. All the participants received dexamethasone peri-operatively to reduce brain edema according to a protocol used at Macquarie University Hospital by the neurosurgery team. Each patient was given 4 mg of dexamethasone 6 hourly at day 0 and one of the surgery. Followed by 4 mg twice daily for the next 3 days. Then, dexamethasone is tapered over 2 weeks. The dose and duration of dexamethasone are not fixed for all patients. Rather, it depends on several factors such as size and site of the tumour, degree of brain edema, and the presence of residual tumour. Insulin was not administered to the patients during the treatment of dexamethasone. In addition, during this phase, each participant was given approximately similar meals during hospital admission. These meals are planned by a specialized dietitian to meet the dietary need for a patient just recovered from a major surgery. Phase III was 6 weeks after stopping dexamethasone when body weight, using the same scale used in phase I, was recorded, and accordingly, BMI was measured. Blood sample to measure fasting plasma insulin (FPI) and C-peptide was collected to calculate HOMA. A 75 g OGTT was also performed.
Oral glucose tolerance test
In phase III, subjects were encouraged to consume generous amounts of carbohydrate for 3 days before the study. Subjects then instructed to fast for 12 h overnight. No smoking, coffee drinking, or physical activity was permitted on the day of the test. Following sampling for the measurement of plasma glucose, insulin, and C-peptide at time 0 (between 8 am and 9 am), subjects were administered 75 g glucose orally (Carbotest, Lomb Scientific, Sydney, Australia). A further sample for plasma glucose and insulin was collected 60 and 120 min after the glucose load. New onset diabetes was defined according to WHO criteria [20].
Laboratory measurements
Plasma glucose concentrations were measured using a hexokinase method (Cobas Integra 800, Roche Diagnostics, Basel, Switzerland). Insulin and C-peptide levels were determined by immune assay (Architect integrated system, Abbott diagnostics, Illinois, USA). Capillary glycaemia was determined with test strips and a glucometer (Free style system, Abbott diagnostics, Illinois, USA). HbA1c was determined by Cation-exchange high-performance liquid chromatography (Bio-Rad Variant D-100 system, Bio-Rad Laboratories, CA, USA).
Data assessment
Glucose tolerance was assessed according to WHO criteria [20]. Normal glucose metabolism was defined as an FPG level of less than 6.1 mmol/L and a glucose value of less than 7.8 mmol/L 2-h post a 75-g OGTT. Impaired glucose metabolism (pre-diabetes) was defined as an FPG level of 6.1–6.9 mmol/L (impaired fasting glycaemia) or a 2-h post a 75 g OGTT glucose value of 7.8–11.0 mmol/L (impaired glucose tolerance). Incidence diabetes was diagnosed if an FPG ≥ 7.0 mmol/L or a plasma glucose ≥ 11.1 mmol/L 2 h after a 75-g OGTT. HOMA values were computed by entering FPG and FPI values into a software calculator [22].
Outcome measures
The primary outcome of this study was the incidence of GCID among patients at 6 weeks after stopping high-dose dexamethasone. The secondary outcomes include the effect of high-dose dexamethasone treatment on glucose homeostasis and identification of risk factors associated with GCID.
Statistical analysis
Descriptive data are presented as the mean ± SD for normally distributed data or, when distribution was skewed, as the median and interquartile range. Changes in the means of HOMA values, C-peptide, FPG, FPI, and BMI before and after dexamethasone treatment were analyzed by paired Student’s t test. The 95% confidence interval (CI) of the difference in the means of the variables was reported. Multiple linear regressions test was performed to examine the association between the changes in HOMA-β and FPG with clinical variables. Potential explanatory variables were age (< 60 or ≥ 60 years), BMI (< 25 kg/m2 or ≥ 25 kg/m2), pre-diabetes (< 6.1 mmol/L or ≥ 6.1 mmol/L), non-Caucasian ethnicity (yes or no), cumulative dose of dexamethasone (< 70 mg or ≥ 70 mg), and history of type 2 DM in a first-degree relative (yes or no). All data analyses were performed with IBM SPSS for Windows 24.0 (SPSS Inc., Chicago, IL, USA). P values < 0.05 were determined as significant.
Ethics approval
The study was approved by Macquarie University Human Research Ethics Committee on 16, June 2016 with a Reference Number: 5201600364. The study was conducted in accordance with the Declaration of Helsinki. All participants provided a written informed consent.
Results
Participants’ characteristics
Between June 2016 and June 2017, a total of 34 participants with no history of diabetes were recruited in this study. Of these, 13 participants were excluded for the following reasons: four died due to the underlying malignant tumour or postoperative complications, four decided to withdraw as they refused to perform the OGTT, and five lost to follow-up due to change in contact details, referral to palliative care or inability to communicate (aphasic). Consequently, 21 participants completed the study (Fig. 1).
Fig. 1.
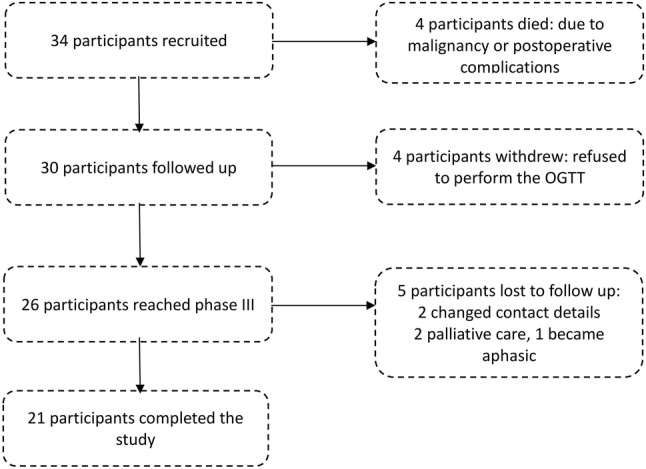
Flow chart of the participants
The baseline characteristic features of these 21 subjects are shown in Table 2. Of these, eleven participants (52.4%) were women. The median age was 63 years (range 30–78 years). Seventeen (81%) were Caucasian. Six subjects (28.5%) had family history of type 2 diabetes in first-degree relatives. Six Participants (28.5%) had meningioma. The mean BMI was 27.5 ± 4.7 kg/m2. Before starting dexamethasone, the mean FPG was 5.7 ± 0.6 mmol/L; nine subjects (42.8%) had pre-diabetes as defined by impaired fasting glucose. The mean HbA1c was 5.3 ± 0.3%. During phase II, the mean cumulative dose of dexamethasone was 96 ± 34 mg with a mean duration of 17 ± 7 days.
Table 2.
Baseline characteristics of the participants
| Characteristic | Total |
|---|---|
| Age, median (range) years | 63 (30–78) |
| < 60, years (%) | 9 (42.8) |
| ≥ 60, years (%) | 12 (57.2) |
| Gender, n (%) | |
| Male | 10 (47.6) |
| Female | 11 (52.4) |
| Ethnicity, n (%) | |
| Caucasian | 17 (81) |
| Other | 4 (19) |
| Family history of diabetes | 6 (28.5) |
| Tumour type, n (%) | |
| Meningioma | 6 (28.5) |
| Brain metastasis | 4 (19) |
| GBM | 4 (19) |
| Glioma | 3 (14) |
| Gliosarcoma | 2 (9.5) |
| Other | 2 (9.5) |
| Weight, mean ± SD (kg) | 78.3 ± 16.5 |
| Height, mean ± SD (m) | 1.7 ± 0.1 |
| BMI, mean ± SD (kg/m2) | 27.5 ± 4.7 |
| < 25, n (%) | 4 (19) |
| ≥ 25, n (%) | 17 (81) |
| FPG, mean ± SD (mmol/L) | 5.7 ± 0.6 |
| IFG (pre-diabetes), n (%) | 9 (42.8) |
| HbA1c, mean ± SD (%) | 5.3 ± 0.3 |
| Cumulative dexamethasone dose/person, mean ± SD (mg) | 96 ± 34 |
| Dexamethasone duration, mean ± SD (days) | 17 ± 7 |
BMI body mass index, FPG fasting plasma glucose, GBM glioblastoma multiforme, HbA1c haemoglobin A1c, IFG impaired fasting glucose
Incidence of glucocorticoid-induced diabetes
The incidence of GCID among people without diabetes varies widely in the literature. We examined the incidence of GCID among neurosurgical patients receiving high-dose dexamethasone. A total of 105 BGL readings were documented, with an average of 5 ± 2 tests/person. The median BGL was 7.0 ± 1.0 mmol/L. Only one reading reached the threshold of 11.1 mmol/L while on dexamethasone therapy in phase II. After 6 weeks of stopping therapy, all subjects had normal 75 g OGTT. The mean plasma glucose 2-h post OGTT was 6.0 ± 1.0 mmol/L. Under these circumstances, none of the participants developed diabetes as defined by the previously mentioned criteria [20].
Effect of dexamethasone on glucose homeostasis
Glucocorticoids can affect glucose homeostasis in several ways. We studied these effects via the utilization of the concept of HOMA. A statistically significant decrease in the mean FPG (from 5.6 ± 0.6 mmol/L to 4.8 ± 0.5 mmol/L, p = 0.001) and increase in HOMA-β (from 81.5 ± 29.0% to 102 ± 40%, p = 0.01) values were observed before and after dexamethasone therapy (Fig. 2; Table 3). However, non-significant decrease in insulin (from 8.9 ± 4.2 mU/L to 7.9 ± 4.4 mU/L), C-peptide (from 0.9 ± 0.4 nmol/L to 0.7 ± 0.2 nmol/L), and HOMA-IR (from 1.2 ± 0.5 to 1.0 ± 0.5) were documented. On the other hand, a non-significant increase in BMI (from 27.5 ± 4.7 kg/m2 to 27.6 ± 4.6 kg/m2) and HOMA-S (from 110.4 ± 66.0% to 128.6 ± 77.3%) was observed (Table 3). An interesting finding of this study is the significant reduction in FPG and improvement in HOMA-β after 6 weeks of stopping dexamethasone therapy.
Fig. 2.
Changes in fasting plasma glucose and HOMA-β before and 6 weeks after dexamethasone. FPG fasting plasma glucose, HOMA-β homeostatic model assessment of β-cells. **p value 0.001 (paired student t test), *p value 0.01
Table 3.
Changes in the means of glucose homeostasis variables and BMI before and 6 weeks after dexamethasone
| Variable | Before DEXA | After DEXA | Diff. in mean | 95% CI of diff. | P value |
|---|---|---|---|---|---|
| BMI (kg/m2) | 27.5 ± 4.7 | 27.6 ± 4.6 | − 0.7 | − 0.3 to 0.1 | 0.5 |
| FPG (mmol/L) | 5.6 ± 0.6 | 4.8 ± 0.5 | 0.8 | 0.4–1.2 | 0.001 |
| Insulin (mU/L) | 8.9 ± 4.2 | 7.9 ± 4.4 | 0.9 | − 1 to 3 | 0.3 |
| C-peptide (nmol/L) | 0.9 ± 0.4 | 0.7 ± 0.2 | 0.1 | 0.5–1 | 0.3 |
| HOMA-IR | 1.2 ± 0.5 | 1.0 ± 0.5 | 0.1 | − 0.1 to 0.4 | 0.2 |
| HOMA-β (%) | 81.5 ± 29 | 102 ± 40 | − 20.5 | − 36.9 to − 4.2 | 0.01 |
| HOMA-S (%) | 110.4 ± 66.0 | 128.6 ± 77.3 | − 18.2 | − 55.5 to 19.1 | 0.3 |
BMI body mass index, CI confidence interval, DEXA dexamethasone, FPG fasting plasma glucose, HOMA homeostatic model assessment
Predictive factors
The previous studies identified different predictive factors for GCID. As this study did not identified one case of GCID after 6 weeks of dexamethasone therapy, a further analysis was conducted to examine for predictive factors of FPG and HOMA-β changes by multiple linear regression instead. Consequently, none of the following were identified as an independent predictor of neither FPG nor HOMA-β changes before and 6 weeks after dexamethasone therapy, age, gender, ethnicity, tumour type, family history of diabetes, pre-diabetes, BMI, dexamethasone dose, and duration.
Extended follow-up
Three participants were followed up for more than 6 months. These participants continued to receive medical care at Macquarie University Hospital due to their underlying malignant or recurrent disease. BGLs remained within the non-diabetic range in the extended follow-up period and none of the three developed diabetes (Table 4).
Table 4.
Characteristics of three participants after 6 months of follow-up
| Participant no. | Age (years) | Gender | Diagnosis | FPG1 (mmol/L) | FPG2 (mmol/L) | BGL (mmol/L) | Wt.1 (kg) | Wt.2 (kg) |
|---|---|---|---|---|---|---|---|---|
| 1 | 55 | Male | GBM | 5.7 | 4.4 | 6.6 | 120 | 118 |
| 2 | 65 | Female | Brain mets | 5.0 | 5.2 | 6.8 | 63 | 65 |
| 3 | 30 | Male | Gliosarcoma | 4.9 | 5.7 | 7.8 | 60 | 64 |
BGL blood glucose level at 6-month post-dexamethasone therapy, GBM glioblastoma multiforme, mets metastasis, FPG1 fasting plasma glucose at baseline, FPG2 fasting plasma glucose after 6 months, Wt.1 body weight at baseline, Wt.2 body weight after 6 months
Conclusions and discussion
The main finding of this study is that the use of high-dose dexamethasone to reduce peri-operative cerebral oedema among people without diabetes who are afflicted by brain tumour did not result in GCID. Even though half of the participants had established risk factors for diabetes, the results of OGTT 6-week post-dexamethasone treatment were normal for all of them. Interestingly, even during dexamethasone use, only one patient recorded a reading of BGL of 11.1 mmol/L or more which is the threshold for diagnosing diabetes. However, exposure to large cumulative dose of dexamethasone resulted in significant changes in some parameters of glucose homeostasis.
To our knowledge, this is the first study that examined the effect of high-dose dexamethasone on glucose homeostasis in people without DM undergoing craniotomy. The results of this study are clinically important, since dexamethasone use in high doses to reduce cerebral oedema has been in clinical practice since the 1960s. Both neurosurgeons and endocrinologists need to know the effect of exposing those patients to high-dose dexamethasone.
The diabetes of injury is a syndrome characterized by insulin resistance, glucose intolerance, and hyperglycaemia secondary to physiological stresses of serious illness, trauma, or surgery [23]. Even though the subjects of this study had physiological stresses of surgery, none of them developed diabetes after exposure to a high cumulative dose of dexamethasone.
The incidence of GCID varies widely in the literature and depends not only on the type, dose, and duration of glucocorticoid used, but also the underlying condition, diagnostic method for diabetes, and presence or absence of risk factors for diabetes.
Similar to our study, Fizazi et al. reported a low incidence of GCID (1% of their participants) after a long period of follow-up (30 months) [24]. In contrast to our study, the study with the highest incidence (52%) was a retrospective series in which all cases of GCID were detected solely by postprandial hyperglycaemia [25]. In general, studies that reported a high incidence of GCID were biased by the presence of major confounders such as the presence of a pre-existing inflammatory condition, a risk factor for diabetes on its own [26], the concomitant use of chemotherapeutic agent known to cause diabetes and the report of hyperglycaemia during GC treatment rather than after stopping it. In the same way, other studies reported the incidence based on random glucose samples without performing an OGTT [8, 9, 12, 16, 25, 27]. Inflammation is a strong risk factor for diabetes. It impairs glucose tolerance both by impairing β-cell function and reducing insulin sensitivity via pro-inflammatory cytokines such as interleukin 6 and tumour necrosis factor α [26]. This is further supported by the findings in Burt et al.’s study [28] when the investigators demonstrated a non-significant difference in the incidence of new onset diabetes between those with rheumatoid arthritis who received high-dose prednisolone compared to those with rheumatoid arthritis who did not; 15% and 10%, respectively, p value 0.45. Majority of participants of our cohort had no or minimal degree of inflammation which may explain the absence of documented cases of diabetes at the end of the study. Moreover, GC might cause transient hyperglycaemia during therapy which may resolve spontaneously upon discontinuation. For instance, in Gonzalez–Gonzalez et al.’s study, most of subjects with pre-diabetes or DM normalized spontaneously by the 12th week (spontaneous remission) despite reporting high incidence (34%) of GCID [12]. Likewise, in Den Uyl et al.’s study, glucose metabolism reverted to normal after short-term glucocorticoid exposure in nine patients with impaired glucose metabolism at baseline [26].
In our study, we did not rely on HbA1c in the diagnosis of GCID as this test has a low sensitivity in diagnosing diabetes in the general population and in those treated with GC specifically, 45% and 11%, respectively [28, 29]. Instead, we utilized the OGTT to diagnose GCID, because it is the only test with sufficient sensitivity to diagnose diabetes in people exposed to GC [28, 30].
An interesting observation in our study is the significant decrease in FPG after dexamethasone therapy, a similar finding to Burt et al.’s study [28]. This finding can be explained by three factors. First, GC therapy causes afternoon and postprandial hyperglycaemia rather than fasting one via inducing a state of relative insulin resistance [16, 28]. For instance, a typical patient with hyperglycaemia after corticosteroid therapy will have elevated glucose values 1–2 h after a meal, which drops to normal overnight. Therefore, we measured BGL at different times of the day including postprandial measurements. Second, the suppression of hypothalamic–pituitary–adrenal (HPA) axis by dexamethasone may blunt the rising of early morning cortisol, thus leading to the relative decrease of FPG. Third, a compensatory increase in β-cell mass, as will be discussed in the next paragraph, resulted in lowering of FPG.
Another important finding in our study is the significant increase in HOMA-β, a surrogate marker of β-cell function [31], after dexamethasone therapy. Similarly, this finding has been demonstrated in one study on human [26] and three on animals [32–34]. Namely, Assefa et al.’s study illustrated that the use of high-dose GC on rats almost doubled the number of β-cells in 2 weeks [32]. Correspondingly, Choi pointed out the use of dexamethasone expanded β-cell mass via hypertrophy and neogenesis from precursor cells, rather than increasing proliferation and decreasing apoptosis [33]. Thus, this effect of GC on β-cell will have deleterious consequences on the long period if exposure continued which will result eventually in failure of the pancreas to overcome the stress induced by GC and hence the development of GCID. Therefore, we speculate that if the subjects in this cohort were exposed to dexamethasone therapy for a longer period, they may develop diabetes.
HOMA-IR, which is based on FPG and insulin levels, has been widely validated and used as a measure of insulin resistance in large epidemiologic studies and in clinical practice [31]. In this study, we demonstrated a non-significant decrease in HOMA-IR. This finding was in line with that of Gonzalez–Gonzalez et al.’s study [12]. Similarly, Choi et al.’s study showed that dexamethasone delayed and decreased first-phase insulin secretion through impairment of the glucose-sensing mechanism in β-cells [33].
C-peptide is co-secreted with insulin on an equimolar basis from pancreatic β-cells, unlike insulin, it is not metabolized by the liver and, therefore, is a more accurate indicator of pancreatic insulin secretion than insulin itself [16]. Interestingly, our study demonstrated a simultaneous non-significant decrease in the level of both C-peptide and insulin after dexamethasone treatment. This finding implies that there was an improvement in the metabolic parameters after dexamethasone treatment supported by the documented, though non-significant improvement in insulin sensitivity as reflected by HOMA-S. Another possibility would be a temporary improvement in the metabolic parameters secondary to the anti-inflammatory effect of dexamethasone. This effect may vanish with a prolonged use of dexamethasone bearing in mind the relatively short period of exposure to dexamethasone and follow-up in this study.
This study was intended to examine risk factors associated with the incidence of GCID. Because we did not document GCID in any of the study subjects, we examined risk factors that predict changes in FPG and HOMA-β alternatively. However, none of the examined risk factors such as age, gender, tumour type, family history of diabetes, pre-diabetes, BMI, dexamethasone dose, or duration, showed a significant correlation.
The strengths of this study include the prospective design, which enables a detailed and systematic follow-up and outcome definition; the utilization of HOMA as a surrogate marker of insulin resistance and β-cell function; the utilization of OGTT as a screening tool and the application of a clear pre-specified criteria for the diagnosis of new onset diabetes.
Several limitations of this study warrant consideration. First, it was intended to assess the incidence of GCID, and was not powered to determine the effect of steroid-induced diabetes or insulin resistance due to small sample size. The sample size was relatively small, because it was designed as a pilot study. Second, study subjects were limited to patients with brain tumour and old age which in addition to the single centre may affect generalizability. Third, the gold standards for measurement of insulin sensitivity and β-cell function are the hyperinsulinaemic–euglycaemic clamp and the hyperglycaemic clamp procedures, respectively. These tests are laborious and were considered too demanding for use in the present study population. Finally, the follow-up period was relatively short. In conclusion, despite there were patients with pre-existing risk factors for diabetes, the peri-operative use of high-dose dexamethasone to reduce cerebral oedema in this cohort of neurosurgical patients did not result in the development of diabetes. Hence, this practice may be considered safe regarding the effect of dexamethasone on glucose homeostasis. The compensatory improvement in β-cell function limited hyperglycaemia, if any, to transient occurrence which resolved spontaneously by the end of 6 weeks. In the same way, the improvement in β-cell function consequently resulted in the reduction of FPG at the end of follow-up period. No particular predictors for the improvement of either FPG or β-cell function were identified in the current study.
We are the first to conduct a prospective study using pre- and post-GC HOMA calculation to examine glucose homeostasis in a cohort of neurosurgical patients receiving high-dose steroids (dexamethasone). Future prospective studies with larger sample size and longer follow-up period may provide further information to confirm our finding of a low incidence of GCID in a similar patient group (with presence of some risk factors for diabetes but the absence of pre-existing diabetes).
Acknowledgements
The authors would like to thank the nursing staff at Ward 2 and the Neurosurgeons working at Macquarie University Hospital for their help in recruiting the patients. Also, many thanks to Douglas-Hanley Moir Pathology for their help in performing the blood tests.
Author contribution
MA recruited the participants, performed the statistical analysis and drafted the manuscript. KH designed the study, reviewed and edited the manuscript. ML reviewed and edited the manuscript.
Funding
This research did not receive any specific grant from any funding agency.
Conflict of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Human rights statement and informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or substitute for it was obtained from all patients for being included in the study.
References
- 1.Abdelmannan D, Tahboub R, Genuth S, Ismail-Beigi F. Effect of dexamethasone on oral glucose tolerance in healthy adults. Endocr Pract. 2010;16(5):770–777. doi: 10.4158/EP09373.OR. [DOI] [PubMed] [Google Scholar]
- 2.Kaal EC, Vecht CJ. The management of brain edema in brain tumors. Curr Opin Oncol. 2004;16(6):593–600. doi: 10.1097/01.cco.0000142076.52721.b3. [DOI] [PubMed] [Google Scholar]
- 3.Mager DE, Lin SX, Blum RA, Lates CD, Jusko WJ. Dose equivalency evaluation of major corticosteroids: pharmacokinetics and cell trafficking and cortisol dynamics. J Clin Pharmacol. 2003;43(11):1216–1227. doi: 10.1177/0091270003258651. [DOI] [PubMed] [Google Scholar]
- 4.Abdelmalak BB, Bonilla AM, Yang D, Chowdary HT, Gottlieb A, Lyden SP, et al. The hyperglycemic response to major noncardiac surgery and the added effect of steroid administration in patients with and without diabetes. Anesth Analg. 2013;116(5):1116–1122. doi: 10.1213/ANE.0b013e318288416d. [DOI] [PubMed] [Google Scholar]
- 5.Lukins MB, Manninen PH. Hyperglycemia in patients administered dexamethasone for craniotomy. Anesth Analg. 2005;100(4):1129–1133. doi: 10.1213/01.ANE.0000146943.45445.55. [DOI] [PubMed] [Google Scholar]
- 6.Hans P, Vanthuyne A, Dewandre P-Y, Brichant J-F, Bonhomme V. Blood glucose concentration profile after 10 mg dexamethasone in non-diabetic and type 2 diabetic patients undergoing abdominal surgery. Br J Anaesth. 2006;97(2):164–170. doi: 10.1093/bja/ael111. [DOI] [PubMed] [Google Scholar]
- 7.Sethi R, Naqash IA, Bajwa SJS, Dutta V, Ramzan AU, Zahoor SA. Evaluation of hyperglycaemic response to intra-operative dexamethasone administration in patients undergoing elective intracranial surgery: a randomised, prospective study. Asian J Neurosurg. 2016;11(2):98–102. doi: 10.4103/1793-5482.177660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abroug F, Ouanes-Besbes L, Fkih-Hassen M, Ouanes I, Ayed S, Dachraoui F, et al. Prednisone in COPD exacerbation requiring ventilatory support: an open-label randomised evaluation. Eur Respir J. 2014;43(3):717–724. doi: 10.1183/09031936.00002913. [DOI] [PubMed] [Google Scholar]
- 9.Alexander TH, Weisman MH, Derebery JM, Espeland MA, Gantz BJ, Gulya AJ, et al. Safety of high-dose corticosteroids for the treatment of autoimmune inner ear disease. Otol Neurotol. 2009;30(4):443–448. doi: 10.1097/MAO.0b013e3181a52773. [DOI] [PubMed] [Google Scholar]
- 10.Fong AC, Cheung NW. The high incidence of steroid-induced hyperglycaemia in hospital. Diabetes Res Clin Pract. 2013;99(3):277–280. doi: 10.1016/j.diabres.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 11.Boloori A, Saghafian S, Chakkera HA, Cook CB. Characterization of remitting and relapsing hyperglycemia in post-renal-transplant recipients. PLoS ONE. 2015;10(11):e0142363. doi: 10.1371/journal.pone.0142363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez-Gonzalez JG, Mireles-Zavala LG, Rodriguez-Gutierrez R, Gomez-Almaguer D, Lavalle-Gonzalez FJ, Tamez-Perez HE, et al. Hyperglycemia related to high-dose glucocorticoid use in noncritically ill patients. Diabetol Metab Syndr. 2013;5(1):18. doi: 10.1186/1758-5996-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pilkey J, Streeter L, Beel A, Hiebert T, Li X. Corticosteroid-induced diabetes in palliative care. J Palliat Med. 2012;15(6):681–689. doi: 10.1089/jpm.2011.0513. [DOI] [PubMed] [Google Scholar]
- 14.Yoo K-E, Kang RY, Lee J-Y, Lee YJ, Suh SY, Kim KS, et al. Awareness of the adverse effects associated with prophylactic corticosteroid use during docetaxel therapy. Support Care Cancer. 2015;23(7):1969–1977. doi: 10.1007/s00520-014-2547-y. [DOI] [PubMed] [Google Scholar]
- 15.Rowbottom L, Stinson J, McDonald R, Emmenegger U, Cheng S, Lowe J, et al. Retrospective review of the incidence of monitoring blood glucose levels in patients receiving corticosteroids with systemic anti-cancer therapy. Ann Palliat Med. 2015;4(2):70–77. doi: 10.3978/j.issn.2224-5820.2015.04.07. [DOI] [PubMed] [Google Scholar]
- 16.Jeong Y, Han HS, Lee HD, Yang J, Jeong J, Choi MK, et al. A Pilot Study evaluating steroid-induced diabetes after antiemetic dexamethasone therapy in chemotherapy-treated cancer patients. Cancer Res Treat. 2016;48(4):1429. doi: 10.4143/crt.2015.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.X-X Liu, X-M Zhu, Miao Q, Ye H-Y, Zhang Z-Y, Li Y-M. Hyperglycemia induced by glucocorticoids in nondiabetic patients: a meta-analysis. Ann Nutr Metab. 2014;65(4):324–332. doi: 10.1159/000365892. [DOI] [PubMed] [Google Scholar]
- 18.Sonabend RY, McKay SV, Okcu MF, Yan J, Haymond MW, Margolin JF. Hyperglycemia during induction therapy is associated with poorer survival in children with acute lymphocytic leukemia. J Pediatr. 2009;155(1):73–78. doi: 10.1016/j.jpeds.2009.01.072. [DOI] [PubMed] [Google Scholar]
- 19.Weiser MA, Cabanillas ME, Konopleva M, Thomas DA, Pierce SA, Escalante CP, et al. Relation between the duration of remission and hyperglycemia during induction chemotherapy for acute lymphocytic leukemia with a hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone/methotrexate–cytarabine regimen. Cancer. 2004;100(6):1179–1185. doi: 10.1002/cncr.20071. [DOI] [PubMed] [Google Scholar]
- 20.Organization WH. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO/IDF consultation. Geneva: World Hearth Organisation; 2006. [Google Scholar]
- 21.World Health Organization . Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus: abbreviated report of a WHO consultation 2011. Geneva: World Health Organization; 2013. [PubMed] [Google Scholar]
- 22.University of Oxford OCfD, endocrinology and metabolism, diabetes trials unit. HOMA calculator. Oxford (England): https://www.dtu.ox.ac.uk/homacalculator/download.php.
- 23.Nazar CE, Lacassie HJ, Lopez RA, Munoz HR. Dexamethasone for postoperative nausea and vomiting prophylaxis: effect on glycaemia in obese patients with impaired glucose tolerance. Eur J Anaesthesiol. 2009;26(4):318–321. doi: 10.1097/EJA.0b013e328319c09b. [DOI] [PubMed] [Google Scholar]
- 24.Fizazi K, Chi KN, de Bono JS, Gomella LG, Miller K, Rathkopf DE, et al. Low incidence of corticosteroid-associated adverse events on long-term exposure to low-dose prednisone given with abiraterone acetate to patients with metastatic castration-resistant prostate cancer. Eur Urol. 2016;70(3):438–444. doi: 10.1016/j.eururo.2016.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwamoto T, Kagawa Y, Naito Y, Kuzuhara S, Kojima M. Steroid induced diabetes mellitus and related risk factors in patients with neurologic diseases. Pharmacotherapy. 2004;24(4):5–514. doi: 10.1592/phco.24.5.508.33355. [DOI] [PubMed] [Google Scholar]
- 26.den Uyl D, van Raalte DH, Nurmohamed MT, Lems WF, Bijlsma JW, Hoes JN, et al. Metabolic effects of high-dose prednisolone treatment in early rheumatoid arthritis: balance between diabetogenic effects and inflammation reduction. Arthritis Rheumatol. 2012;64(3):639–646. doi: 10.1002/art.33378. [DOI] [PubMed] [Google Scholar]
- 27.Pirsch J, Henning A, First M, Fitzsimmons W, Gaber AO, Reisfield R, et al. New-onset diabetes after transplantation: results from a double-blind early corticosteroid withdrawal trial. Am J Transplant. 2015;15(7):1982–1990. doi: 10.1111/ajt.13247. [DOI] [PubMed] [Google Scholar]
- 28.Burt MG, Willenberg VM, Petersons CJ, Smith MD, Ahern MJ, Stranks SN. Screening for diabetes in patients with inflammatory rheumatological disease administered long-term prednisolone: a cross-sectional study. Rheumatology. 2012;51(6):1112–1119. doi: 10.1093/rheumatology/kes003. [DOI] [PubMed] [Google Scholar]
- 29.Kumar PR, Bhansali A, Ravikiran M, Bhansali S, Dutta P, Thakur J, et al. Utility of glycated hemoglobin in diagnosing type 2 diabetes mellitus: a community-based study. J Clin Endocrinol Metab. 2010;95(6):2832–2835. doi: 10.1210/jc.2009-2433. [DOI] [PubMed] [Google Scholar]
- 30.Hoes J, Van Der Goes M, Van Raalte D, Van Der Zijl N, Den Uyl D, Lems W, et al. Glucose tolerance, insulin sensitivity and β-cell function in patients with rheumatoid arthritis treated with or without low-to-medium dose glucocorticoids. Ann Rheum Dis. 2011;70(11):1887–1894. doi: 10.1136/ard.2011.151464. [DOI] [PubMed] [Google Scholar]
- 31.Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 32.Assefa Z, Akbib S, Lavens A, Stangé G, Ling Z, Hellemans KH, et al. Direct effect of glucocorticoids on glucose-activated adult rat β-cells increases their cell number and their functional mass for transplantation. Am J Physiol-Endocrinol Metab. 2016;311(4):E698–E705. doi: 10.1152/ajpendo.00070.2016. [DOI] [PubMed] [Google Scholar]
- 33.Choi SB, Jang JS, Hong SM, Jun DW, Park S. Exercise and dexamethasone oppositely modulate β-cell function and survival via independent pathways in 90% pancreatectomized rats. J Endocrinol. 2006;190(2):471–482. doi: 10.1677/joe.1.06400. [DOI] [PubMed] [Google Scholar]
- 34.Ogawa A, Johnson JH, Ohneda M, McAllister CT, Inman L, Alam T, et al. Roles of insulin resistance and beta-cell dysfunction in dexamethasone-induced diabetes. J Clin Invest. 1992;90(2):497. doi: 10.1172/JCI115886. [DOI] [PMC free article] [PubMed] [Google Scholar]