Abstract
Background
Dipeptidyl peptidase-4 (DPP-4) inhibitors are commonly used for the treatment of type 2 diabetes and have been previously shown to prevent diabetic renal injury via various mechanisms, including the attenuation of oxidative stress. Therefore, we hypothesized that linagliptin, a DPP-4 inhibitor, attenuates oxidized stress and diabetic renal injury.
Methods
In total, 30 patients with type 2 diabetes who were undergoing treatment with linagliptin (5 mg) during the 3-month study period were enrolled. Oxidative stress markers [serum malondialdehyde-modified LDL (MDA-LDL) and urinary 8-hydroxydeoxyguanosine (8-OHdG)], an inflammatory marker (high-sensitive CRP), urinary albumin excretion, estimated GFR, and a urinary tubulointerstitial injury marker [urinary liver-type fatty acid-binding protein (L-FABP)] were evaluated at baseline and after 3 months of treatment.
Results
Following linagliptin treatment, serum MDA-LDL, serum HbA1c, and urinary L-FABP levels significantly decreased, while urinary 8-OHdG tended to decrease. In contrast, 1,5-AG levels increased, and high-sensitive CRP and urinary albumin excretion remained unchanged.
Conclusion
In this study, we demonstrated that linagliptin partially attenuated oxidative stress. We also demonstrated that linagliptin treatment reduced urinary L-FABP excretion, suggesting that renal tubule-interstitial injury may be attenuated by linagliptin (UMIN 000015308).
Keywords: Diabetic kidney disease, DPP-4 inhibitor, Oxidative stress, Tubulointerstitial injury
Introduction
Diabetic kidney disease (DKD) is a leading cause of end-stage renal disease, along with the increasing rate of type 2 diabetes worldwide. DKD causes proteinuria, leading to renal dysfunction, and is an important risk factor for cardiovascular diseases [1]. Therefore, improvements in the clinical outcomes of diabetic patients are crucial to prevent DKD progression in addition to preventive measures, such as strict glycemic and blood pressure control using renin–angiotensin system inhibitors [2]. Dipeptidyl peptidase-4 (DPP-4) inhibitors are hypoglycemic agents that enhance the action of endogenous glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). GLP-1 primarily acts in the pancreatic islets, wherein it stimulates insulin secretion and inhibits glucagon secretion [3]. In addition, previous experimental studies have demonstrated that GLP-1 shows direct protective action on the cardiovascular system and kidneys [4]. Therefore, DPP-4 inhibitors can prevent DKD progression by enhancing GLP-1 action. Linagliptin, a DPP-4 inhibitor, has a xanthine-based molecular structure [5] and, therefore, may diminish oxidative stress, which plays a key role in DKD progression [6].
Therefore, we investigated the effects of linagliptin on oxidative stress markers and DKD progression in patients with type 2 diabetes in a single-arm prospective interventional study.
Methods
Study subjects
Diabetic patients aged 20–80 years were eligible for inclusion in this study. Exclusion criteria included the start or withdrawal of statins, pioglitazone, or renin–angiotensin system inhibitors within 1 month prior to enrollment. Finally, 30 patients (24 males and 6 females; age, 68 ± 7 years) were enrolled.
Study design
This study was single-center single-arm interventional study. Study subjects were treated with linagliptin (5 mg). Other medications for diabetes, hypertension, and hyperlipidemia were unchanged throughout the 3-month study period.
Study endpoint
The primary endpoint was changes from baseline in serum malondialdehyde-modified LDL (MDA-LDL) levels, high-sensitive CRP levels, urinary 8-hydroxydeoxyguanosine (8-OHdG) excretion, and urinary liver-type fatty acid-binding protein (L-FABP) excretion to the end of 3-month linagliptin treatment. The secondary endpoint was changes from baseline in urinary albumin/creatinine ratio (ACR) and estimated glomerular filtration rate (eGFR) to the end of 3-month linagliptin treatment.
Laboratory analysis
Blood samples were obtained after 12-h fasting to measure MDA-LDL and high-sensitive CRP levels. Urinary 8-OHdG, L-FABP, albumin, and creatinine levels were measured using single-voided urine samples. Plasma glucose, 1,5-anhydroglucitol (1,5-AG), HbA1c, LDL cholesterol, HDL cholesterol, triglyceride, and creatinine levels were also measured.
Statistical analysis
Data are expressed as mean ± standard deviation (SD). Student’s t test was used to compare changes in parameters from baseline to the end of the 3-month study period. The strength of correlation between variables was determined using Spearman’s rank correlation coefficient. All statistical analyses were performed using the JMP 8.0 (SAS Institute, Inc., Cary, NC, USA).
Results
Baseline patient characteristics are summarized in Table 1. Mean patient BMI was 24.8 ± 3.7; mean systemic blood pressure was 125 ± 15/73 ± 11 mmHg. The following medications were administered during the study period: sulfonylurea (n = 12), biguanide (n = 20), alpha-glucosidase inhibitors (n = 10), insulin (n = 12), angiotensin-converting enzyme inhibitor and/or angiotensin receptor blockers (n = 9), and statin (n = 16). No patient was administered SGLT2 inhibitor. After 3-month linagliptin treatment, mean HbA1c level significantly decreased, and mean 1,5-AG level significantly increased, reflecting the postprandial hyperglycemic status of the patients. Mean TG level significantly decreased after 3 months, whereas mean LDLC and HDLC levels did not significantly change. MDA-LDL, an oxidized LDL, significantly decreased (Fig. 1a), while urinary 8-OHdG levels tended to decrease after 3 months although this change was not significant (Fig. 1b). However, uric acid was not changed significantly although linagliptin has a xanthine-based molecular structure [5]. Both ΔMDA-LDL and Δurinary 8OHdG were not significantly associated with ΔHbA1c (Table 3). There were no significant changes in high-sensitive CRP (Fig. 1c), ACR, and eGFR levels (Table 2), whereas urinary L-FABP, a tubulointerstitial injury marker, significantly decreased after 3 months (Fig. 2). Furthermore, the Δurinary L-FABP was not significantly associated with ΔHbA1c (Table 3). Metformin was reported to enhance the effect of DPP4 inhibitor on GLP-1 level [7], suggesting that DPP4 inhibitor plus metformin may be more potent GLP-1 action. However, there was no significant difference between presence and absence of metformin in ΔHbA1c (presence; − 1.07 ± 1.56, absence; − 0.86 ± 0.60), ΔMDA-LDL (presence; − 49.9 ± 46.72, absence; − 1.33 ± 35.6), Δurinary 8OhDG (presence; − 1.47 ± 5.27, absence; 0.70 ± 3.37), and Δurinary L-FABP (presence; − 1.26 ± 4.42, absence; − 0.86 ± 1.96).
Table 1.
Baseline characteristics
| Age (years) | 68 ± 7 |
| M/F | 24/6 |
| Body weight (kg) | 66.4 ± 11.5 |
| BMI | 24.8 ± 3.7 |
| SBP (mmHg) | 125 ± 15 |
| DBP (mmHg) | 73 ± 11 |
| HbA1c (%) | 7.9 ± 1.2 |
| FPG (mg/dl) | 149 ± 32 |
| TG (mg/dl) | 162 ± 100 |
| HDLC (mg/dl) | 46 ± 15 |
| LDLC (mg/dl) | 104 ± 24 |
| eGFR (ml/min/1.73 m2) | 63.9 ± 20.1 |
| 1,5-AG (mg/ml) | 7.8 ± 4.2 |
| Log ACR (mg/gCr) | 1.34 ± 0.71 |
| Hypertension (Y/N) | 25/5 |
| Dyslipidemia (Y/N) | 25/5 |
| History of CVD (Y/N) | 16/14 |
| Statin (Y/N) | 16/14 |
| RAS inhibitor (Y/N) | 11/19 |
| Sulfonylurea (Y/N) | 12/18 |
| Biguanide (Y/N) | 20/10 |
| Α-glucosidase inhibitor (Y/N) | 10/20 |
| Insulin (Y/N) | 2/28 |
| Pioglitazone (Y/N) | 1/29 |
| Sodium glucose transporter 2 inhibitor | 0/30 |
Mean ± SD
BMI body mass index, SBP systolic blood pressure, DBP diastolic blood pressure, HbA1c hemoglobin A1c, FPG fasting plasma glucose, TG triglyceride, HDLC high-density lipoprotein cholesterol, LDLC low-density lipoprotein cholesterol, eGFR estimated glomerular filtration rate, 1,5-AG 1,5-anhydroglucitol, ACR albumin/creatinine ratio, CVD cardiovascular disease, RAS rennin–angiotensin system
Fig. 1.
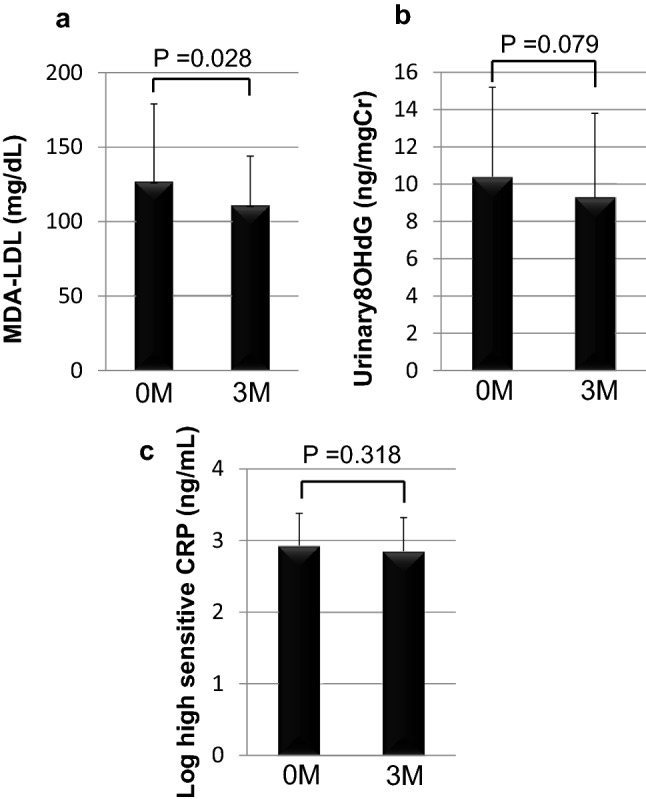
Effect of linagliptin on oxidative stress and inflammation markers after 3 months of treatment: a MDA-LDL (mg/dl), b urinary 8-OHdG excretion (ng/gCr), and c high-sensitive CRP ng/ml levels at baseline (0 M) and after 3 months of treatment (3 M). Data express mean ± SD
Table 3.
Association between ΔHbA1c and ΔMDA-LDL, Δurinary 8OHdG, and Δurinary L-FABP
| R | P | |
|---|---|---|
| ΔMDA-LDL | 0.21 | 0.258 |
| Δurinary 8OHdG | − 0.204 | 0.316 |
| Δurinary L-FABP | 0.216 | 0.258 |
Table 2.
Changes in glucose and lipid profiles
| Baseline | 3 months | |
|---|---|---|
| Body weight (kg) | 66.4 ± 11.5 | 64.5 ± 11.4* |
| SBP (mmHg) | 125 ± 15 | 128 ± 12 |
| DBP (mmHg) | 73 ± 11 | 74 ± 7 |
| HbA1c (%) | 7.9 ± 1.2 | 6.9 ± 0.6** |
| FPG (mg/dl) | 149 ± 32 | 134 ± 23* |
| TG (mg/dl) | 162 ± 100 | 134 ± 95* |
| HDLC (mg/dl) | 46 ± 15 | 48 ± 14 |
| LDLC (mg/dl) | 104 ± 24 | 97 ± 26 |
| 1,5-AG (mg/ml) | 7.8 ± 4.2 | 12.7 ± 6.0** |
| Uric acid (mg/dl) | 5.8 ± 1.4 | 5.9 ± 1.1 |
| Estimated GFR (ml/min/1.73 m2) | 63.9 ± 20.1 | 62.3 ± 20.2 |
| Urinary albumin excretion (mg/gCr) | 200.6 ± 876.0 | 149.1 ± 608.7 |
Mean ± SD
SBP systolic blood pressure, DBP diastolic blood pressure, HbA1c hemoglobin A1c, FPG fasting plasma glucose, TG triglyceride, HDLC high-density lipoprotein cholesterol, LDLC low-density lipoprotein cholesterol, 1,5-AG 1,5-anhydroglucitol, GFR glomerular filtration rate
*P < 0.05 vs. baseline, **P < 0.01 vs. baseline
Fig. 2.
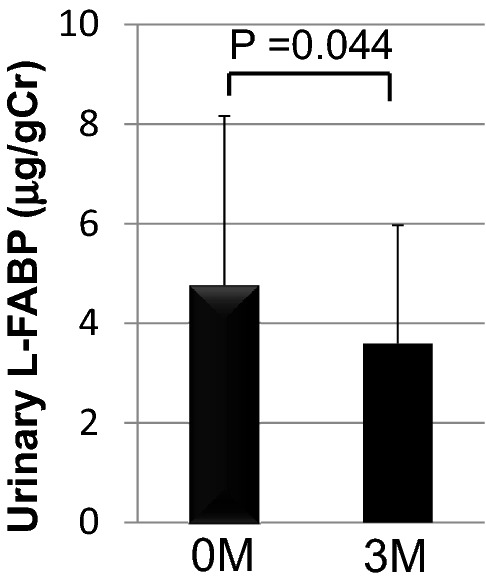
Effect of linagliptin on urinary L-FABP excretion after 3 months of treatment: data express mean ± SD
Discussion
Tubulointerstitial injury rather than glomerular injury is strongly associated with chronic kidney disease progression [8, 9]. L-FABP is a 15-kD fatty acid-binding protein expressed in the proximal tubules of the human kidneys and reflects the tubulointerstitial injury of the kidneys [10]. Urinary L-FABP has been reported to be a predictive marker for renal prognosis in the early stage of DKD, likely reflecting tubulointerstitial injury during the early disease stage [11, 12]. The present study demonstrated that urinary L-FABP excretion decreased following linagliptin treatment in patients with type 2 diabetes. To the best of our knowledge, this is the first clinical report demonstrating the preventive effect of linagliptin treatment on tubulointerstitial injury in DKD. In this study, the change rate of L-FABP was not associated with change rate of HbA1c. These results suggest that reduction of L-FABP may be not due to glycemic control improvement. A recent clinical study showed that switch from other DPP4 inhibitors including linagliptin (subjects with linagliptin was only two) to anagliptin treatment significantly decreased urinary L-FABP excretion in spite of HbA1c level was not changed [13]. These suggests that each DPP4 inhibitor may have different effect on urinary L-FABP excretion.
A previous study has demonstrated that GLP-1 receptors are expressed in the glomeruli and small vessels of the kidneys and that a GLP-1 analog ameliorated diabetic renal injury in diabetic mice [14]. In the clinical setting, the LEADER trial has demonstrated that liraglutide, a GLP-1 analog, improved renal prognosis [15]. Thus, the enhancement of GLP-1 action can prevent DKD progression, and the renal protective effect of linagliptin may, at least in part, be due to the enhancement of GLP-1 action.
Inflammation plays a key role in DKD progression [6]. DPP-4 is highly expressed in the kidneys [16] and has been reported to directly enhance inflammation [17]. These data indicate that DPP-4 inhibitors can prevent diabetic renal injury by inhibiting inflammation. However, our results showed no decline in high-sensitive CRP levels following linagliptin treatment although linagliptin might suppress local inflammation in the kidneys.
Linagliptin has a xanthine-based molecular structure [5], and, therefore, may exert anti-oxidant effects. Oxidative stress plays a key role in the progression of diabetic renal injury. Indeed, an experimental study has demonstrated that linagliptin prevented the progression of diabetic renal injury by inhibiting oxidative stress in a rat model [18]. Our study demonstrated that the levels of oxidized LDL and urinary 8-OHdG decreased following linagliptin treatment and change rate of these oxidative stress markers was not associated with change rate of HbA1c, suggesting that linagliptin may reduce oxidative stress independent glucose lowering effect, although serum uric acid level was not decreased by linagliptin treatment in this study.
A previous clinical study has demonstrated that urinary albumin excretion was attenuated by sitagliptin treatment [19]. Moreover, the SAVOR-TIMI 53 trial has shown that saxagliptin treatment prevented the progression of diabetic nephropathy stage, as defined by urinary albumin excretion [20]. However, in the present study, ACR did not change following linagliptin treatment despite the attenuation of tubulointerstitial injury marker. The duration of this study may have been inadequate to investigate the effect of linagliptin on urinary albumin excretion. Indeed, the MARLINA-T2D trial has shown that linagliptin could not significantly improve albuminuria compared with placebo when examined over a relatively short duration (24 weeks) [21]. Furthermore, an experimental study has shown that linagliptin treatment prevented tubulointerstitial injury by inhibiting kidney fibrosis via endothelial–mesenchymal transition in diabetic mice [22], suggesting that linagliptin might be more effective in protecting the tubulointerstitial injury than the glomerular injury of DKD.
This study has several limitations. This was a small single-center single-arm study conducted over relatively short duration. Therefore, this study could not demonstrate the effect of linagliptin precisely. Furthermore, we did not evaluate other standard markers of tubule-interstitial injury such as urinary NAG and β2 microglobulin. Therefore, a large randomized control trial over long duration which evaluates tubule-interstitial injury markers as primary outcome is warranted to confirm the effects of linagliptin on diabetic tubulointerstitial injury.
In conclusion, we demonstrated that linagliptin treatment partially attenuated oxidative stress in this single-arm pilot study. Furthermore, we demonstrated that urinary L-FABP excretion was decreased by linagliptin, suggesting that linagliptin might have preventive effect on tubule-interstitial injury in patients with type 2 diabetes. We posit that the large clinical trial, CAROLINA [23] and CARMELINA [24], which are currently in progress, may demonstrate the effects of linagliptin on diabetic vascular complications, including DKD, although outcomes of those trials do not include renal tubulointerstitial injury markers.
Acknowledgements
This study was funded by the Japanese Circulation Foundation (Grant no. J026).
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
All subjects provided written informed consent.
Ethical standards
The study was approved by the ethics committee of National Cerebral and Cardiovascular Center (approved at December 9, 2013, approval number: M25-095) and performed in accordance with Helsinki Declaration of 1964 and later versions. This study was registered under UMIN (ID UMIN 000015308).
References
- 1.Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Hallé JP, Young J, Rashkow A, Joyce C, Nawaz S, Yusuf S, HOPE Study Investigators Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association Microvascular complications and foot care: standards of medical care on diabetes—2018. Diabetes Care. 2018;41:S105–S118. doi: 10.2337/dc18-S010. [DOI] [PubMed] [Google Scholar]
- 3.Drucker DJ. Dipeptidyl peptidase-4 inhibition and the treatment of type 2 diabetes: preclinical biology and mechanisms of action. Diabetes Care. 2007;30:1335–1343. doi: 10.2337/dc07-0228. [DOI] [PubMed] [Google Scholar]
- 4.Saraiva FK, Sposito AC. Cardiovascular effects of glucagon-like peptide 1 (GLP-1) receptor agonists. Cardiovasc Diabetol. 2014;13:142–153. doi: 10.1186/s12933-014-0142-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghatak SB, Patel DS, Shanker N, Srivastava A, Deshpande SS, Panchal SJ. Linagliptin: a novel xanthine-based dipeptidyl peptidase-4 inhibitor for treatment of type II diabetes mellitus. Curr Diabetes Rev. 2011;7:325–335. doi: 10.2174/157339911797415648. [DOI] [PubMed] [Google Scholar]
- 6.Magee C, Grieve DJ, Watson CJ, Brazil DP. Diabetic nephropathy: a tangled web to unweave. Cardiovasc Drugs Ther. 2017;31:579–592. doi: 10.1007/s10557-017-6755-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solis-Herrera C, Triplitt C, de Garduno-Garcia J, Adams J, DeFronzo RA, Cersosimo E. Mechanisms of glucose lowering of dipeptidyl peptidase-4 inhibitor sitagliptin when used alone or with metformin in type 2 diabetes: a double-tracer study. Diabetes Care. 2013;36:2756–2762. doi: 10.2337/dc12-2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis. 1992;20:1–17. doi: 10.1016/S0272-6386(12)80312-X. [DOI] [PubMed] [Google Scholar]
- 9.Mise K, Hoshino J, Ueno T, Hazue R, Hasegawa J, Sekine A, Sumida K, Hiramatsu R, Hasegawa E, Yamanouchi M, Hayami N, Suwabe T, Sawa N, Fujii T, Hara S, Ohashi K, Takaichi K, Ubara Y. Prognostic value of tubulointerstitial lesions, urinary N-acetyl-β-d-glucosaminidase, and urinary β2-microglobulin in patients with type 2 diabetes and biopsy-proven diabetic nephropathy. Clin J Am Soc Nephrol. 2016;11:593–601. doi: 10.2215/CJN.04980515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamijo A, Sugaya T, Hikawa A, Okada M, Okumura F, Yamanouchi M, Honda A, Okabe M, Fujino T, Hirata Y, Omata M, Kaneko R, Fujii H, Fukamizu A, Kimura K. Urinary excretion of fatty acid-binding protein reflects stress overload on the proximal tubules. Am J Pathol. 2004;165:1243–1255. doi: 10.1016/S0002-9440(10)63384-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Araki S, Haneda M, Koya D, Sugaya T, Isshiki K, Kume S, Kashiwagi A, Uzu T, Maegawa H. Predictive effects of urinary liver-type fatty acid-binding protein for deteriorating renal function and incidence of cardiovascular disease in type 2 diabetic patients without advanced nephropathy. Diabetes Care. 2013;36:1248–1253. doi: 10.2337/dc12-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamijo-Ikemori A, Sugaya T, Yasuda T, Kawata T, Ota A, Tatsunami S, Kaise R, Ishimitsu T, Tanaka Y, Kimura K. Clinical significance of urinary liver-type fatty acid-binding protein in diabetic nephropathy of type 2 diabetic patients. Diabetes Care. 2011;34:691–696. doi: 10.2337/dc10-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitada M, Tsuda SI, Konishi K, Takeda-Watanabe A, Fujii M, Kanasaki K, Nishizawa M, Nakagawa A, Koya D. Anagliptin ameliorates albuminuria and urinary liver-type fatty acid-binding protein excretion in patients with type 2 diabetes with nephropathy in a glucose-lowering-independent manner. BMJ Open Diabetes Res Care. 2017;5:e000391. doi: 10.1136/bmjdrc-2017-000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujita H, Morii T, Fujishima H, Sato T, Shimizu T, Hosoba M, Tsukiyama K, Narita T, Takahashi T, Drucker DJ, Seino Y, Yamada Y. The protective roles of GLP-1R signaling in diabetic nephropathy: possible mechanism and therapeutic potential. Kidney Int. 2014;85:579–589. doi: 10.1038/ki.2013.427. [DOI] [PubMed] [Google Scholar]
- 15.Mann JFE, Ørsted DD, Brown-Frandsen K, Marso SP, Poulter NR, Rasmussen S, Tornøe K, Zinman B, Buse JB, LEADER Steering Committee and Investigators Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377:839–848. doi: 10.1056/NEJMoa1616011. [DOI] [PubMed] [Google Scholar]
- 16.von Websky K, Reichetzeder C, Hocher B. Physiology and pathophysiology of incretins in the kidney. Curr Opin Nephrol Hypertens. 2014;23:54–60. doi: 10.1097/01.mnh.0000437542.77175.a0. [DOI] [PubMed] [Google Scholar]
- 17.Zhuge F, Ni Y, Nagashimada M, Nagata N, Xu L, Mukaida N, Kaneko S, Ota T. DPP-4 inhibition by linagliptin attenuates obesity-related inflammation and insulin resistance by regulating M1/M2 macrophage polarization. Diabetes. 2016;65:2966–2979. doi: 10.2337/db16-0317. [DOI] [PubMed] [Google Scholar]
- 18.Nakashima S, Matsui T, Takeuchi M, Yamagishi SI. Linagliptin blocks renal damage in type 1 diabetic rats by suppressing advanced glycation end products-receptor axis. Horm Metab Res. 2014;46:717–721. doi: 10.1055/s-0034-1371892. [DOI] [PubMed] [Google Scholar]
- 19.Mori H, Okada Y, Arao T, Tanaka Y. Sitagliptin improves albuminuria in patients with type 2 diabetes mellitus. J Diabetes Investig. 2014;5:313–319. doi: 10.1111/jdi.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mosenzon O, Leibowitz G, Bhatt DL, Cahn A, Hirshberg B, Wei C, Im K, Rozenberg A, Yanuv I, Stahre C, Ray KK, Iqbal N, Braunwald E, Scirica BM, Raz I. Effect of saxagliptin on renal outcomes in the SAVOR-TIMI 53 trial. Diabetes Care. 2017;40:69–76. doi: 10.2337/dc16-0621. [DOI] [PubMed] [Google Scholar]
- 21.Groop PH, Cooper ME, Perkovic V, Hocher B, Kanasaki K, Haneda M, Schernthaner G, Sharma K, Stanton RC, Toto R, Cescutti J, Gordat M, Meinicke T, Koitka-Weber A, Thiemann S, von Eynatten M. Linagliptin and its effects on hyperglycemia and albuminuria in patients with type 2 diabetes and renal dysfunction: the randomized MARLINA-T2D trial. Diabetes Obes Metab. 2017;19:1610–1619. doi: 10.1111/dom.13041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanasaki K, Shi S, Kanasaki M, He J, Nagai T, Nakamura Y, Ishigaki Y, Kitada M, Srivastava SP, Koya D. Linagliptin-mediated DPP-4 inhibition ameliorates kidney fibrosis in streptozotocin-induced diabetic mice by inhibiting endothelial-to-mesenchymal transition in a therapeutic regimen. Diabetes. 2014;63:2120–2131. doi: 10.2337/db13-1029. [DOI] [PubMed] [Google Scholar]
- 23.Marx N, Rosenstock J, Kahn SE, Zinman B, Kastelein JJ, Lachin JM, Espeland MA, Bluhmki E, Mattheus M, Ryckaert B, Patel S, Johansen OE, Woerle HJ. Design and baseline characteristics of the CARdiovascular Outcome Trial of LINAgliptin versus glimepiride in type 2 diabetes (CAROLINA®) Diabetes Vasc Dis Res. 2015;12:164–174. doi: 10.1177/1479164115570301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenstock J, Perkovic V, Alexander JH, Cooper ME, Marx N, Pencina MJ, Toto RD, Wanner C, Zinman B, Baanstra D, Pfarr E, Mattheus M, Broedl UC, Woerle HJ, George JT, von Eynatten M, McGuire DK, CARMELINA® Investigators Rationale, design, and baseline characteristics of the CArdiovascular safety and Renal Microvascular outcomE study with LINAgliptin (CARMELINA®): a randomized, double-blind, placebo-controlled clinical trial in patients with type 2 diabetes and high cardio-renal risk. Cardiovasc Diabetol. 2018;17:39. doi: 10.1186/s12933-018-0682-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


