Abstract
The experimental aim of this study was to determine, in vitro, the effects of glucose-induced EMPs on endothelial cell expression of E-selectin, intercellular cell adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 and platelet cell adhesion molecule-1 (PECAM-1). Human umbilical vein endothelial cells (HUVECs) were cultured (3rd passage) and plated in 6-well plates at a density of 5.0 × 105 cells/condition. HUVECs were incubated with media containing either 25 mM d-glucose (concentration representing a hyperglycemic state) or 5 mM d-glucose (normoglycemic condition) for 48 h to generate EMPs. EMP identification (CD144+) and concentration were determined by flow cytometry. HUVECs (3 × 106 cells/condition) were treated with either high glucose-derived EMPs (hgEMPs) or normal glucose-derived (ngEMPs) for 24 h and surface expression of E-selectin (CD62E-PE), ICAM-1 (CD54-FITC), VCAM-1 (CD106-APC) and PECAM-1 (CD31-BV) was assessed by flow cytometry and reported as mean fluorescent intensity (MFI). Hyperglycemic-derived EMPs induced significantly higher surface expression of E-selectin (2614 ± 132 vs. 2010 ± 204 MFI), ICAM-1 (2110 ± 81 vs. 1688 ± 152 MFI), VCAM-1 (3589 ± 431 vs. 2134 ± 386) and PECAM-1 (4237 ± 395 vs. 2525 ± 269 MFI) on endothelial cells than EMPs from normoglycemic conditions. Microparticle-induced cell adhesion molecule expression provides potential novel mechanistic insight regarding the accelerated risk of atherosclerotic vascular disease associated with hyperglycemia.
Keywords: Glucose, Endothelial cells, Microparticles, Cell adhesion molecules
Introduction
Hyperglycemia is associated with an increased risk and prevalence of atherosclerotic cardiovascular disease (CVD) [1]. A major factor underlying the increased CVD burden associated with hyperglycemia is glucose-induced endothelial cell activation and dysfunction [2, 3]. A hallmark characteristic of endothelial cell activation, and in turn atherogenesis, is increased surface expression of cell adhesion molecules [4, 5]. Indeed, the adherence and subsequent transendothelial migration of circulating leukocytes and other inflammatory cells into the intima are predominantly mediated by the surface expression of adhesion molecules such as E-selectin, intracellular adhesion molecule (ICAM)-1, vascular cell adhesion molecule (VCAM)-1 and platelet endothelial cell adhesion molecule (PECAM)-1 on the vascular endothelium. Morigi et al. [6] and others [7, 8] have demonstrated, in vitro, that high glucose concentrations upregulate the surface expression of these adhesion molecules, establishing glucose as a potent promoter of leukocyte adhesion and intimal infiltration [7]. High glucose also stimulates microparticle release from endothelial cells [9]. These endothelial-derived microparticles (EMPs) can confer deleterious autocrine effects on the endothelium [10–12]. For example, hyperglycemic-related EMPs have been shown to promote endothelial cell inflammation and apoptosis [11, 13, 14]. Moreover, EMPs have emerged as mediators and biomarkers of diabetes-related CVD risk and development [15, 16]. The effects of glucose-derived EMPs on endothelial adhesion molecule expression are not well defined. Accordingly, the aim of this study was to determine the effects of glucose-stimulated EMPs on endothelial cell surface expression of adhesion molecules.
Methods
Cell culture, EMP generation and enumeration
Human umbilical vein endothelial cells (HUVECs) were purchased from Life Technologies (ThermoFisher, Waltham, MA), and cultured in endothelial growth media (EBM-2 Bulletkit, Clonetics Lonza, Walkersville, MD), supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin (Life Technologies, Carlsbad, CA) under standard cell culture conditions (37 °C and 5% CO2). HUVECs were cultured to the 3rd passage after reaching ~ 90% confluence and treated with media (RPMI 1640) containing 25 mM mannitol (to control for osmolality) and either 5 mM d-glucose (normal glucose) or 25 mM d-glucose (high glucose; representative of a diabetic glycemic state) for 48 h as previously described [14]. Thereafter, the supernatant from each condition was collected, centrifuged at 13,000 × g at room temperature for 2 min to pellet and discard cellular debris, transferred to TruCount™ tubes (BD Biosciences, San Jose, CA) and incubated with CD144-PE (VE-Cadherin) for EMP determination. EMP size threshold was established using Megamix-Plus SSC calibrator beads (Biocytex, Marseille, France) [17]. EMP concentration (MP/μL) in the media from both conditions was determined by flow cytometry using the formula: [(number of events in region containing EMPs/number of events in absolute count bead region) × (total number of beads per test/total volume of sample)].
EMP-treated HUVECs
HUVECs were cultured as described above. Media containing EMPs harvested from either the normal glucose or high glucose conditions were centrifuged at 20,500 × g for 30 min at 4 °C to pellet EMPs [11, 12, 14]. The pelleted EMPs were then re-suspended in EBM-2 at a concentration of 1.0 × 107 MPs/mL. HUVECs were then treated with an equal number of EMPs derived from either the normal glucose (ngEMPs) or high glucose (hgEMPs) conditions for 24 h.
Endothelial cell adhesion molecule surface expression
Cell surface expression of cell adhesion molecules was determined by flow cytometry. After EMP treatment, harvested HUVECs were incubated with CD62E-PE (E-selectin); CD54-FITC (ICAM-1); CD106-APC (VCAM-1); and CD31-BV (PECAM-1). Briefly, endothelial cells (2 × 105 per condition) were gated by their forward and side scatter profile. Biexponential scaling was used in all dot plots. Fluorescence minus one or isotype controls were used in all experiments. Mean fluorescence intensity (MFI) was standardized using Cytometer Setup and Tracking Beads (BD, Franklin Lakes, NJ, USA) and isotype negative control antibodies [18, 19].
Statistical analysis
Differences in glucose-treatment derived EMP number and adhesion molecule expression were determined by two-tailed, unpaired Student’s t test. Data are reported as mean ± SEM for four independent HUVEC experiments. Statistical significance was set a priori at P < 0.05.
Results
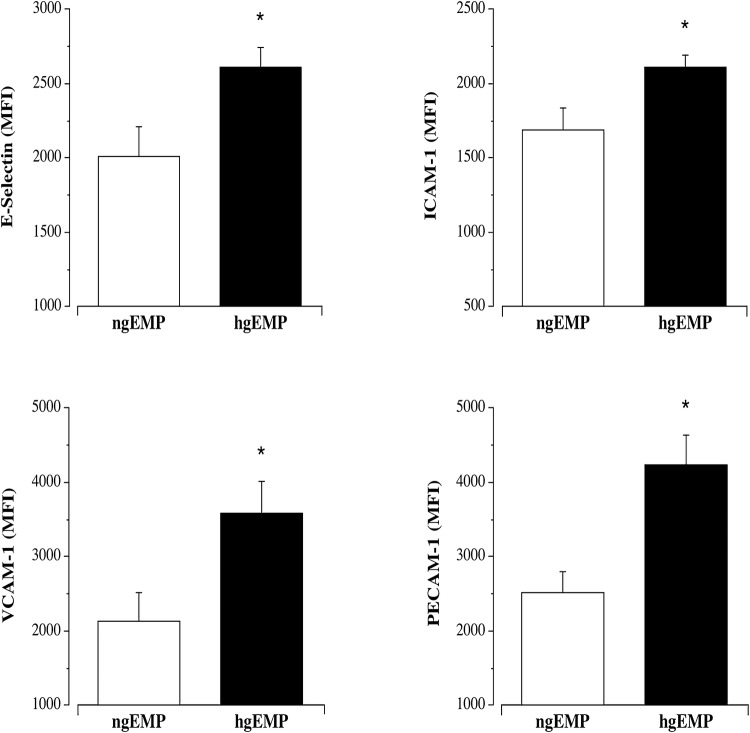
The hyperglycemic condition elicited markedly higher (~ 260%; P < 0.05) EMP release compared with the normoglycemic condition (136 ± 38 vs. 37 ± 13 MP/µL) (Fig. 1). hgEMP exposure induced greater endothelial cell expression of each adhesion molecule. Surface expression of E-selectin (2614 ± 132 vs. 2010 ± 204 MFI), ICAM-1 (2110 ± 81 vs. 1688 ± 152 MFI), VCAM-1 (3590 ± 431 vs. 2134 ± 386 MFI) and PECAM-1 (4237 ± 395 vs. 2525 ± 269 MFI) was significantly higher in the hgEMP treated compared with ngEMP-treated cells (Fig. 2). Representative flow cytometry side scatter and histograms for EMP quantification and adhesion molecule expression, respectively, are shown in Fig. 3.
Fig. 1.
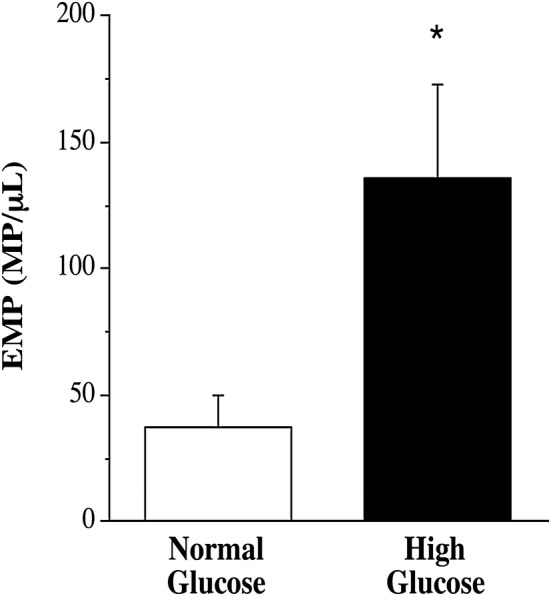
EMP release in response to normal and high glucose concentrations. Values are mean ± SEM (N = 4). *P < 0.05
Fig. 2.
Effect of EMPs generated from normal (ngEMPs) and high glucose (hgEMPs) concentrations on surface expression of E-selectin, ICAM-1, VCAM-1 and PECAM-1. Values are mean ± SEM (N = 4). *P < 0.05
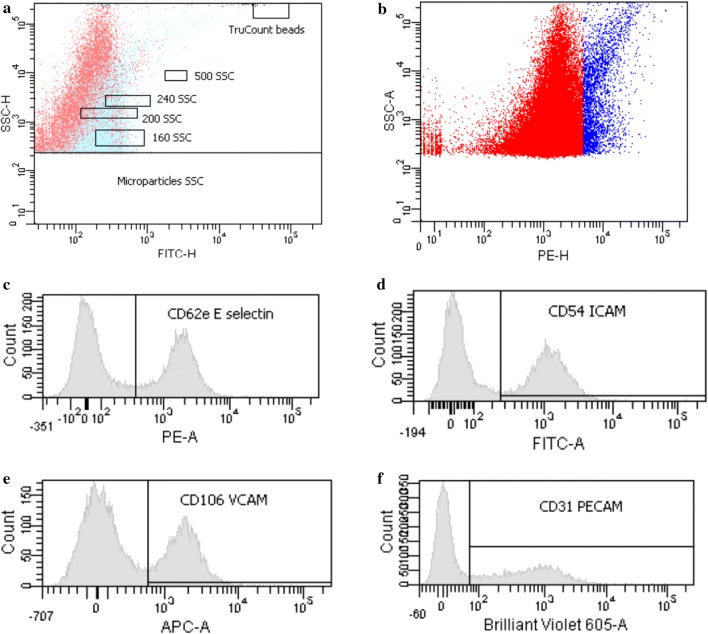
Fig. 3.
Representative side scatter (SSC-H) by FITC height (FITC-H) (panel A) and PE height (PE-H) (panel B) dot plots showing the total number of microparticles and CD144+ microparticles. In addition, representative flow cytometric histograms showing cell surface expression of CD31-PECAM (panel B), CD62e-E-selectin (panel C), CD54-ICAM (panel D) and CD106-VCAM (panel. Mean fluorescence intensity (MFI) was established using Cytometer Setup and Tracking Beads (BD) and isotype negative controls
Discussion
The novel finding of the present study is that high glucose-derived EMPs markedly increase endothelial cell surface expression of E-selectin, ICAM-1, VCAM-1 and PECAM-1. Elevated endothelial surface expression of these adhesion molecules is known to be an integral, precipitating event in the pathogenesis of atherosclerosis as well as an important contributor to plaque development and rupture [20, 21]. Several experimental [8, 22, 23] and clinical [24, 25] studies have demonstrated that hyperglycemia is associated with enhanced expression of cell adhesion molecules. While the direct effects of glucose on cell adhesion molecules are clear, to our knowledge, this is the first study to demonstrate that EMPs produced in response to high glucose stimulation also promote overexpression of adhesion molecules on the surface of endothelial cells.
Increased expression of cell adhesion molecules on the surface of endothelial cells has been shown to facilitate greater luminal leukocyte interaction, adhesion and migration into the subendothelial space, in turn, increasing the propensity for atherosclerosis [5]. Indeed, the tethering and rolling of leukocytes on the endothelial surface are controlled by selectins such as E-selectin, whereas leukocyte adhesion is mediated largely by immunoglobulins (e.g., ICAM-1 and VCAM-1) and transmigration through gap junctions is facilitated by PECAM-1 [26]. The pathologic consequence of this coordinated, complex interaction on the endothelial cell surface depends on the extent of adhesion molecule availability and presentation on the surface of endothelial cells [24, 25]. Surface expression of adhesion molecules is requisite for these interactions; as such, it is the evaluation of cell surface expression of adhesion molecules rather than general, non-location-specific cellular protein levels that is most important and physiologically relevant. The seminal and novel finding of the present study is that hgEMPs significantly increased the expression of E-selectin (~ 30%), ICAM-1 (~ 25%), VCAM-1 (~ 70%) and PECAM-1 (~ 70%) on the surface of endothelial cells. This finding is consistent with the notion that glucose-stimulated EMPs likely contribute to the increased risk of atherosclerotic vascular disease associated with hyperglycemic conditions [12]. The mechanisms underlying the hgEMP-induced increase in the surface expression of adhesion molecules are not clear. However, EMPs are important transport vectors for a myriad of proteins, cytokines and miRNAs that can disrupt target cell homeostasis. For example, Jansen et al. [11] demonstrated that EMPs-derived under high glucose conditions (similar to the present study) are pro-oxidative, with a cargo rich in NADPH oxidase and reactive oxygen species. This atherogenic microparticle phenotype was suggested to promote endothelial activation and adhesion molecule expression. Indeed, in their study, Jansen and colleagues [11] reported an increase in endothelial cell ICAM-1 and VCAM-1 protein levels in response to high glucose-derived EMPs. Our findings confirm increased expression of these adhesion molecules and significantly extend these findings demonstrating, for the first time, that increased expression involves greater endothelial cell surface density of not only ICAM-1 and VCAM-1 but also E-selectin and PECAM-1 [27]. The expression of cell adhesion molecules on the surface of endothelial cells is critical to atherogenesis. As such, our findings provide novel, more specific insight regarding the proatherogenic effects of EMPs that are produced under hyperglycemic conditions.
In summary, greater cell adhesion molecule expression on the surface of the endothelium heightens atherogenic potential and thrombotic risk [5]. Importantly, herein we demonstrate that EMPs generated from high glucose exposure induce an increase in cell adhesion molecules on the surface of endothelial cells. Our results provide additional, specific support for the notion that hyperglycemic-related EMPs contribute to the pathogenesis of vascular disease associated with diabetes. EMPs may represent an important target for therapeutic intervention aimed at reducing the risk of vascular disease in diabetic individuals.
Acknowledgements
We would like to thank all the staff at the University of Colorado Anschutz Medical Campus ACI/ID Flow Core for their technical assistance. This study was supported in part by the National Institutes of Health (NIH) awards HL131458 and HL135598.
Ethical standards
This study does not involve either human or animal subjects.
Conflict of interest
Each author affirms no conflicts of interest in the conduct or reporting of this study.
References
- 1.Ding H, Triggle CR. Endothelial cell dysfunction and the vascular complications associated with type 2 diabetes: assessing the health of the endothelium. Vasc Health Risk Manag. 2005;1(1):55–71. doi: 10.2147/vhrm.1.1.55.58939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffman RP. Hyperglycemic endothelial dysfunction: does it happen and does it matter? J Thorac Dis. 2015;7(10):1693–1695. doi: 10.3978/j.issn.2072-1439.2015.10.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azcutia V, Abu-Taha M, Romacho T, Vazquez-Bella M, Matesanz N, Luscinskas FW, et al. Inflammation determines the pro-adhesive properties of high extracellular d-glucose in human endothelial cells in vitro and rat microvessels in vivo. PLoS One. 2010;5(4):e10091. doi: 10.1371/journal.pone.0010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh RB, Mengi SA, Xu YJ, Arneja AS, Dhalla NS. Pathogenesis of atherosclerosis: a multifactorial process. Exp Clin Cardiol. 2002;7(1):40–53. [PMC free article] [PubMed] [Google Scholar]
- 5.Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis. 2003;170(2):191–203. doi: 10.1016/S0021-9150(03)00097-2. [DOI] [PubMed] [Google Scholar]
- 6.Morigi M, Angioletti S, Imberti B, Donadelli R, Micheletti G, Figliuzzi M, et al. Leukocyte-endothelial interaction is augmented by high glucose concentrations and hyperglycemia in a NF-kB-dependent fashion. J Clin Invest. 1998;101(9):1905–1915. doi: 10.1172/JCI656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JA, Berliner JA, Natarajan RD, Nadler JL. Evidence that glucose increases monocyte binding to human aortic endothelial cells. Diabetes. 1994;43(9):1103–1107. doi: 10.2337/diab.43.9.1103. [DOI] [PubMed] [Google Scholar]
- 8.Kado S, Wakatsuki T, Yamamoto M, Nagata N. Expression of intercellular adhesion molecule-1 induced by high glucose concentrations in human aortic endothelial cells. Life Sci. 2001;68(7):727–737. doi: 10.1016/S0024-3205(00)00968-1. [DOI] [PubMed] [Google Scholar]
- 9.Li S, Wei J, Zhang C, Li X, Meng W, Mo X, et al. Cell-derived microparticles in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Cell Physiol Biochem. 2016;39(6):2439–2450. doi: 10.1159/000452512. [DOI] [PubMed] [Google Scholar]
- 10.Santilli F, Marchisio M, Lanuti P, Boccatonda A, Miscia S, Davi G. Microparticles as new markers of cardiovascular risk in diabetes and beyond. Thromb Haemost. 2016;116(2):220–234. doi: 10.1160/TH08-12-0796. [DOI] [PubMed] [Google Scholar]
- 11.Jansen F, Yang X, Franklin BS, Hoelscher M, Schmitz T, Bedorf J, et al. High glucose condition increases NADPH oxidase activity in endothelial microparticles that promote vascular inflammation. Cardiovasc Res. 2013;98(1):94–106. doi: 10.1093/cvr/cvt013. [DOI] [PubMed] [Google Scholar]
- 12.Burger D, Turner M, Xiao F, Munkonda MN, Akbari S, Burns KD. High glucose increases the formation and pro-oxidative activity of endothelial microparticles. Diabetologia. 2017;60:1791–1800. doi: 10.1007/s00125-017-4331-2. [DOI] [PubMed] [Google Scholar]
- 13.Said AS, Rogers SC, Doctor A. Physiologic impact of circulating rbc microparticles upon blood-vascular interactions. Fron Physiol. 2017;8:1120. doi: 10.3389/fphys.2017.01120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bammert TD, Hijmans JG, Reiakvam WR, Levy MV, Brewster LM, Goldthwaite ZA, et al. High glucose derived endothelial microparticles increase active caspase-3 and reduce microRNA-Let-7a expression in endothelial cells. Biochem Biophys Res Commun. 2017;493:1026–1029. doi: 10.1016/j.bbrc.2017.09.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu CC, Ma KL, Ruan XZ, Liu BC. The emerging roles of microparticles in diabetic nephropathy. Int J Biol Sci. 2017;13(9):1118–1125. doi: 10.7150/ijbs.21140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng B, Chen Y, Luo Y, Chen M, Li X, Ni Y. Circulating level of microparticles and their correlation with arterial elasticity and endothelium-dependent dilation in patients with type 2 diabetes mellitus. Atherosclerosis. 2010;208(1):264–269. doi: 10.1016/j.atherosclerosis.2009.06.037. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen MH, Beck-Nielsen H, Andersen MN, Handberg A. A flow cytometric method for characterization of circulating cell-derived microparticles in plasma. J Extracell Vesicles. 2014 doi: 10.3402/jev.v3.20795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mittag A, Tarnok A. Basics of standardization and calibration in cytometry—a review. J Biophotonics. 2009;2(8–9):470–481. doi: 10.1002/jbio.200910033. [DOI] [PubMed] [Google Scholar]
- 19.Imanparast F, Paknejad M, Faramarzi MA, Kobarfard F, Amani A, Doosti M. Potential of mZD7349-conjugated PLGA nanoparticles for selective targeting of vascular cell-adhesion molecule-1 in inflamed endothelium. Microvasc Res. 2016;106:110–116. doi: 10.1016/j.mvr.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Davies MJ, Gordon JL, Gearing AJ, Pigott R, Woolf N, Katz D, et al. The expression of the adhesion molecules ICAM-1, VCAM-1, PECAM, and E-selectin in human atherosclerosis. J Pathol. 1993;171(3):223–229. doi: 10.1002/path.1711710311. [DOI] [PubMed] [Google Scholar]
- 21.Nakashima Y, Raines EW, Plump AS, Breslow JL, Ross R. Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the ApoE-deficient mouse. Arterioscler Thromb Vasc Biol. 1998;18(5):842–851. doi: 10.1161/01.ATV.18.5.842. [DOI] [PubMed] [Google Scholar]
- 22.Baumgartner-Parzer SM, Wagner L, Pettermann M, Grillari J, Gessl A, Waldhausl W. High-glucose–triggered apoptosis in cultured endothelial cells. Diabetes. 1995;44(11):1323–1327. doi: 10.2337/diab.44.11.1323. [DOI] [PubMed] [Google Scholar]
- 23.Rattan V, Shen Y, Sultana C, Kumar D, Kalra VK. Glucose-induced transmigration of monocytes is linked to phosphorylation of PECAM-1 in cultured endothelial cells. Am J Physiol. 1996;271(4 Pt 1):E711–E717. doi: 10.1152/ajpendo.1996.271.4.E711. [DOI] [PubMed] [Google Scholar]
- 24.Devaraj S, Jialal I. Low-density lipoprotein postsecretory modification, monocyte function, and circulating adhesion molecules in type 2 diabetic patients with and without macrovascular complications: the effect of alpha-tocopherol supplementation. Circulation. 2000;102(2):191–196. doi: 10.1161/01.CIR.102.2.191. [DOI] [PubMed] [Google Scholar]
- 25.Kado S, Nagata N. Circulating intercellular adhesion molecule-1, vascular cell adhesion molecule-1, and E-selectin in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 1999;46(2):143–148. doi: 10.1016/S0168-8227(99)00083-2. [DOI] [PubMed] [Google Scholar]
- 26.Khodabandehlou K, Masehi-Lano JJ, Poon C, Wang J, Chung EJ. Targeting cell adhesion molecules with nanoparticles using in vivo and flow-based in vitro models of atherosclerosis. Exp Biol Med (Maywood) 2017;242(8):799–812. doi: 10.1177/1535370217693116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osborn L, Hession C, Tizard R, Vassallo C, Luhowskyj S, Chi-Rosso G, et al. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989;59(6):1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]