Abstract
The prevalence of non-alcoholic fatty liver disease (NAFLD) is rising exponentially worldwide. The spectrum of NAFLD includes non-alcoholic fatty liver, non-alcoholic steatohepatitis, liver cirrhosis, and even hepatocellular carcinoma. Evidence shows that microbial metabolites play pivotal roles in the onset and progression of NAFLD. In this review, we discuss how microbe-derived metabolites, such as short-chain fatty acids, endogenous ethanol, bile acids and so forth, contribute to the pathogenesis of NAFLD.
Keywords: Microbial metabolites, Non-alcoholic steatohepatitis, Short-chain fatty acids
Core tip: Non-alcoholic fatty liver disease (NAFLD) is a global epidemic metabolic disease lacking effective therapeutic strategies and the internal pathogenesis is still uncertain. Gut microbiota-derived metabolites have attracted much attention for its association with the onset and progression of NAFLD. In this review, we mainly elucidate the diverse roles of microbe-derived metabolites in the development of NAFLD, which is conducive to better understanding the biological functions of microbial metabolites in NAFLD via the gut-liver axis and facilitating the excavation of potential therapeutic approaches for NAFLD.
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is a chronic metabolic disorder that is strongly associated with obesity and metabolic syndrome. NAFLD has become the most common chronic liver disease worldwide[1,2], causing a substantial global health burden. Although the exact pathogenesis of NAFLD is uncertain, in addition to the well-known “two-hit” theory or the multiple-parallel-hits hypothesis[3,4], the dysbiosis of the gut microbiota also promotes the development of NAFLD by mediating the processes of energy metabolism, insulin resistance, immunity, and inflammation[5-7].
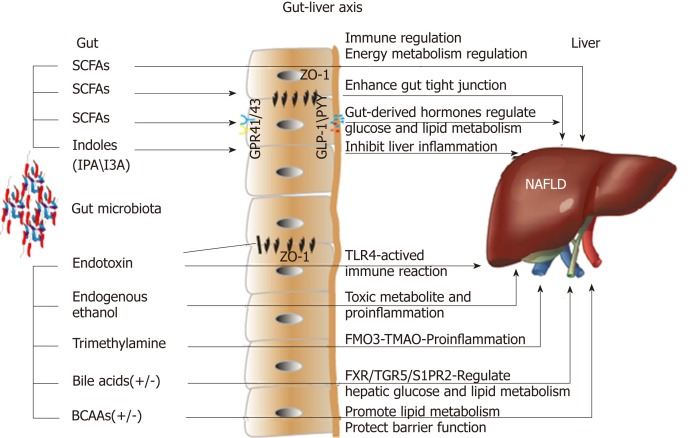
The gut flora in the intestinal tract exhibits high diversity and distinct differences, and the total number of bacterial cells can reach 1014[8]. The intestinal bacteria mainly belong to the following phyla: Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Verrucomicrobia, and Fusobacteria; together, Firmicutes and Bacteroidetes account for up to 90% of all bacterial cells in the human intestine. The gut microbiota is deemed a special "organ" in human beings; bacterial genes are approximately 100-fold more abundant than human genes, and they encode more functional genes[8]. A large proportion of bacterial genes and their biological functions are specific, and the metabolic potential related to the capacity for the conversion and degradation of host-derived substances is strong. Therefore, the gut microbiota exhibits a profound capacity to synthesize or produce many metabolites. Recently, increasing evidence has shown that these metabolites play pivotal roles in the interactions between the gut microbiota and the host in various ways, and the gut-liver axis is the main link between the gut and the liver (Figure 1). Naturally, an imbalance in the intestinal microbiome and the related metabolites contributes to the onset and progression of NAFLD[9,10]. The accurate pathological diagnosis of NAFLD relies on a liver biopsy; however, with further investigation, the gut microbiota and its metabolites may serve as potential biomarkers for NAFLD and non-alcoholic steatohepatitis (NASH). A clinical study demonstrated that certain gut microbiome-derived metabolites shared gene-effects with hepatic steatosis and liver fibrosis[11,12]. In addition, another study used targeted metagenomics and metabolomics analysis to demonstrate that a decrease in Oscillospira accompanied by upregulation of 2-butanone and an increase in Ruminococcus and Dorea were signatures of non-alcoholic fatty liver (NAFL) onset and NAFL-NASH progression[13]. However, additional validations with clinical samples are needed.
Figure 1.
Effects of microbial metabolites on non-alcoholic fatty liver disease via the gut-liver axis. SCFAs: Short-chain fatty acids; I3A: Indole-3-acetic acid; IPA: Indole propionic acid; GPR41/43: G-protein-coupled receptors 41/43; ZO-1: Zonula occludens 1; GLP-1: Glucagon-like peptide-1; PYY: Peptide YY; TLR4: Toll-like receptor 4; FMO3: Flavin-containing monooxygenase 3; TMAO: Trimethylamine-N-oxide; FXR: Farnesoid X receptor; TGR5: Takeda G-protein-coupled receptor 5; S1PR2: Sphingosine 1-phosphate receptor 2; BCAAs: Branched-chain amino acids.
Recently, several original investigations showed that the severity of NAFLD is associated with changes in the levels of certain metabolites in the serum; although not all such metabolites are synthesized or produced by gut bacteria[12,14-16], a better understanding of the role of these metabolites in the development of NAFLD will be valuable for the discovery of new non-invasive diagnostic and treatment options for NAFLD.
SHORT-CHAIN FATTY ACIDS (SCFAS)
The most important bacterial metabolites are SCFAs, which contain fewer than six carbon atoms and have become an increasingly studied gut metabolite due to their multiple biological functions in the liver[17]. The fermentation of dietary fibers by gut bacteria, including Roseburia, Ruminococcus, Salmonella, Blautia, Eubacterium, Anaerostipes, Coprococcus, Faecalibacterium, Marvinbryantia, and Megasphaera, is the main source of SCFAs. The most abundant SCFAs present in the colon lumen are acetate, propionate, and butyrate[18]. SCFAs not only provide energy for the intestinal epithelium, but they also have many bioactive roles, such as the regulation of immunity, lipometabolism, and glycometabolism, and the maintenance of gut microbiota homeostasis. SCFAs are involved in the pathogenesis of NAFLD after their absorption and delivery to the liver via the portal vein. A clinical study showed that propionate supplementation significantly reduced weight gain and intrahepatocellular lipid content, prevented deterioration in the case of insulin sensitivity, and significantly stimulated the release of peptide-YY and glucagon-like peptide-1 (GLP-1) from human colonic cells; these hormones are closely related to energy metabolism[19]. Another clinical study showed that the total amount of SCFAs was higher in obese subjects compared with lean subjects and, moreover, the ratio of the phyla Firmicutes to Bacteroidetes was altered in favor of Bacteroidetes in obese humans[20]. Basic studies have shown that butyrate-producing probiotics corrected high-fat diet (HFD)-induced enterohepatic immunologic dissonance and attenuated steatohepatitis in mice, which is mediated in part through SCFAs[21-23]. A clinical study showed that a select group of SCFAs-producing bacterial strains played pivotal roles in regulating glucose and lipid metabolism, in part through increased GLP-1 production; therefore, the targeted restoration of these SCFA producers may present a novel ecological approach for managing metabolic syndrome and NAFLD[24].
Increasing studies have revealed that SCFAs exert their biological functions mainly via activating the G-protein-coupled receptor (GPR) 41/43 or through the inhibition of histone deacetylase (HDAC). Animal experiments showed that GPR41 and GPR43 were involved in lipid and immune regulation, and GPR41/43 deficiency protected against HFD-induced obesity, insulin resistance, and dyslipidemia, in part via increased energy expenditure and the promotion of gut-derived hormone GLP-1[25-27]. In addition, the activation of GPR41/43 has been suggested to participate in the pathogenesis of NAFLD. As mentioned above, except for the activation of GPRs, SCFAs can inhibit HDAC directly and regulate the transcriptional activation of genes; among the SCFAs, butyrate is the most powerful HDAC inhibitor[28]. Previous animal studies showed that sodium butyrate supplementation could attenuate HFD-induced NASH, and the underlying mechanisms were associated with restoring the dysbiosis of gut microbiota and improving the gastrointestinal barrier, thereby inhibiting the delivery of gut-derived endotoxin into the liver[29]. Recently, an investigation found that the expression of hepatic GLP-1 receptor was significantly down-regulated in patients with NAFLD, and supplementation with butyrate enhanced hepatic GLP-1R expression in an NASH mouse model by inhibiting histone deacetylase-2 and activating AMP-activated protein kinase (AMPK). These findings indicated that butyrate could be a GLP-1 sensitizer and could prevent the progression of NAFL to NASH via promoting the expression of hepatic GLP-1R[30].
In general, SCFAs are thought to be beneficial prebiotics; however, a recent study demonstrated that the soluble fiber inulin, when fermented by gut bacteria into SCFAs, could induce icteric hepatocellular carcinoma (HCC)[31], which was an astounding finding. However, SCFA-induced HCC was shown to be conditional and microbiota-dependent, and this condition was observed in dysbiotic mice; meanwhile, the inhibition of fermentation reduced intestinal SCFAs and prevented HCC. Thus, the enrichment of fermentable fiber should be promoted with caution, and the intake of diverse types of dietary fiber should be emphasized to establish and maintain healthy gut microbiota. This topic still requires further investigation for the design, production, and supply of rational food additives to improve human health. According to various studies, it is very difficult to draw accurate conclusions about the roles of gut microbiota and SCFAs in NAFLD because confounding factors are extensive and cannot be ignored[32].
ENDOGENOUS ETHANOL AND ENDOTOXINS
It is well known that ethanol is a substance that can contribute to hepatic steatosis and inflammation and increase the risk of liver fibrosis and HCC[33]. Endogenous ethanol produced by bacterial fermentation (mainly by Ruminococcus) stimulates oxidative stress and aggravates liver inflammation in NAFLD, which was confirmed in animal experiments[34]. Zhu et al[35] showed that children and adolescents with NASH harbored more ethanol-producing bacteria in the gut and exhibited higher serum levels of ethanol; moreover, the expression of genes involved in alcohol metabolism was enhanced. Another clinical study showed that children with NAFLD had significantly higher serum levels of ethanol, which were associated with a greater abundance of Gammaproteobacteria and Prevotella[36]. However, another study demonstrated that the increased blood ethanol levels in patients with NAFLD might result from insulin-dependent impairments of ethanol dehydrogenase activity in the liver rather than an increase in endogenous ethanol synthesis[37]. Although these studies did not all produce consistent results, endogenous ethanol might play a pivotal role in the pathogenesis of NASH. Future investigations are required to determine the exact influence of endogenous ethanol on NAFLD and NASH.
Systemic low inflammatory state is related to the insulin resistance (IR) which contributes to the onset of NAFLD. Gram-negative bacteria-derived endotoxins such as lipopolysaccharide (LPS) were proved to stimulate and aggravate hepatic necroinflammation. The increased intestinal permeability and dysbiosis of gut microbiota promote the translocation of microbial products from intestinal lumen to the liver via the portal vein[38]. Toll-like receptors (TLRs), including TLR4, were involved in the LPS-induced liver damage, and LPS could activate TLR4-myeloid differentiation primary-response gene 88 (Myd88) signaling pathway, causing IR, hepatic steatosis, liver inflammation, and fibrosis[39,40]. In addition, Kupffer cells positive for cluster of differentiation 14 (CD14) could enhance LPS-TLR4 response in the liver[41].
BRANCHED-CHAIN AMINO ACIDS (BCAAS)
BCAAs are produced by proteolytic fermentation in the colon. Species implicated in proteolytic fermentation include Clostridium, Fusobacterium, Bacteroides, Actinomyces, Propionibacterium, and Peptostreptococci[42]. Patients with NAFLD have dysregulation of BCAA metabolism, the BCAAs including leucine, valine, and isoleucine were higher both in the blood and urine samples from NAFLD patients[16], the circulating BCAAs were negatively correlated with hepatic insulin sensitivity, and the baseline valine level was identified to be predictive of liver fat accumulation[43]. Meanwhile, BCAAs could reflect hepatic steatosis independently of routine metabolic risk factors, and the metabolic aberrations of BCAAs may precede the development of NAFLD to a certain extent[44]. The increased BCAA levels (valine, leucine, and isoleucine) and downstream BCAA metabolites, such as branched-chain keto acids and short-chain acylcarnitines, were associated with a greater body mass index (BMI)[45]. Further animal experiments showed that BCAA supplementation reduced HFD-induced overweight, but caused obvious liver damage in HFD mice, which was associated with the abnormal lipolysis[46]. Oppositely, several studies indicated that BCAA intervention could alleviate NASH in animal models via inhibiting triglyceride deposition in hepatocytes and reducing oxidative and endoplasmic reticulum stress[47-50]. BCAAs were found to have the ability to improve immune function, decrease susceptibility to pathogens, promote the growth of intestinal beneficial bacteria, and enhance the intestinal barrier function[51], all of which appear to prevent the gut-derived toxic substances into the liver.
According to the inconsistent results, limited information is available about the accurate role of BCAAs in the metabolic diseases; it may be valuable to develop diagnostic biomarkers for NAFLD. Further research examining proteolytic fermentation may be vital to understand the interaction between the BCAAs and NAFLD.
BILE ACIDS
Bile acids are not directly produced by the intestinal microbiota; rather, they are mainly synthesized in the liver by using cholesterol as the substrate. Bile acids can be deconjugated and dehydroxylated by the gut microbiota, and the enterohepatic circulation of bile acids, which are reabsorbed and returned to the liver via the portal vein, perform many biological functions involved in lipid and glucose metabolism, and are linked to the pathogenesis and treatment of NASH. In an early clinical trial using Danning Pian (a traditional Chinese medicine that regulates bile acid metabolism) to treat NAFLD, clinical symptoms, serum alanine transaminase levels, blood lipid profiles, and ultrasound-based fatty liver were significantly improved after three months of treatment[52]. Studies show that patients with NASH exhibit alterations in their bile acid profile. The serum levels of bile acids were shown to be elevated in patients with NASH, including the more hydrophobic and cytotoxic secondary species; this increased bile acid exposure may be involved in the pathogenesis of NAFLD[53]. Furthermore, another clinical study demonstrated that increased bile acids were significantly associated with higher grades of heaptic steatosis (taurocholate), lobular (glycocholate) and portal inflammation (taurolithocholate), and hepatocyte ballooning (taurocholate), while the conjugated cholate and taurocholate directly and secondary to primary bile acid ratio was inversely correlated to NAFLD activity score[54]. These results indicated a relationship between the specific bile acids and the histological features of NASH.
Obesity is strongly associated with NAFLD and HCC[55]. Recently, deoxycholicacid (DCA) and the senescence-associated secretory phenotype (SASP) axis were found to have crucial roles in promoting obesity-associated HCC in mice; obesity induces alterations in the gut microbiota and contributes to the increase in DCA, which can cause DNA damage. Moreover, the enterohepatic circulation of DCA promotes the SASP in hepatic stellate cells, which consequently secrete various inflammatory and tumor-promoting factors in the liver. Hence, HCC development was exacerbated in mice after exposure to this chemical carcinogen[56]. This work inferred that maintaining a balanced intestinal microbiota should be advocated, and weight loss may be an effective method.
In addition, bile acids are ligands for the nuclear receptor farnesoid X receptor (FXR). FXR-mediated signaling has beneficial effects on hepatic lipid and carbohydrate metabolism, and this signaling pathway also modulates primary bile acid synthesis in the liver. A previous study found that the serum concentration of bile acids was increased in patients with NAFLD, and the FXR antagonistic deoxycholic acid was also increased, whereas the agonistic chenodeoxycholic acid and the serum level of fibroblast growth factor 19 (FGF 19) were decreased in NAFLD; these alterations contribute to the suppression of hepatic FXR-mediated and fibroblast growth factor receptor 4-mediated signaling, thereby exacerbating NAFLD[57]. This study indicated that targeting FXR signaling might be helpful to the intervention of NAFLD. Fan et al. reported that ursodeoxycholic acid (UDCA) combined with a low-calorie diet had therapeutic effects on steatohepatitis in rats[58]. A clinical study demonstrated that patients with NASH who were treated with obeticholic acid (an activator of FXR) exhibited improvements in liver fibrosis, hepatocellular ballooning, steatosis, and lobular inflammation, although the long-term benefits and safety of this drug treatment require further clarification[59]. Newer synthetic FXR agonists that are currently being investigated might cause fewer side effects and exert more powerful effects against NASH[60]. Except for FXR, bile acids are ligands for the cell membrane G-protein-coupled bile acid receptor 1 (known as Takeda G-protein-coupled receptor 5 [TGR5]). TGR5 can regulate inflammation and glucose homeostasis in the liver, which may be associated with the release of GLP-1 and the inhibition of the NLRP3 inflammasome; meanwhile, the activation of TGR5 results in sustained weight loss, improved hepatic steatosis, remitted insulin resistance, and increased energy expenditure in mice[61,62]. Nagahashi et al[63] found that the conjugated bile acids can activate the ERK1/2 and AKT signaling pathways via sphingosine 1-phosphate receptor 2 (S1PR2) in rodent hepatocytes and in vivo to regulate hepatic lipid metabolism. Overall, bile acids are important substances for communication between the liver and the gut; therefore, therapeutically targeting bile acid-related pathways warrants further exploration.
TRIMETHYLAMINE
The nutrient choline was first classified as an essential nutrient due to its physiological function in the prevention of NAFLD[64]. Choline deficiency can lead to NAFLD; thus, a choline-deficient diet is widely used in animal models of NASH[65]. Choline is mainly obtained from the diet, and studies have shown that choline was metabolized to trimethylamine (TMA) by the gut microbiota including Proteus penneri, Escherichia fergusonii, Proteus mirabilis, and other bacteria which can cut the C-N bond of choline[66,67]. TMA is absorbed into the liver via the portal vein and oxidized by hepatic flavin-containing monooxygenases into trimethylamine-N-oxide (TMAO)[68]. TMAO is found to contribute to many metabolic diseases, such as cardiovascular diseases, type 2 diabetes mellitus, and NAFLD. A clinical study found that the circulating levels of TMAO were inversely associated with the severity of NAFLD; in particular, the serum levels of TMAO, choline, and the betaine/choline ratio were shown to be adversely associated with the scores of steatosis and total NAFLD activity. Moreover, the severity of NAFLD was independently correlated with higher serum levels of TMAO, lower levels of betaine, and a lower ratio of betaine/choline[69]. Although the direct mechanisms through which TMA is involved in the onset and progression of NAFLD require further investigation, another clinical study demonstrated that the serum levels of TMAO increased along with BMI and were strongly associated with the fatty liver index, suggesting that a specific cut-off value of serum TMAO might help to identify subjects who are at high risk for NAFLD[70]. Animal experiments showed that in HFD-fed 129S6 mice, the impaired glucose homeostasis and NAFLD occurred, which were associated with disruptions in choline metabolism; meanwhile, the circulating plasma levels of phosphatidylcholine were lower, and the urinary excretion of methylamines was higher, indicating the crucial role of the metabolic balance of choline by the gut microbiota[71]. In addition, previous experiments demonstrated that supplementation with TMAO along with an HFD exacerbated impaired glucose tolerance, obstructed the hepatic insulin signaling pathway, and caused adipose tissue inflammation in mice[72]. Moreover, blocking the TMAO-producing enzyme flavin-containing monooxygenase 3 (FMO3) can regulate obesity and the beiging of white adipose tissue[73]. There are also inconsistent results showing that supplementation with TMAO in HFD-fed mice attenuated impaired glucose tolerance and increased insulin secretion. Therefore, the effects of TMAO on NAFLD might be a double-edged sword[74]. In addition, TMAO can influence cholesterol transport, thereby reducing the synthesis of bile acids and decreasing the production of very low-density lipoprotein (VLDL)[75-77]. In research on other disease, the inhibition of TMA production exhibited beneficial effects on cardiometabolic diseases[67]. Therefore, further well-designed studies are needed to explore the effects of TMA on metabolic syndrome and NAFLD.
TRYPTOPHAN METABOLITES
In addition to the aforementioned gut microbiota-derived metabolites, tryptophan metabolites have been shown to affect the development of NAFLD. Indoles are the main tryptophan-derived gut bacterial products, which include indole-3-acetic acid (I3A), indole propionic acid (IPA), indole-3-lactic acid, indole-3-carboxylic acid, and tryptamine, mainly produced by Bacteroides, Eubacterium, and Clostridium[18]. I3A and tryptamine reduced the production of pro-inflammatory cytokines by macrophages and inhibited macrophage migration to monocyte chemoattractant protein-1. In addition, I3A could alleviate cytokine-mediated lipogenesis in hepatocytes via the activation of the aryl-hydrocarbon receptor[78]. This study suggests that I3A and tryptamine are crucial metabolites that mediate host-microbiota crosstalk. Further studies are warranted, including animal experiments and clinical investigations, to determine whether I3A and tryptamine can effectively alleviate NAFLD.
Obesity is definitely associated with the morbidity of NAFLD, and supplement of IPA obviously reduced weight gain in animal experiments[79]. Previous work showed that IPA could scavenge free radicals and reduce oxidative stress[79], and IPA was thought to be a candidate for treatment of metabolic disorders as for its beneficial effects on glucose metabolism and insulin resistance[80]. Besides, IPA was found to be lower in obese subjects, and the elevation of plasma IPA level improved intestinal barrier function in vitro and in vivo through the combination of IPA and pregnane X receptor[81,82], which in turn inhibited the endotoxin-induced TLR4 signaling and improved tissue inflammation. Taken all together, further basic and clinical research on the tryptophan-derived microbial metabolite may be crucial for understanding their implications in obesity and NAFLD.
CONCLUSION
NAFLD has become the most common chronic liver disease worldwide. The interactions between the gut microbiota and NAFLD have been widely investigated, and advances in understanding the molecular mechanisms underlying the gut-liver interactions are critical to the development of non-invasive serum biomarkers and targeted therapies for NAFLD and NASH. To date, the precise association between the gut microbiota and NAFLD, as well as an accurate definition of a healthy gut microbiota, are still difficult to conclude; however, the gut microbiota is undoubtedly a contributing pathogenic factor in NAFLD, and the microbial metabolites serve as a key bridge between the gut microbiota and NAFLD via the gut-liver axis.
Although alterations in microbial metabolites may be remarkable therapeutic targets or excellent biomarkers for NAFLD, conclusions from different studies are inconsistent due to numerous uncontrollable factors that influence the results. Therefore, unified research standards, detection methods and conditions, and evaluation approaches should be established. On the other hand, translational and precision studies including a single species or a specific bacterial group, and a single signaling pathway molecule associated with bacterial metabolism should be employed for improvements of human health. In addition, a larger and more comprehensive clinical cohort from the world is indispensable. Different races, environments, and genetic backgrounds should be effectively distinguished, which would help to obtain more accurate, stable, and applicable results. Hopefully, individualized treatment for NAFLD targeting the gut microbiota or microbial metabolites will be revealed in the near future.
Footnotes
Conflict-of-interest statement: No potential conflicts of interest.
Peer-review started: February 22, 2019
First decision: March 20, 2019
Article in press: March 30, 2019
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Milovanovic T, Sitkin S S-Editor: Ma RY L-Editor: Wang TQ E-Editor:Ma YJ
Contributor Information
Da Zhou, Department of Gastroenterology and Hepatology, Zhongshan Hospital, Fudan University, Shanghai Institute of Liver Disease, Shanghai 200032, China.
Jian-Gao Fan, Center for Fatty Liver, Department of Gastroenterology, Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai Key Lab of Pediatric Gastroenterology and Nutrition, Shanghai 200092, China. fanjiangao@xinhuamed.com.cn.
References
- 1.Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology. 2014;60:2099–2108. doi: 10.1002/hep.27406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fan JG, Kim SU, Wong VW. New trends on obesity and NAFLD in Asia. J Hepatol. 2017;67:862–873. doi: 10.1016/j.jhep.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 4.Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836–1846. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- 5.Diehl AM, Day C. Cause, Pathogenesis, and Treatment of Nonalcoholic Steatohepatitis. N Engl J Med. 2017;377:2063–2072. doi: 10.1056/NEJMra1503519. [DOI] [PubMed] [Google Scholar]
- 6.Le Roy T, Llopis M, Lepage P, Bruneau A, Rabot S, Bevilacqua C, Martin P, Philippe C, Walker F, Bado A, Perlemuter G, Cassard-Doulcier AM, Gérard P. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2013;62:1787–1794. doi: 10.1136/gutjnl-2012-303816. [DOI] [PubMed] [Google Scholar]
- 7.Khan R, Bril F, Cusi K, Newsome PN. Modulation of Insulin Resistance in NAFLD. Hepatology. 2018 doi: 10.1002/hep.30429. [DOI] [PubMed] [Google Scholar]
- 8.Blaut M. Gut microbiota and energy balance: role in obesity. Proc Nutr Soc. 2015;74:227–234. doi: 10.1017/S0029665114001700. [DOI] [PubMed] [Google Scholar]
- 9.Shen F, Zheng RD, Sun XQ, Ding WJ, Wang XY, Fan JG. Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis Int. 2017;16:375–381. doi: 10.1016/S1499-3872(17)60019-5. [DOI] [PubMed] [Google Scholar]
- 10.Cao Y, Pan Q, Cai W, Shen F, Chen GY, Xu LM, Fan JG. Modulation of Gut Microbiota by Berberine Improves Steatohepatitis in High-Fat Diet-Fed BALB/C Mice. Arch Iran Med. 2016;19:197–203. [PubMed] [Google Scholar]
- 11.Chu H, Duan Y, Yang L, Schnabl B. Small metabolites, possible big changes: a microbiota-centered view of non-alcoholic fatty liver disease. Gut. 2019;68:359–370. doi: 10.1136/gutjnl-2018-316307. [DOI] [PubMed] [Google Scholar]
- 12.Caussy C, Hsu C, Lo MT, Liu A, Bettencourt R, Ajmera VH, Bassirian S, Hooker J, Sy E, Richards L, Schork N, Schnabl B, Brenner DA, Sirlin CB, Chen CH, Loomba R Genetics of NAFLD in Twins Consortium. Link between gut-microbiome derived metabolite and shared gene-effects with hepatic steatosis and fibrosis in NAFLD. Hepatology. 2018 doi: 10.1002/hep.29892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Del Chierico F, Nobili V, Vernocchi P, Russo A, Stefanis C, Gnani D, Furlanello C, Zandonà A, Paci P, Capuani G, Dallapiccola B, Miccheli A, Alisi A, Putignani L. Gut microbiota profiling of pediatric nonalcoholic fatty liver disease and obese patients unveiled by an integrated meta-omics-based approach. Hepatology. 2017;65:451–464. doi: 10.1002/hep.28572. [DOI] [PubMed] [Google Scholar]
- 14.Loomba R, Seguritan V, Li W, Long T, Klitgord N, Bhatt A, Dulai PS, Caussy C, Bettencourt R, Highlander SK, Jones MB, Sirlin CB, Schnabl B, Brinkac L, Schork N, Chen CH, Brenner DA, Biggs W, Yooseph S, Venter JC, Nelson KE. Gut Microbiome-Based Metagenomic Signature for Non-invasive Detection of Advanced Fibrosis in Human Nonalcoholic Fatty Liver Disease. Cell Metab. 2017;25:1054–1062.e5. doi: 10.1016/j.cmet.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boursier J, Mueller O, Barret M, Machado M, Fizanne L, Araujo-Perez F, Guy CD, Seed PC, Rawls JF, David LA, Hunault G, Oberti F, Calès P, Diehl AM. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63:764–775. doi: 10.1002/hep.28356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoyles L, Fernández-Real JM, Federici M, Serino M, Abbott J, Charpentier J, Heymes C, Luque JL, Anthony E, Barton RH, Chilloux J, Myridakis A, Martinez-Gili L, Moreno-Navarrete JM, Benhamed F, Azalbert V, Blasco-Baque V, Puig J, Xifra G, Ricart W, Tomlinson C, Woodbridge M, Cardellini M, Davato F, Cardolini I, Porzio O, Gentileschi P, Lopez F, Foufelle F, Butcher SA, Holmes E, Nicholson JK, Postic C, Burcelin R, Dumas ME. Molecular phenomics and metagenomics of hepatic steatosis in non-diabetic obese women. Nat Med. 2018;24:1070–1080. doi: 10.1038/s41591-018-0061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panasevich MR, Peppler WT, Oerther DB, Wright DC, Rector RS. Microbiome and NAFLD: potential influence of aerobic fitness and lifestyle modification. Physiol Genomics. 2017;49:385–399. doi: 10.1152/physiolgenomics.00012.2017. [DOI] [PubMed] [Google Scholar]
- 18.Canfora EE, Meex RCR, Venema K, Blaak EE. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev Endocrinol. 2019 doi: 10.1038/s41574-019-0156-z. [DOI] [PubMed] [Google Scholar]
- 19.Chambers ES, Viardot A, Psichas A, Morrison DJ, Murphy KG, Zac-Varghese SE, MacDougall K, Preston T, Tedford C, Finlayson GS, Blundell JE, Bell JD, Thomas EL, Mt-Isa S, Ashby D, Gibson GR, Kolida S, Dhillo WS, Bloom SR, Morley W, Clegg S, Frost G. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 2015;64:1744–1754. doi: 10.1136/gutjnl-2014-307913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C, Hardt PD. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 2010;18:190–195. doi: 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- 21.Zhou D, Pan Q, Liu XL, Yang RX, Chen YW, Liu C, Fan JG. Clostridium butyricum B1 alleviates high-fat diet-induced steatohepatitis in mice via enterohepatic immunoregulation. J Gastroenterol Hepatol. 2017;32:1640–1648. doi: 10.1111/jgh.13742. [DOI] [PubMed] [Google Scholar]
- 22.Liang Y, Lin C, Zhang Y, Deng Y, Liu C, Yang Q. Probiotic mixture of Lactobacillus and Bifidobacterium alleviates systemic adiposity and inflammation in non-alcoholic fatty liver disease rats through Gpr109a and the commensal metabolite butyrate. Inflammopharmacology. 2018;26:1051–1055. doi: 10.1007/s10787-018-0479-8. [DOI] [PubMed] [Google Scholar]
- 23.Zhou D, Pan Q, Shen F, Cao HX, Ding WJ, Chen YW, Fan JG. Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci Rep. 2017;7:1529. doi: 10.1038/s41598-017-01751-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao L, Zhang F, Ding X, Wu G, Lam YY, Wang X, Fu H, Xue X, Lu C, Ma J, Yu L, Xu C, Ren Z, Xu Y, Xu S, Shen H, Zhu X, Shi Y, Shen Q, Dong W, Liu R, Ling Y, Zeng Y, Wang X, Zhang Q, Wang J, Wang L, Wu Y, Zeng B, Wei H, Zhang M, Peng Y, Zhang C. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018;359:1151–1156. doi: 10.1126/science.aao5774. [DOI] [PubMed] [Google Scholar]
- 25.Bjursell M, Admyre T, Göransson M, Marley AE, Smith DM, Oscarsson J, Bohlooly-Y M. Improved glucose control and reduced body fat mass in free fatty acid receptor 2-deficient mice fed a high-fat diet. Am J Physiol Endocrinol Metab. 2011;300:E211–E220. doi: 10.1152/ajpendo.00229.2010. [DOI] [PubMed] [Google Scholar]
- 26.Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M, Gordon JI. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci USA. 2008;105:16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaji I, Karaki S, Kuwahara A. Short-chain fatty acid receptor and its contribution to glucagon-like peptide-1 release. Digestion. 2014;89:31–36. doi: 10.1159/000356211. [DOI] [PubMed] [Google Scholar]
- 28.Schilderink R, Verseijden C, de Jonge WJ. Dietary inhibitors of histone deacetylases in intestinal immunity and homeostasis. Front Immunol. 2013;4:226. doi: 10.3389/fimmu.2013.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou D, Pan Q, Xin FZ, Zhang RN, He CX, Chen GY, Liu C, Chen YW, Fan JG. Sodium butyrate attenuates high-fat diet-induced steatohepatitis in mice by improving gut microbiota and gastrointestinal barrier. World J Gastroenterol. 2017;23:60–75. doi: 10.3748/wjg.v23.i1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou D, Chen YW, Zhao ZH, Yang RX, Xin FZ, Liu XL, Pan Q, Zhou H, Fan JG. Sodium butyrate reduces high-fat diet-induced non-alcoholic steatohepatitis through upregulation of hepatic GLP-1R expression. Exp Mol Med. 2018;50:157. doi: 10.1038/s12276-018-0183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh V, Yeoh BS, Chassaing B, Xiao X, Saha P, Aguilera Olvera R, Lapek JD, Jr, Zhang L, Wang WB, Hao S, Flythe MD, Gonzalez DJ, Cani PD, Conejo-Garcia JR, Xiong N, Kennett MJ, Joe B, Patterson AD, Gewirtz AT, Vijay-Kumar M. Dysregulated Microbial Fermentation of Soluble Fiber Induces Cholestatic Liver Cancer. Cell. 2018;175:679–694.e22. doi: 10.1016/j.cell.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cani PD. Microbiota and metabolites in metabolic diseases. Nat Rev Endocrinol. 2019;15:69–70. doi: 10.1038/s41574-018-0143-9. [DOI] [PubMed] [Google Scholar]
- 33.Fuster D, Samet JH. Alcohol Use in Patients with Chronic Liver Disease. N Engl J Med. 2018;379:1251–1261. doi: 10.1056/NEJMra1715733. [DOI] [PubMed] [Google Scholar]
- 34.Cope K, Risby T, Diehl AM. Increased gastrointestinal ethanol production in obese mice: implications for fatty liver disease pathogenesis. Gastroenterology. 2000;119:1340–1347. doi: 10.1053/gast.2000.19267. [DOI] [PubMed] [Google Scholar]
- 35.Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, Gill SR. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601–609. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 36.Michail S, Lin M, Frey MR, Fanter R, Paliy O, Hilbush B, Reo NV. Altered gut microbial energy and metabolism in children with non-alcoholic fatty liver disease. FEMS Microbiol Ecol. 2015;91:1–9. doi: 10.1093/femsec/fiu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Engstler AJ, Aumiller T, Degen C, Dürr M, Weiss E, Maier IB, Schattenberg JM, Jin CJ, Sellmann C, Bergheim I. Insulin resistance alters hepatic ethanol metabolism: studies in mice and children with non-alcoholic fatty liver disease. Gut. 2016;65:1564–1571. doi: 10.1136/gutjnl-2014-308379. [DOI] [PubMed] [Google Scholar]
- 38.Dornas W, Lagente V. Intestinally derived bacterial products stimulate development of nonalcoholic steatohepatitis. Pharmacol Res. 2019;141:418–428. doi: 10.1016/j.phrs.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 39.Sharifnia T, Antoun J, Verriere TG, Suarez G, Wattacheril J, Wilson KT, Peek RM, Jr, Abumrad NN, Flynn CR. Hepatic TLR4 signaling in obese NAFLD. Am J Physiol Gastrointest Liver Physiol. 2015;309:G270–G278. doi: 10.1152/ajpgi.00304.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duparc T, Plovier H, Marrachelli VG, Van Hul M, Essaghir A, Ståhlman M, Matamoros S, Geurts L, Pardo-Tendero MM, Druart C, Delzenne NM, Demoulin JB, van der Merwe SW, van Pelt J, Bäckhed F, Monleon D, Everard A, Cani PD. Hepatocyte MyD88 affects bile acids, gut microbiota and metabolome contributing to regulate glucose and lipid metabolism. Gut. 2017;66:620–632. doi: 10.1136/gutjnl-2015-310904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawaratani H, Tsujimoto T, Kitazawa T, Kitade M, Yoshiji H, Uemura M, Fukui H. Innate immune reactivity of the liver in rats fed a choline-deficient L-amino-acid-defined diet. World J Gastroenterol. 2008;14:6655–6661. doi: 10.3748/wjg.14.6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diether NE, Willing BP. Microbial Fermentation of Dietary Protein: An Important Factor in Diet⁻Microbe⁻Host Interaction. Microorganisms. 2019;7 doi: 10.3390/microorganisms7010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goffredo M, Santoro N, Tricò D, Giannini C, D'Adamo E, Zhao H, Peng G, Yu X, Lam TT, Pierpont B, Caprio S, Herzog RI. A Branched-Chain Amino Acid-Related Metabolic Signature Characterizes Obese Adolescents with Non-Alcoholic Fatty Liver Disease. Nutrients. 2017:9. doi: 10.3390/nu9070642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaikkonen JE, Würtz P, Suomela E, Lehtovirta M, Kangas AJ, Jula A, Mikkilä V, Viikari JS, Juonala M, Rönnemaa T, Hutri-Kähönen N, Kähönen M, Lehtimäki T, Soininen P, Ala-Korpela M, Raitakari OT. Metabolic profiling of fatty liver in young and middle-aged adults: Cross-sectional and prospective analyses of the Young Finns Study. Hepatology. 2017;65:491–500. doi: 10.1002/hep.28899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ottosson F, Brunkwall L, Ericson U, Nilsson PM, Almgren P, Fernandez C, Melander O, Orho-Melander M. Connection Between BMI-Related Plasma Metabolite Profile and Gut Microbiota. J Clin Endocrinol Metab. 2018;103:1491–1501. doi: 10.1210/jc.2017-02114. [DOI] [PubMed] [Google Scholar]
- 46.Zhang F, Zhao S, Yan W, Xia Y, Chen X, Wang W, Zhang J, Gao C, Peng C, Yan F, Zhao H, Lian K, Lee Y, Zhang L, Lau WB, Ma X, Tao L. Branched Chain Amino Acids Cause Liver Injury in Obese/Diabetic Mice by Promoting Adipocyte Lipolysis and Inhibiting Hepatic Autophagy. EBioMedicine. 2016;13:157–167. doi: 10.1016/j.ebiom.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li T, Geng L, Chen X, Miskowiec M, Li X, Dong B. Branched-chain amino acids alleviate nonalcoholic steatohepatitis in rats. Appl Physiol Nutr Metab. 2013;38:836–843. doi: 10.1139/apnm-2012-0496. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka H, Fukahori S, Baba S, Ueno T, Sivakumar R, Yagi M, Asagiri K, Ishii S, Tanaka Y. Branched-Chain Amino Acid-Rich Supplements Containing Microelements Have Antioxidant Effects on Nonalcoholic Steatohepatitis in Mice. JPEN J Parenter Enteral Nutr. 2016;40:519–528. doi: 10.1177/0148607114555160. [DOI] [PubMed] [Google Scholar]
- 49.Honda T, Ishigami M, Luo F, Lingyun M, Ishizu Y, Kuzuya T, Hayashi K, Nakano I, Ishikawa T, Feng GG, Katano Y, Kohama T, Kitaura Y, Shimomura Y, Goto H, Hirooka Y. Branched-chain amino acids alleviate hepatic steatosis and liver injury in choline-deficient high-fat diet induced NASH mice. Metabolism. 2017;69:177–187. doi: 10.1016/j.metabol.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 50.Anzai A, Marcondes RR, Gonçalves TH, Carvalho KC, Simões MJ, Soares JM Jr, Padmanabhan V, Baracat EC, da Silva IDCG, Maciel GAR. Impaired branched-chain amino acid metabolism may underlie the nonalcoholic fatty liver disease-like pathology of neonatal testosterone-treated female rats. Sci Rep. 2017;7:13167. doi: 10.1038/s41598-017-13451-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou H, Yu B, Gao J, Htoo JK, Chen D. Regulation of intestinal health by branched-chain amino acids. Anim Sci J. 2018;89:3–11. doi: 10.1111/asj.12937. [DOI] [PubMed] [Google Scholar]
- 52.Fan JG Shanghai Multicenter Clinical Cooperative Group of Danning Pian Trial. Evaluating the efficacy and safety of Danning Pian in the short-term treatment of patients with non-alcoholic fatty liver disease: a multicenter clinical trial. Hepatobiliary Pancreat Dis Int. 2004;3:375–380. [PubMed] [Google Scholar]
- 53.Ferslew BC, Xie G, Johnston CK, Su M, Stewart PW, Jia W, Brouwer AS 4th. Altered Bile Acid Metabolome in Patients with Nonalcoholic Steatohepatitis. Dig Dis Sci. 2015;60:3318–3328. doi: 10.1007/s10620-015-3776-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Puri P, Daita K, Joyce A, Mirshahi F, Santhekadur PK, Cazanave S, Luketic VA, Siddiqui MS, Boyett S, Min HK, Kumar DP, Kohli R, Zhou H, Hylemon PB, Contos MJ, Idowu M, Sanyal AJ. The presence and severity of nonalcoholic steatohepatitis is associated with specific changes in circulating bile acids. Hepatology. 2017 doi: 10.1002/hep.29359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer. 2011;11:886–895. doi: 10.1038/nrc3174. [DOI] [PubMed] [Google Scholar]
- 56.Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, Honda K, Ishikawa Y, Hara E, Ohtani N. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 57.Jiao N, Baker SS, Chapa-Rodriguez A, Liu W, Nugent CA, Tsompana M, Mastrandrea L, Buck MJ, Baker RD, Genco RJ, Zhu R, Zhu L. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut. 2018;67:1881–1891. doi: 10.1136/gutjnl-2017-314307. [DOI] [PubMed] [Google Scholar]
- 58.Fan JG, Zhong L, Tia LY, Xu ZJ, Li MS, Wang GL. Effects of ursodeoxycholic acid and/or low-calorie diet on steatohepatitis in rats with obesity and hyperlipidemia. World J Gastroenterol. 2005;11:2346–2350. doi: 10.3748/wjg.v11.i15.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy S, Diehl AM, Hameed B, Kowdley KV, McCullough A, Terrault N, Clark JM, Tonascia J, Brunt EM, Kleiner DE, Doo E NASH Clinical Research Network. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arab JP, Karpen SJ, Dawson PA, Arrese M, Trauner M. Bile acids and nonalcoholic fatty liver disease: Molecular insights and therapeutic perspectives. Hepatology. 2017;65:350–362. doi: 10.1002/hep.28709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ding L, Sousa KM, Jin L, Dong B, Kim BW, Ramirez R, Xiao Z, Gu Y, Yang Q, Wang J, Yu D, Pigazzi A, Schones D, Yang L, Moore D, Wang Z, Huang W. Vertical sleeve gastrectomy activates GPBAR-1/TGR5 to sustain weight loss, improve fatty liver, and remit insulin resistance in mice. Hepatology. 2016;64:760–773. doi: 10.1002/hep.28689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo C, Xie S, Chi Z, Zhang J, Liu Y, Zhang L, Zheng M, Zhang X, Xia D, Ke Y, Lu L, Wang D. Bile Acids Control Inflammation and Metabolic Disorder through Inhibition of NLRP3 Inflammasome. Immunity. 2016;45:802–816. doi: 10.1016/j.immuni.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 63.Nagahashi M, Takabe K, Liu R, Peng K, Wang X, Wang Y, Hait NC, Wang X, Allegood JC, Yamada A, Aoyagi T, Liang J, Pandak WM, Spiegel S, Hylemon PB, Zhou H. Conjugated bile acid-activated S1P receptor 2 is a key regulator of sphingosine kinase 2 and hepatic gene expression. Hepatology. 2015;61:1216–1226. doi: 10.1002/hep.27592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smallwood T, Allayee H, Bennett BJ. Choline metabolites: gene by diet interactions. Curr Opin Lipidol. 2016;27:33–39. doi: 10.1097/MOL.0000000000000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908–922. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, Gu X, Huang Y, Zamanian-Daryoush M, Culley MK, DiDonato AJ, Fu X, Hazen JE, Krajcik D, DiDonato JA, Lusis AJ, Hazen SL. Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell. 2015;163:1585–1595. doi: 10.1016/j.cell.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bennett BJ, de Aguiar Vallim TQ, Wang Z, Shih DM, Meng Y, Gregory J, Allayee H, Lee R, Graham M, Crooke R, Edwards PA, Hazen SL, Lusis AJ. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013;17:49–60. doi: 10.1016/j.cmet.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen YM, Liu Y, Zhou RF, Chen XL, Wang C, Tan XY, Wang LJ, Zheng RD, Zhang HW, Ling WH, Zhu HL. Associations of gut-flora-dependent metabolite trimethylamine-N-oxide, betaine and choline with non-alcoholic fatty liver disease in adults. Sci Rep. 2016;6:19076. doi: 10.1038/srep19076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barrea L, Annunziata G, Muscogiuri G, Di Somma C, Laudisio D, Maisto M, de Alteriis G, Tenore GC, Colao A, Savastano S. Trimethylamine-N-oxide (TMAO) as Novel Potential Biomarker of Early Predictors of Metabolic Syndrome. Nutrients. 2018:10. doi: 10.3390/nu10121971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dumas ME, Barton RH, Toye A, Cloarec O, Blancher C, Rothwell A, Fearnside J, Tatoud R, Blanc V, Lindon JC, Mitchell SC, Holmes E, McCarthy MI, Scott J, Gauguier D, Nicholson JK. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci USA. 2006;103:12511–12516. doi: 10.1073/pnas.0601056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gao X, Liu X, Xu J, Xue C, Xue Y, Wang Y. Dietary trimethylamine N-oxide exacerbates impaired glucose tolerance in mice fed a high fat diet. J Biosci Bioeng. 2014;118:476–481. doi: 10.1016/j.jbiosc.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 73.Schugar RC, Shih DM, Warrier M, Helsley RN, Burrows A, Ferguson D, Brown AL, Gromovsky AD, Heine M, Chatterjee A, Li L, Li XS, Wang Z, Willard B, Meng Y, Kim H, Che N, Pan C, Lee RG, Crooke RM, Graham MJ, Morton RE, Langefeld CD, Das SK, Rudel LL, Zein N, McCullough AJ, Dasarathy S, Tang WHW, Erokwu BO, Flask CA, Laakso M, Civelek M, Naga Prasad SV, Heeren J, Lusis AJ, Hazen SL, Brown JM. The TMAO-Producing Enzyme Flavin-Containing Monooxygenase 3 Regulates Obesity and the Beiging of White Adipose Tissue. Cell Rep. 2017;19:2451–2461. doi: 10.1016/j.celrep.2017.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dumas ME, Rothwell AR, Hoyles L, Aranias T, Chilloux J, Calderari S, Noll EM, Péan N, Boulangé CL, Blancher C, Barton RH, Gu Q, Fearnside JF, Deshayes C, Hue C, Scott J, Nicholson JK, Gauguier D. Microbial-Host Co-metabolites Are Prodromal Markers Predicting Phenotypic Heterogeneity in Behavior, Obesity, and Impaired Glucose Tolerance. Cell Rep. 2017;20:136–148. doi: 10.1016/j.celrep.2017.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mehedint MG, Zeisel SH. Choline's role in maintaining liver function: new evidence for epigenetic mechanisms. Curr Opin Clin Nutr Metab Care. 2013;16:339–345. doi: 10.1097/MCO.0b013e3283600d46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tang WH, Hazen SL. Microbiome, trimethylamine N-oxide, and cardiometabolic disease. Transl Res. 2017;179:108–115. doi: 10.1016/j.trsl.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krishnan S, Ding Y, Saedi N, Choi M, Sridharan GV, Sherr DH, Yarmush ML, Alaniz RC, Jayaraman A, Lee K. Gut Microbiota-Derived Tryptophan Metabolites Modulate Inflammatory Response in Hepatocytes and Macrophages. Cell Rep. 2018;23:1099–1111. doi: 10.1016/j.celrep.2018.03.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Konopelski P, Konop M, Gawrys-Kopczynska M, Podsadni P, Szczepanska A, Ufnal M. Indole-3-Propionic Acid, a Tryptophan-Derived Bacterial Metabolite, Reduces Weight Gain in Rats. Nutrients. 2019;11 doi: 10.3390/nu11030591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Abildgaard A, Elfving B, Hokland M, Wegener G, Lund S. The microbial metabolite indole-3-propionic acid improves glucose metabolism in rats, but does not affect behaviour. Arch Physiol Biochem. 2018;124:306–312. doi: 10.1080/13813455.2017.1398262. [DOI] [PubMed] [Google Scholar]
- 81.Jennis M, Cavanaugh CR, Leo GC, Mabus JR, Lenhard J, Hornby PJ. Microbiota-derived tryptophan indoles increase after gastric bypass surgery and reduce intestinal permeability in vitro and in vivo. Neurogastroenterol Motil. 2018:30. doi: 10.1111/nmo.13178. [DOI] [PubMed] [Google Scholar]
- 82.Venkatesh M, Mukherjee S, Wang H, Li H, Sun K, Benechet AP, Qiu Z, Maher L, Redinbo MR, Phillips RS, Fleet JC, Kortagere S, Mukherjee P, Fasano A, Le Ven J, Nicholson JK, Dumas ME, Khanna KM, Mani S. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity. 2014;41:296–310. doi: 10.1016/j.immuni.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]