Abstract
Aldosterone contributes to end-organ damage in heart failure and chronic kidney disease. Mineralocorticoid-receptor inhibitors limit activation of the receptor by aldosterone and slow disease progression, but side effects, including hyperkalemia, limit their clinical use. Damage to the endothelial glycocalyx (a luminal biopolymer layer) has been implicated in the pathogenesis of endothelial dysfunction and albuminuria, but to date no one has investigated whether the glomerular endothelial glycocalyx is affected by aldosterone. In vitro, human glomerular endothelial cells exposed to 0.1 nM aldosterone and 145 mMol NaCl exhibited reduced cell surface glycocalyx components (heparan sulfate and syndecan-4) and disrupted shear sensing consistent with damage of the glycocalyx. In vivo, administration of 0.6 μg/g/d of aldosterone (subcutaneous minipump) and 1% NaCl drinking water increased glomerular matrix metalloproteinase 2 activity, reduced syndecan 4 expression, and caused albuminuria. Intravital multiphoton imaging confirmed that aldosterone caused damage of the glomerular endothelial glycocalyx and increased the glomerular sieving coefficient for albumin. Targeting matrix metalloproteinases 2 and 9 with a specific gelatinase inhibitor preserved the glycocalyx, blocked the rise in glomerular sieving coefficient, and prevented albuminuria. Together these data suggest that preservation of the glomerular endothelial glycocalyx may represent a novel strategy for limiting the pathological effects of aldosterone.
Keywords: albuminuria, aldosterone, cardiovascular disease, endothelium, inflammation
Chronic kidney disease (CKD) affects 1 in 8 adults globally and its prevalence is increasing owing to the increasing incidence of diabetes, hypertension, and obesity.1 Blocking the renin-angiotensin-aldosterone pathway, blood pressure control, and salt restriction delay the progression of CKD and are widely recommended in clinical guidelines.2 High aldosterone levels are seen commonly in Conn’s disease and syndrome,3 but they also are seen in a subset of patients taking angiotensin-converting enzyme inhibitors or angiotensin-receptor blocker medications,4 and in patients with hypertension,3 obesity,3 CKD,5 and obstructive sleep apnea.3 In patients consuming a salt-rich Western diet, aldosterone levels higher than the population median value are associated with an increased risk of developing albuminuria, systemic endothelial dysfunction, and CKD, even when adjustments are made for other known risk factors.6–8 Indigenous seasonal diets with low sodium content result in very high serum aldosterone levels, however, they do not result in demonstrable end-organ damage.9,10 Thus, it appears that the balance between aldosterone and salt may alter the result of aldosterone exposure. Blockade of aldosterone systemically via mineralocorticoid receptor (MR) inhibition reduces albuminuria, prevents cardiac fibrosis, and protects individuals with heart failure from progressive myocardial damage.11 However, because of the phenomenon of aldosterone escape, blockade of the renin-angiotensin-aldosterone at levels upstream of the MR may not be effective at limiting MR stimulation.12 The addition of the MR blockade to angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers in CKD currently is under investigation,13 however, side effects, including potentially life-threatening hyperkalemia, limit the use of MR antagonists and have made clinicians reluctant to adopt this therapeutic strategy.14–16 Thus, novel tissue-specific therapeutic targets downstream of MR stimulation need to be sought.
MRs are widely expressed outside of the renal tubules.17–25 One site of action that has generated considerable research interest is the vascular endothelium.18,26–30 However, the actions of aldosterone on these specialized cells have yet to be fully investigated. The endothelial glycocalyx is a potential downstream target of aldosterone. This anionic biopolymer on the luminal surface of endothelial cells consists of anchored components including proteoglycans and sialoproteins, with adsorbed elements from the circulating plasma.31 The anionic charge largely is owing to the expression of the glycosaminoglycans: heparan sulfate (HS) and chondroitin sulphate, which are bound covalently to core proteins including syndecans.31 Many of the specialist functions performed by the endothelium are dependent on a healthy glycocalyx.31 To date, the endothelial glycocalyx has been found to function as a permeability regulator, shear sensor, immune cell regulator, and clotting modulator, but further roles are under investigation.31
We hypothesized that glomerular endothelial cells (highly specialized fenestrated vascular endothelial cells) expressed MR, and that salt and aldosterone exposure would lead to pathologic remodeling of the glomerular glycocalyx and contribute to albuminuria.
RESULTS
Glomerular endothelial cells expressed MR and lost key glycocalyx components in response to salt and aldosterone
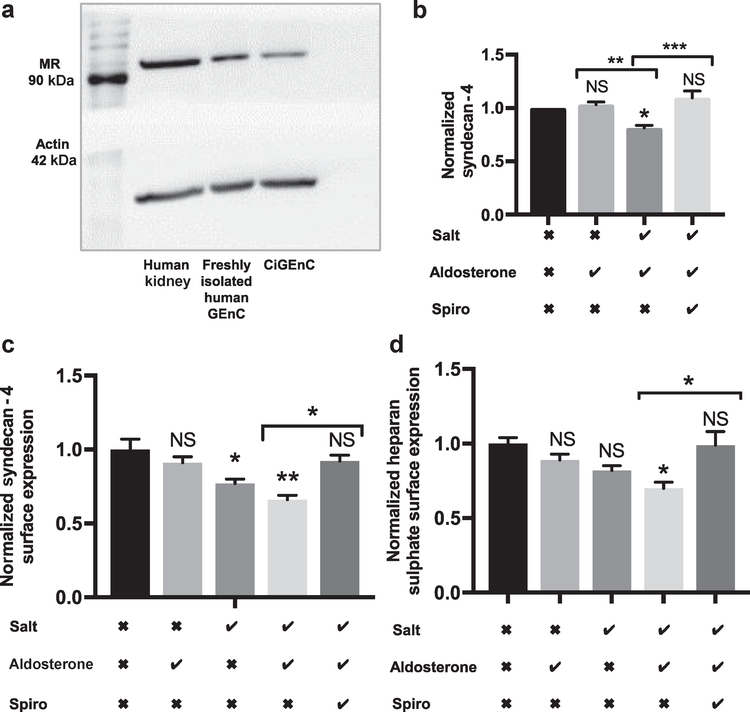
MRs are expressed by freshly isolated human glomerular endothelial cells and conditionally immortalized human glomerular endothelial cells (CiGEnCs) (Figure 1a). Cultured monolayers of CiGEnC swapped from osmotically balanced media (containing mannitol and 125 mmol/l NaCl) to media containing 145 mmol/l NaCl and 0.1 nmol/l aldosterone for 5 days expressed significantly lower concentrations of syndecan-4. This effect was prevented by spironolactone, suggesting the pathway was dependent on MR activation (Figure 1b). Glycocalyx components HS and the HS proteoglycan syndecan-4 can be detected on the surface of CiGEnCs (Supplementary Figure S1). Under iso-osmotic conditions, CiGEnC exposed to 145 mmol/l NaCl and 0.1 nmol/l aldosterone for 5 days lost significant quantities of syndecan-4 and HS from their cell surface (Figure 1c and d, respectively). In contrast, the addition of aldosterone to osmotically balanced media containing 125 mmol/l NaCl had no measurable effect. Media containing 145 mmol/l NaCl had only a small effect in the absence of aldosterone (Figure 1c and d).
Figure 1 |. Glomerular endothelial cells express mineralocorticoid receptor (MR) and loose key glycocalyx components in response to salt and aldosterone.
(a) Western blot confirming MR expression in renal cortex, fluorescence-activated cell sorting isolated human glomerular endothelial cells, and conditionally immortalized human glomerular endothelial cells (CiGEnCs). (b) Syndecan-4 enzyme-linked immunosorbent assay confirming that salt and aldosterone in combination result in syndecan-4 loss from CiGEnC lysates (analysis of variance P = 0.0011; Tukey correction shown; n = 4). (c) Syndecan-4 surface expression on CiGEnCs decreased after 5 days of exposure to salt and aldosterone (analysis of variance P = 0.0021; Tukey correction shown; n = 5). (d) Heparan sulfate (HS) surface expression on CiGEnC decreased after 5 days of exposure to 145 mmol/l NaCl and combined 145 mmol/l NaCl and 0.1 nmol/l aldosterone exposure (n = 5) (analysis of variance P = 0.012; Tukey correction shown; n = 5). Salt row: the checkmark indicates the NaCl concentration increased to 145 mmol; the X indicates mannitol was added to balance osmolarity. Aldosterone row: the checkmark indicates 0.1 nmol/l aldosterone was added to media; the X indicates vehicle alone was added to media. Spironolactone (Spiro) row: the checkmark indicates 0.1 μm spironolactone was added to media; the X indicates vehicle alone was added to media. Significance was relative to control unless indicated. All error bars = SEM. *P < 0.05; **P < 0.01; ***P < 0.005. To optimize viewing of this image, please see the online version of this article at www.kidney-international.org.
Exposing CiGEnC to salt and aldosterone resulted in matrix metalloproteinase up-regulation
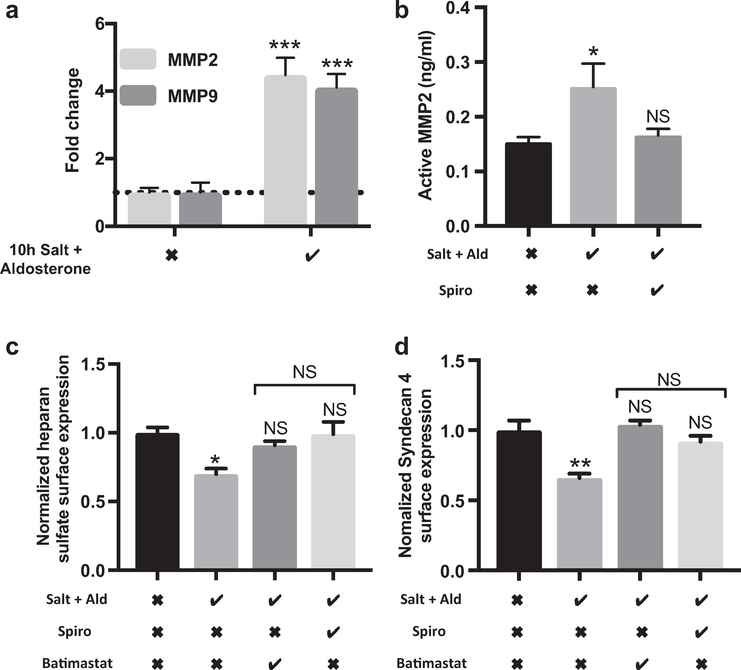
Matrix metalloproteinase (MMP) inhibition and MR inhibition were equally effective in preserving the glycocalyx. MMP2 and MMP9 cleave syndecan-4 from the endothelial cell surface and have been implicated in glomerular disease.32 We have shown that MMPs are important in glycocalyx shedding in response to tumor necrosis factor-α.33 We therefore conducted time-course experiments to study changes at the mRNA level for these key enzymes. Under iso-osmotic conditions, 145 mmol/l NaCl and 0.1 nmol/l aldosterone for 10 hours increased MMP2 and MMP9 mRNA expression significantly (Figure 2a). A total of 145 mmol/l NaCl and 0.1 nmol/l aldosterone exposure for 5 days also significantly increased MMP2 activity in conditioned cell media. This effect was prevented by MR antagonism with spironolactone (Figure 2b). HS and syndecan-4 loss from the CiGEnC glycocalyx was prevented by MR antagonism or MMP inhibition with batimastat. The effects on glycocalyx preservation of these 2 remote drug classes were equivalent; suggesting that the MMP inhibition may be effective in preserving the glycocalyx from MR-mediated damage.
Figure 2 |. Exposing conditionally immortalized human glomerular endothelial cells (CiGEnCs) to salt and aldosterone (Ald) results in matrix metalloproteinase (MMP) up-regulation while MMP inhibition and mineralocorticoid receptor (MR) inhibition are equally effective at preserving the glycocalyx.
(a) mRNA response, assessed by quantitative polymerase chain reaction, after 10 hours of salt and aldosterone showing significant up-regulation of MMP2 and 9 (analysis of variance P < 0.0001; Tukey correction shown; n = 3). (b) MMP2 activity assay confirmed increased MMP2 activity after salt and aldosterone. The increased activity was prevented by MR inhibition (analysis of variance P = 0.02; Tukey correction shown; n = 5). (c and d) Cells maintained in media containing batimastat (MMP inhibitor) or spironolactone (Spiro) did not lose significant heparan sulfate (HS) (analysis of variance P = 0.0164; Tukey correction shown; n = 3) or syndecan-4 from their surface (analysis of variance P = 0.0015; Tukey correction; n = 3). Salt row: the checkmark indicates the NaCl concentration increased to 145 mmol; the X indicates mannitol was added to balance osmolarity. Aldosterone row: the checkmark indicates 0.1 nmol/l aldosterone was added to media; the X indicates vehicle alone was added to media. Spironolactone row: the checkmark indicates 0.1 μm spironolactone was added to media. Batimastat row: the checkmark indicates 5 μm batimastat was added to media. Spironolactone and Batimastat rows: the X indicates vehicle alone was added to media (*P < 0.05; **P < 0.01; ***P < 0.005).
MMP inhibition maintained glycocalyx function
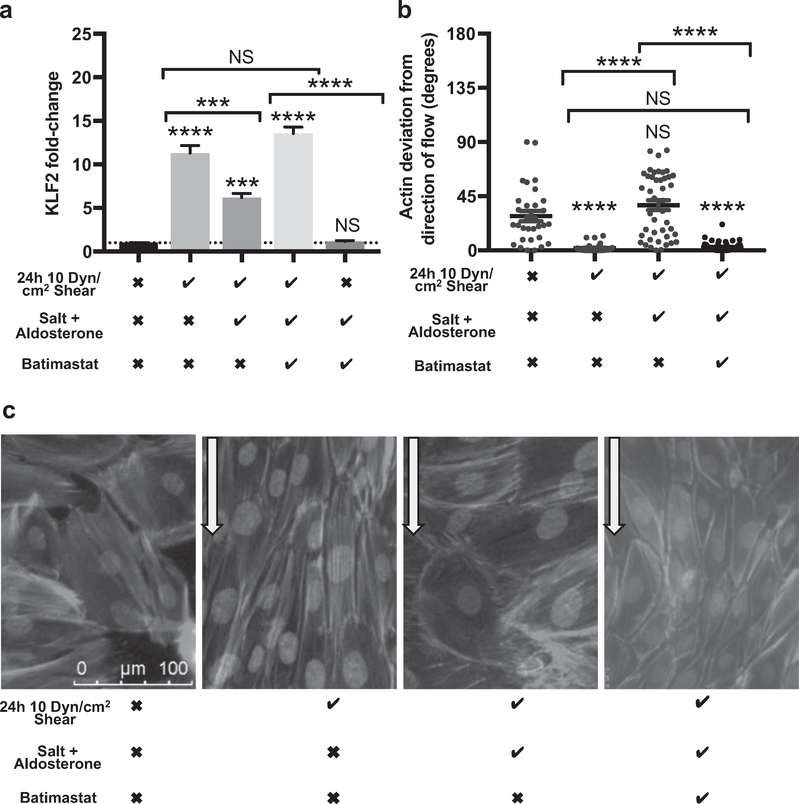
Endothelial glycocalyx contributes to the transduction of laminar shear stress (LSS) to induce intracellular signaling events and cellular responses.31 KLF2 is a key gene associated with cellular remodeling in response to LSS, and we used this as a functional measure of glycocalyx integrity.34 Under standard conditions, CiGEnCs respond to 10 dyn/cm2 LSS with a dramatic increase in KLF2 mRNA expression at 24 hours.35 After 5 days, 145 mmol/l NaCl and 0.1 nmol/l aldosterone KLF2 up-regulation in response to shear was significantly impaired relative to cells maintained in osmotically balanced control media, however, this effect was prevented in the presence of batimastat (Figure 3a). Enzymatic degradation of the glycocalyx, with a corresponding reduction in syndecan-4, had the same effect on CiGEnCs, confirming that glycocalyx damage resulted in impairment of KLF2 up-regulation (Supplementary Figure S2). Syndecan-4 contributes to endothelial cell alignment in response to LSS.36 We therefore investigated if the observed glycocalyx damage also affected CiGEnC alignment. After 24 hours of 10 dyn/cm2 LSS, CiGEnCs maintained in osmotically balanced control media aligned their actin fibers parallel to the flowing media but cells maintained for 5 days in media containing 145 mmol/l NaCl and 0.1 nmol/l aldosterone failed to significantly align. Batimastat restored actin alignment (Figure 3b and c). Together these results suggest that MMP inhibition preserved CiGEnC glycocalyx function.
Figure 3 |. Matrix metalloproteinase (MMP) inhibition using batimastat preserved the conditionally immortalized human glomerular endothelial cell (CiGEnC) glycocalyx-dependent response to shear.
(a) In cells exposed to salt and aldosterone for 5 days, the Inclusion of 5 mmol/l batimastat In the cell media significantly Increased cells’ ability to up-regulate KLF2 mRNA In response to 24 hours of laminar shear stress (LSS). Batimastat did not affect KLF2 expression under static conditions (first column and dotted line, comparison with static controls) (n = 3) (analysis of variance with Tukey correction). (b) Measurement of actin alignment relative to the direction of applied LSS. Actin alignment was not altered after 24 hours LSS in CiGEnCs exposed to salt and aldosterone for 5 days. Control cells, and cells exposed to salt and aldosterone in the presence of batimastat, showed significant actin alignment in response to shear with no detectable difference in the response (n = 3) (analysis of variance with Tukey correction). (c) CiGEnCs with nuclear staining (4′,6-diamidino-2-phenylindole), and actin staining (Alexa Fluor 568 phaloidin) highlighting actin cytoskeleton alignment. This process is visibly impaired in cells previously exposed to salt and aldosterone for 5 days, but was restored in the presence of batimastat (arrows indicate direction of flow). Shear row: the checkmark indicates 10 dyn/cm2 shear stress for 24 hours; the X indicates static conditions in the same incubator. Salt + aldosterone row: the checkmark indicates 0.1 nmol/l aldosterone was added to media containing 145 mmol NaCl; the X indicates vehicle alone was added to media with mannitol to balance osmolarity. Batimastat row: the checkmark indicates 5 μmol/l batimastat was added to media; the X indicates vehicle only was added to media (***p < 0.005, ****P < 0.001). To optimize viewing of this image, please see the online version of this article at www.kidney-international.org.
Mice given salt and aldosterone developed significant albuminuria and glomerular inflammation in the absence of detectable hypertension or other detectable alterations to the glomerular filtration barrier
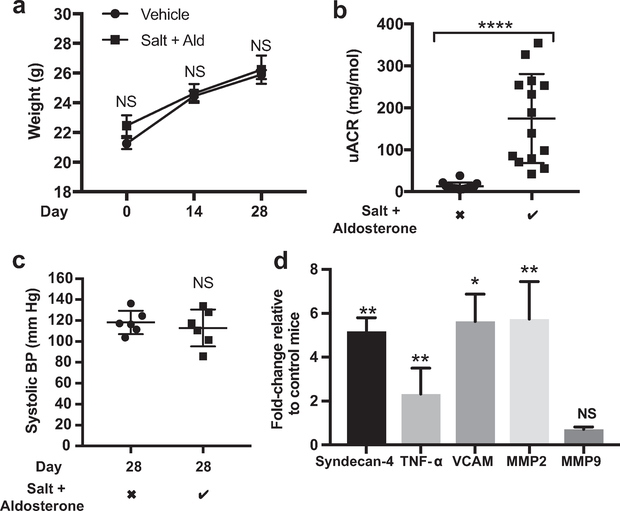
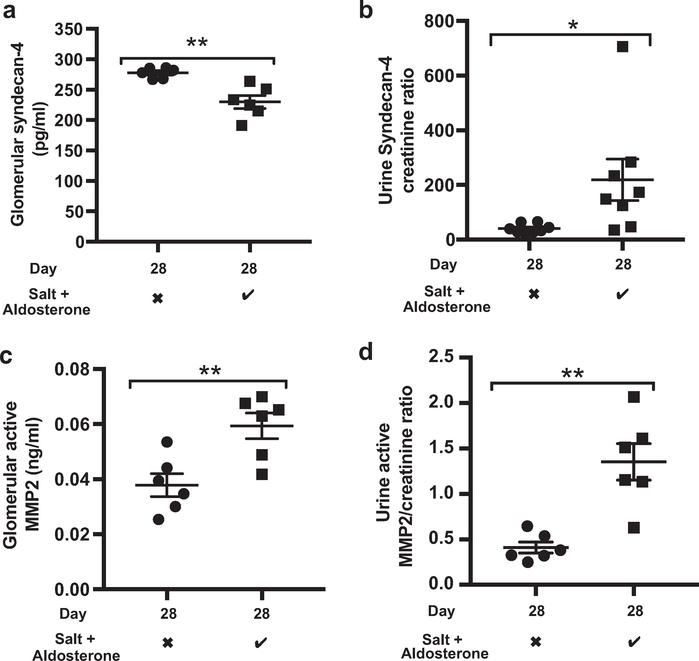
Mice receiving 1% NaCl drinking water and 0.6 μg/g/d aldosterone delivered subcutaneously via minipump continued to gain weight at a normal rate (Figure 4a). However, after 28 days of salt and aldosterone, adult male DBA2J mice developed significant albuminuria (Figure 4b). The systolic blood pressure of awake, trained mice did not increase over this period (Figure 4c). The glomerular expression of syndecan-4, tumor necrosis factor-α, vascular cell adhesion molecule-1, and MMP2 mRNA all increased significantly relative to controls by day 28, suggesting glomerular inflammation (Figure 4d). A syndecan-4 ectodomain enzyme-linked immunosorbent assay confirmed that glomerular syndecan-4 expression was reduced despite the increased mRNA expression (Figure 5a). These findings are consistent with an increased rate of shedding of syndecan-4 from the glomerular glycocalyx. Syndecan-4 levels did not increase measurably in the plasma, however, increased ectodomain concentrations in the urine were detected (Figure 5b). Syndecan-4 ectodomains have been shown to act as signaling molecules, altering podocyte mobilization via transient receptor potential cation 6 channels,37 whether this observation represents direct endothelial-podocyte communication warrants further investigation. Consistent with the glomerular mRNA results, a MMP2 activity assay confirmed an increased level of activated MMP2 within the glomeruli and urine of mice exposed to salt and aldosterone (Figure 5c and d). We also found that glomerular and urine heparanase activity increased in response to salt and aldosterone exposure (Supplementary Figure S3A and B). However, CiGEnCs exposed to salt and aldosterone in vitro did not increase heparanase activity, and batimastat had no effect on heparanase activity (Supplementary Figure S3C). It seemed unlikely therefore that the effects on the glycocalyx seen in response to salt and aldosterone were the result of increased heparanase activity in this model. In addition, after blinded analysis of electron micrographs, we found no evidence of damage to the glomerular basement membrane or podocyte foot process effacement. These findings suggest that damage may be limited to the glycocalyx at this early (28 days) time point (Supplementary Figure S4), however, other undetectable damage to the glomerular filtration barrier cannot be excluded. Images suggested that glycocalyx depth may have been reduced after salt and aldosterone exposure, but this was not quantified owing to inconsistent staining in this group of animals.
Figure 4 |. Male DBA2J mice given salt and aldosterone (Ald) developed significant albuminuria and glomerular inflammation in the absence of detectable systolic hypertension.
(a) The weight of mice receiving salt and aldosterone remained equivalent to control mice (analysis of variance, time-matched comparison; P = 0.232) (b) Mice given 28 days of salt and aldosterone developed significant albuminuria (t-test; P < 0.0001). (c) Mice were trained for 5 days before daily blood pressure (BP) measurements were taken on days 24 to 28, salt and aldosterone did not increase systolic BPs measured using the tail-cuff method (t test; P = 0.55; n = 6). (d) Key glomerular mRNA changes occurred after 28 days of exposure to salt and aldosterone with significant up-regulation of matrix metalloproteinase (MMP)2, vascular cell adhesion molecule (VCAM), tumor necrosis factor-α (TNF- α), and syndecan-4. Fold-change relative to control mice; t test shown; n = 8). Salt + aldosterone row: the checkmark indicates 1% NaCl in drinking water (ad libitum) and 0.6 μg/g/d aldosterone delivered subcutaneously via minipump; the X indicates standard drinking water (ad libitum) and a vehicle (ethanol saline)-filled minipump (*P < 0.05; **P < 0.01; ****P < 0.001).
Figure 5 |. Salt and aldosterone result in loss of glomerular syndecan-4 and an increase in glomerular matrix metalloproteinase (MMP)2 activity.
(a) Glomerular syndecan-4, measured by enzyme-linked Immunosorbent assay, decreased significantly after 28 days of salt and aldosterone (t test; P = 0.0016; n = 6). (b) Urine syndecan-4 creatinine ratios Increased significantly (t test; P = 0.0339; n = 8). (c) Active glomerular MMP2, measured by activity assay, increased after 28 days of salt and aldosterone (t test; P = 0.0061; N = 6). (d) Urine active MMP2/creatinine ratios increased after salt and aldosterone (t test; P = 0.0012; n = 6). Salt and aldosterone: the checkmark indicates 1% NaCl in drinking water (ad libitum) and 0.6 μg/g/d aldosterone delivered subcutaneously via minipump; the X indicates standard drinking water (ad libitum) and a vehicle (ethanol saline)-filled minipump (*P < 0.05; **P < 0.01).
Multiphoton microscopy can be used to directly measure changes in glomerular albumin leakage over time
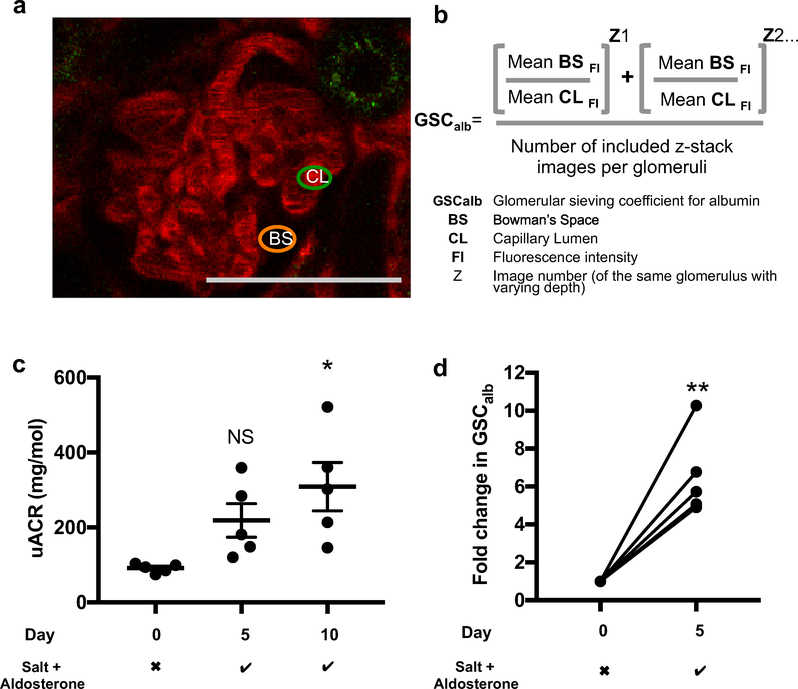
To confirm that detected albuminuria was owing to increased glomerular albumin leakage (and not alterations in tubular albumin uptake), multiphoton microscopy was used. Mice were lightly anesthetized before Z-stack images of the same glomeruli were acquired on day 0, day 5, and day 10 (when applicable). The glomerular sieving coefficient for albumin (GSCalb) was calculated as the ratio of Alexa 594 albumin signal intensity within the glomerular capillaries to that in Bowman’s space (Figure 6a and b). Young DBA2J mice developed significant albuminuria by day 10 of salt and aldosterone exposure (Figure 6c). The GSCalb was increased significantly by day 5 (Figure 6d). These effects were not due to alterations in systolic blood pressure under anesthesia (Supplementary Figure S5). Glomerular depth is known to increase in mice with age and vary between strains.38 We found that DBA2J mice were not ideal for multiphoton glomerular imaging because of the scarcity of superficial glomeruli. A total of 20 male DBA2J mice were imaged but only 5 mice had sufficiently superficial glomeruli on days 0 and 5 (and only 1 mouse on day 10, data not shown) to be included in the study. For this reason, we used C57 BL/6 mice for subsequent experiments.
Figure 6 |. Multiphoton microscopy can be used to measure changes in glomerular albumin leakage.
(a) Simultaneous measurement of Alexa 594 albumin florescence intensity in the Bowman’s space (BS) and within plasma-filled glomerular capillary loops (CL) allowed calculation of the glomerular sieving coefficient for albumin (GSCalb). Multiphoton images were selected for analysis provided the BS could be clearly defined and 2 sites with no capillary loops within 4.5 μm (X, Y, and Z plane) could be sampled, minimizing background florescence. The same glomeruli were imaged on day 0 and day 5, allowing calculation of the fold-change in GSCalb in each glomerulus before calculating the mean for each mouse. Bar = 50 μm. (b) The formula used to calculate the GSCalb for each glomerulus. Fluorescence intensity (FI) was measured at 2 points within the BS and at 3 points within the capillary lumens on each image, 4 images were analyzed for each glomerulus. The final GSCalb for each mouse was taken as the average result from all included glomeruli. (c) Young DBA2J mice developed significant albuminuria (elevated urine albumin creatinine ratio [uACR]) after 10 days of salt and aldosterone exposure (analysis of variance; P = 0.176; Tukey correction shown; n = 5). (d) Fold-change in the glomerular albumin sieving coefficients for young DBA2J mice after 5 days of salt and aldosterone (Mann-Whitney; P = 0.0079; n = 5). Day 10 measurements were attempted, but glomeruli were too deep for reliable assessment. Salt + aldosterone row: the checkmark indicates 1% NaCl in drinking water (ad libitum) and 0.6 μg/g/d aldosterone delivered subcutaneously via a minipump; the X indicates standard drinking water (ad libitum) and a vehicle (ethanol saline)-filled minipump (*P < 0.05; **P < 0.01). To optimize viewing of this image, please see the online version of this article at www.kidney-international.org.
Salt and aldosterone caused albuminuria in C57 BL/6 mice with an associated increase in glomerular albumin leakage and glomerular glycocalyx damage caused by MMPs
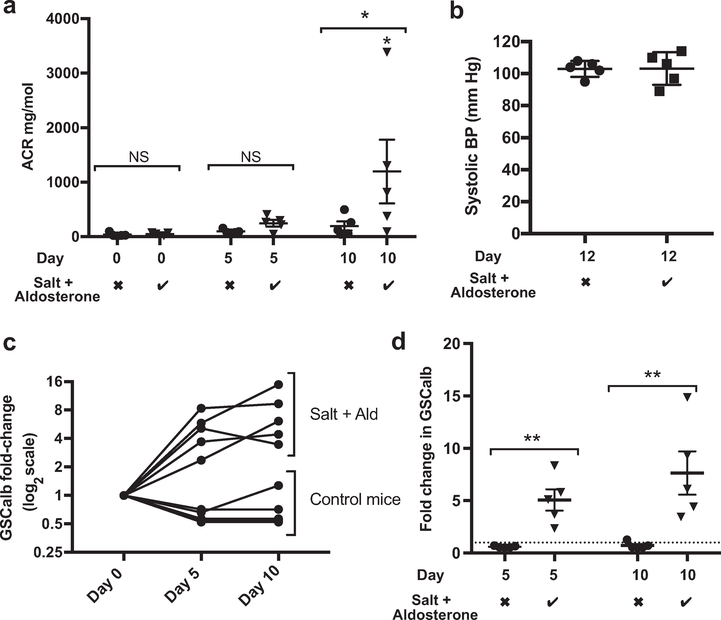
After 10 days of exposure to 1% NaCl (drinking water) and 0.6 μg/g/d aldosterone (via subcutaneous minipump), the urinary albumin creatinine ratio (uACR) had increased significantly (Figure 7a). No detectable systolic blood pressure change had occurred by day 12 of salt and aldosterone exposure (Figure 7b). Figure 7c shows the changes in recorded GSCalb for each individual mouse with time on a logarithmic scale. Figure 7d presents the same data with statistical comparisons highlighting the significantly increased GSCalb at days 5 and 10.
Figure 7 |. Salt and aldosterone exposure resulted in albuminuria and increased glomerular albumin leakage with associated glycocalyx damage in young C57 BL/6 mice.
(a) Young C57 BL/6 mice developed significant albuminuria after 10 days of exposure to salt and aldosterone (Ald) compared with controls and baseline values (analysis of variance; P = 0.0191; Sidak comparison used for matched time points and shown). (b) Male C57 BL6 mice receiving salt and aldosterone do not develop significant systolic hypertension by day 12 (measured via tail-cuff plethysmography in awake trained mice) (t test; P = 0.9694; n = 5). (c) Glomerular sieving coefficient for albumin (GSCalb) values for control mice are not altered significantly (nonparametric Friedman test of paired data with repeated measures; P = 0.0864), although there was a trend for GSCalb to decrease between day 0 and day 5. GSCalb values for mice receiving salt and aldosterone increased significantly (nonparametric Friedman test of paired data with repeated measures; P = 0.0085; n = 5). (b) Time-match comparisons between control mice and those receiving salt and aldosterone confirmed significant increases in GSCalb (Mann-Whitney day 5; P = 0.0079; day 10; P = 0.0079; n = 5). Salt + aldosterone row: the checkmark indicates 1% NaCl in drinking water (ad libitum) and 0.6 μg/g/d aldosterone delivered subcutaneously via a minipump; the X indicates standard drinking water (ad libitum) and a vehicle (ethanol saline)-filled minipump. BP, blood pressure (*P < 0.05; **P < 0.01).
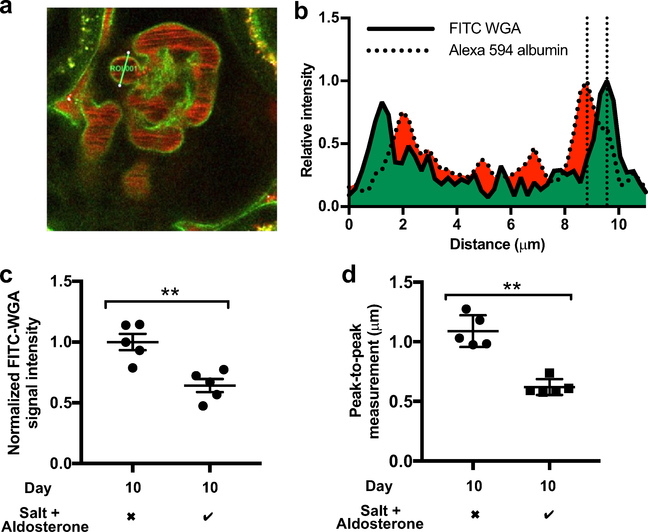
Wheat germ agglutinin (WGA) binds to the glomerular endothelial glycocalyx in rats.39 WGA also has been shown to bind to the glycocalyx in systemic vessels in mice.40 Consistent with these findings, we found that i.v. administered fluorescein isothiocyanate (FITC)-WGA bound to the endothelial glycocalyx within mouse glomeruli (Figure 8a). We developed 2 methods to quantify changes to the glycocalyx using multiphoton images. First, the fluorescence intensity of FITC-WGA bound to GEnC glycocalyx was taken to represent the number of available binding sites for WGA and therefore the amount of glycocalyx.40,41 Second, measurement of the anatomic distance between peak FITC-WGA fluorescence and peak plasma albumin intensity (peak-to-peak measurement), representing the restriction of albumin’s access to the endothelial cell membrane by the glycocalyx, provided an index of glycocalyx thickness (Figure 8b). Significant reductions in the glomerular WGA signal intensity and peak-to-peak measurement were evident by day 10 of salt and aldosterone exposure (Figure 8c and d).
Figure 8 |. Multiphoton microscopy confirmed that the glomerular endothelial glycocalyx is damaged after 10 days of salt and aldosterone exposure.
(a) A multiphoton image of a fluorescein isothiocyanate (FITC)-wheat germ agglutinin (WGA)-perfused control glomerulus on day 10 (after glomerular sieving coefficient for albumin [GSCalb] images had been taken) (region of interest = 11 μm). Glycocalyx measurement relied on the selection of capillary segments where a clear plasma peak signal could be seen (free from circulating erythrocytes). Only cross-sectioned (round) capillary profiles, and the level at one-half the capillary depth (deduced from the Z-stack) were analyzed to ensure the glycocalyx was measured perpendicular to the endothelial membrane. (b) Plasma Alexa 594 albumin concentrations are maximal toward the periphery of glomerular capillaries (Fahraeus-Lindqvist effect). The plasma concentration of labeled albumin decreases as it meets the glycocalyx (the first component of the glomerular filtration barrier). The distance between the maximal FITC-WGA glycocalyx signal and the adjacent peak Alexa 594 plasma albumin signal (indicated by the 2 vertical dotted lines) therefore can be used as an index of glycocalyx depth. (c) The intensity of the glomerular capillary FITC-WGA signal was assessed using standardized settings and normalized to adjacent tubular autofluorescence. Ten days of exposure to salt and aldosterone reduced glomerular glycocalyx florescence intensity (Mann-Whitney; P = 0.0079; n = 5). (d) Peak-to-peak assessment of the glomerular endothelial glycocalyx on day 10 showed a significant reduction in glycocalyx thickness in mice exposed to salt and aldosterone (t test; P = 0.0013; n = 5) (**P < 0.01). To optimize viewing of this image, please see the online version of this article at www.kidney-international.org.
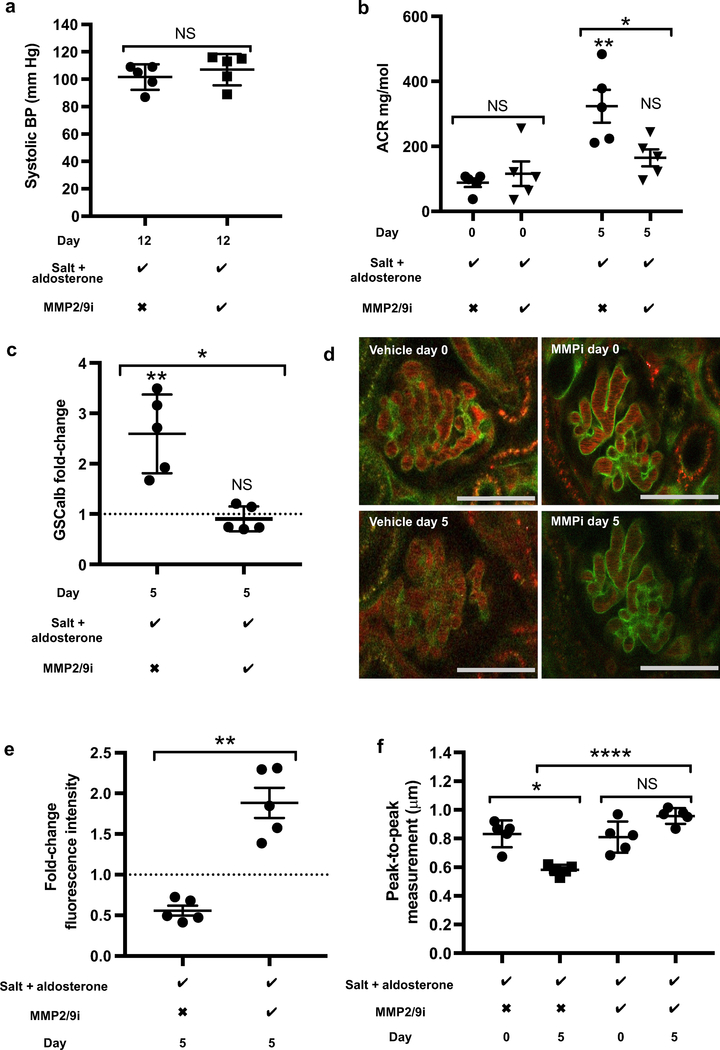
Following earlier work that highlighted increased glomerular MMP2 mRNA and activity after 28 days of salt and aldosterone exposure, we investigated if MMP2 blockade could prevent glycocalyx damage in vivo. Again, male C57 BL/ 6 mice were given salt and aldosterone but with either MMP2/9 inhibitor or vehicle (dimethylsulfoxide saline) administered daily via i.p. injection. Mice were injected with FITC-WGA to image the glycocalyx on day 0 and day 5, allowing us to study changes in the glomerular endothelial glycocalyx from baseline (in the same glomeruli) as well as between groups. This shortened protocol ensured images were taken while glomeruli were very superficial to maximize the resolution of generated images. The MMP2/9 inhibitor did not measurably alter the systolic blood pressure of mice by day 12 in mice receiving salt and aldosterone (Figure 9a). However, the MMP2/9 inhibitor successfully prevented significant increases in the uACR and GSCalb from occurring (Figure 9b and c). Significant glycocalyx damage was visible in vehicle-treated mice by day 5 (relative to baseline and relative to the MMP2/9-treated group) (Figure 9d). The MMP2/9 inhibitor prevented any detectable decrease in glomerular capillary WGA intensity and peak-to-peak measurement (Figure 9e and f).
Figure 9 |. Matrix Metaloprotease 2 and 9 (MMP2/9) inhibitor prevented the effects of salt and aldosterone in vivo, without affecting animals’ blood pressure.
(a) MMP2/9 inhibitor did not alter the systolic blood pressure (BP) of male C57 BL6 mice receiving salt and aldosterone measured on day 12 using tail-cuff plethysmography (t test; P = 0.435; n = 5). (b) Urine albumin creatinine ratio (uACR) values increased significantly in mice receiving salt, aldosterone, and vehicle by day 5. No significant change was detected in mice receiving salt, aldosterone, and MMP2/9 inhibitor. Comparison between the groups on day 0 was not significant, but comparison on day 5 confirmed that mice receiving salt, aldosterone, and MMP2/9 inhibitor had significantly lower uACR levels (analysis of variance; P = 0.0010; Tukey analysis shown; n = 5). (c) Mice receiving salt, aldosterone, and MMP2/9 inhibitor had significantly lower glomerular sieving coefficient for albumin (GSCalb) fold-change values by day 5 compared with mice receiving salt, aldosterone, and vehicle (Mann-Whitney; P = 0.0079). (d) Representative images of the same glomeruli on day 0 and day 5 with FITC-wheat germ agglutinin (WGA)-labeled glomerular glycocalyx (green) and circulating Alexa 594 albumin-labeled plasma. (Bar = 50 μm) (e) Quantitative analysis of FITC-WGA florescence intensity confirmed MMP2/9 inhibition preserved the endothelial glycocalyx (Mann-Whitney; P = 0.0079; n = 5). (f) Peak-to-peak measurement of glomerular endothelial glycocalyx depth showed a significant thinning of glycocalyx during the first 5 days of exposure to salt and aldosterone. In the presence of MMP2/9 inhibitor no alteration was seen (analysis of variance; P < 0.0001; Tukey correction shown; n = 5). Salt + aldosterone row: the checkmark indicates 1% NaCl in drinking water (ad libitum) and 0.6 μg/g/d aldosterone delivered subcutaneously via minipump; the X indicates standard drinking water (ad libitum) and a vehicle (ethanol saline)-filled minipump. MMP2/9i row: the checkmark indicates 5 mg/kg i.p. injection once daily; the X indicates daily vehicle (dimethylsulfoxide saline) (*P < 0.05; **P < 0.01, ****P < 0.001). To optimize viewing of this image, please see the online version of this article at www.kidney-international.org.
DISCUSSION
We have shown that up-regulation of MMPs in response to salt and aldosterone resulted in GEnC glycocalyx damage and a detectable impairment of glycocalyx function both in vitro and in vivo. To achieve this we used cutting-edge techniques to simultaneously study the glomerular endothelial glycocalyx and glomerular albumin permeability in live perfused mice. In addition, we tracked how the GSCalb in individual glomeruli changed with time and correlated these changes with albuminuria, providing unique insight into how glomerular and tubular albumin handling change within this model. Together these data suggest that direct glycocalyx protection could be a novel therapeutic strategy in patients unable to tolerate mineralocorticoid inhibition.
Glomerular endothelial cells expressed MR and responded to aldosterone. In vitro the combination of sodium chloride (145 mmol/l) and aldosterone (0.1 nmol/l) resulted in the loss of key glycocalyx components. Atomic force microscopy previously has shown that the glycocalyx on ex vivo human umbilical artery endothelial cells was reduced in height by 50% after salt and aldosterone exposure, closely matching the 53% reduction seen in glycocalyx depth on day 10 in the current study.42 The combination of increased salt intake and exogenous aldosterone has been noted previously to cause albuminuria before alterations in blood pressure occur.43–45 We saw no detectable changes in systolic blood pressure in either mouse strain, or when the matrix metaloprotease inhibitor was used, however, tail-cuff photoplethysmography cannot be used for 24-hour BP recording and so intermittent or nocturnal hypertension cannot be excluded. Our data are consistent with that generated by other groups using this model and with clinical studies suggesting that aldosterone can mediate damage via hypertension-independent mechanisms.8,45
The glycocalyx limits albumin permeability and acts as a mechanosensor.35,39,46,47 Enzymatic degradation of the glycocalyx in vitro impaired cells’ ability to remodel in response to physiological shear stress, suggesting that this function is glycocalyx-dependent in CiGEnC. Consistent with our findings murine syndecan-4 knock-down prevented endothelial cell alignment, suggesting that syndecan-4 may be a key glycocalyx mechanosensor.36 In addition to MMPs, heparanase exposure has been shown to result in syndecan-4 loss, this action is thought to be owing to increased MMP access to the syndecan-4 cleavage site.48 Interestingly, glomerular heparanase activity did increase in this model, but we found no evidence that it was produced from glomerular endothelial cells. Podocytes have been shown to produce heparanase in response to aldosterone, so it is possible that other cell types within the glomerulus were responsible for this increase.49 The possibility that remotely produced heparanase contributed to the loss of HS and syndecan-4 from the glycocalyx in this model warrants further investigation, but the effectiveness of MMP inhibition in this model suggests that heparanase had a limited direct effect. Syndecan-4 levels dramatically increased in the urine, while decreasing in the glomeruli. We could not detect a significant increase in plasma levels but this may have been confounded by the increased urinary protein leakage seen after 4 weeks of salt and aldosterone. With the increased glomerular syndecan-4 mRNA level seen in these mice, and based on our previous studies in vitro,33 we believe it is likely that syndecan-4 is shed from the glycocalyx in this model and largely excreted in the urine, however, rapid metabolism of the ectodomain in the plasma cannot be excluded.
The key mechanism of damage in this model appears to be MMP induction. Salt and aldosterone have been shown to up-regulate MMP2 mRNA, and protein expression in murine hearts and renin-overexpressing rats have been shown to have increased glomerular levels of MMP2 and MMP9 mRNA suppression, consistent with our findings.50,51 We have shown that salt and aldosterone increased glomerular MMP2 activity in mice. MMP2 is a member of the gelatinase class of MMPs. Its substrates include syndecan-4 (a core protein for HS) and so the loss of HS seen in vitro could be explained by syndecan-4 loss.33,52 At the early time point studied we did not see evidence of damage to other components of the glomerular filtration barrier, suggesting the glycocalyx damage may be an early initiating step in the development of albuminuria in this model. MMP inhibition prevented detectable damage to the glycocalyx, reduced glomerular albumin leakage, and prevented significant increases in uACR. The use of FITC-WGA in mice with kidney imaging windows facilitated repeated measurements of glomerular glycocalyx depth and WGA binding site density to be made in the same glomeruli over time. When combined with serial GSCalb measurements in the same glomeruli these measurements provided a unique insight into how remodeling of the glomerular glycocalyx affected the glomerular filtration of albumin. Measuring GSCalb also allowed comparisons to be made with uACR results. By using these data it is possible to derive a measure of how tubular albumin uptake/degradation may have affected the uACR. Comparison of the changes in sieving coefficient between days 0 and 5 (5-fold) and days 5 and 10 (a further 1.4-fold) with the corresponding changes in uACR highlighted a possible role for early tubular albumin re-uptake/degradation in this model, a finding that warrants further investigation and validation.
In summary, we have shown that MMP inhibition prevented damage to the glomerular endothelial glycocalyx in response to increased salt and aldosterone. More broadly, we have shown that preserving the glycocalyx appears to limit glomerular albumin leakage. In patients in whom MR blockade is deemed unsafe, novel approaches to protect the glomerular endothelial glycocalyx may have exciting therapeutic potential.
METHODS
The CiGEnCs used in this study have been used extensively to study the glomerular endothelial glycocalyx.33,35,53 CiGEnC expresses fenestrations, which increase in response to vascular endothelial growth factor and endothelial surface proteins.54 At the permissive temperature of 33 °C, cells divide to form 80% confluent monolayers before terminally differentiating at 37 °C. All cells (passages 20–26) were cultured in EGM2 media (Lonza, Slough, Berkshire, UK) with the EGM2-MV bullet kit (Lonza) in the absence of supplied vascular endothelial growth factor and gentamicin. To avoid acute osmotic stress, all cells were swapped into media containing mannitol 5 days before experiments began. During the experimental phase, mannitol (to maintain osmolarity) was continued or NaCl (to make a final concentration of 145 mmol/l) was added to cell media ±0.1 nmol/l aldosterone (A9477; Sigma, Gillingham, Dorset, UK). When used, 0.1 μmol/l spironolactone (S3378; Sigma) or 5 μmol/l batimastat (SML0041; Sigma) was given 2 hours before, and during, salt and aldosterone exposure. Experiments typically lasted 5 days (with the exception of RNA harvest, which was conducted at 10 hours). When indicated, 10 dyn/cm2 shear stress was applied to cells cultured in 10-cm round dishes using an orbital shaker (SSM1; Stuart, Stone, Staffordshire, UK) for 24 hours.35 To image actin alignment after shear stress, cells were cultured on fibronectin-coated coverslips at the periphery of 10-cm tissue culture dishes. Shear stress was applied for 24 hours before cells were stained using phaloidin conjugate (1:200, 10135092; Invitrogen, Loughborough, Leicestershire, UK) diluted in 1% bovine serum albumin solution and imaged using a Leica DMI6000B microscope (Leica, Cambridge, Cambridgeshire, UK). All experiments were conducted using matched controls cultured simultaneously and maintained in the same incubators. A standard technique for Western blot was used with a MR antibody (ab62532; Abcam, Cambridge, Cambridgeshire, UK).35 To quantify glycocalyx components using immunofluorescence, cells were grown in 96-well plates or on fibronectin-coated coverslips (for representative images). After 5 days of experimental exposure, cells were incubated at room temperature with 4% paraformaldehyde before blocking with 5% bovine serum albumin. Primary antibodies (anti-heparan sulfate [1:100, 1698; Bio-Rad, Watford, Hertfordshire, UK] and anti-syndecan-4 antibody [1:100, ABT157; Millipore, Watford, Hertfordshire, UK]) were incubated at 4 °C for 12 hours. Secondary antibodies were applied for 1 hour at room temperature. Cells were imaged using an automated plate reader (96-well) (Dynex Opsys MR, Worthing, West Sussex, UK), or fixed on coverslips using Vectashield (cat. no. H-1000; Vectashield Laboratories, Peterborough, Cambridgshire, UK) and imaged manually (Leica DMI6000B, with Leica CTR7000) to confirm cell surface staining. A comparison was made with cells incubated with heparatinase III (1 mU/ml) (UK H-8891; Sigma) for 3 hours to confirm HS antibody specificity and IgG controls (Supplementary Figure S1).
RNA was extracted from lysed conditionally immortalized glomerular endothelial cells using the standard protocol supplied with the Qiagen RNeasy Kit (cat. no. 74104; Quiagen, Manchester, UK). To collect RNA from mice, sections of renal cortex were passed through sequential sieves to extract glomeruli before they were lysed in the supplied buffer. To aid cellular lysis the glomeruli were drawn repeatedly into a 1-ml syringe via sequentially smaller needles to break the glomeruli into single-cell fragments. After lysis, RNA was extracted using the Qiagen RNeasy Kit (cat. no. 74104). RNA concentrations were measured using a nanophotometer (Pearl Implen, München, Germany) and normalized before cDNA conversion. To convert RNA to cDNA a standard protocol was used with a highcapacity mRNA-to-cDNA kit (ref 4387406; Applied Biosystems, Foster City, California, USA). Once primer specificity and optimal concentrations had been established, a standard protocol was used for all quantitative polymerase chain reaction using the Fast Sybr Green master mix (ref 438612; Applied Biosystems) (sequences are listed in Supplementary Figure S6). All statistics and errors were calculated on delta cycle threshold values, all data are shown as fold-change + SEM. A human syndecan-4 enzyme-linked immunosorbent assay (SEB939Hu; USCN Life Science, Inc., Houston, Texas, USA) was used to quantify the syndecan-4 loss in cell culture. Mouse syndecan-4 levels were assessed using a mouse enzyme-linked immunosorbent assay kit (CSB-EL020891MO; Cusabio, Houston, Texas, USA). MMP2 activity was studied using the MMP-2 Biotrack Activity assay (RPN2631; GE Healthcare, Little Chalfont, Buckinghamshire, UK). Heparanase activity was studied using a heparanase degrading enzyme assay kit (MK412; Takara, Shiga, Japan). For all commercially available assays the manufacturer’s instructions were followed in full.
All animal protocols were approved by the UK Government Home Office or the University of Southern California animal care committee and conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Time lines of the experimental protocols used are included (Supplementary Figure S7). All mice were male and purchased from Jackson Laboratory (Sacramento, California, USA) (C57 BL/6 aged 2 weeks were purchased with nursing mothers, remaining mice were purchased aged 3 weeks). All mice had free access to food and water/saline throughout the study. To administer aldosterone 0.6 μg/g/d (A9477; Sigma) or vehicle (ethanol saline), Alzet minipumps (models 2004 or 1002; Alzet, Cupertino, California, USA) were implanted subcutaneously under isoflurane anesthesia according to the manufacturer’s guidelines. The dose selected has been shown previously to increase blood aldosterone levels in mice from 4.42 ± 0.83 nmol/l to 11.09 ± 2.07 nmol/l.45 One percent NaCl solution was given as the only source of drinking water to all mice receiving aldosterone. To confirm that MMP induction mediated the glycocalyx damage seen in vivo, MMP2/9 inhibitor (5 mg/kg) (444241; EMD Millipore, Temecula, California, USA) was dissolved in dimethylsulfoxide saline and given via daily i.p. injection and comparisons were made with vehicle-treated mice (dimethylsulfoxide saline). Blood pressure was recorded after 5 days of training using the Visitech BP2000 system (Apex, North Carolina, USA) and the protocols used are outlined in Supplementary Figure S7. Intravital multiphoton microscopy was performed using a Leica TCS SP5 multiphoton confocal fluorescence imaging system powered by a Chameleon Ultra-II MP laser (Coherent, Inc., Santa Clara, CA). Standardized settings were used to gain 12-bit, 1.5-μm Z-stack images of all superficial glomeruli. The imaging duration for glomerular permeability was limited to 30 minutes. Animals were anesthetized using isoflurane before 40 μl of Alexa Flour 594 bovine serum albumin (1812249; Invitrogen, Waltham, Massachusetts, USA) was injected into the retro-orbital sinus (prepared using Nanosep 30K omega spin columns (Sigma) to remove free dye). Z-stack images (1.5 μm) then were taken of all superficial glomeruli. Images were selected for blinded analysis provided the Bowman’s space could be clearly defined and there was sufficient area to analyze florescence at 2 sites with no capillary loops within 4.5 μm (3 frames in z-stack). Imaging the same glomeruli on day 0, day 5, and day 10 allowed us to focus on glomerular changes resulting from salt and aldosterone exposure (fold-change from baseline permeability for each glomerulus was calculated before averages were calculated for each mouse). FITC-WGA (2 μg/g, L4895 in phosphate-buffered saline; Sigma, St. Louis, Missouri, USA) was given as a 20-μl bolus via the retro-orbital sinus only after glomerular sieving coefficients had been calculated. Images were taken 10 minutes after the bolus, up to 40 minutes after the bolus. WGA staining intensity was quantified within the glomerular capillaries and normalized to adjacent proximal tubular autofluorescence to negate the effects of tissue depth/overlying light absorption. In addition, peak-to-peak analysis of the WGA-labeled glycocalyx was used to derive a direct measure of glycocalyx depth. Having established that the glomerular sieving coefficient rapidly increased (within 5 days) in response to salt and aldosterone exposure, the protocol was amended before testing the effectiveness of the MMP2/9 inhibitor. Amendments included baseline assessment of the glycocalyx using WGA, and a shortened exposure to salt and aldosterone to optimize image resolution for glycocalyx depth calculations (Supplementary Figure S7A and B, and Supplementary Figure S8A, B, and C).
All image analysis was conducted using Leica Application Suite. All statistics were calculated using Prism 7 (GraphPad, La Jolla, California, USA). Normality was assessed visually and using the Shapiro-Wilk test. Normally distributed data were compared using the t test and analysis of variance, where normality could not be assumed, and the Mann-Whitney, Wilcoxon, Kruskal-Wallis, and Friedman tests had been used. Significance was set at P < 0.05.
Supplementary Material
Figure S1. Immunofluorescence on nonpermeabilized conditionally immortalized human glomerular endothelial cells. The surface expression of syndecan-4 (A). The surface expression of heparan sulfate (HS) (B). Surface expression of HS after 3 hours of incubation with 1 mU/ml heparatinase III (taken with identical settings to panel B) (C). Digitally enhanced version of panel C, showing the presence of an endothelial cell with minimal cell surface staining, confirming the anti-HS antibody’s specificity (D). IgG control for syndecan-4 staining (E). IgG control for HS staining (F). Bar = 25 μm.
Figure S2. Validation of KLF2 response to shear stress. A low-dose enzyme cocktail previously shown to remove glycocalyx components55 was used to confirm that an intact glycocalyx is needed for optimal KLF2 up-regulation in response to shear (A). Removal of syndecan-4 was confirmed using cell surface immunofluorescence (t test P = 0.0073; N = 6 controls; N = 14 enzyme treated). Fold-change in KLF2 in response to shear stress normalized to matched controls confirmed that enzymatic damage to the glycocalyx was associated with a significant impairment in cells’ ability to up-regulate KLF2 in response to shear stress (paired t test; N = 7; P = 0.045) (B). Significance relative to control. All error bars = SEM. *P < 0.05; **P < 0.01; ***P < 0.005; ****P < 0.001. The checkmark indicates glycocalyx degrading enzymes with 2 hours of pretreatment and ongoing exposure during shear stress. Enzymes used were as follows: hyaluronidase (2.5 mg/ml), chondroitinase (1 mu/ml), and neuraminidase (1 mu/ml). X indicates enzyme used was vehicle only.
Figure S3. Heparanase activity increased in glomeruli and urine but not in conditionally immortalized human glomerular endothelial cells (CiGEnCs) after salt and aldosterone exposure. Urine heparanase activity (normalized to urine creatinine) increased after 28 days of salt and aldosterone (t test; P = 0.0012; n = 5) (A). Glomerular lysate heparanase activity increased after 28 days of salt and aldosterone (t test; P = 0.0059; n = 6) (B). Conditioned media from CiGEnCs exposed to salt and aldosterone for 5 days remained equivalent to control levels (C). Batimastat had no measurable effect on heparanase activity.
Figure S4. Analysis of the glomerular filtration barrier using electron microscopy confirmed that glomerular basement membrane (GBM) thickness and podocyte foot process width had not altered. Representative electron micrograph of the filtration barrier of a control mouse. The white arrow indicates the glycocalyx labeled with Alcian Blue. Bar = 500 nmol/l (A). Representative electron micrograph of the filtration barrier of a mouse exposed to 28 days of salt and aldosterone. The white arrow indicates the glycocalyx labeled with Alcian Blue. Bar = 500 nmol/l (B). Glomeruli from 4 control mice and 3 mice exposed to salt and aldosterone were analyzed (total, 20 glomeruli; 60 capillary loops). No significant difference in GBM thickness was detected (t test P = 0.72; N = 4+3) (C). Podocyte foot process width did not increase significantly (t test P = 0.31) (D). Fenestration density did not alter significantly (t test P = 0.7) (E). Checkmark indicates salt + aldosterone: 1% NaCl in drinking water (ad libitum) and 0.6 μg/g/d aldosterone delivered subcutaneously via minipump. X indicates salt and aldosterone in standard drinking water (ad libitum) and vehicle (ethanol saline)-filled minipump. Ald, aldosterone.
Figure S5. Systolic BP under anesthesia. There were no significant differences in mouse (DBA2J) blood pressures from basal values or between groups under isoflurane anesthesia (equivalent to that used during multiphoton microscopy) (analysis of variance, NS).
Figure S6. Quantitative polymerase chain reaction primer sequences. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; KLF2, Krupple-like factor 2; MMP, matrix metalloproteinase; TNF-α, tumor necrosis factor-α; VCAM, vascular cell adhesion molecule.
Figure S7. Blood pressure (BP) training consisted of 5 days of daily handling and mock blood pressure measurement (5 inflation cycles repeated twice on days 1, 2, and 3 before increasing to 3 repeats on days 4 and 5). For the final BP measurement, 5 inflation cycles were used to acclimatize the mice, before 10 inflation cycles were used to determine the BP for each mouse. For inclusion, the computer had to accept 6 of the 10 readings (no systolic time out). A single day of recording was used for C57 BL6 mice on day 12 (because of the smaller minipump size used multiple recordings were not possible), whereas the mean value after 4 sequential days of recording (days 24–27) was used in DBA2J mice. Having established that the glomerular sieving coefficient rapidly increased (within 5 days) in response to salt and aldosterone exposure, the protocol initially used (Supplementary Figure S7B) was amended (Supplementary Figure S7C) before testing the effectiveness of the MMP2/9 inhibitor. Amendments included baseline assessment of the glycocalyx using WGA and a shortened exposure to salt and aldosterone to optimize image resolution. FITC, fluorescein isothiocyanate; PFA, paraformaldehyde.
Figure S8. Example normalized line profiles and the images from which they were taken. Images were taken after the perfusion of Alexa 594 albumin (red) and fluorescein isothiocyanate (FITC)-WGA (green). Line profiles have been generated by measuring the florescence intensity along the regions of interest represented by the yellow lines. The length of each regions of interest is given as the x-axis on the corresponding graphs. Peak-to-peak measurements provide an index of glycocalyx thickness. Salt and aldosterone exposed glomerulus day 5 (this image has been digitally enhanced to aid visualization of the glycocalyx for illustration purposes) (A). Control glomerulus on day 10 (B). MMP2/9 inhibitor-treated glomerulus on day 5 (C).
ACKNOWLEDGMENTS
We would like to thank the kidney donors and their families for consenting to research on donated organs that were not suitable for transplantation.
This work was supported by Medical Research Council Clinical Research Training Fellowship grant MR/M018237/1 (M.J.B.) and Kidney Research UK grants S/RP/2015/10 (J.K.F.) and ID_004_20170330 (A.S.O.).
Footnotes
DISCLOSURE
All the authors declared no competing interests.
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at www.kidney-international.org.
REFERENCES
- 1.Kovesdy CP, Furth S, Zoccali C. Obesity and kidney disease: hidden consequences of the epidemic. Rev Med Chil. 2017;145:281–291. [DOI] [PubMed] [Google Scholar]
- 2.Lamb EJ, Levey AS, Stevens PE. The Kidney Disease Improving Global Outcomes (KDIGO) guideline update for chronic kidney disease: evolution not revolution. Clin Chem. 2013;59:462–465. [DOI] [PubMed] [Google Scholar]
- 3.Shibata H, Itoh H. Mineralocorticoid receptor-associated hypertension and its organ damage: clinical relevance for resistant hypertension. Am J Hypertens. 2012;25:514–523. [DOI] [PubMed] [Google Scholar]
- 4.Bomback AS, Klemmer PJ. The incidence and implications of aldosterone breakthrough. Nat Clin Pract Nephrol. 2007;3:486–492. [DOI] [PubMed] [Google Scholar]
- 5.Bomback AS, Kshirsagar AV, Ferris ME, et al. Disordered aldosteronevolume relationship in end-stage kidney disease. J Renin Angiotensin Aldosterone Syst. 2009;10:230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox CS, Gona P, Larson MG, et al. A multi-marker approach to predict incident CKD and microalbuminuria. J Am Soc Nephrol. 2010;21:2143–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sechi LA, Novello M, Lapenna R, et al. Long-term renal outcomes in patients with primary aldosteronism. JAMA. 2006;295:2638–2645. [DOI] [PubMed] [Google Scholar]
- 8.Liu G, Yin GS, Tang JY, et al. Endothelial dysfunction in patients with primary aldosteronism: a biomarker of target organ damage. J Hum Hypertens. 2014;28:711–715. [DOI] [PubMed] [Google Scholar]
- 9.Rikimaru T, Fujita Y, Okuda T, et al. Responses of sodium balance, blood pressure, and other variables to sodium loading in Papua New Guinea highlanders. Am J Clin Nutr. 1988;47:502–508. [DOI] [PubMed] [Google Scholar]
- 10.Funder JW. Aldosterone and mineralocorticoid receptors-physiology and pathophysiology. Int J Mol Sci. 2017;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolignano D, Palmer SC, Navaneethan SD, et al. Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev. 2014;4:CD007004. [DOI] [PubMed] [Google Scholar]
- 12.Schjoedt KJ. The renin-angiotensin-aldosterone system and its blockade in diabetic nephropathy: main focus on the role of aldosterone. Dan Med Bull. 2011;58:B4265. [PubMed] [Google Scholar]
- 13.Hill NR, Lasserson D, Thompson B, et al. Benefits of Aldosterone Receptor Antagonism in Chronic Kidney Disease (BARACK D) trial-a multi-centre, prospective, randomised, open, blinded end-point, 36-month study of 2, 616 patients within primary care with stage 3b chronic kidney disease to compare the efficacy of spironolactone 25 mg once daily in addition to routine care on mortality and cardiovascular outcomes versus routine care alone: study protocol for a randomized controlled trial. Trials. 2014; 15:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pitt B, Rossignol P. Mineralocorticoid receptor antagonists in patients with end-stage renal disease on chronic hemodialysis. J Am Coll Cardiol. 2014;63:537–538. [DOI] [PubMed] [Google Scholar]
- 15.Ng KP, Jain P, Gill PS, et al. Results and lessons from the Spironolactone To Prevent Cardiovascular Events in Early Stage Chronic Kidney Disease (STOP-CKD) randomised controlled trial. BMJ Open. 2016;6:e010519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Currie G, Taylor AH, Fujita T, et al. Effect of mineralocorticoid receptor antagonists on proteinuria and progression of chronic kidney disease: a systematic review and meta-analysis. BMC Nephrol. 2016;17:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weber KT. Aldosteronism revisited: perspectives on less well-recognized actions of aldosterone. J Lab Clin Med. 2003;142:71–82. [DOI] [PubMed] [Google Scholar]
- 18.McGraw AP, McCurley A, Preston IR, et al. Mineralocorticoid receptors in vascular disease: connecting molecular pathways to clinical implications. Curr Atheroscler Rep. 2013;15:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galmiche G, Pizard A, Gueret A, et al. Smooth muscle cell mineralocorticoid receptors are mandatory for aldosterone-salt to induce vascular stiffness. Hypertension. 2014;63:520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koenig JB, Jaffe IZ. Direct role for smooth muscle cell mineralocorticoid receptors in vascular remodeling: novel mechanisms and clinical implications. Curr Hypertens Rep. 2014;16:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tarjus A, Belozertseva E, Louis H, et al. Role of smooth muscle cell mineralocorticoid receptor in vascular tone. Pflugers Arch. 2015;467:1643–1650. [DOI] [PubMed] [Google Scholar]
- 22.Chadwick JA, Hauck JS, Lowe J, et al. Mineralocorticoid receptors are present in skeletal muscle and represent a potential therapeutic target. FASEB J. 2015;29:4544–4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilet A, Zou F, Boumenir M, et al. Aldosterone up-regulates MMP-9 and MMP-9/NGAL expression in human neutrophils through p38, ERK½ and PI3K pathways. Exp Cell Res. 2015;331:152–163. [DOI] [PubMed] [Google Scholar]
- 24.Bienvenu LA, Reichelt ME, Delbridge LM, et al. Mineralocorticoid receptors and the heart, multiple cell types and multiple mechanisms: a focus on the cardiomyocyte. Clin Sci (Lond). 2013;125:409–421. [DOI] [PubMed] [Google Scholar]
- 25.Armani A, Marzolla V, Fabbri A, et al. Cellular mechanisms of MR regulation of adipose tissue physiology and pathophysiology. J Mol Endocrinol. 2015;55:R1–R10. [DOI] [PubMed] [Google Scholar]
- 26.Jia G, Habibi J, DeMarco VG, et al. Endothelial mineralocorticoid receptor deletion prevents diet-induced cardiac diastolic dysfunction in females. Hypertension. 2015;66:1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen Dinh Cat A, Griol-Charhbili V, Loufrani L, et al. The endothelial mineralocorticoid receptor regulates vasoconstrictor tone and blood pressure. FASEB J. 2010;24:2454–2463. [DOI] [PubMed] [Google Scholar]
- 28.Lang F Stiff endothelial cell syndrome in vascular inflammation and mineralocorticoid excess. Hypertension. 2011;57:146–147. [DOI] [PubMed] [Google Scholar]
- 29.Rickard AJ, Morgan J, Chrissobolis S, et al. Endothelial cell mineralocorticoid receptors regulate deoxycorticosterone/salt-mediated cardiac remodeling and vascular reactivity but not blood pressure. Hypertension. 2014;63:1033–1040. [DOI] [PubMed] [Google Scholar]
- 30.Sekizawa N, Yoshimoto T, Hayakawa E, et al. Transcriptome analysis of aldosterone-regulated genes in human vascular endothelial cell lines stably expressing mineralocorticoid receptor. Mol Cell Endocrinol. 2011;341:78–88. [DOI] [PubMed] [Google Scholar]
- 31.Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Ann Rev Biomed Eng. 2007;9:121–167. [DOI] [PubMed] [Google Scholar]
- 32.Cheng Z, Limbu MH, Wang Z, et al. MMP-2 and 9 in chronic kidney disease. Int J Mol Sci. 2017;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramnath R, Foster RR, Qiu Y, et al. Matrix metalloproteinase 9-mediated shedding of syndecan 4 in response to tumor necrosis factor alpha: a contributor to endothelial cell glycocalyx dysfunction. FASEB J. 2014;28: 4686–4699. [DOI] [PubMed] [Google Scholar]
- 34.Satchell SC. The glomerular endothelium emerges as a key player in diabetic nephropathy. Kidney Int. 2012;82:949–951. [DOI] [PubMed] [Google Scholar]
- 35.Slater SC, Ramnath RD, Uttridge K, et al. Chronic exposure to laminar shear stress induces Kruppel-like factor 2 in glomerular endothelial cells and modulates interactions with co-cultured podocytes. Int J Biochem Cell Biol. 2012;44:1482–1490. [DOI] [PubMed] [Google Scholar]
- 36.Baeyens N, Mulligan-Kehoe MJ, Corti F, et al. Syndecan 4 is required for endothelial alignment in flow and atheroprotective signaling. Proc Natl Acad Sci U S A. 2014;111:17308–17313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim EY, Roshanravan H, Dryer SE. Syndecan-4 ectodomain evokes mobilization of podocyte TRPC6 channels and their associated pathways: an essential role for integrin signaling. Biochim Biophys Acta. 2015;1853: 2610–2620. [DOI] [PubMed] [Google Scholar]
- 38.Schiessl IM, Bardehle S, Castrop H. Superficial nephrons in BALB/c and C57BL/6 mice facilitate in vivo multiphoton microscopy of the kidney. PLoS One. 2013;8:e52499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salmon AH, Ferguson JK, Burford JL, et al. Loss of the endothelial glycocalyx links albuminuria and vascular dysfunction. J Am Soc Nephrol. 2012;23:1339–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kataoka H, Ushiyama A, Kawakami H, et al. Fluorescent imaging of endothelial glycocalyx layer with wheat germ agglutinin using intravital microscopy. Microsc Res Tech. 2016;79:31–37. [DOI] [PubMed] [Google Scholar]
- 41.Fukui M, Yamada M, Akune Y, et al. Fluorophotometric analysis of the ocular surface glycocalyx in soft contact lens wearers. Curr Eye Res. 2016;41:9–14. [DOI] [PubMed] [Google Scholar]
- 42.Oberleithner H, Peters W, Kusche-Vihrog K, et al. Salt overload damages the glycocalyx sodium barrier of vascular endothelium. Pflugers Arch. 2011;462:519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nugrahaningsih DA, Emoto N, Vignon-Zellweger N, et al. Chronic hyperaldosteronism in cryptochrome-null mice induces high-salt- and blood pressure-independent kidney damage in mice. Hypertens Res. 2014;37:202–209. [DOI] [PubMed] [Google Scholar]
- 44.Ogawa Y, Mukoyama M, Yokoi H, et al. Natriuretic peptide receptor guanylyl cyclase-A protects podocytes from aldosterone-induced glomerular injury. J Am Soc Nephrol. 2012;23:1198–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schreier B, Rabe S, Schneider B, et al. Aldosterone/NaCl-induced renal and cardiac fibrosis is modulated by TGF-beta responsiveness of T cells. Hypertens Res. 2011;34:623–629. [DOI] [PubMed] [Google Scholar]
- 46.Altshuler AE, Morgan MJ, Chien S, et al. Proteolytic activity attenuates the response of endothelial cells to fluid shear stress. Cell Mol Bioeng. 2012;5: 82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bartosch AMW, Mathews R, Tarbell JM. Endothelial glycocalyx-mediated nitric oxide production in response to selective AFM pulling. Biophys J. 2017;113:101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramani VC, Pruett PS, Thompson CA, et al. Heparan sulfate chains of syndecan-1 regulate ectodomain shedding. J Biol Chem. 2012;287:9952–9961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van den Hoven MJ, Waanders F, Rops AL, et al. Regulation of glomerular heparanase expression by aldosterone, angiotensin II and reactive oxygen species. Nephrol Dial Transplant. 2009;24: 2637–2645. [DOI] [PubMed] [Google Scholar]
- 50.Sakamuri SS, Valente AJ, Siddesha JM, et al. TRAF3IP2 mediates aldosterone/salt-induced cardiac hypertrophy and fibrosis. Mol Cell Endocrinol. 2016;429:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bolbrinker J, Markovic S, Wehland M, et al. Expression and response to angiotensin-converting enzyme inhibition of matrix metalloproteinases 2 and 9 in renal glomerular damage in young transgenic rats with renin-dependent hypertension. J Pharmacol Exp Ther. 2006;316:8–16. [DOI] [PubMed] [Google Scholar]
- 52.Manon-Jensen T, Multhaupt HA, Couchman JR. Mapping of matrix metalloproteinase cleavage sites on syndecan-1 and syndecan-4 ectodomains. FEBS J. 2013;280:2320–2331. [DOI] [PubMed] [Google Scholar]
- 53.Singh A, Ramnath RD, Foster RR, et al. Reactive oxygen species modulate the barrier function of the human glomerular endothelial glycocalyx. PLoS One. 2013;8:e55852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Satchell SC, Tasman CH, Singh A, et al. Conditionally immortalized human glomerular endothelial cells expressing fenestrations in response to VEGF. Kidney int. 2006;69:1633–1640. [DOI] [PubMed] [Google Scholar]
- 55.Foster RR, Armstrong L, Baker S, et al. Glycosaminoglycan regulation by VEGFA and VEGFC of the glomerular microvascular endothelial cell glycocalyx in vitro. Am J Pathol. 2013;183:604–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Immunofluorescence on nonpermeabilized conditionally immortalized human glomerular endothelial cells. The surface expression of syndecan-4 (A). The surface expression of heparan sulfate (HS) (B). Surface expression of HS after 3 hours of incubation with 1 mU/ml heparatinase III (taken with identical settings to panel B) (C). Digitally enhanced version of panel C, showing the presence of an endothelial cell with minimal cell surface staining, confirming the anti-HS antibody’s specificity (D). IgG control for syndecan-4 staining (E). IgG control for HS staining (F). Bar = 25 μm.
Figure S2. Validation of KLF2 response to shear stress. A low-dose enzyme cocktail previously shown to remove glycocalyx components55 was used to confirm that an intact glycocalyx is needed for optimal KLF2 up-regulation in response to shear (A). Removal of syndecan-4 was confirmed using cell surface immunofluorescence (t test P = 0.0073; N = 6 controls; N = 14 enzyme treated). Fold-change in KLF2 in response to shear stress normalized to matched controls confirmed that enzymatic damage to the glycocalyx was associated with a significant impairment in cells’ ability to up-regulate KLF2 in response to shear stress (paired t test; N = 7; P = 0.045) (B). Significance relative to control. All error bars = SEM. *P < 0.05; **P < 0.01; ***P < 0.005; ****P < 0.001. The checkmark indicates glycocalyx degrading enzymes with 2 hours of pretreatment and ongoing exposure during shear stress. Enzymes used were as follows: hyaluronidase (2.5 mg/ml), chondroitinase (1 mu/ml), and neuraminidase (1 mu/ml). X indicates enzyme used was vehicle only.
Figure S3. Heparanase activity increased in glomeruli and urine but not in conditionally immortalized human glomerular endothelial cells (CiGEnCs) after salt and aldosterone exposure. Urine heparanase activity (normalized to urine creatinine) increased after 28 days of salt and aldosterone (t test; P = 0.0012; n = 5) (A). Glomerular lysate heparanase activity increased after 28 days of salt and aldosterone (t test; P = 0.0059; n = 6) (B). Conditioned media from CiGEnCs exposed to salt and aldosterone for 5 days remained equivalent to control levels (C). Batimastat had no measurable effect on heparanase activity.
Figure S4. Analysis of the glomerular filtration barrier using electron microscopy confirmed that glomerular basement membrane (GBM) thickness and podocyte foot process width had not altered. Representative electron micrograph of the filtration barrier of a control mouse. The white arrow indicates the glycocalyx labeled with Alcian Blue. Bar = 500 nmol/l (A). Representative electron micrograph of the filtration barrier of a mouse exposed to 28 days of salt and aldosterone. The white arrow indicates the glycocalyx labeled with Alcian Blue. Bar = 500 nmol/l (B). Glomeruli from 4 control mice and 3 mice exposed to salt and aldosterone were analyzed (total, 20 glomeruli; 60 capillary loops). No significant difference in GBM thickness was detected (t test P = 0.72; N = 4+3) (C). Podocyte foot process width did not increase significantly (t test P = 0.31) (D). Fenestration density did not alter significantly (t test P = 0.7) (E). Checkmark indicates salt + aldosterone: 1% NaCl in drinking water (ad libitum) and 0.6 μg/g/d aldosterone delivered subcutaneously via minipump. X indicates salt and aldosterone in standard drinking water (ad libitum) and vehicle (ethanol saline)-filled minipump. Ald, aldosterone.
Figure S5. Systolic BP under anesthesia. There were no significant differences in mouse (DBA2J) blood pressures from basal values or between groups under isoflurane anesthesia (equivalent to that used during multiphoton microscopy) (analysis of variance, NS).
Figure S6. Quantitative polymerase chain reaction primer sequences. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; KLF2, Krupple-like factor 2; MMP, matrix metalloproteinase; TNF-α, tumor necrosis factor-α; VCAM, vascular cell adhesion molecule.
Figure S7. Blood pressure (BP) training consisted of 5 days of daily handling and mock blood pressure measurement (5 inflation cycles repeated twice on days 1, 2, and 3 before increasing to 3 repeats on days 4 and 5). For the final BP measurement, 5 inflation cycles were used to acclimatize the mice, before 10 inflation cycles were used to determine the BP for each mouse. For inclusion, the computer had to accept 6 of the 10 readings (no systolic time out). A single day of recording was used for C57 BL6 mice on day 12 (because of the smaller minipump size used multiple recordings were not possible), whereas the mean value after 4 sequential days of recording (days 24–27) was used in DBA2J mice. Having established that the glomerular sieving coefficient rapidly increased (within 5 days) in response to salt and aldosterone exposure, the protocol initially used (Supplementary Figure S7B) was amended (Supplementary Figure S7C) before testing the effectiveness of the MMP2/9 inhibitor. Amendments included baseline assessment of the glycocalyx using WGA and a shortened exposure to salt and aldosterone to optimize image resolution. FITC, fluorescein isothiocyanate; PFA, paraformaldehyde.
Figure S8. Example normalized line profiles and the images from which they were taken. Images were taken after the perfusion of Alexa 594 albumin (red) and fluorescein isothiocyanate (FITC)-WGA (green). Line profiles have been generated by measuring the florescence intensity along the regions of interest represented by the yellow lines. The length of each regions of interest is given as the x-axis on the corresponding graphs. Peak-to-peak measurements provide an index of glycocalyx thickness. Salt and aldosterone exposed glomerulus day 5 (this image has been digitally enhanced to aid visualization of the glycocalyx for illustration purposes) (A). Control glomerulus on day 10 (B). MMP2/9 inhibitor-treated glomerulus on day 5 (C).