Abstract
BACKGROUND
The methylated septin 9 (mSEPT9) assay was the first blood-based test approved by the United States Food and Drug Administration as a colorectal screening test. However, the diagnostic and prognostic role of preoperative mSEPT9 for colorectal cancer (CRC) in Chinese patients is still unknown.
AIM
To improve the understanding of diagnostic and prognostic factors, serum mSEPT9 was detected in Chinese CRC patients.
METHODS
A retrospective analysis of 354 cases, of which 300 had CRC and 54 were normal, was performed in China. Patients’ characteristics, treatments, and laboratory data, including age, the date of surgery, Union for International Cancer Control (UICC) stages, distant metastasis (M), and so on, were collected. Methylation levels of SEPT9 were quantified by quantitative, methylation-specific polymerase chain reaction before surgery. In addition, the effects of mSEPT9 on the occurrence and prognosis of 330 CRC cases from The Cancer Genome Atlas (TCGA) database were evaluated using bioinformatics analyses. Potential prognostic factors for overall survival (OS) and progression-free survival (PFS) were evaluated by Kaplan-Meier univariate analysis.
RESULTS
In Chinese CRC patients, positive mSEPT9 was strongly associated with advanced UICC stages, deeper invasion by the primary tumor, and more distant metastasis. Methylation levels of SEPT9 were stage-dependent and showed a stepwise increase in UICC stages (I–IV), primary tumor categories (T1–T4), regional node categories (N0–N2), and distant metastasis categories (M0–M1). The patients with positive mSEPT9 showed a tendency toward lower PFS. After analyzing TCGA clinical data, the high mSEPT9 group was found to be obviously correlated only with more distant metastasis. The patients with high mSEPT9 levels showed a tendency toward lower OS. Besides, nine meaningful mSEPT9 sites were found to provide guidance for the follow-up studies.
CONCLUSION
MSEPT9 analysis may add valuable information to current tumor staging. Serum mSEPT9 in Chinese CRC patients appears to offer promising novel prognostic markers and might be considered for monitoring CRC recurrence.
Keywords: Methylated septin 9, Methylated, Colorectal cancer, Diagnosis, Prognosis
Core tip: This study retrospectively explored the value of serum septin 9 methylation (mSEPT9) in the diagnosis and prognosis of colorectal cancer in a Chinese population. Preoperative mSEPT9 levels in 354 enrolled patients were retrospectively analyzed. In addition, the effects of mSEPT9 on the occurrence and prognosis of 330 colorectal cancer cases from The Cancer Genome Atlas database were evaluated using bioinformatics analyses. Besides, nine meaningful mSEPT9 sites were found to provide guidance for the follow-up studies.
INTRODUCTION
Colorectal cancer (CRC) remains the third most common cancer expected to occur in men and women[1], accounting for approximately 10% of the global cancer burden. To date, more than 90% of patients with early CRC have survived five years after diagnosis[2,3]. However, in the case of regional spread to lymph nodes or adjacent organs, the five-year relative survival rate decreases to 69%, and when there is distant metastasis, it drops sharply to approximately 10%[3,4]. Despite significant recent achievements in the diagnosis and treatment of these patients, resulting in partial reductions in overall incidence and mortality, there is no effective diagnostic assay so far for tumor progression or recurrence monitoring, especially in vitro.
Detection of CRC recurrences or metastases in the early stage by constant monitoring may improve long-term outcomes through timely treatment. The American Joint Committee on Cancer (AJCC) Cancer Staging, seventh edition has accepted clinically useful carcinoembryonic antigen (CEA) serum tumor marker as a site-specific prognostic factor in CRC[5]. However, the low detection sensitivity of CEA hinders its use for many surgical patients, because patients with negative CEA results before surgery usually cannot be monitored after surgery[6,7]. In addition, periodic computed tomography (CT) scanning is another noninvasive method for surgical therapeutic effect assessment[8]. However, CT scans have limited sensitivity and high false positive rates, and cannot be used routinely as a monitoring examination due to the danger of long-term radiation[9]. Therefore, development of novel, sensitive biomarkers for monitoring recurrences or metastases of CRC is urgently needed.
Hypermethylation of the promoter of septin 9 (SEPT9) has previously been shown to be a sensitive and specific biomarker in various cancers including CRC[10-13] and its precursor lesions[14-16]. As a result, the methylated SEPT9 (mSEPT9) assay became the first blood-based test approved by the United States Food and Drug Administration as a CRC screening test[17]. A study of Korean CRC patients found that serum mSEPT9 had a tendency to show metastasis and a low disease-free survival rate[18]. In a recent study of German CRC patients, mSEPT9 was significantly associated with Union for International Cancer Control (UICC) stages both before and after therapy[19]. In addition, quantitative mSEPT9 levels have been successfully applied for the diagnosis of CRC[19-22], and for the screening, diagnosis, monitoring, prognosis, and molecular staging of head and neck squamous cell carcinomas (HNSCC)[19]. However, the diagnostic and prognostic role of preoperative mSEPT9 for CRC in Chinese patients is still unknown.
This study assessed the correlation between clinicopathological characteristics and preoperative serum mSEPT9 in Chinese CRC patients and, further, to confirm the correlation between mSEPT9 levels and CRC prognosis by bioinformatics analyses. In addition, we analyzed methylated sites that were co-upregulated or codownregulated in colon and rectum tumors, to provide the theoretical guidance for further research.
MATERIALS AND METHODS
Patients and samples
This present study was conducted from December 2017 to November 2018 among patients at the Department of Hepatobiliary and Enteric Surgery in Xiangya Hospital. A total of 354 subjects with mSEPT9 serum detection before surgery were recruited from a medicine-pharmacy-nursing integrative parenteral medication rational use and safety early warning platform, the Parenteral Prescription Early Warning and Assessment System, including 300 CRC patients and 54 normal subjects. This study was approved by the Ethical Committee of Xiangya Hospital of Central South University (Approval No. 2018111100).
Three hundred patients presented with histologically confirmed primary CRC. Recurrences or metastases were determined from diagnostic tests (CT scan, magnetic resonance imaging, or colonoscopy) and confirmed through tissue pathology when available[7]. Clinical parameters, including mSEPT9 detection results, gender, age, UICC stage, histologic grade, primary tumor (T) categories, regional node (N) categories, distant metastasis categories (M), lymphatic invasion (L), lymph nodal status, vascular invasion (V), and tumor site, were collected. The UICC stage, tumor node metastasis (TNM) categories and histologic differentiation were graded on the basis of the eighth edition of the AJCC[23]. Progression-free survival (PFS) time was calculated from the CRC patients’ date of surgery to presentation of clinical or pathological evidence of cancer recurrence.
In addition, 330 colorectal adenocarcinomas from The Cancer Genome Atlas (TCGA) Research Network (http://cancergenome.nih.gov/.) were selected and analyzed retrospectively. Patients whose mSEPT9 levels were less than or equal to median were assigned to the low mSEPT9 group, whereas others were assigned to the high mSEPT9 group. The overall survival (OS) time was calculated from the CRC patients’ date of surgery to the date of dead or to the last contact date.
Methylated SEPT9 detection
A 10 mL peripheral blood sample was collected with a 10 mL K2EDTA anticoagulant tube for the SEPT9 assay [BioChain (Beijing) Science and Technology, Inc., Beijing, China]. Peripheral blood sample storage and transportation, DNA extraction, and bisulfite conversion were performed manually following the manufacturer’s instructions of the Epi proColon 2.0 kit (Epigenomics AG, Berlin, Germany). The mSEPT9 was assayed with the Epi proColon 2.0 kit on an AB7500 Fast Dx Real Time polymerase chain reaction device (Life Technologies) in the Clinical Laboratory of Xiangya Hospital, Central South University. Briefly, a polymerase chain reaction (PCR) test was performed in triplicate with 15 μL template DNA per well and run for 45 cycles[24]. The instrument software was used to record the PCR results for β-actin (ACTB) and methylated SEPT9 from each of the triplicate reactions. The validity of each sample batch was determined according to methylated SEPT9 and ACTB threshold count (Ct) values for the positive and negative controls. ACTB served as an internal reference to assess the integrity of each sample. According to the instructions, Ct value was less than 41.1 was assigned to the positive mSEPT9 group, whereas those whose Ct value was over 41.1 were assigned to the negative mSEPT9 group.
Statistical analyses
All statistical analyses were performed using SPSS 18 software (SPSS Inc, Chicago, United States). The measurable data was expressed as the mean and standard deviation (SD). Differences of clinicopathological characteristics and Ct values between groups were compared via t-tests and χ2 test. The univariate analysis was performed to assess the effect of mSEPT9 to predict PFS and OS by the Kaplan-Meier method. Binary logistic regression was used to analyze the association between each genetic biomarker (e.g., mismatched repair proteins) and mSEPT9. All the statistical tests were bilateral, and P < 0.05 was considered statistically significant.
RESULTS
The mSEPT9 in CRC patients and normal subjects
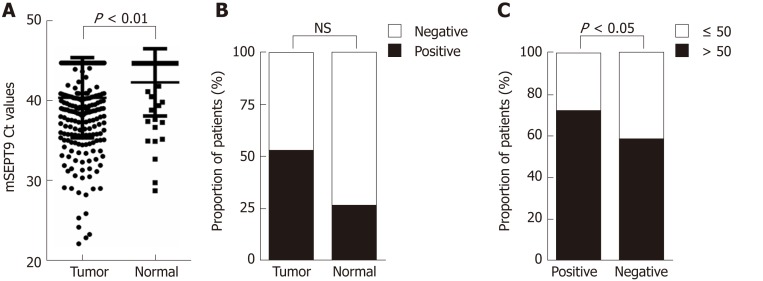
Among Chinese CRC patients, the methylated Ct values of 300 primary CRC patients and 54 normal subjects were analyzed. Based on contradictory trends for Ct value and expression, the preoperative serum mSEPT9 levels were significantly higher in CRC patients than in the normal subjects (P = 0.008) (Figure 1A). The positive rate of mSEPT9 was 52.3% for CRC patients and 25.9% for normal subjects (P = 0.102) (Figure 1B).
Figure 1.
Graphical representations of difference in methylated septin 9 Ct values or proportion of patients between different groups. A: Methylated septin 9 (mSEPT9) Ct values in tumor group and normal group; B: Proportion of patients with positive and negative mSEPT9 in tumor group and normal group; C: Proportion of patients older than 50 and aged 50 or younger in positive group and negative group. The statistical significance for difference of means is shown in P values, t-test, or χ2 test. mSEPT9: Methylated septin 9.
Among 351 patients from the TCGA database, the mSEPT9 levels of 330 CRC patients and 21 normal subjects were analyzed. The serum mSEPT9 levels of the CRC patients were higher than those of the normal subjects, but were not statistically significant (P = 0.530) (Supplemental Figure 1).
Clinicopathological characteristics and mSEPT9 in CRC
The Chinese CRC patients, clinicopathological features of 300 CRC patients are described in Table 1. As shown in Figure 1C, patients older than 50 years were statistically more numerous than those aged 50 or younger in both positive and negative groups (P = 0.016). Through analyzing UICC stages, we found that the positive rate of stage III was observably higher than stage I (46.6% vs 31.0%, P = 0.012) (Figure 2A); mSEPT9 levels showed a significant increase from UICC stages II to III (P = 0.033) and stages III to IV (P < 0.0001), but no obvious difference was detected between stages I to II (P = 0.898, Figure 3A).
Table 1.
Clinicopathological characteristics based on methylated septin 9 status in 300 colorectal cancer patients
| Parameter | Positive group (%) | Negative group (%) | P | |
| Gender | Male | 84 (28.0) | 81 (27.0) | 0.585 |
| Female | 73 (24.3) | 62 (20.7) | ||
| Age | ≤ 50 yr | 45 (15.0) | 60 (20.0) | 0.016 |
| > 50 yr | 112 (37.3) | 83 (27.7) | ||
| UICC stage | I | 9 (3.0) | 20 (6.7) | 0.020 |
| II | 46 (15.3) | 32 (10.7) | ||
| III | 62 (20.6) | 71 (23.7) | ||
| IV | 13 (4.3) | 6 (2.0) | ||
| Unknown | 27 (9.0) | 14 (4.7) | ||
| Histologic grade | Low level | 115 (38.3) | 103 (34.3) | 0.972 |
| High level | 18 (6.0) | 17 (5.7) | ||
| Not recorded | 24 (8.0) | 23 (7.7) | ||
| Primary tumor (T) category | T1 | 2 (0. 7) | 3 (1.0) | 0.002 |
| T2 | 15 (5.0) | 23 (7.7) | ||
| T3 | 70 (23.3) | 67 (22.3) | ||
| T4 | 40 (13.3) | 34 (11.3) | ||
| Not recorded | 30 (10.0) | 16 (5.3) | ||
| Regional node (N) category | N0 | 57 (19.0) | 23 (7.7) | 0.852 |
| N1 | 32 (10.7) | 67 (22.3) | ||
| N2 | 37 (12.3) | 34 (11.3) | ||
| Not recorded | 31 (10.3) | 16 (5.3) | ||
| Distant metastasis (M) | Absent | 141 (47.0) | 137 (45.7) | 0.015 |
| Present | 16 (5.3) | 6 (2.0) | ||
| Lymphatic invasion (L) | Absent | 58 (19.3) | 60 (20.0) | 0.217 |
| Present | 51 (17.0) | 52 (17.3) | ||
| Not recorded | 48 (16.0) | 31 (10.3) | ||
| Lymph nodal status | No node involved | 51 (17.0) | 52 (17.3) | 0.048 |
| 1-3 lymph node involved | 27 (9.0) | 40 (13.3) | ||
| > 4 lymph node involved | 31 (10.3) | 20 (6.7) | ||
| Not recorded | 48 (16) | 31 (10.3) | ||
| Vascular invasion (V) | Absent | 36 (12.0) | 31 (10.3) | 0.278 |
| Present | 70 (23.3) | 76 (25.3) | ||
| Not recorded | 51 (17.0) | 36 (12.0) | ||
| Tumor sitea | Left colon | 44 (14.7) | 31 (10.3) | 0.022 |
| Right colon | 27 (9.0) | 11 (3.7) | ||
| Rectum | 74 (24.7) | 86 (28.6) | ||
| Unable to distinguish | 12 (4.0) | 15 (5.0) |
Right colon includes cecum through transverse colon, whereas left colon includes splenic flexure, descending colon, and sigmoid colon. Clinicopathological characteristics between positive group and negative group were analyzed using χ2 test. UICC: Union for International Cancer Control.
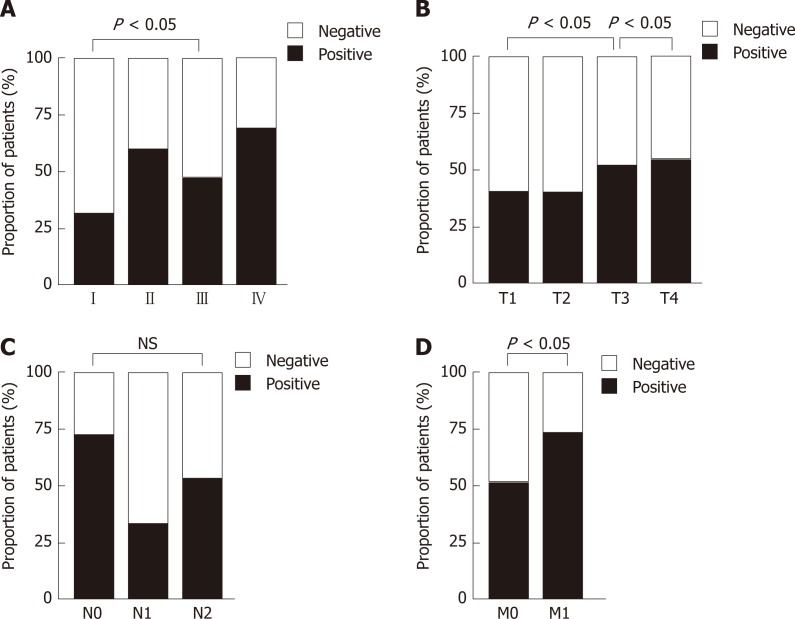
Figure 2.
Graphical representations of the proportion of patients with positive and negative methylated septin 9 with different tumor status. A: Union for International Cancer Control stages; B: Primary tumor categories; C: Regional node categories; D: Distant metastasis categories. The statistical significance for difference of means is shown in P values and χ2 test).
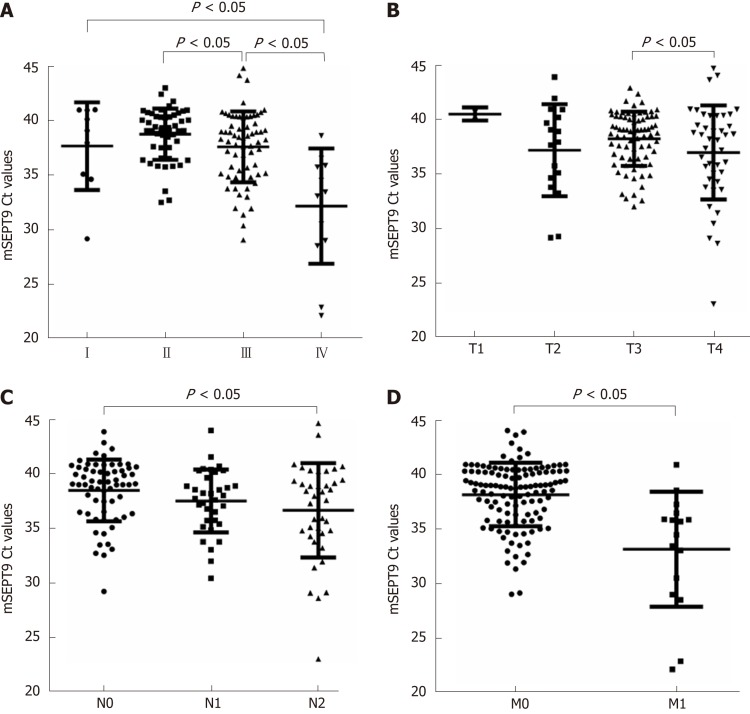
Figure 3.
Graphical representations of methylated septin 9 Ct values in different tumor status. A: Union for International Cancer Control stages; B: Primary tumor categories; C: Regional node categories; D: Distant metastasis categories. The statistical significance for difference of means is shown in P values and t-test. mSEPT9: Methylated septin 9.
In addition, the association of mSEPT9 levels and rate of positive mSEPT9 among primary tumor categories (T1–T4), regional node categories (N0–N2) and distant metastasis categories (M0–M1) were also analyzed. The detection rate of positive T3 was observably higher than that of T1 (51.1% vs 40.0%, P = 0.019) (Figure 2B). Positive rate and levels of mSEPT9 revealed a significant increase from T3 to T4 (P = 0.030, P = 0.046, respectively) (Figure 2B, Figure 3B). In terms of regional node categories, N0 to N2 showed a gradual increase in mSEPT9 levels (P = 0.012) (Figure 3C), but did not show any association with the rate of positive mSEPT9 (Figure 2C). As shown in Figures 2D and 3D, mSEPT9 showed the best ability to discriminate between local and metastatic CRC (P = 0.015, P < 0.0001, respectively). However, higher mSEPT9 levels were not found in CRC patients with lymphatic or vascular invasion than in those without invasion (all P > 0.05). We also failed to find association among MLH1, MSH2 (25D12), MSH6, PMS2, and Ki67 and mSEPT9 (all P > 0.05) (Supplemental Table 1).
The clinicopathological features of 330 CRC patients from the TCGA database are described in detail in Supplemental Table 2. Similarly, there was a tendency for more distant metastasis (P = 0.0001) and more CRC patients older than 50 (P < 0.0001) in the high mSEPT9 group, but no significant difference was found in UICC stages, primary tumor categories, or regional node categories (all P > 0.05).
Prognostic significance of mSEPT9 in CRC patients
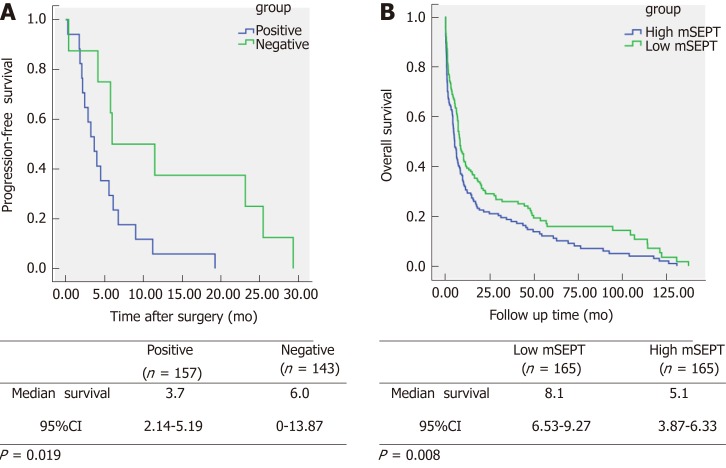
Kaplan-Meier univariate analysis showed that positive mSEPT9 was obviously associated with shorter PFS among the Chinese CRC patients (P = 0.019, Figure 4A). The positive mSEPT9 CRC cases were estimated to have an mean PFS duration of 3.7 mo [95% confidence interval (CI): 2.14-5.19] compared with the 6.0 mo (95%CI: 0-13.87) in the negative mSEPT9 CRC cases.
Figure 4.
Kaplan-Meier univariate survival curves according to methylated septin 9 status. A: Progression-free survival time; B: Overall survival. The statistical significance for difference of means shown in P values and Kaplan-Meier univariate analysis. mSEPT: Methylated septin; CI: Confidence interval.
In addition, serum mSEPT9 showed prognostic significance for the CRC patients from the TCGA database (P = 0.008, Figure 4B). CRC patients with low mSEPT9 levels were found to be correlated with longer OS. The low mSEPT9 CRC cases had an estimated mean OS duration of 8.1 months (95%CI: 6.53-9.27) compared with the 5.1 mo (95%CI: 3.87-6.33) in the high mSEPT9 CRC cases.
Significant methylation sites for SEPT9
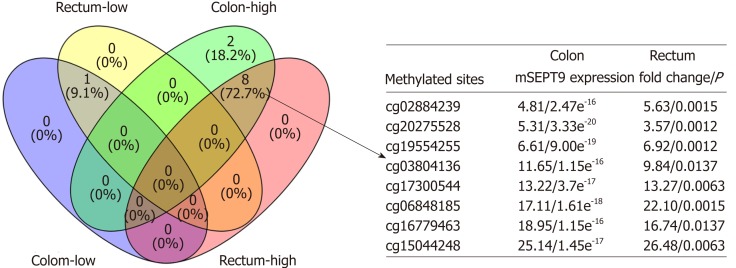
In further analyzed TCGA clinical data, 124 mSEPT9 sites were found that showed differential expression among normal subjects and those with colon and rectum adenocarcinoma, respectively (all P < 0.05) (Supplemental Figure 2). After analyzing the detailed information of these 124 mSEPT9 sites, 68 co-upregulated and 36 co-downregulated mSEPT9 sites in CRC adenocarcinoma were further observed. We finally confirmed that there were eight co-upregulated mSEPT9 sites (Figure 5) and one co-downregulated mSEPT9 site (cg02975107) through setting a cut-off of a two-fold expression change of mSEPT9.
Figure 5.
Venn diagram of eight co-upregulated methylated septin 9 sites and one co-downregulated methylated septin 9 site in colon and rectum adenocarcinoma. “Rectum-low” and “Rectum-high” represented sites that showed low or high expression in rectum adenocarcinoma, and “Colon-low” and “Colon-high” represented sites that showed low or high expression in colon adenocarcinoma. Eight co-upregulated methylated septin 9 (mSEPT9) sites also showed mSEPT9 expression fold of rectum/ colon adenocarcinoma compared to normal subjects and corresponding P value. mSEPT: Methylated septin.
DISCUSSION
Most patients with early CRC undergo curatively intended surgery to clear up primary lesions and local lymph node metastasis up. However, 30%-50% of patients would still confront tumor recurrence and might die from metastasis[25]. Timely monitoring of recurrence and metastasis is of great significance to the prognosis and survival of patients. In our study, mSEPT9 was proved to be an effective bio-marker for diagnosis, recurrence, and prognosis of CRC in Chinese patients, and nine significant mSEPT9 sites were confirmed for further in-depth consideration.
Our study confirmed the value of serum mSEPT9 for CRC diagnosis. Compared with normal tissues in Chinese and TCGA data, serum SEPT9 was found to be hypermethylated in tumor tissues, which was consistent with previous studies[18,19,26]. Studies showed that age affected the detection rate of the SEPT9 assay[27,28], and we found that a positive rate of mSEPT9 was strongly associated with CRC patients aged over 50 years both in Chinese and TCGA data. This accords with the definition of an average risk population in National Comprehensive Cancer Network Guidelines for CRC[29]. Remarkably, we reported that SEPT9 performs outstandingly as an auxiliary molecular staging parameter in the Chinese population, especially because mSEPT9 levels could distinguish between pathological UICC and TNM stages in an incremental fashion. In addition, our data demonstrated that CRC patients in earlier tumor stages showed lower mSEPT9 levels compared to those with more advanced lesions, which is consistent with studies in German CRC patients[19,30-32]. Most importantly, its ability to identify patients with distant metastases emphasizes the potential of mSEPT9 as a bio-marker, which adds valuable information to the classification of tumors[33-35]. However, high mSEPT9 group did not show any association with UICC, T, or N stages in patients from the TGGA database, who were from American Indian, Asian, Black, or African American populations. This might be explained by the different study populations. Previous studies found that the incidence of CRC and the sensitivity to the mSEPT9 test assay in different ethnic groups were different[14,36].
In addition, serum mSEPT9 were proved to be an independent predictors of CRC recurrence and unfavorable cancer-specific survival in Chinese and TCGA data, which is consistent with previous studies in Singapore and Germany[19,37,38]. The study was performed with a large number of prognostic features and patients; however, much longer prognosis and follow-up time are necessary before final conclusions can be made, and the increasing number of patients with earlier-stage CRC demands a widening of the clinical importance of predictive value for prognosis.
After further analysis of the TCGA clinical data, we obtained nine SEPT9 methylation sites that show two-fold higher or lower mSEPT9 levels in CRC than normal tissues. However, no studies were found at present that investigate the prognosis of these methylation sites and CRC was found. Surprisingly, cg12783819, which only shows 1.5-fold higher mSEPT9 levels in CRC than in normal tissues, has been proven to be able to assess the diagnosis, prognosis, and molecular staging of German HNSCC and CRC patients[19,20]. The result prompts us to explore the potential association between these nine methylation sites and in Chinese CRC patients in the future.
In conclusions, serum SEPT9 methylation testing is a powerful additional diagnostic tool and promising, novel prognostic markers. Patients with initially high mSEPT9 levels may benefit from intensive therapy and close monitoring of disease development, thereby improving outcomes for CRC patients. These patients may benefit from early systemic treatment.
ARTICLE HIGHLIGHTS
Research background
The methylated septin 9 (mSEPT9) assay was the first blood-based test approved by the United States Food and Drug Administration as a colorectal screening test. Previous researchers found that mSEPT9 was a powerful screening, diagnostic, monitoring, and prognostic tool for German colorectal cancer (CRC) patients. However, the diagnostic and prognostic value of mSEPT9 in Chinese CRC patients is still unknown, and may be affected by differences in ethnicity and socioeconomic status.
Research motivation
To explore the diagnostic and prognostic value of serum mSEPT9 for Chinese CRC patients.
Research objectives
This study aimed to explore the diagnostic value of preoperative serum mSEPT9 in the Chinese population, and then assess the value of quantitative mSEPT9 levels for CRC staging. In addition, Chinese population and TCGA database information were combined to determine the prognostic significance of mSEPT9 by bioinformatics analyses.
Research methods
Three hundred fifty-four subjects (300 CRC, 54 normal) from China and 351 subjects (330 CRC, 21 normal) from the TCGA database including American Indian, Asian, Black, and African American populations were retrospectively analyzed. Preoperative mSEPT9 levels were quantified by quantitative methylation-specific polymerase chain reaction. Kaplan-Meier univariate assay was performed to analyze potential prognostic factors including overall survival (OS) and progression-free survival (PFS).
Research results
In Chinese CRC patients, positive mSEPT9 and quantitative mSEPT9 levels were strongly associated with clinico-pathological parameters. The patients with positive mSEPT9 showed a tendency toward lower PFS. Higher mSEPT9 levels were correlated with more distant metastasis among the TCGA database patients, and patients with high mSEPT9 levels showed a tendency toward lower OS.
Research conclusions
Testing for mSEPT9 is a powerful diagnostic and promising prognostic tool for Chinese CRC patients; it may add valuable information to current tumor staging and holds the potential to monitor CRC recurrence.
Research perspectives
This study assessed the correlation between clinicopathological characteristics and preoperative serum mSEPT9 in Chinese CRC patients and, further, to confirm the correlation between mSEPT9 levels and CRC prognosis by bioinformatics analyses. In addition, we analyzed methylated sites that were co-upregulated or co-downregulated in colon and rectum tumors, to provide the theoretical guidance for further research.
Footnotes
Institutional review board statement: This study was reviewed and approved by the Ethical Committee of Xiangya Hospital of Central South University (Approval No. 2018111100).
Informed consent statement: According to the “Human Biomedical Research Ethical Review Procedures” approved by the National Health and Family Planning Committee of China (No. 11, Section 39), informed consent was waived because of the retrospective nature of the study. After the following circumstances have been reviewed and approved by the ethics committee, the informed consent form can be waived if: Research is conducted using human body materials or data that can identify information, and the subjects can’t be found, and the research project does not involve personal privacy and commercial interests.
Conflict-of-interest statement: The authors have no conflicts of interest to disclose.
Peer-review started: February 15, 2019
First decision: March 5, 2019
Article in press: April 10, 2019
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Matsui T, Vetvicka V S-Editor: Yan JP L-Editor: A E-Editor: Ma YJ
Contributor Information
Xue Yang, Department of Pharmacy, Institute for Rational and Safe Medication Practices, National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha 410008, Hunan Province, China.
Zhi-Jie Xu, Department of Pathology, Xiangya Hospital, Central South University, Changsha 410008, Hunan Province, China.
Xi Chen, Department of Pharmacy, Institute for Rational and Safe Medication Practices, National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha 410008, Hunan Province, China.
Shuang-Shuang Zeng, Department of Pharmacy, Institute for Rational and Safe Medication Practices, National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha 410008, Hunan Province, China.
Long Qian, Department of Pharmacy, Institute for Rational and Safe Medication Practices, National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha 410008, Hunan Province, China.
Jie Wei, Department of Pharmacy, Institute for Rational and Safe Medication Practices, National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha 410008, Hunan Province, China.
Mei Peng, Department of Pharmacy, Institute for Rational and Safe Medication Practices, National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha 410008, Hunan Province, China.
Xiang Wang, Department of Pharmacy, Institute for Rational and Safe Medication Practices, National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha 410008, Hunan Province, China.
Wan-Li Liu, Department of Pharmacy, Institute for Rational and Safe Medication Practices, National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha 410008, Hunan Province, China.
Hong-Ying Ma, Department of Pharmacy, Institute for Rational and Safe Medication Practices, National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha 410008, Hunan Province, China.
Zhi-Cheng Gong, Department of Pharmacy, Institute for Rational and Safe Medication Practices, National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha 410008, Hunan Province, China. gongzhicheng@csu.edu.cn.
Yuan-Liang Yan, Department of Pharmacy, Institute for Rational and Safe Medication Practices, National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha 410008, Hunan Province, China.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Bresalier RS, Kopetz S, Brenner DE. Blood-based tests for colorectal cancer screening: Do they threaten the survival of the FIT test? Dig Dis Sci. 2015;60:664–671. doi: 10.1007/s10620-015-3575-2. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, Lin C, Leach C, Cannady RS, Cho H, Scoppa S, Hachey M, Kirch R, Jemal A, Ward E. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Song L, Gong Y, He B. Detection of colorectal cancer by DNA methylation biomarker SEPT9: Past, present and future. Biomark Med. 2014;8:755–769. doi: 10.2217/bmm.14.8. [DOI] [PubMed] [Google Scholar]
- 5.Cuccurullo V, Mansi L. AJCC Cancer Staging Handbook: from the AJCC Cancer Staging Manual (7th edition) Eur J Nucl Med Mol I. 2011;38:408–408. [Google Scholar]
- 6.Young GP, Pedersen SK, Mansfield S, Murray DH, Baker RT, Rabbitt P, Byrne S, Bambacas L, Hollington P, Symonds EL. A cross-sectional study comparing a blood test for methylated BCAT1 and IKZF1 tumor-derived DNA with CEA for detection of recurrent colorectal cancer. Cancer Med. 2016;5:2763–2772. doi: 10.1002/cam4.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicholson BD, Shinkins B, Pathiraja I, Roberts NW, James TJ, Mallett S, Perera R, Primrose JN, Mant D. Blood CEA levels for detecting recurrent colorectal cancer. Cochrane Database Syst Rev. 2015:CD011134. doi: 10.1002/14651858.CD011134.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng SB, Chua C, Ng M, Gan A, Poon PS, Teo M, Fu C, Leow WQ, Lim KH, Chung A, Koo SL, Choo SP, Ho D, Rozen S, Tan P, Wong M, Burkholder WF, Tan IB. Individualised multiplexed circulating tumour DNA assays for monitoring of tumour presence in patients after colorectal cancer surgery. Sci Rep. 2017;7:40737. doi: 10.1038/srep40737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metser U, You J, McSweeney S, Freeman M, Hendler A. Assessment of tumor recurrence in patients with colorectal cancer and elevated carcinoembryonic antigen level: FDG PET/CT versus contrast-enhanced 64-MDCT of the chest and abdomen. AJR Am J Roentgenol. 2010;194:766–771. doi: 10.2214/AJR.09.3205. [DOI] [PubMed] [Google Scholar]
- 10.Song L, Jia J, Peng X, Xiao W, Li Y. The performance of the SEPT9 gene methylation assay and a comparison with other CRC screening tests: A meta-analysis. Sci Rep. 2017;7:3032. doi: 10.1038/s41598-017-03321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song L, Li Y. Progress on the clinical application of the SEPT9 gene methylation assay in the past 5 years. Biomark Med. 2017;11:415–418. doi: 10.2217/bmm-2017-0091. [DOI] [PubMed] [Google Scholar]
- 12.Warren JD, Xiong W, Bunker AM, Vaughn CP, Furtado LV, Roberts WL, Fang JC, Samowitz WS, Heichman KA. Septin 9 methylated DNA is a sensitive and specific blood test for colorectal cancer. BMC Med. 2011;9:133. doi: 10.1186/1741-7015-9-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song LL, Li YM. Current noninvasive tests for colorectal cancer screening: An overview of colorectal cancer screening tests. World J Gastrointest Oncol. 2016;8:793–800. doi: 10.4251/wjgo.v8.i11.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song L, Li Y. SEPT9: A Specific Circulating Biomarker for Colorectal Cancer. Adv Clin Chem. 2015;72:171–204. doi: 10.1016/bs.acc.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Liu S, Xiao Z, Ai F, Liu F, Chen X, Cao K, Ren W, Zhang X, Shu P, Zhang D. Corrigendum to "miR-142-5p promotes development of colorectal cancer through targeting SDHB and facilitating generation of aerobic glycolysis" [Biomed. Pharmacother. 92 (2017) 1119-1127] Biomed Pharmacother. 2018;99:1033–1036. doi: 10.1016/j.biopha.2018.01.031. [DOI] [PubMed] [Google Scholar]
- 16.Semaan A, van Ellen A, Meller S, Bergheim D, Branchi V, Lingohr P, Goltz D, Kalff JC, Kristiansen G, Matthaei H, Pantelis D, Dietrich D. SEPT9 and SHOX2 DNA methylation status and its utility in the diagnosis of colonic adenomas and colorectal adenocarcinomas. Clin Epigenetics. 2016;8:100. doi: 10.1186/s13148-016-0267-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Church TR, Wandell M, Lofton-Day C, Mongin SJ, Burger M, Payne SR, Castaños-Vélez E, Blumenstein BA, Rösch T, Osborn N, Snover D, Day RW, Ransohoff DF PRESEPT Clinical Study Steering Committee, Investigators and Study Team. Prospective evaluation of methylated SEPT9 in plasma for detection of asymptomatic colorectal cancer. Gut. 2014;63:317–325. doi: 10.1136/gutjnl-2012-304149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Li Y, Liu Y, Xie P, Li F, Li G. PAX6, a novel target of microRNA-7, promotes cellular proliferation and invasion in human colorectal cancer cells. Dig Dis Sci. 2014;59:598–606. doi: 10.1007/s10620-013-2929-x. [DOI] [PubMed] [Google Scholar]
- 19.Lee HS, Hwang SM, Kim TS, Kim DW, Park DJ, Kang SB, Kim HH, Park KU. Circulating methylated septin 9 nucleic Acid in the plasma of patients with gastrointestinal cancer in the stomach and colon. Transl Oncol. 2013;6:290–296. doi: 10.1593/tlo.13118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergheim J, Semaan A, Gevensleben H, Groening S, Knoblich A, Dietrich J, Weber J, Kalff JC, Bootz F, Kristiansen G, Dietrich D. Potential of quantitative SEPT9 and SHOX2 methylation in plasmatic circulating cell-free DNA as auxiliary staging parameter in colorectal cancer: A prospective observational cohort study. Br J Cancer. 2018;118:1217–1228. doi: 10.1038/s41416-018-0035-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schröck A, Leisse A, de Vos L, Gevensleben H, Dröge F, Franzen A, Wachendörfer M, Schröck F, Ellinger J, Teschke M, Wilhelm-Buchstab T, Landsberg J, Holdenrieder S, Hartmann G, Field JK, Bootz F, Kristiansen G, Dietrich D. Free-Circulating Methylated DNA in Blood for Diagnosis, Staging, Prognosis, and Monitoring of Head and Neck Squamous Cell Carcinoma Patients: An Observational Prospective Cohort Study. Clin Chem. 2017;63:1288–1296. doi: 10.1373/clinchem.2016.270207. [DOI] [PubMed] [Google Scholar]
- 22.Wu A, He S, Li J, Liu L, Liu C, Wang Q, Peng X, Zhou J, Cao PG, Cao K. Colorectal cancer in cases of multiple primary cancers: Clinical features of 59 cases and point mutation analyses. Oncol Lett. 2017;13:4720–4726. doi: 10.3892/ol.2017.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao H, Wu H, Liu Y. [Improvement of prognostic and predictive network of colorectal cancer based upon the 8th edition of AJCC colorectal cancer staging system] Zhonghua Wei Chang Wai Ke Za Zhi. 2017;20:24–27. [PubMed] [Google Scholar]
- 24.Wu D, Zhou G, Jin P, Zhu J, Li S, Wu Q, Wang G, Sheng J, Wang J, Song L, Han X, Qian J. Detection of Colorectal Cancer Using a Simplified SEPT9 Gene Methylation Assay Is a Reliable Method for Opportunistic Screening. J Mol Diagn. 2016;18:535–545. doi: 10.1016/j.jmoldx.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Allemani C, Rachet B, Weir HK, Richardson LC, Lepage C, Faivre J, Gatta G, Capocaccia R, Sant M, Baili P, Lombardo C, Aareleid T, Ardanaz E, Bielska-Lasota M, Bolick S, Cress R, Elferink M, Fulton JP, Galceran J, Gózdz S, Hakulinen T, Primic-Zakelj M, Rachtan J, Diba CS, Sánchez MJ, Schymura MJ, Shen T, Tagliabue G, Tumino R, Vercelli M, Wolf HJ, Wu XC, Coleman MP. Colorectal cancer survival in the USA and Europe: A CONCORD high-resolution study. BMJ Open. 2013;3:e003055. doi: 10.1136/bmjopen-2013-003055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu B, Yan P, Zhang S, Lu Y, Pan L, Tang W, Chen S, Chen S, Zhang A, Liu W. Cell-Free Circulating Methylated SEPT9 for Noninvasive Diagnosis and Monitoring of Colorectal Cancer. Dis Markers. 2018;2018:6437104. doi: 10.1155/2018/6437104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song L, Jia J, Yu H, Peng X, Xiao W, Gong Y, Zhou G, Han X, Li Y. The performance of the mSEPT9 assay is influenced by algorithm, cancer stage and age, but not sex and cancer location. J Cancer Res Clin Oncol. 2017;143:1093–1101. doi: 10.1007/s00432-017-2363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ren W, Shen S, Sun Z, Shu P, Shen X, Bu C, Ai F, Zhang X, Tang A, Tian L, Li G, Li X, Ma J. Jak-STAT3 pathway triggers DICER1 for proteasomal degradation by ubiquitin ligase complex of CUL4A(DCAF1) to promote colon cancer development. Cancer Lett. 2016;375:209–220. doi: 10.1016/j.canlet.2016.02.055. [DOI] [PubMed] [Google Scholar]
- 29.Provenzale D, Gupta S, Ahnen DJ, Markowitz AJ, Chung DC, Mayer RJ, Regenbogen SE, Blanco AM, Bray T, Cooper G, Early DS, Ford JM, Giardiello FM, Grady W, Hall MJ, Halverson AL, Hamilton SR, Hampel H, Klapman JB, Larson DW, Lazenby AJ, Llor X, Lynch PM, Mikkelson J, Ness RM, Slavin TP, Sugandha S, Weiss JM, Dwyer MA, Ogba N. NCCN Guidelines Insights: Colorectal Cancer Screening, Version 1.2018. J Natl Compr Canc Netw. 2018;16:939–949. doi: 10.6004/jnccn.2018.0067. [DOI] [PubMed] [Google Scholar]
- 30.Tóth K, Sipos F, Kalmár A, Patai AV, Wichmann B, Stoehr R, Golcher H, Schellerer V, Tulassay Z, Molnár B. Detection of methylated SEPT9 in plasma is a reliable screening method for both left- and right-sided colon cancers. PLoS One. 2012;7:e46000. doi: 10.1371/journal.pone.0046000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tóth K, Wasserkort R, Sipos F, Kalmár A, Wichmann B, Leiszter K, Valcz G, Juhász M, Miheller P, Patai ÁV, Tulassay Z, Molnár B. Detection of methylated septin 9 in tissue and plasma of colorectal patients with neoplasia and the relationship to the amount of circulating cell-free DNA. PLoS One. 2014;9:e115415. doi: 10.1371/journal.pone.0115415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen J, Wang W, Zhang Y, Hu T, Chen Y. The roles of miR-200c in colon cancer and associated molecular mechanisms. Tumour Biol. 2014;35:6475–6483. doi: 10.1007/s13277-014-1860-x. [DOI] [PubMed] [Google Scholar]
- 33.Choi AH, Nelson RA, Schoellhammer HF, Cho W, Ko M, Arrington A, Oxner CR, Fakih M, Wong J, Sentovich SM, Garcia-Aguilar J, Kim J. Accuracy of computed tomography in nodal staging of colon cancer patients. World J Gastrointest Surg. 2015;7:116–122. doi: 10.4240/wjgs.v7.i7.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sjövall A, Blomqvist L, Egenvall M, Johansson H, Martling A. Accuracy of preoperative T and N staging in colon cancer--a national population-based study. Colorectal Dis. 2016;18:73–79. doi: 10.1111/codi.13091. [DOI] [PubMed] [Google Scholar]
- 35.Brouwer NPM, Stijns RCH, Lemmens VEPP, Nagtegaal ID, Beets-Tan RGH, Fütterer JJ, Tanis PJ, Verhoeven RHA, de Wilt JHW. Clinical lymph node staging in colorectal cancer; a flip of the coin? Eur J Surg Oncol. 2018;44:1241–1246. doi: 10.1016/j.ejso.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Ollberding NJ, Nomura AM, Wilkens LR, Henderson BE, Kolonel LN. Racial/ethnic differences in colorectal cancer risk: The multiethnic cohort study. Int J Cancer. 2011;129:1899–1906. doi: 10.1002/ijc.25822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tham C, Chew M, Soong R, Lim J, Ang M, Tang C, Zhao Y, Ong SY, Liu Y. Postoperative serum methylation levels of TAC1 and SEPT9 are independent predictors of recurrence and survival of patients with colorectal cancer. Cancer. 2014;120:3131–3141. doi: 10.1002/cncr.28802. [DOI] [PubMed] [Google Scholar]
- 38.Song L, Guo S, Wang J, Peng X, Jia J, Gong Y, Yang B, Xiao W, Dong C, Liu H, Li Y. The blood mSEPT9 is capable of assessing the surgical therapeutic effect and the prognosis of colorectal cancer. Biomark Med. 2018;12:961–973. doi: 10.2217/bmm-2018-0012. [DOI] [PubMed] [Google Scholar]