Abstract
Proton pump inhibitors (PPIs) are one of the most frequently used medications for upper gastrointestinal diseases. However, a number of physicians have raised concern about the serious side effects of long-term use of PPIs, including the development of gastric cancer. Recent epidemiological studies have reported a significant association between long-term PPI intake and the risk of gastric cancer, even after successful Helicobacter pylori eradication. However, the effects of PPIs on the development of pre-malignant conditions such as atrophic gastritis or intestinal metaplasia are not fully known, suggesting the need for comprehensive and confirmative studies are needed in the future. Meanwhile, several experimental studies have demonstrated the effects of PPIs in reducing chemoresistance in gastric cancer cells by modulating the acidic microenvironment, cancer stemness and signal transducer and activator of transcription 3 (STAT3) signaling pathway. The inhibitory effects of PPIs on STAT3 activity may overcome drug resistance and enhance the efficacy of conventional or targeted chemotherapeutic agents. Taken together, PPIs may “play dual role” in gastric carcinogenesis and treatment of gastric cancer.
Keywords: Proton pump inhibitor, Gastric cancer, Drug resistance, Signal transducer and activator of transcription 3
Core tip: Recent epidemiological studies have demonstrated a significant increase in gastric cancer risk following the long-term use of proton pump inhibitors (PPIs). However, observational studies have fundamental limitations. PPIs may affect gastric cancer cells and the microenvironment by modifying the acidic conditions and inhibiting the cancer stemness via various signaling pathways including signal transducer and activator of transcription 3, which in turn, reduces drug resistance to chemotherapy. In this review, we briefly summarize the current clinical outcomes of the effects of long-term PPI use and the development of gastric cancer, as well as experimental studies showing enhanced chemosensitivity in gastric cancer.
INTRODUCTION
Gastric cancer is one of the most frequently found malignant solid tumors worldwide, and is the third leading cancer-related cause of mortality[1]. Advances in technical and clinical knowledge have increased the early detection of gastric cancer for prompt intervention and successful management[2]. However, a significant number of gastric cancer cases are still diagnosed in advanced stages with distant metastasis, resulting in poor prognosis. A recent pivotal prospective randomized study showed that the overall survival in gastric cancer with a single metastasis was not significantly different between patients receiving chemotherapy alone and patients treated with gastrectomy combined with chemotherapy. The study also reported that the median overall survival was less than 18 mo[3].
Significant risk factors for gastric cancer include male gender; old age; ethnicity; Helicobacter pylori (H. pylori) infection; dietary factors, such as smoked food, high salt intake, pickled vegetables and nitrated meat; smoking; and family history. In terms of gastric factors, atrophic gastritis and intestinal metaplasia are proven pre-cancerous conditions[4]. A South Korean study showed that the risk of developing gastric cancer was increased more than 10 fold among subjects who had intestinal metaplasia compared with subjects who did not[5]. Thus, avoidance and optimal surveillance of risk factors are mandatory for the prevention of gastric cancer.
Proton pump inhibitors (PPIs) are the most potent acid inhibitors ever developed: they act by blocking the H+/K+ ATPase of parietal cells[6]. PPIs are powerful acid inhibitors and there, are widely used as drugs of choice for the treatment of gastroesophageal reflux disease (GERD) and drug-induced peptic ulcers. Long-term use of PPIs may facilitate the optimal management of GERD combined with severe complications such as esophageal stricture[7], and in practice, the long-term prescription of a PPI is often preferred as maintenance therapy, even for uncomplicated GERD patients[7]. However, there are rising concerns about the potential side effects of long-term PPI intake which include Clostridium difficile infection, pneumonia, bone fractures, dementia, chronic renal disease and small intestinal bacterial overgrowth[8]. Recent observational studies demonstrated a positive association between PPI use and malignant or pre-malignant tumors of the gastrointestinal tract. Reflecting recent trends, a recent expert opinion suggests that the dose of long-term PPIs should be periodically re-evaluated and that the lowest possible effective dose needs to be prescribed[9]. However, several experimental studies showed significant anti-tumor effects of PPI in cancer cells such as Barrett’s adenocarcinoma and melanoma cells[10,11], and suggested that PPIs may contribute to reducing of tumor resistance to chemotherapy[12]. Several experimental studies have demonstrated this “unexpected” effect of PPIs in gastric cancer cells. In this review, we focused on the dual action of PPIs in gastric cancer. We not only summarized the clinical outcomes correlating the development of gastric malignancy with long-term use of PPI but presented an experimental hypothesis and experimental evidence supporting the anti-tumorigenic, drug-sensitizing effects of PPI in gastric cancer cells.
PPI AND GASTRIC CARCINOGENESIS
Hypothesis for causality of PPI and gastric cancer
The most plausible hypothesis for the association between long-term PPI intake and the development of gastric cancer is mediated via hypergastrinemia due to the reduced secretion of gastric acid[13]. This reduced acidity, in turn, triggers a proliferation of enterochromaffin-like cells (ECL cells), which express gastric cholecystokinin-2 (CCK-2) receptors and are the target cells of gastrin in the oxyntic mucosa, and formation of neuroendocrine tumors (NETs)[14]. The somatostatin-mediated negative feedback of gastrin release on antral G-cells is frequently inhibited by gastric hypochlorhydria caused by long-term PPI use and other anti-acidic drugs, which leads to hypergastrinemia and hyperplasia of the gastric mucosa or ECL-cells[15]. The second hypothesis is that gastrin per se has a trophic effect on the oxyntic mucosa, as well as on ECL cells, under hypergastrinemic conditions such as chronic atrophic gastritis or prolonged PPI use[16]. A previous animal study showed that a high salt diet administered to H. pylori-infected Mongolian gerbils significantly increased serum gastrin levels and mucosal inflammation, which were ameliorated by a gastrin antagonist[17]. A recent case-control study showed that the subgroup with the highest quartile of serum gastrin levels was significantly associated with gastric non-cardia adenocarcinoma [fully adjusted odds ratio (OR) = 1.92; 95% confidence interval (CI): 1.21-3.05], as well as NET (age-adjusted continuous model OR = 4.67; 95%CI: 2.67-8.15)[18].
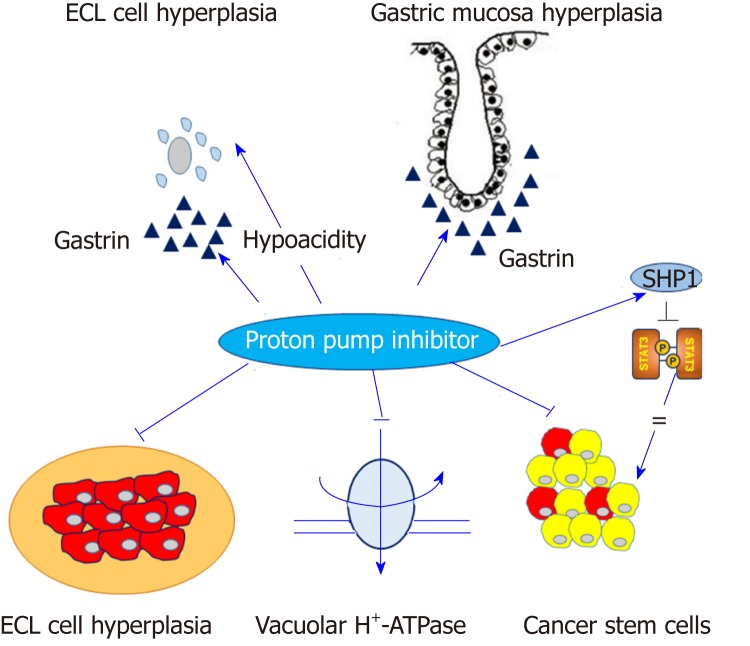
However, a molecular link between ECL cell hyperplasia and gastric adenocarcinoma is less relevant than gastric NET in general[14]. Nevertheless, a fraction of gastric adenocarcinomas originates from ECL cells. A previous study using human gastric carcinoma tissues showed that ECL cell markers, such as chromogranin A, synaptophysin, histidine decarboxylase and neuron specific enolase, were predominantly expressed in diffuse type gastric cancer rather than intestinal type gastric cancer[19]. Moreover, several pathologic studies have shown that most periodic acid-Schiff (PAS)-positive signet ring cell carcinomas abundantly expressed ECL-cell markers, but not mucin, suggesting that signet ring cell carcinoma might be a consequence of dedifferentiation from ECL cells toward signet ring cells with PAS-positive cytoplasm[20,21]. At the present stage, the effect of PPIs might be summarized by the following statement. PPIs reduce gastric acid secretion and lead to hypergastrinemia with the proliferation of ECL cells in the oxyntic gland, partially and theoretically explaining the potential association between PPI and gastric cancer, or at least, the enhancement of H. pylori-associated gastric carcinogenesis[22] (Figure 1). However, this hypothesis is often insufficient to elucidate the mechanism of PPI-induced gastric carcinogenesis. Moreover, a recent pivotal translational study demonstrated that PPI-treated patients showed similar microbial diversity compared with normal subjects while patients with H. pylori-induced atrophic gastritis manifested a lower bacterial abundance and diversity. This finding suggested that PPIs do not significantly alter gastric microbiota nor do they contribute significantly to the development of gastric cancer[23].
Figure 1.
Contrast effects of proton pump inhibitors in normal gastric mucosa and gastric cancer cells. Proton pump inhibitors (PPIs) induce hypergastrinemia and hypochlorhydria, which may contribute to enterochromaffin-like cell hyperplasia and proliferation of gastric mucosa. Conversely, PPIs may modify the acidic tumor microenvironment and inhibit vacuolar H+-ATPase or signal transducer and activator of transcription 3 activity in gastric cancer cells. Arrow indicates the positive effect and straight line indicates the inhibitory effect. ECL: Enterochromaffin-like; SHP1: Src homology 2 domain-containing protein tyrosine phosphatase 1; STAT3: Signal transducer and activator of transcription 3.
Clinical evidence supporting the association of PPI and development of gastric cancer
Previously, three retrospective, case-control studies from databases of Western countries analyzed the increased risk of gastric cancer with PPI intake[24-26]. These studies included relatively small number of gastric cancer cases (approximately 2000) and missed several major confounding factors, such as H. pylori infection status, dietary patterns or family history of gastric cancer. A meta-analysis which included the above three case-control studies, showed that the pooled relative risk (RR) of gastric cancer following PPI use was 1.43 (95%CI: 1.23-1.66) using both fixed- and random-effects models. However, the subgroup analysis failed to show a dose-dependent relationship between PPI and gastric cancer (PPI < 12 mo: pooled RR = 1.73, 95%CI: 1.24-2.52; > 12 mo: pooled RR = 1.42, 95%CI: 0.98-2.07; > 36 mo: pooled RR = 2.45, 95%CI: 1.41-4.25). The authors stated that colonization with H. pylori and adequate long-term use of PPI synergistically increased the risk of gastric cancer[27]. Another previous meta-analysis showed a similar effect of acid suppressive drugs on gastric cancer (adjusted OR = 1.42; 95%CI: 1.29-1.56); however, the pooled effect was confounded by H2RA, and was not solely due to PPI[28].
Recently, Cheung et al[29] showed a positive correlation between PPI and gastric cancer in H. pylori- infected patients who underwent eradication therapy. In this large-scale, population-based study involving a Hong Kong health database, the authors enrolled more than 63000 adult patients who were prescribed with a clarithromycin-based triple therapy. Current H. pylori infection was diagnosed by an invasive or non-invasive study. To eliminate protopathic bias, patients who were diagnosed with gastric cancer within six months before the study or within 12 mo after H. pylori eradication therapy were excluded[30]. Furthermore, to minimize the effect of H. pylori-induced gastric carcinogenesis, only patients successfully treated with eradication therapy were enrolled. Failure of H. pylori eradication was therapy identified if patients were prescribed subsequent medication of (1) repeated standard triple therapy, (2) bismuth-containing second-line quadruple therapy, or (3) rifabutin-based third-line therapy. During a median follow-up of 7.6 years, 153 patients (0.24%) developed gastric cancer. PPI use significantly increased the risk of gastric cancer [hazard ratio (HR) = 2.44; 95%CI: 1.42-4.20], unlike H2RA (HR = 0.72; 95%CI: 0.48-1.07). Moreover, the positive association between PPI and gastric cancer showed dose- and duration-dependent relationship[29]. This study was significant in that it demonstrated the increased risk of gastric cancer with long-term use of PPIs, even after successful eradication of H. pylori. However, it had several important limitations. First, due to the fundamental limitations of observational studies, several baseline characteristics such as age, metabolic diseases (diabetes, hypertension, and dyslipidemia) and other major comorbidities (ischemic heart disease, stroke, congestive heart failure, and chronic renal failure) were significantly biased between the case and control groups. Consequently, gastric atrophy, salty food intake or obesity, which are related to gastric cancer development, may have occurred more frequently in the PPI user group, even after statistically sophisticated propensity-score matching[31]. Second, important confounding factors of gastric cancer such as gastric atrophy, intestinal metaplasia and dietary patterns were excluded[32]. Third, the authors determined success or failure of H. pylori eradication only based on prescription histories. Thus, a portion of the enrolled patients may have continued to harbor H. pylori infection, even after eradication, and the carcinogenic effect of H. pylori may not have been completely eliminated.
A Swedish nationwide population-based cohort study recruited almost 800000 Swedish adults who were undergoing maintenance therapy with PPIs, and the significance incidence ratio (SIR) of gastric cancer was 3.38 (95%CI: 3.23-3.53), which was consistent regardless of gender, age, indications for PPIs (i.e., GERD), concomitant use of anti-inflammatory drugs such as aspirin or nonsteroidal anti-inflammatory drugs (NSAIDs) and the subsite of gastric cancer (cardia and non-cardia cancer)[33]. In this study, the authors restricted enrollment to subjects who were exposed to PPI maintenance therapy, defined as a cumulative defined daily dose of at least 180 d during the study period, to reduce the possibility of reverse causality of PPI and gastric cancer. However, this study also failed to establish a causal relationship between gastric cancer and long-term use of PPI, in that the SIR of gastric cancer did not show any duration-dependent pattern. Furthermore, crucial information such as the current H. pylori infection status was missing. Clinical studies correlating the long-term use of PPIs with gastric cancer are summarized in Table 1.
Table 1.
Summary of clinical studies associating gastric cancer with long-term use of proton pump inhibitors
| Author, year | Study design, country | Study period | Source of database | No. of case and control | Information of PPI | Adjustment | Main outcomes |
| Garcia-Rodriguez et al[24], 2006 | Nested case-control, retrospective, United Kingdom | 1994-2001 | The general practitioners research database in the United Kingdom | 522/10000 | Duration, indication | Age, sex, calendar year, smoking, alcohol consumption, body mass index, gastro-esophageal reflux, hiatal hernia, peptic ulcer, and dyspepsia | OR for gastric cardia adenocarcinoma: 1.06 (0.57-2.00); gastric non-cardia adenocarcinoma: 1.75 (1.10-2.79) |
| Tamim et al[25], 2008 | Case control, retrospective, Canada | 1995-2003 | Quebec health insurance plan | 1598/12991 | Type, dose, exposure time | Number of drug prescriptions, total length of hospitalizations, number of visits to GPs, specialists, and emergency rooms during the year before the diagnosis | Adjusted OR: 1.40 (1.08-1.51); 1st quartile: 1.66 (1.24-2.23); 2nd quartile: 1.37 (1.00-1.88); 3rd quartile: 1.57 (1.17-2.10); 4th quartile: 1.20 (0.85-1.70) |
| Poulsen et al[26], 2009 | Population-based cohort, retrospective, Denmark | 1990-2003 | Danish National Health-care System | 109/not reported | Type, year of follow-up, no. of prescription | Age, gender, calendar period, gastroscopy (≥ 1 yr before censoring events), use of NSAIDs and H. pylori eradication | IRR for gastric cancer: 1.2 (0.8-2.0) among PPI users with the largest number of prescriptions (15+) or the longest follow-up (5+) |
| Cheung et al[29], 2018 | Population-based cohort study, retrospective, Hong Kong | 2003-2012 | Clinical Data Analysis and Reporting System of the Hong Kong Hospital Authority | 153/63397 | Frequency, duration | Age of receiving H. pylori eradication therapy, sex, smoking, alcohol use, comorbidities, concomitant medications | HR for gastric cancer: 2.44 (1.42-4.20); ≥ 1 yr: 5.04 (1.23-20.61); ≥ 2 yr: 6.65 (1.62-27.26); ≥ 3 yr: 8.34 (2.02-34.41). The adjusted absolute risk difference for PPIs vs nonPPIs use: 4.29 (1.25-9.54) per 10000 person-yr. |
| Brusselaers et al[33], 2017 | Population-based cohort study, retrospective, Sweden | 2005-2012 | The Swedish Prescribed Drug Registry | 2219/794848 | Indication, cumulative defined daily dosages, estimated number of days | Age, sex, calendar period, indication of PPI, maintenance use (≥ 180 d) of aspirin or other NSAIDs | SIR: 3.38 (3.23-3.53) in both sexes, all age groups and all indication groups; < 1 yr: 12.82 (12.19-13.47); 1.0-2.9 yr: 2.19 (1.98 to 2.42); 3.0-4.9 yr: 1.10 (0.91-1.31); ≥ 2 yr: 0.61 (0.52-0.72) |
PPI: Proton pump inhibitor; OR: Odds ratio; GP: General physician; NSAID: Nonsteroidal anti-inflammatory drug; H. pylori: Helicobacter pylori; IRR: Incidence rate ratio; HR: Hazard ratio; SIR: Standardized incidence ratio.
Interestingly, clinical outcomes supporting the effect of long-term PPI use on the development of pre-cancerous conditions, such as atrophic gastritis or intestinal metaplasia, are lacking. A previous cohort study showed that 30% (18/59) of patients who were treated with long-term omeprazole and H. pylori infection at baseline developed atrophic gastritis, which was significantly higher than non-omeprazole group[34]. Meanwhile, previous randomized controlled trials (RCTs) showed that the proportion of patients who progressed to gastric corpus glandular atrophy and intestinal metaplasia was not significantly different between the long-term omeprazole-treated group and the control group[35,36]. A previous study based on histopathologic evaluation of gastric biopsy samples showed that only a small number of patients had worsening of their gastritis score for gastric atrophy and intestinal metaplasia following 12 mo of esomeprazole therapy: 1.4% had atrophy and 0.5% had intestinal metaplasia on the antrum, and 1.2% had atrophy and 0.8% had intestinal metaplasia on the corpus[37]. Recently, the Cochrane Database systematically reviewed four RCTs for the effects of long-term PPI intake on corporal atrophy and intestinal metaplasia. The meta-analysis showed that OR for corporal atrophy was 1.50 (95%CI: 0.59-3.80; P = 0.39), and the OR for intestinal metaplasia was 1.46 (95%CI: 0.43-5.03; P = 0.55), both of which failed to reach statistical significance[38]. Clinical studies associating the long-term use of PPIs with pre-malignant conditions of gastric cancer are summarized in Table 2.
Table 2.
Summary of clinical studies associating of gastric pre-malignant conditions with long-term use of proton pump inhibitors
| Author, year | Study design, country | Source of database | No. of PPI and control group | Information of PPI | Aims | Main outcomes |
| Kuipers et al[34], 1996 | Prospective cohort, Netherland/ Sweden | Reflux esophagitis cohort (fundoplication/ omeprazole) | 105 (PPI)/ 72 (fundoplication) | Type (omeprazole only), dose (20 and 40mg), duration (5 years) | Corpus gastritis, atrophic gastritis | Atrophic gastritis: 0/31 (fundoplication group) vs 18/59 (omeprazole group) with H. pylori infection at baseline (P < 0.001); 0/41 (fundoplication group) vs 2/46 (omeprazole group) without H. pylori infection at baseline (P = 0.62) |
| Lundell et al[35], 1999 | RCT, Sweden | RCT comparing the efficacy of omeprazole and ARS | 155 (PPI)/155 (ARS) | Type (omeprazole only), duration (3 years) | Gastric corpus glandular atrophy, intestinal metaplasia of corpus mucosa | No difference in glandular atrophy between H. pylori-infected omeprazole and ARS group (P = 0.57); No difference in intestinal metaplasia between H. pylori-infected omeprazole and ARS group. |
| Lundell et al[36], 2006 | RCT, Sweden | RCT comparing the efficacy of omeprazole and ARS | 117 (PPI)/98 (surgical arm) | Type (omeprazole only), duration (7 years) | Gastric corpus glandular atrophy | No significant change of gastric atrophy between H. pylori-negative omeprazole and ARS group; Two patients developed severe atrophy from none at baseline in H. pylori-infected omeprazole group, three patients developed mild atrophy from none at baseline in H. pylori-infected ARS group, no statistical difference. |
| Gental et al[37], 2003 | Two RCTs, United States | Maintenance trial (n = 519), Safety trial (n = 807) | Maintenance trial: 519 (PPI)/169 (placebo); Safety trial: 807/PPI | Type (esomeprazole only), duration (6 months: maintenance trial; 12 months: safety trial) | Atrophy (antrum and corpus), intestinal metaplasia (antrum and corpus) | In the maintenance studies, the majority of omeprazole group had no change in the extent of atrophy and intestinal metaplasia. In the safety study, > 98% of omeprazole had either no change or improved atrophy scores in antrum and corpus, and intestinal metaplasia scores remained unchanged or improved compared with those that worsened. |
PPI: Proton pump inhibitor; RCT: Randomized controlled trial; ARS: Anti-reflux surgery; H. pylori: Helicobacter pylori.
In summary, several studies have shown a significant relationship between long-term PPI use and the risk of gastric cancer. However, the evidence is far from definitive because of limitations of research design and omission of several major confounding variables. Furthermore, conflicting data also exist. For example, although United States is one of the countries with the most frequent and long-term use of PPI, the incidence of gastric cancer is relatively low[39]. Thus, robust evidence including well-designed, large-scale prospective studies are needed to support the potential association between long-term PPI use and gastric cancer
PARADOXICAL ACTION OF PPI IN GASTRIC CANCER CELLS
Effects of PPI on tumor resistance
The unexpected effects of PPIs on solid tumors, including gastric cancer occur by several potential mechanisms (Figure 1).
First, a change of acidity occurs in the tumor microenvironment, for instance in solid tumors, the extracellular pH is acidic and the intracellular pH is neutral-to-alkaline, whereas the pH of the microenvironment in normal tissue usually remains alkaline[40]. This phenomenon leads to decreased intracellular concentrations of cytotoxic drugs that are weakly basic, such as cisplatin, 5-fluorouracil, vinblastine or doxorubicin[12]. PPIs contribute to overcoming drug resistance and enhance chemosensitivity by inhibiting the vacuolar H+-ATPase (V-H+-ATPase) of tumor cells, alkalizing the tumor microenvironment and retaining weakly basic cytotoxic drugs within the intracellular targets[11]. An in vitro study showed that pretreatment with omeprazole and esomeprazole significantly increased the sensitivity of cytotoxic drugs, such as cisplatin, 5-fluorouracil and vinblastine in various solid cancer cell lines with multi-drug resistance phenotypes[41].
Second, the modulation of cancer stemness plays a role. Cancer stem cells (CSCs) play a key role in the development of chemoresistance as well as cancer metastasis[42]. Several family proteins of ATP binding cassette (ABC) transporters such as P-glycoprotein, multi-drug resistance (MDR) associated protein-1 (MRP-1), lung resistance protein (LRP) and breast cancer resistance protein (BCRP) are highly expressed in CSCs and contribute to MDR by enhancing the activity of drug efflux pumps[43]. PPIs reduce chemoresistance via modification of anaerobic glycolysis and ABC transporters in solid cancer cells[44].
Several experimental studies have demonstrated the anti-tumor effects and the ability of PPIs to overcome MDR in gastric cancer. An in vitro and in vivo study showed that pantoprazole treatment selectively induced apoptotic cell death in gastric cancer cells, while normal gastric epithelial cells were resistant to pantoprazole[45]. Pretreatment of PPIs effectively inhibited the activity of V-H+-ATPase, which resulted in an increased concentration of cytotoxic drugs in gastric cancer cells[46]. Several in vitro studies demonstrated putative downstream effectors following the inhibition of V-H+-ATPase by PPIs in gastric cancer cells, such as the dephosphorylation of LRP6 and the inhibition of Wnt/β-catenin signaling[47] or PI3K/Akt/mTOR/HIF-1α signaling pathways[48]. A study showed that high-dose esomeprazole inhibited the release of exosomes and exosome-related micro-RNAs such as miR-494-3p, miR-6126 and miR-3934-5p, which are closely associated with tumor invasion, metastasis, adhesion and migration, and in turn, regulated the HIF-1α-FOXO1 axis to induce apoptosis and inhibit cellular migration and invasion in gastric cancer cells[49]. In summary, PPIs modulate the acidic microenvironment, and regulate V-H+-ATPase and cancer stemness of various cancer cells including gastric cancer, and contribute to the reduction of tumor resistance to chemotherapeutic agents.
PPI modulating SHP-1/STAT3 signaling axis
It is well known that signal transducer and activator of transcription 3 (STAT3) signaling pathway plays a pivotal role in the invasion of gastric cancer[50]. In brief, phosphorylated STAT3 forms a homodimer for nuclear translocation, where it acts as a transcription factor to activate various target genes including cellular migration and invasion in epithelial cells. It also activates surrounding immune cells to regulate various immunologic reactions favoring cancer cell survival, such as the production of inflammatory cytokines and formation of pre-metastatic niches[51]. Various in vitro and in vivo studies have demonstrated that fully activated STAT3 induced epithelial-mesenchymal transition (EMT) via upregulation of relevant target genes such as vimentin and survivin in gastric cancer cells[52-54]. Furthermore, clinical outcomes also showed that high level of phosphorylated STAT3 were significantly associated with regional lymph node metastasis and poor prognosis in gastric cancer patients[55-57]. STAT3 also plays as a key role in the activation of CSCs. A previous study showed that gastric cancer-derived mesenchymal stem cells (GC-MSCs) secreted interleukin (IL)-6 and activated STAT3 in neutrophils. These GC-MSCs-primed neutrophils induced transdifferentiation of normal MSCs to cancer associated fibroblasts[58]. Thus, STAT3 may present a primary target for the inhibition of gastric cancer invasion.
Several inhibitors of STAT3 including direct STAT3 inhibitors or inhibitors of upstream kinases, such as janus kinase 2 (JAK2) or Src kinase have been introduced and evaluated in experimental studies[59-61]. However, clinical studies involving gastric cancer patients are lacking, and technical limitations due to large surface of the target area have demonstrated the need for more stable and effective direct STAT3 inhibitors[62]. Src homology 2 (SH2) domain-containing protein tyrosine phosphatase 1 (SHP-1), a non-receptor type protein Tyr phosphatase (PTPase), has attracted attention as an effective inhibitor of STAT3 activity[63]. SHP-1 acts as a protein Tyr PTPase and induces the dephosphorylation of STAT3 in various cell types. It is abundantly expressed and has been mostly evaluated in cells of hematopoietic lineage, such as macrophages, neutrophils, monocytes and mast cells[64]. Pivotal studies demonstrated that the expression of SHP-1 was aberrantly reduced by CpG island hypermethylation in lymphoma and leukemia[65,66]. Recently, the suppressive effect of STAT3 by SHP-1 has been evaluated in solid tumors. Chen et al. showed that several multiple kinase inhibitors such as sorafenib, dovitinib and regorafenib effectively induced SHP-1 in hepatocellular carcinoma, and in turn, suppressed STAT3 activity via dephosphorylation[67-69]. The function of SHP-1 has been recently evaluated in gastric cancer. Sun et al. showed that the expression of SHP-1 was the highest in normal gastric epithelium, followed by intestinal metaplasia and dysplasia and was the lowest in gastric cancer tissues[70], SHP-1 combines with a transmembrane protein with epidermal growth factor and two follistatin motifs 2 (TMEFF2) to inhibit STAT3 phosphorylation in gastric cancer cells and H. pylori-infected gastric epithelial cells[71].
We also previously showed that the expression of SHP-1 was aberrantly reduced following CpG island hypermethylation in various gastric cancer cell lines, and enhanced expression of SHP-1 in gastric cancer cells effectively dephosphorylated STAT3, resulting in downregulation of various target genes involved in cellular migration and invasion[72]. An in vitro study reported that PPIs exhibited a dose-dependent cytotoxicity and enhanced the sensitivity of cisplatin via inhibition of IL-6-stimulated STAT3 activity and its target genes[73]. Recently, we demonstrated that pantoprazole, a well-known PPI, effectively induced SHP-1 and downregulated phosphorylated-STAT3 levels in gastric cancer cells in a dose-dependent manner and modulated EMT markers[74]. Thus, we suggest that PPIs may act as effective STAT3 inhibitors via induction of SHP-1 in gastric cancer cells and play a role in the inhibition of progression of gastric cancer.
Application of PPIs in overcoming chemoresistance
Previous studies have demonstrated that the constitutive expression of STAT3 in gastric cancer was closely associated with the MDR of chemotherapeutic agents via enhanced expression of various oncogenes and downregulation of apoptotic genes[75]. Enhanced STAT3 activity also induced V-H+-ATPase in gastric cancer cells, which abrogated the uptake of chemotherapeutic agents and contributed to the development of chemoresistance, as mentioned above[76]. Furthermore, recent studies showed that STAT3 activation reduced the efficacy of trastuzumab, a promising therapeutic antibody targeting HER2, via upregulation of MUC1 and MUC4[77], or the positive feedback loop of IL-6/STAT3/Jagged-1/Notch[78]. Thus, effective inhibition of STAT3 activity is considered the mainstay of intervention to overcome chemoresistance and effective management of advanced gastric cancer patients. A previous study demonstrated that pantoprazole effectively inhibited invasion and EMT of adriamycin-resistant gastric cancer cells via suppression of the Akt/GSK-β/β-catenin signaling pathway[79]. We recently found that a minimal dose of pantoprazole combined with docetaxel significantly induced SHP-1 expression, downregulated phosphorylation of STAT3, modulated EMT markers, and inhibited cellular migration and invasion in gastric cancer cells. Injection of both pantoprazole and docetaxel into nude mice significantly reduced the tumor volume of xenograft tumors of gastric cancer cells, compared with single administration of each drug[80]. Taken together, we suggest that a combination of PPIs during chemotherapy may play a role in enhancing the sensitivity and efficacy of chemotherapeutic agents including trastuzumab. Experimental studies reporting the effects of PPIs in gastric cancer cells and chemotherapeutic agents are summarized in Table 3. However, the lack of human studies and limited clinical relevance represent challenges that need to be addressed before PPIs are used to increase the effectiveness of chemotherapy for actual gastric cancer and improve patient prognosis. Further pre-clinical and clinical studies that are relevant to this hypothesis are needed.
Table 3.
Summary of experimental studies investigating the effects of proton pump inhibitors in gastric cancer cells
| Author, year | Study design | Type of PPI | Cell type | Main outcomes | Underlying hypothesis |
| Yeo et al[45], 2004 | In vitro, in vivo | Pantoprazole | MKN 45, MKN 28, AGS, SNU 601, RGM-1 (normal gastric mucosa cell) | Apoptotic cell death in gastric cancer cells, but not in normal gastric mucosal cells, induced by pantoprazole | Modulation of heat-shock proteins (HSP 70, HSP 27) |
| Chen et al[46], 2009 | In vitro | Pantoprazole | SGC7901 | Inhibition of V-H+-ATPase expression in a dose-dependent manner; enhancement of efficacy of anti-tumor drug (cisplatin) and increased apoptosis rate | Change of pH gradient (decrease of intracellular pH and reverse of the transmembrane pH gradient) |
| Shen et al[47], 2013 | In vitro | Pantoprazole | SGC7901 | Anti-proliferation, anti-invasive and pro-apoptotic effects, decrease of V-H+-ATPase expression | Inhibition of LRP6 in Wnt/β-catenin signaling |
| Chen et al[48], 2018 | In vitro, in vivo | Pantoprazole | SGC7901, SGC7901/MDR | Inhibition of V-H+-ATPase expression in, SGC7901/MDR cells | Inhibition of P-gp and MRP1, and downregulation of PI3K/Akt/mTOR/HIF-1α signaling pathway |
| Guan et al[49], 2017 | In vitro, in vivo | Esomeprazole | SGC7901 | Enhancement of efficacy of anti-tumor drugs (cisplatin, paclitaxel, 5-FU); Inhibition of transformation of CAF | Regulation of HIF-1α-FOXO1 axis and inhibition of release of exosome and exosome-related microRNAs (tumor invasion, metastasis and TGF-beta pathway) |
| Huang et al[73], 2013 | In vitro | Pantoprazole | SGC7901, GBC823, AGS | Inhibition of cellular proliferation and increase in the number of apoptotic cells | Inhibition of STAT3 |
| Koh et al[74], 2018 | In vitro, in vivo | Pantoprazole | AGS, MKN-28 | Inhibition of cellular invasion, migration and modulation of EMT markers | Induction of SHP-1 and inhibition of JAK2/STAT3 |
| Zhang et al[79], 2015 | In vitro | Pantoprazole | Adriamycin-resistant SGC7901 (SGC7901/ADR) | Inhibition of cellular migration/invasion and modulation of EMT markers in SGC7901/ADR cells | Inhibition of Akt/GSK-β/βcatenin signaling |
| Joo et al[80], 2018 | In vitro, in vivo | Pantoprazole | AGS | Enhanced cellular migration/invasion and anti-tumor effect of docetaxel by combination with minimal dose pantoprazole | Induction of SHP-1 and inhibition of JAK2/STAT3 |
PPI: Proton pump inhibitor; V-H+-ATPase: Vacuolar-H+-ATPase; LRP6: Low-density lipoprotein receptor related protein 6; MDR: Multidrug resistance; MRP1: Multidrug resistance-associated protein 1; mTOR: Mammalian target of rapamycin; HIF-1α: Hypoxia-inducible factor 1alpha; CAF: Cancer associated fibroblast; FOXO1: Forkhead box protein O1; TGF-beta: Tumor growth factor-beta; EMT: Epithelial-mesenchymal transition; STAT3: Signal transducer and activator of transcription 3; JAK2: Janus kinase 2; ADR: Adriamycin; GSK-β: Glycogen synthetase kinase-3-β.
CONCLUSION
Many physicians have raised concerns that long-term PPI use may be a significant risk factor for GI tract neoplasia, including gastric cancer, and data from recent clinical studies support this hypothesis. However, from a methodological point of view, application of the results from observational clinical studies is limited until solid evidence is available to establish the long-term use of PPI and its association with gastric cancer. However, in patients with pre-malignant lesions such as atrophic gastritis or intestinal metaplasia, it may be necessary to restrict long-term PPI administration, even after H. pylori eradication, to prevent gastric cancer. By contrast, theoretical investigations and experimental findings suggest that PPIs may play an adjunct role of in improving the efficacy of chemotherapy for malignant tumors including stomach cancer. Currently, PPIs might play a “dual role” in gastric carcinogenesis and management of advanced gastric cancer.
Footnotes
Conflict-of-interest statement: None of the authors have potential conflicts (financial, professional, or personal) that are relevant to this publication.
Peer-review started: February 20, 2019
First decision: March 5, 2019
Article in press: March 25, 2019
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chiu CC, Link A, Mastoraki A, Tan HJ S-Editor: Ma RY L-Editor: A E-Editor: Ma YJ
Contributor Information
Moon Kyung Joo, Division of Gastroenterology, Department of Internal Medicine, Korea University College of Medicine Guro Hospital, Seoul 08308, South Korea. latyrx@korea.ac.kr.
Jong-Jae Park, Division of Gastroenterology, Department of Internal Medicine, Korea University College of Medicine Guro Hospital, Seoul 08308, South Korea.
Hoon Jai Chun, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Institute of Digestive Disease and Nutrition, Korea University College of Medicine, Seoul 02841, South Korea.
References
- 1.Herrero R, Park JY, Forman D. The fight against gastric cancer - the IARC Working Group report. Best Pract Res Clin Gastroenterol. 2014;28:1107–1114. doi: 10.1016/j.bpg.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Shin DW, Hwang HY, Jeon SW. Comparison of Endoscopic Submucosal Dissection and Surgery for Differentiated Type Early Gastric Cancer within the Expanded Criteria. Clin Endosc. 2017;50:170–178. doi: 10.5946/ce.2016.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujitani K, Yang HK, Mizusawa J, Kim YW, Terashima M, Han SU, Iwasaki Y, Hyung WJ, Takagane A, Park DJ, Yoshikawa T, Hahn S, Nakamura K, Park CH, Kurokawa Y, Bang YJ, Park BJ, Sasako M, Tsujinaka T REGATTA study investigators. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. 2016;17:309–318. doi: 10.1016/S1470-2045(15)00553-7. [DOI] [PubMed] [Google Scholar]
- 4.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–1186. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 5.Kim N, Park RY, Cho SI, Lim SH, Lee KH, Lee W, Kang HM, Lee HS, Jung HC, Song IS. Helicobacter pylori infection and development of gastric cancer in Korea: long-term follow-up. J Clin Gastroenterol. 2008;42:448–454. doi: 10.1097/MCG.0b013e318046eac3. [DOI] [PubMed] [Google Scholar]
- 6.Yang YX, Metz DC. Safety of proton pump inhibitor exposure. Gastroenterology. 2010;139:1115–1127. doi: 10.1053/j.gastro.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 7.Vigneri S, Termini R, Leandro G, Badalamenti S, Pantalena M, Savarino V, Di Mario F, Battaglia G, Mela GS, Pilotto A. A comparison of five maintenance therapies for reflux esophagitis. N Engl J Med. 1995;333:1106–1110. doi: 10.1056/NEJM199510263331703. [DOI] [PubMed] [Google Scholar]
- 8.Malfertheiner P, Kandulski A, Venerito M. Proton-pump inhibitors: understanding the complications and risks. Nat Rev Gastroenterol Hepatol. 2017;14:697–710. doi: 10.1038/nrgastro.2017.117. [DOI] [PubMed] [Google Scholar]
- 9.Freedberg DE, Kim LS, Yang YX. The Risks and Benefits of Long-term Use of Proton Pump Inhibitors: Expert Review and Best Practice Advice From the American Gastroenterological Association. Gastroenterology. 2017;152:706–715. doi: 10.1053/j.gastro.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 10.Chueca E, Apostolova N, Esplugues JV, García-González MA, Lanas Á, Piazuelo E. Proton Pump Inhibitors Display Antitumor Effects in Barrett's Adenocarcinoma Cells. Front Pharmacol. 2016;7:452. doi: 10.3389/fphar.2016.00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Milito A, Canese R, Marino ML, Borghi M, Iero M, Villa A, Venturi G, Lozupone F, Iessi E, Logozzi M, Della Mina P, Santinami M, Rodolfo M, Podo F, Rivoltini L, Fais S. pH-dependent antitumor activity of proton pump inhibitors against human melanoma is mediated by inhibition of tumor acidity. Int J Cancer. 2010;127:207–219. doi: 10.1002/ijc.25009. [DOI] [PubMed] [Google Scholar]
- 12.De Milito A, Fais S. Proton pump inhibitors may reduce tumour resistance. Expert Opin Pharmacother. 2005;6:1049–1054. doi: 10.1517/14656566.6.7.1049. [DOI] [PubMed] [Google Scholar]
- 13.Lundell L, Vieth M, Gibson F, Nagy P, Kahrilas PJ. Systematic review: the effects of long-term proton pump inhibitor use on serum gastrin levels and gastric histology. Aliment Pharmacol Ther. 2015;42:649–663. doi: 10.1111/apt.13324. [DOI] [PubMed] [Google Scholar]
- 14.Waldum HL, Sørdal Ø, Fossmark R. Proton pump inhibitors (PPIs) may cause gastric cancer - clinical consequences. Scand J Gastroenterol. 2018;53:639–642. doi: 10.1080/00365521.2018.1450442. [DOI] [PubMed] [Google Scholar]
- 15.Feng J, Petersen CD, Coy DH, Jiang JK, Thomas CJ, Pollak MR, Wank SA. Calcium-sensing receptor is a physiologic multimodal chemosensor regulating gastric G-cell growth and gastrin secretion. Proc Natl Acad Sci U S A. 2010;107:17791–17796. doi: 10.1073/pnas.1009078107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fossmark R, Rao S, Mjønes P, Munkvold B, Flatberg A, Varro A, Thommesen L, Nørsett KG. PAI-1 deficiency increases the trophic effects of hypergastrinemia in the gastric corpus mucosa. Peptides. 2016;79:83–94. doi: 10.1016/j.peptides.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 17.Kato S, Tsukamoto T, Mizoshita T, Tanaka H, Kumagai T, Ota H, Katsuyama T, Asaka M, Tatematsu M. High salt diets dose-dependently promote gastric chemical carcinogenesis in Helicobacter pylori-infected Mongolian gerbils associated with a shift in mucin production from glandular to surface mucous cells. Int J Cancer. 2006;119:1558–1566. doi: 10.1002/ijc.21810. [DOI] [PubMed] [Google Scholar]
- 18.Murphy G, Abnet CC, Choo-Wosoba H, Vogtmann E, Weinstein SJ, Taylor PR, Männistö S, Albanes D, Dawsey SM, Rehfeld JF, Freedman ND. Serum gastrin and cholecystokinin are associated with subsequent development of gastric cancer in a prospective cohort of Finnish smokers. Int J Epidemiol. 2017;46:914–923. doi: 10.1093/ije/dyx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waldum HL, Aase S, Kvetnoi I, Brenna E, Sandvik AK, Syversen U, Johnsen G, Vatten L, Polak JM. Neuroendocrine differentiation in human gastric carcinoma. Cancer. 1998;83:435–444. [PubMed] [Google Scholar]
- 20.Bakkelund K, Fossmark R, Nordrum I, Waldum H. Signet ring cells in gastric carcinomas are derived from neuroendocrine cells. J Histochem Cytochem. 2006;54:615–621. doi: 10.1369/jhc.5A6806.2005. [DOI] [PubMed] [Google Scholar]
- 21.Sørdal Ø, Qvigstad G, Nordrum IS, Sandvik AK, Gustafsson BI, Waldum H. The PAS positive material in gastric cancer cells of signet ring type is not mucin. Exp Mol Pathol. 2014;96:274–278. doi: 10.1016/j.yexmp.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 22.Waldum HL, Hauso Ø, Sørdal ØF, Fossmark R. Gastrin May Mediate the Carcinogenic Effect of Helicobacter pylori Infection of the Stomach. Dig Dis Sci. 2015;60:1522–1527. doi: 10.1007/s10620-014-3468-9. [DOI] [PubMed] [Google Scholar]
- 23.Parsons BN, Ijaz UZ, D'Amore R, Burkitt MD, Eccles R, Lenzi L, Duckworth CA, Moore AR, Tiszlavicz L, Varro A, Hall N, Pritchard DM. Comparison of the human gastric microbiota in hypochlorhydric states arising as a result of Helicobacter pylori-induced atrophic gastritis, autoimmune atrophic gastritis and proton pump inhibitor use. PLoS Pathog. 2017;13:e1006653. doi: 10.1371/journal.ppat.1006653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.García Rodríguez LA, Lagergren J, Lindblad M. Gastric acid suppression and risk of oesophageal and gastric adenocarcinoma: a nested case control study in the UK. Gut. 2006;55:1538–1544. doi: 10.1136/gut.2005.086579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamim H, Duranceau A, Chen LQ, Lelorier J. Association between use of acid-suppressive drugs and risk of gastric cancer. A nested case-control study. Drug Saf. 2008;31:675–684. doi: 10.2165/00002018-200831080-00004. [DOI] [PubMed] [Google Scholar]
- 26.Poulsen AH, Christensen S, McLaughlin JK, Thomsen RW, Sørensen HT, Olsen JH, Friis S. Proton pump inhibitors and risk of gastric cancer: a population-based cohort study. Br J Cancer. 2009;100:1503–1507. doi: 10.1038/sj.bjc.6605024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tran-Duy A, Spaetgens B, Hoes AW, de Wit NJ, Stehouwer CD. Use of Proton Pump Inhibitors and Risks of Fundic Gland Polyps and Gastric Cancer: Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2016;14:1706–1719.e5. doi: 10.1016/j.cgh.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 28.Ahn JS, Eom CS, Jeon CY, Park SM. Acid suppressive drugs and gastric cancer: a meta-analysis of observational studies. World J Gastroenterol. 2013;19:2560–2568. doi: 10.3748/wjg.v19.i16.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheung KS, Chan EW, Wong AYS, Chen L, Wong ICK, Leung WK. Long-term proton pump inhibitors and risk of gastric cancer development after treatment for Helicobacter pylori: a population-based study. Gut. 2018;67:28–35. doi: 10.1136/gutjnl-2017-314605. [DOI] [PubMed] [Google Scholar]
- 30.Suissa S, Suissa A. Proton-pump inhibitors and increased gastric cancer risk: time-related biases. Gut. 2018;67:2228–2229. doi: 10.1136/gutjnl-2017-315729. [DOI] [PubMed] [Google Scholar]
- 31.Laterza L, Scaldaferri F, Gasbarrini A. Risk factors for gastric cancer: is it time to discard PPIs? Gut. 2019;68:176–177. doi: 10.1136/gutjnl-2017-315621. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki H, Matsuzaki J. Helicobacter pylori eradication failure may have confounded the recent large-scale health database study that showed proton pump inhibitors increase gastric cancer risk. Gut. 2018;67:2071–2072. doi: 10.1136/gutjnl-2017-315698. [DOI] [PubMed] [Google Scholar]
- 33.Brusselaers N, Wahlin K, Engstrand L, Lagergren J. Maintenance therapy with proton pump inhibitors and risk of gastric cancer: a nationwide population-based cohort study in Sweden. BMJ Open. 2017;7:e017739. doi: 10.1136/bmjopen-2017-017739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuipers EJ, Lundell L, Klinkenberg-Knol EC, Havu N, Festen HP, Liedman B, Lamers CB, Jansen JB, Dalenback J, Snel P, Nelis GF, Meuwissen SG. Atrophic gastritis and Helicobacter pylori infection in patients with reflux esophagitis treated with omeprazole or fundoplication. N Engl J Med. 1996;334:1018–1022. doi: 10.1056/NEJM199604183341603. [DOI] [PubMed] [Google Scholar]
- 35.Lundell L, Miettinen P, Myrvold HE, Pedersen SA, Thor K, Andersson A, Hattlebakk J, Havu N, Janatuinen E, Levander K, Liedman B, Nyström P. Lack of effect of acid suppression therapy on gastric atrophy. Nordic Gerd Study Group. Gastroenterology. 1999;117:319–326. doi: 10.1053/gast.1999.0029900319. [DOI] [PubMed] [Google Scholar]
- 36.Lundell L, Havu N, Miettinen P, Myrvold HE, Wallin L, Julkunen R, Levander K, Hatlebakk JG, Liedman B, Lamm M, Malm A, Walan A Nordic Gerd Study Group. Changes of gastric mucosal architecture during long-term omeprazole therapy: results of a randomized clinical trial. Aliment Pharmacol Ther. 2006;23:639–647. doi: 10.1111/j.1365-2036.2006.02792.x. [DOI] [PubMed] [Google Scholar]
- 37.Genta RM, Rindi G, Fiocca R, Magner DJ, D'Amico D, Levine DS. Effects of 6-12 months of esomeprazole treatment on the gastric mucosa. Am J Gastroenterol. 2003;98:1257–1265. doi: 10.1111/j.1572-0241.2003.07489.x. [DOI] [PubMed] [Google Scholar]
- 38.Song H, Zhu J, Lu D. Long-term proton pump inhibitor (PPI) use and the development of gastric pre-malignant lesions. Cochrane Database Syst Rev. 2014:CD010623. doi: 10.1002/14651858.CD010623.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strand DS, Kim D, Peura DA. 25 Years of Proton Pump Inhibitors: A Comprehensive Review. Gut Liver. 2017;11:27–37. doi: 10.5009/gnl15502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee AH, Tannock IF. Heterogeneity of intracellular pH and of mechanisms that regulate intracellular pH in populations of cultured cells. Cancer Res. 1998;58:1901–1908. [PubMed] [Google Scholar]
- 41.Cianfriglia M, Cenciarelli C, Tombesi M, Barca S, Mariani M, Morrone S, Santoni A, Samoggia P, Alessio M, Malavasi F. Murine monoclonal antibody recognizing a 90-kDa cell-surface determinant selectively lost by multi-drug-resistant variants of CEM cells. Int J Cancer. 1990;45:95–103. doi: 10.1002/ijc.2910450118. [DOI] [PubMed] [Google Scholar]
- 42.Chen D, Wu M, Li Y, Chang I, Yuan Q, Ekimyan-Salvo M, Deng P, Yu B, Yu Y, Dong J, Szymanski JM, Ramadoss S, Li J, Wang CY. Targeting BMI1+ Cancer Stem Cells Overcomes Chemoresistance and Inhibits Metastases in Squamous Cell Carcinoma. Cell Stem Cell. 2017;20:621–634.e6. doi: 10.1016/j.stem.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han YM, Park JM, Kangwan N, Jeong M, Lee S, Cho JY, Ko WJ, Hahm KB. Role of proton pump inhibitors in preventing hypergastrinemia-associated carcinogenesis and in antagonizing the trophic effect of gastrin. J Physiol Pharmacol. 2015;66:159–167. [PubMed] [Google Scholar]
- 44.Kim YS, Lee HJ, Park JM, Han YM, Kangwan N, Oh JY, Lee DY, Hahm KB. Targeted molecular ablation of cancer stem cells for curing gastrointestinal cancers. Expert Rev Gastroenterol Hepatol. 2017;11:1059–1070. doi: 10.1080/17474124.2017.1356224. [DOI] [PubMed] [Google Scholar]
- 45.Yeo M, Kim DK, Kim YB, Oh TY, Lee JE, Cho SW, Kim HC, Hahm KB. Selective induction of apoptosis with proton pump inhibitor in gastric cancer cells. Clin Cancer Res. 2004;10:8687–8696. doi: 10.1158/1078-0432.CCR-04-1065. [DOI] [PubMed] [Google Scholar]
- 46.Chen M, Zou X, Luo H, Cao J, Zhang X, Zhang B, Liu W. Effects and mechanisms of proton pump inhibitors as a novel chemosensitizer on human gastric adenocarcinoma (SGC7901) cells. Cell Biol Int. 2009;33:1008–1019. doi: 10.1016/j.cellbi.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 47.Shen W, Zou X, Chen M, Shen Y, Huang S, Guo H, Zhang L, Liu P. Effect of pantoprazole on human gastric adenocarcinoma SGC7901 cells through regulation of phosphoLRP6 expression in Wnt/β-catenin signaling. Oncol Rep. 2013;30:851–855. doi: 10.3892/or.2013.2524. [DOI] [PubMed] [Google Scholar]
- 48.Chen M, Lu J, Wei W, Lv Y, Zhang X, Yao Y, Wang L, Ling T, Zou X. Effects of proton pump inhibitors on reversing multidrug resistance via downregulating V-ATPases/PI3K/Akt/mTOR/HIF-1α signaling pathway through TSC1/2 complex and Rheb in human gastric adenocarcinoma cells in vitro and in vivo. Onco Targets Ther. 2018;11:6705–6722. doi: 10.2147/OTT.S161198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guan XW, Zhao F, Wang JY, Wang HY, Ge SH, Wang X, Zhang L, Liu R, Ba Y, Li HL, Deng T, Zhou LK, Bai M, Ning T, Zhang HY, Huang DZ. Tumor microenvironment interruption: a novel anti-cancer mechanism of Proton-pump inhibitor in gastric cancer by suppressing the release of microRNA-carrying exosomes. Am J Cancer Res. 2017;7:1913–1925. [PMC free article] [PubMed] [Google Scholar]
- 50.Judd LM, Bredin K, Kalantzis A, Jenkins BJ, Ernst M, Giraud AS. STAT3 activation regulates growth, inflammation, and vascularization in a mouse model of gastric tumorigenesis. Gastroenterology. 2006;131:1073–1085. doi: 10.1053/j.gastro.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 51.Yu H, Lee H, Herrmann A, Buettner R, Jove R. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer. 2014;14:736–746. doi: 10.1038/nrc3818. [DOI] [PubMed] [Google Scholar]
- 52.Yoon J, Ko YS, Cho SJ, Park J, Choi YS, Choi Y, Pyo JS, Ye SK, Youn HD, Lee JS, Chang MS, Kim MA, Lee BL. Signal transducers and activators of transcription 3-induced metastatic potential in gastric cancer cells is enhanced by glycogen synthase kinase-3β. APMIS. 2015;123:373–382. doi: 10.1111/apm.12370. [DOI] [PubMed] [Google Scholar]
- 53.Chen G, Tang N, Wang C, Xiao L, Yu M, Zhao L, Cai H, Han L, Xie C, Zhang Y. TNF-α-inducing protein of Helicobacter pylori induces epithelial-mesenchymal transition (EMT) in gastric cancer cells through activation of IL-6/STAT3 signaling pathway. Biochem Biophys Res Commun. 2017;484:311–317. doi: 10.1016/j.bbrc.2017.01.110. [DOI] [PubMed] [Google Scholar]
- 54.Xu YY, Guo M, Yang LQ, Zhou F, Yu C, Wang A, Pang TH, Wu HY, Zou XP, Zhang WJ, Wang L, Xu GF, Huang Q. Regulation of CD44v6 expression in gastric carcinoma by the IL-6/STAT3 signaling pathway and its clinical significance. Oncotarget. 2017;8:45848–45861. doi: 10.18632/oncotarget.17435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim DY, Cha ST, Ahn DH, Kang HY, Kwon CI, Ko KH, Hwang SG, Park PW, Rim KS, Hong SP. STAT3 expression in gastric cancer indicates a poor prognosis. J Gastroenterol Hepatol. 2009;24:646–651. doi: 10.1111/j.1440-1746.2008.05671.x. [DOI] [PubMed] [Google Scholar]
- 56.Deng JY, Sun D, Liu XY, Pan Y, Liang H. STAT-3 correlates with lymph node metastasis and cell survival in gastric cancer. World J Gastroenterol. 2010;16:5380–5387. doi: 10.3748/wjg.v16.i42.5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deng J, Liang H, Zhang R, Sun D, Pan Y, Liu Y, Zhang L, Hao X. STAT3 is associated with lymph node metastasis in gastric cancer. Tumour Biol. 2013;34:2791–2800. doi: 10.1007/s13277-013-0837-5. [DOI] [PubMed] [Google Scholar]
- 58.Zhu Q, Zhang X, Zhang L, Li W, Wu H, Yuan X, Mao F, Wang M, Zhu W, Qian H, Xu W. The IL-6-STAT3 axis mediates a reciprocal crosstalk between cancer-derived mesenchymal stem cells and neutrophils to synergistically prompt gastric cancer progression. Cell Death Dis. 2014;5:e1295. doi: 10.1038/cddis.2014.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim MJ, Nam HJ, Kim HP, Han SW, Im SA, Kim TY, Oh DY, Bang YJ. OPB-31121, a novel small molecular inhibitor, disrupts the JAK2/STAT3 pathway and exhibits an antitumor activity in gastric cancer cells. Cancer Lett. 2013;335:145–152. doi: 10.1016/j.canlet.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 60.Oh DY, Lee SH, Han SW, Kim MJ, Kim TM, Kim TY, Heo DS, Yuasa M, Yanagihara Y, Bang YJ. Phase I Study of OPB-31121, an Oral STAT3 Inhibitor, in Patients with Advanced Solid Tumors. Cancer Res Treat. 2015;47:607–615. doi: 10.4143/crt.2014.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wong AL, Soo RA, Tan DS, Lee SC, Lim JS, Marban PC, Kong LR, Lee YJ, Wang LZ, Thuya WL, Soong R, Yee MQ, Chin TM, Cordero MT, Asuncion BR, Pang B, Pervaiz S, Hirpara JL, Sinha A, Xu WW, Yuasa M, Tsunoda T, Motoyama M, Yamauchi T, Goh BC. Phase I and biomarker study of OPB-51602, a novel signal transducer and activator of transcription (STAT) 3 inhibitor, in patients with refractory solid malignancies. Ann Oncol. 2015;26:998–1005. doi: 10.1093/annonc/mdv026. [DOI] [PubMed] [Google Scholar]
- 62.Yue P, Turkson J. Targeting STAT3 in cancer: how successful are we? Expert Opin Investig Drugs. 2009;18:45–56. doi: 10.1517/13543780802565791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bousquet C, Susini C, Melmed S. Inhibitory roles for SHP-1 and SOCS-3 following pituitary proopiomelanocortin induction by leukemia inhibitory factor. J Clin Invest. 1999;104:1277–1285. doi: 10.1172/JCI7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu C, Sun M, Liu L, Zhou GW. The function of the protein tyrosine phosphatase SHP-1 in cancer. Gene. 2003;306:1–12. doi: 10.1016/s0378-1119(03)00400-1. [DOI] [PubMed] [Google Scholar]
- 65.Koyama M, Oka T, Ouchida M, Nakatani Y, Nishiuchi R, Yoshino T, Hayashi K, Akagi T, Seino Y. Activated proliferation of B-cell lymphomas/leukemias with the SHP1 gene silencing by aberrant CpG methylation. Lab Invest. 2003;83:1849–1858. doi: 10.1097/01.lab.0000106503.65258.2b. [DOI] [PubMed] [Google Scholar]
- 66.Han Y, Amin HM, Franko B, Frantz C, Shi X, Lai R. Loss of SHP1 enhances JAK3/STAT3 signaling and decreases proteosome degradation of JAK3 and NPM-ALK in ALK+ anaplastic large-cell lymphoma. Blood. 2006;108:2796–2803. doi: 10.1182/blood-2006-04-017434. [DOI] [PubMed] [Google Scholar]
- 67.Chen KF, Tai WT, Liu TH, Huang HP, Lin YC, Shiau CW, Li PK, Chen PJ, Cheng AL. Sorafenib overcomes TRAIL resistance of hepatocellular carcinoma cells through the inhibition of STAT3. Clin Cancer Res. 2010;16:5189–5199. doi: 10.1158/1078-0432.CCR-09-3389. [DOI] [PubMed] [Google Scholar]
- 68.Tai WT, Cheng AL, Shiau CW, Liu CY, Ko CH, Lin MW, Chen PJ, Chen KF. Dovitinib induces apoptosis and overcomes sorafenib resistance in hepatocellular carcinoma through SHP-1-mediated inhibition of STAT3. Mol Cancer Ther. 2012;11:452–463. doi: 10.1158/1535-7163.MCT-11-0412. [DOI] [PubMed] [Google Scholar]
- 69.Tai WT, Chu PY, Shiau CW, Chen YL, Li YS, Hung MH, Chen LJ, Chen PL, Su JC, Lin PY, Yu HC, Chen KF. STAT3 mediates regorafenib-induced apoptosis in hepatocellular carcinoma. Clin Cancer Res. 2014;20:5768–5776. doi: 10.1158/1078-0432.CCR-14-0725. [DOI] [PubMed] [Google Scholar]
- 70.Sun T, Du W, Xiong H, Yu Y, Weng Y, Ren L, Zhao H, Wang Y, Chen Y, Xu J, Xiang Y, Qin W, Cao W, Zou W, Chen H, Hong J, Fang JY. TMEFF2 deregulation contributes to gastric carcinogenesis and indicates poor survival outcome. Clin Cancer Res. 2014;20:4689–4704. doi: 10.1158/1078-0432.CCR-14-0315. [DOI] [PubMed] [Google Scholar]
- 71.Sun TT, Tang JY, Du W, Zhao HJ, Zhao G, Yang SL, Chen HY, Hong J, Fang JY. Bidirectional regulation between TMEFF2 and STAT3 may contribute to Helicobacter pylori-associated gastric carcinogenesis. Int J Cancer. 2015;136:1053–1064. doi: 10.1002/ijc.29061. [DOI] [PubMed] [Google Scholar]
- 72.Joo MK, Park JJ, Yoo HS, Lee BJ, Chun HJ, Lee SW, Bak YT. Epigenetic regulation and anti-tumorigenic effects of SH2-containing protein tyrosine phosphatase 1 (SHP1) in human gastric cancer cells. Tumour Biol. 2016;37:4603–4612. doi: 10.1007/s13277-015-4228-y. [DOI] [PubMed] [Google Scholar]
- 73.Huang S, Chen M, Ding X, Zhang X, Zou X. Proton pump inhibitor selectively suppresses proliferation and restores the chemosensitivity of gastric cancer cells by inhibiting STAT3 signaling pathway. Int Immunopharmacol. 2013;17:585–592. doi: 10.1016/j.intimp.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 74.Koh JS, Joo MK, Park JJ, Yoo HS, Choi BI, Lee BJ, Chun HJ, Lee SW. Inhibition of STAT3 in gastric cancer: role of pantoprazole as SHP-1 inducer. Cell Biosci. 2018;8:50. doi: 10.1186/s13578-018-0248-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Song S, Min H, Niu M, Wang L, Wu Y, Zhang B, Chen X, Liang Q, Wen Y, Wang Y, Yi L, Wang H, Gao Q. S1PR1 predicts patient survival and promotes chemotherapy drug resistance in gastric cancer cells through STAT3 constitutive activation. EBioMedicine. 2018;37:168–176. doi: 10.1016/j.ebiom.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang S, Chen M, Shen Y, Shen W, Guo H, Gao Q, Zou X. Inhibition of activated Stat3 reverses drug resistance to chemotherapeutic agents in gastric cancer cells. Cancer Lett. 2012;315:198–205. doi: 10.1016/j.canlet.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 77.Li G, Zhao L, Li W, Fan K, Qian W, Hou S, Wang H, Dai J, Wei H, Guo Y. Feedback activation of STAT3 mediates trastuzumab resistance via upregulation of MUC1 and MUC4 expression. Oncotarget. 2014;5:8317–8329. doi: 10.18632/oncotarget.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang Z, Guo L, Liu D, Sun L, Chen H, Deng Q, Liu Y, Yu M, Ma Y, Guo N, Shi M. Acquisition of resistance to trastuzumab in gastric cancer cells is associated with activation of IL-6/STAT3/Jagged-1/Notch positive feedback loop. Oncotarget. 2015;6:5072–5087. doi: 10.18632/oncotarget.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang B, Yang Y, Shi X, Liao W, Chen M, Cheng AS, Yan H, Fang C, Zhang S, Xu G, Shen S, Huang S, Chen G, Lv Y, Ling T, Zhang X, Wang L, Zhuge Y, Zou X. Proton pump inhibitor pantoprazole abrogates adriamycin-resistant gastric cancer cell invasiveness via suppression of Akt/GSK-β/β-catenin signaling and epithelial-mesenchymal transition. Cancer Lett. 2015;356:704–712. doi: 10.1016/j.canlet.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 80.Joo MK, Park J-J, Koh JS, Yoo HS, Lee BJ, Chun HJ, Lee SW. Synergic effect of pantoprazole and docetaxel to inhibit EMT via induction of SHP‐1 in gastric cancer cells. Helicobacter. 2018;23 Suppl 1:e12525. [Google Scholar]