Abstract
Despite advances in antifungal therapy, invasive fungal infections remain a significant cause of morbidity and mortality worldwide. One important factor contributing to the relative ineffectiveness of existing antifungal drugs is insufficient drug exposure at the site of infection. Despite the importance of this aspect of antifungal therapy, we generally lack a full appreciation of how antifungal drugs distribute, penetrate, and interact with their target organisms in different tissue subcompartments. A better understanding of drug distribution will be critical to guide appropriate use of currently available antifungal drugs, as well as to aid development of new agents. Herein we briefly review current perspectives of antifungal drug exposure at the site of infection and describe a new technique, matrix-assisted laser desorption ionization (MALDI) mass spectrometry imaging, which has the potential to greatly expand our understanding of drug penetration.
Keywords: antifungals, tissue penetration, site of infection, MALDI imaging, drug quantification, posaconazole
Introduction
Invasive fungal infections (IFIs) pose a significant threat to global health, particularly in critically ill patients and those with underlying immunosuppression, accounting for more than 1.35 million deaths per year.1 The predominant causative agents include species of Candida, Aspergillus, and Cryptococcus.1 Successful patient management requires early and effective antifungal therapy. Currently, only four classes of antifungal agents are available to treat systemic fungal infections: polyenes, azoles, echinocandins, and flucytosine (5-FC). Polyenes were the first antifungal agents developed and have a long-standing history in the treatment of IFIs. The use of conventional amphotericin B has been limited due to toxic side effects, which have been reduced by the lipid formulations of amphotericin B. Treatment options for invasive mycoses expanded with the introduction of the second-generation triazoles (itraconazole, voriconazole, posaconazole, and isavuconazole) and the echinocandins (caspofungin, micafungin, anidulafungin). Triazole drugs are the mainstay in the treatment of all forms of Aspergillus infections. Azoles exert their primary action by interrupting biosynthesis of ergosterol via inhibition of the enzyme lanosterol 14α-demethylase. Echinocandins are recommended as first-line therapies for most types of invasive candidiasis (IC).2,3 5-FC was first synthesized in 1957 as a potential anti-tumor agent.4 Later, it was found that 5-FC can be quickly taken up by fungal cells and converted into 5-fluorouracil, resulting in antifungal activity against Cryptococcus neoformans and Candida spp.5,6 Each of these antifungal classes has its advantages; however, their use is often limited by either toxicities, nonlinear pharmacokinetics (PK) and other PK restrictions, drug-drug interactions, or inadequate killing and subsequent resistance development. For example, the clinical use of the 5-FC has been largely limited to combination therapy with other antifungals (primarily amphotericin B) to treat cryptococcosis, due to the rapid development of resistance during 5-FC treatment.6,7 Despite the significant roles of triazole drugs in the treatment of invasive aspergillosis (IA), the mortality rates of IA remain unacceptably high, especially in heavily immunosuppressed hosts such as leukemia patients and transplant recipients.8,9 Meanwhile, echinocandin treatment failures occur in up to 40% of IC cases.10 The relative ineffectiveness of existing antifungal drugs reflects the interplay of factors involving both the drug and the host. Perhaps one of the most important but poorly understood factors underlying the failure of antifungal therapy is inadequate local drug exposure at the site of infection. Sufficient penetration into infected tissue compartments is a key requirement for efficacy of all antimicrobial agents.11,12 However, our knowledge of the mechanisms by which drugs distribute, penetrate, and interact with their target organisms in different tissue subcompartments at the site of infection remains limited. A better understanding of antifungal pharmacokinetics at the site of infection will not only help understand different therapeutic effects of antifungal drugs currently in use but will be critical for the development of new antifungal agents. In this article, we briefly review current perspectives of antifungal drug exposure at the site of infection, as well as provide descriptions of some new frontiers that we believe will largely advance this area in the near future.
Serum or tissue concentrations do not tell the complete story of drug penetration
Sufficient drug exposure at the site of infection is a key determinant of antimicrobial efficacy. As antifungal agents must exert their effects within the tissues infected by fungal cells, tissue concentrations are believed to be an informative measure of effective drug exposure.13 Indeed, the distribution process of most antifungal drugs is characterized by considerable variability among different target tissues, and drug levels at the target site may substantially differ from those in plasma.14-22 For example, Conte et al. studied intrapulmonary PK of itraconazole at steady state in healthy human subjects and found that the area under the concentration-time curves (AUC) of itraconazole in plasma, epithelial lining fluid (ELF), and alveolar cells (AC) were very different, measured as 34.4, 7.4, and 101 μg·hr/ml.14 One study using rat model to evaluate tissue distributions of posaconazole reported that posaconazole lung concentrations were several-fold higher than in serum samples for all tested single or repeated doses.16 Different PK parameters for plasma and target tissues were also reported from the study investigating intrapulmonary PK of micafungin in adult lung transplant patients.19 Therefore, the concern has been raised that plasma drug levels may not serve reliably as a surrogate measure of drug exposure at the site of infection. Although tissue antifungal levels may provide a better estimate of fungal drug delivery, the type and location of fungal lesions can greatly impact the degree to which organisms are exposed to antifungal agents.13,23 Fungal infections can manifest as disseminated, single- or multifocal diseases, such as intra-abdominal candidiasis,24–26 pulmonary aspergilloma27,28 or cerebral cryptococcoma.29,30 Drug penetration in areas of tissue infection may differ markedly from healthy tissue as a result of altered tissue structure and permeability associated with tissue necrosis and/or fungal biofilm formation. Additionally, as fungi grow both extracellularly or intracellularly (e.g., Histoplasma capsulatum) during infection, the accumulation of antifungals within different intracellular compartments may influence their activity. Therefore, the location of infecting fungi and drug distribution within host cells themselves must also be considered.
New frontiers in drug development research
Although plasma PK of most of the currently available antifungal drugs have been well described, antifungal distribution within specific target tissues is less well understood. Lately, the cutting edge technology matrix-assisted laser desorption ionization (MALDI) mass spectrometry (MS) imaging has entered the field of tissue-based research by providing unique advantages for analyzing tissue specimen in an unprecedented detail.31 Due to the ability to directly detect analytes at high specificity based on their molecular weight, no labeling is required for using MALDI imaging to visualize the localization of a broad range of molecules including peptides, lipids, proteins, drugs, and their metabolites within tissue.32,33 Therefore, this in situ technology is a powerful tool to acquire spatial molecular information in tissues and has been increasingly adopted in drug discovery and development research to investigate distributions of drugs in relation to tissue morphology and histopathology.31,34–36 The principles of MALDI imaging and varieties of applications have been reviewed in a number of articles31,37–39 and will not be discussed in detail. Briefly, four sequential steps are involved in the MALDI imaging analysis. First, sample preparation must be carefully performed to maintain the spatial arrangement of compounds and avoid delocalization and degradation of the analytes. Careful handling of tissue samples immediately after sample procurement includes procedural freezing the tissue in liquid nitrogen, proper storage at −80°C, and delicate cryosectioning. Once the frozen tissue sections are mounted onto a MALDI plate, the second step follows with the application of an ionization-enhancing matrix. The choice of matrix and optimization of the coating procedure is extremely important for a successful imaging experiment.40 Next, MALDI-MS analysis is performed to acquire the spatial distribution of molecules in a defined mass range present within a given tissue section. During this phase, the laser beam is rastered across the surface of the matrix coated tissue, allowing desorption and ionization of biomolecules, and generating and collecting mass spectra at each measuring position (defined by x,y coordinates) in a serial manner. The final step of MALDI imaging is data analysis and image construction. These analyses can be extremely complicated due to the large data sets acquired for a large number of molecules ionized from the target tissue section during the process.41 Using imaging analysis software, ion intensity maps (images) can be plotted for any selected peak in the acquired mass spectrum to visualize the corresponding spatial distribution of the target peak. This ion intensity map can be overlaid on tissue histology images, obtained from consecutive tissue sections or, less commonly, the same section after the MALDI measurement.42 In contrast to traditional immunohistochemistry approaches, MALDI imaging permits the simultaneous detection and mapping of multiple molecules in a single measurement. A reasonable yet not very compelling part of this technique is the high demand on well-qualified personnel. Nevertheless, MALDI imaging has provided invaluable insights into drug distribution studies, particularly for anticancer drugs.43–45 An increasing number of studies utilizing MALDI imaging have also been seen in the field of antimicrobial agent development.35,46,47 These studies to date have mainly focused on antibacterial drugs and have only recently been applied to study the tissue distribution of antifungal agents.
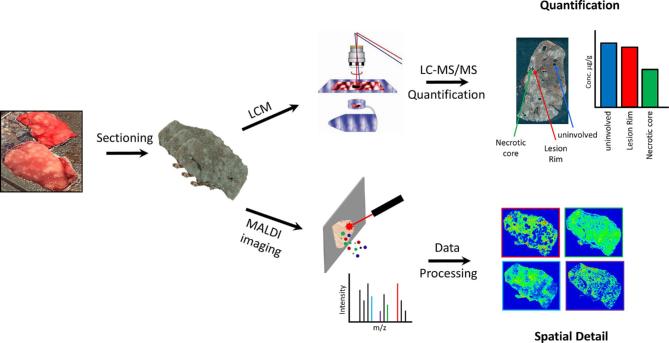
While providing excellent molecular specificity and spatial information, absolute quantification by MALDI imaging mass spectrometry remains challenging, due to the very large data sets and complicated statistical analysis.41 Accordingly, the majority of current MALDI imaging studies are only semi-quantitative in spite of great efforts put into developing quantitative MALDI imaging analysis.48,49 In order to overcome this limitation, a complementary technique laser-capture microdissection (LCM) can be utilized to procure subpopulations of cells from heterogeneous tissues under direct microscopic visualization.50–52 LCM is compatible with a variety of downstream analyses, including liquid chromatography-tandem mass spectrometry (LC-MS/MS), in order to accurately measure drug concentrations within distinct tissue subcompartments (Fig. 1). By combining LCM and LC-MS/MS, drug exposure at the exact location of microbial organisms within tissue lesions can be quantified directly and followed over time. This strategy has been applied in a few recent studies investigating drug penetration in relation to efficacy.53,54
Figure 1.
Workflow of tissue drug exposure analysis by integrating MALDI imaging with LCM directed LC-MS/MS. This Figure is reproduced in color in the online version of Medical Mycology.
What we have learned for antifungals at the site of infection
Despite the fact that antifungal delivery to the site of infection is critical to treatment success, our understanding of drug penetration of fungal lesions within tissues is limited by the fact that most studies have measured antifungal tissue levels in whole organ homogenates.13 Thus far, only a few studies have addressed deciphering drug concentrations in histologically distinct tissue compartments. A prime example is a study of human lung tissues isolated postmortem from patients with invasive aspergillosis receiving amphotericin B therapy.55 While amphotericin B levels within healthy lung tissues were found to have sufficient drug levels (0.67 μg/g) to inhibit fungal growth, amphotericin B levels within infected, infarcted lung tissues were significantly lower (0.1 μg/g). All patients failed the therapy in this study with all Aspergillus isolates susceptible to amphotericin B, suggesting that poor penetration of infected tissue by amphotericin B was likely a key contributor to treatment failure in these patients. This study highlighted the importance of aligning tissue concentrations within the context of tissue histopathology. The development of MALDI-MS imaging has now opened the door for a new generation of studies to expand our understanding of the delivery of antifungal drugs at the site of infection by direct visualization and quantification of drug levels in distinct tissue subcompartments.
Penetration of echinocandin drugs in intra-abdominal abscesses
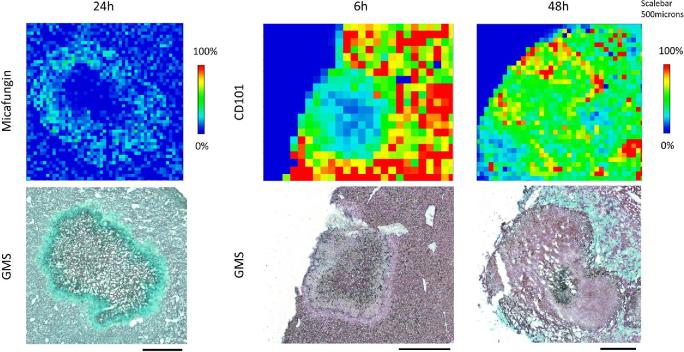
Intra-abdominal candidiasis (IAC) is a common but poorly understood56 invasive fungal infection associated with high mortality.24–26 Scattered abscesses and microlesions are the predominant histopathological findings within abdominal organs from humans with IAC. Prompt antifungal therapy and source control are crucial for successful treatment.25,56 Echinocandin antifungal drugs are first-line agents, yet their clinical effectiveness is highly variable.57–59 Little is known about echinocandin exposure at the site of infection, although it has been postulated that restricted drug penetration into abscesses is the main cause of antifungal treatment failure and favors the emergence of antifungal resistance.60 A recent study has reported the use of MALDI imaging and LCM-directed LC-MS/MS to investigate the spatial and quantitative tissue distribution of two echinocandin drugs, micafungin and rezafungin (CD101), in a clinically relevant IAC mouse model.53 The two echinocandin drugs exhibited notably different tissue pharmacokinetics. Micafungin at 5 mg/kg quickly distributed into liver tissue, reached peak intensity at 1 h postdose, and then decreased through the entire tissue. Drug penetration into the necrotic lesion was observed beginning at 6 h postdose but was not clearly visualized until 24 h postdose. Co-localization of the drug (MALDI imaging) and fungal cells (Gomori Methenamine Silver [GMS] staining) inside of the lesion at 24 h demonstrated a “donut” like distribution pattern of micafungin within the lesion, where a portion of fungal cells were not exposed to significant amounts of micafungin (Fig. 2). Micafungin penetration into fungal lesions improved at steady-state, with drug reaching the necrotic core. In comparison, while rezafunfin also had a swift liver distribution after single dosing, drug retention in the tissue and penetration into the lesions were remarkably different. Rapid and persistent lesion penetration of rezafungin was revealed by the co-localization analysis (Fig. 2), with homogeneous distribution being observed by 48 h. Consistent with the imaging results, drug quantification in tissues recovered by LCM showed that drug levels in lesions were 3.4 μg/g for micafungin and 44.5 μg/g for rezafungin at 24 h postdose. Antifungal activity in tissues was also measured. Not surprisingly, the fungal burden in the livers of mice treated with rezafungin was significantly lower than in those treated with micafungin. Sterilization of liver tissue was achieved in 80% of the mice in the rezafungin treatment arm compared to none in the micafungin group. The authors estimated that rezafungin levels within Candida lesions were sufficiently high to prevent the development of echinocandin resistance, although this hypothesis awaits experimental testing. Collectively, the results of this first successful application of MALDI imaging in the antifungal field establish this technique as a powerful tool to probe tissue pharmacokinetics.
Figure 2.
Close up examination of drug penetration for micafungin at 24 h, and CD101 at 6 and 48 h post single dosing. Enlarged view of drug distribution in a single lesion at pixel level. Matched GMS staining of adjacent sections was placed on the bottom. Signal intensity is fixed for CD101 and micafungin, respectively. Scale bars, 5 mm. Figure reprinted by permission: Zhao et al.53Antimicrob Agents Chemother. 2017; 61: e01009-17. Copyright (2017) American Society for Microbiology (ASM). This Figure is reproduced in color in the online version of Medical Mycology.
Penetration of triazole antifungals in Aspergillus infected lungs
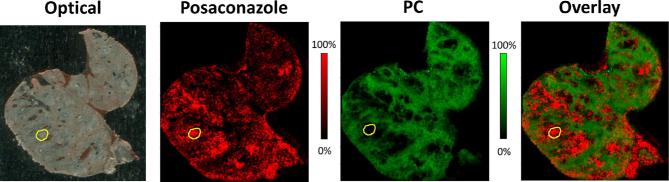
More recently, our group has extended these studies to examine the tissue pharmacokinetics of triazole drugs in experimental Aspergillus infection. Preliminary studies of posaconazole distribution within pulmonary tissue have provided important proof-of-concept data demonstrating the utility of this technique for the study of this condition. In a mouse model of chronic Aspergillus colonization and infection,61,62 mice were treated with two doses of 20 mg/kg posaconazole at 60 h and 68 h following intratracheal infection with agar beads containing Aspergillus fumigatus conidia. Mice were euthanized, and lungs were collected at 4 h after the second dose of posaconazole. MALDI imaging visualized a heterogeneous distribution of posaconazole in the infected lung tissue. Drug signals were preferentially concentrated in areas of infection, which was characterized by small round lesions with A. fumigatus growing within agar beads surrounded by a large number of various inflammatory cell populations including neutrophils, macrophages, and other mononuclear cells and a large zone of tissue necrosis. Overlay of the ion map of posaconazole (m/z 701.337) with that of the healthy lung tissue marker (phosphatidylcholine 32:1, m/z 732.552)63,64 acquired from the same tissue section clearly demonstrated preferential accumulation of posaconazole within necrotic tissues (Fig. 3). Tissues recovered by LCM from lesions displayed average posaconazole levels of 15.6 μg/g, about 38% higher than that measured in unaffected lung tissue. An enlarged view of the MALDI image and the adjacent GMS-stained section indicated that posaconazole was mostly accumulated in lipid rich macrophage cells, which were located in the outer part of the lesion rather than the center where Aspergillus cells aggregated. This observation is consistent with previous findings of high intracellular concentration of posaconazole in human peripheral blood cells or pulmonary cells.65–67 These promising results are being extended to compare the distribution of posaconazole with other triazole agents to better understand drug exposure at the site of Aspergillus infection.
Figure 3.
MALDI imaging analysis of posaconazole and phosphatidylcholine (32:1) distribution in mouse lung infected with A. fumigatus embedded agar beads and collected at 4 h post two doses of posaconazole at 20 mg/kg. Images from left to right were acquired from the same tissue section: optical scan, ion map of posaconazole (m/z 701.337), ion map of phosphatidylcholine 32:1 (PC) (m/z 732.552), and corresponding overlay. Signal intensity color bar is fixed for posaconazole (red) and PC (green), respectively, with gradually increased intensity from black (no signal) to red or green (max signal). Yellow Outlines highlight one typical lesion formed with the A. fumigatus agar beads. This Figure is reproduced in color in the online version of Medical Mycology.
Despite the need for antifungal drugs to act upon organisms growing within tissues, therapeutic drug monitoring of antifungal agents continues to rely on plasma or serum drug concentrations. Even when data regarding tissue antimicrobial levels are available, these studies rely on whole tissue extraction protocols to measure antifungal levels and rarely taken into account regional or subcellular differences in drug distribution, nor the relative location of organisms within these compartments. A much more detailed understanding and delicate dissection of the drug behavior at the site of infection is an unmet need for current antifungal research. MALDI imaging offers a promising tool to meet this need. The ample spatial information captured by MALDI imaging can improve our current appreciation of drug penetration at the site of infection. When coupled with routine analytical methodology and LCM, this technique can provide novel insights to predict antifungal efficacy and may also guide strategies to prevent the emergence of antifungal resistance.
Declaration of interest
D.S.P. has received research support from and served on advisory boards for Astellas, Amplyx, Cidara, and Merck. D.C.S. has received research support and served on advisory boards for Astellas, and Merck. All other authors report no conflicts of interest. The authors alone are responsible for the content and the writing of the paper.
References
- 1. Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Sci Transl Med. 2012; 4: 165rv113. [DOI] [PubMed] [Google Scholar]
- 2. Pappas PG, Kauffman CA, Andes D et al. . Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009; 48: 503–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pappas PG, Kauffman CA, Andes DR et al. . Executive summary: Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016; 62: 409–417. [DOI] [PubMed] [Google Scholar]
- 4. Heidelberger C, Chaudhuri NK, Danneberg P et al. . Fluorinated pyrimidines, a new class of tumour-inhibitory compounds. Nature. 1957; 179: 663–666. [DOI] [PubMed] [Google Scholar]
- 5. Grunberg E, Titsworth E, Bennett M. Chemotherapeutic activity of 5-fluorocytosine. Antimicrob Agents Chemother (Bethesda). 1963; 161: 566–568. [PubMed] [Google Scholar]
- 6. Vermes A, Guchelaar HJ, Dankert J. Flucytosine: a review of its pharmacology, clinical indications, pharmacokinetics, toxicity and drug interactions. J Antimicrob Chemother. 2000; 46: 171–179. [DOI] [PubMed] [Google Scholar]
- 7. Loyse A, Dromer F, Day J, Lortholary O, Harrison TS. Flucytosine and cryptococcosis: time to urgently address the worldwide accessibility of a 50-year-old antifungal. J Antimicrob Chemother. 2013; 68: 2435–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Verweij PE, Chowdhary A, Melchers WJG, Meis JF. Azole resistance in Aspergillus fumigatus: can we retain the clinical use of mold-active antifungal azoles? Clin Infect Dis. 2016; 62: 362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Osherov N, Kontoyiannis DP. The anti-Aspergillus drug pipeline: is the glass half full or empty? Med Mycol. 2017; 55: 118–124. [DOI] [PubMed] [Google Scholar]
- 10. Wiederhold NP, Lewis JS 2nd. The echinocandin micafungin: a review of the pharmacology, spectrum of activity, clinical efficacy and safety. Expert Opin Pharmacother. 2007; 8: 1155–1166. [DOI] [PubMed] [Google Scholar]
- 11. Rodvold KA, Yoo L, George JM. Penetration of anti-infective agents into pulmonary epithelial lining fluid: focus on antifungal, antitubercular and miscellaneous anti-infective agents. Clin Pharmacokinet. 2011; 50: 689–704. [DOI] [PubMed] [Google Scholar]
- 12. Stein GE, Wells EM. The importance of tissue penetration in achieving successful antimicrobial treatment of nosocomial pneumonia and complicated skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus: vancomycin and linezolid. Curr Med Res Opin. 2010; 26: 571–588. [DOI] [PubMed] [Google Scholar]
- 13. Felton T, Troke PF, Hope WW. Tissue penetration of antifungal agents. Clin Microbiol Rev. 2014; 27: 68–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Conte JE Jr., Golden JA, Kipps J, McIver M, Zurlinden E. Intrapulmonary pharmacokinetics and pharmacodynamics of itraconazole and 14-hydroxyitraconazole at steady state. Antimicrob Agents Chemother. 2004; 48: 3823–3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Conte JE Jr., Golden JA, Krishna G, McIver M, Little E, Zurlinden E. Intrapulmonary pharmacokinetics and pharmacodynamics of posaconazole at steady state in healthy subjects. Antimicrob Agents Chemother. 2009; 53: 703–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cendejas-Bueno E, Forastiero A, Ruiz I, Mellado E, Gavalda J, Gomez-Lopez A. Blood and tissue distribution of posaconazole in a rat model of invasive pulmonary aspergillosis. Diagn Microbiol Infect Dis. 2017; 87: 112–117. [DOI] [PubMed] [Google Scholar]
- 17. Schmitt-Hoffmann AH, Kato K, Townsend R et al. . Tissue distribution and elimination of isavuconazole following single and repeat oral-dose administration of isavuconazonium sulfate to rats. Antimicrob Agents Chemother. 2017; 61: e01292–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lat A, Thompson GR 3rd, Rinaldi MG, Dorsey SA, Pennick G, Lewis JS 2nd. Micafungin concentrations from brain tissue and pancreatic pseudocyst fluid. Antimicrob Agents Chemother. 2010; 54: 943–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Walsh TJ, Goutelle S, Jelliffe RW et al. . Intrapulmonary pharmacokinetics and pharmacodynamics of micafungin in adult lung transplant patients. Antimicrob Agents Chemother. 2010; 54: 3451–3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fischman AJ, Alpert NM, Livni E et al. . Pharmacokinetics of 18F-labeled fluconazole in healthy human subjects by positron emission tomography. Antimicrob Agents Chemother. 1993; 37: 1270–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thaler F, Bernard B, Tod M et al. . Fluconazole penetration in cerebral parenchyma in humans at steady state. Antimicrob Agents Chemother. 1995; 39: 1154–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vogelsinger H, Weiler S, Djanani A et al. . Amphotericin B tissue distribution in autopsy material after treatment with liposomal amphotericin B and amphotericin B colloidal dispersion. J Antimicrob Chemother. 2006; 57: 1153–1160. [DOI] [PubMed] [Google Scholar]
- 23. Mouton JW, Theuretzbacher U, Craig WA, Tulkens PM, Derendorf H, Cars O. Tissue concentrations: do we ever learn? J Antimicrob Chemother. 2008; 61: 235–237. [DOI] [PubMed] [Google Scholar]
- 24. Carneiro HA, Mavrakis A, Mylonakis E. Candida peritonitis: an update on the latest research and treatments. World J Surg. 2011; 35: 2650–2659. [DOI] [PubMed] [Google Scholar]
- 25. Bassetti M, Righi E, Ansaldi F et al. . A multicenter multinational study of abdominal candidiasis: epidemiology, outcomes and predictors of mortality. Intensive Care Med. 2015; 41: 1601–1610. [DOI] [PubMed] [Google Scholar]
- 26. Andes DR, Safdar N, Baddley JW et al. . The epidemiology and outcomes of invasive Candida infections among organ transplant recipients in the United States: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Transpl Infect Dis. 2016; 18: 921–931. [DOI] [PubMed] [Google Scholar]
- 27. Denning DW, Cadranel J, Beigelman-Aubry C et al. . Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016; 47: 45–68. [DOI] [PubMed] [Google Scholar]
- 28. Mohan A, Tiwari P, Madan K et al. . Intrabronchial voriconazole is a safe and effective measure for hemoptysis control in pulmonary aspergilloma. J Bronchology Interv Pulmonol. 2017; 24: 29–34. [DOI] [PubMed] [Google Scholar]
- 29. Chen SC, Korman TM, Slavin MA et al. . Antifungal therapy and management of complications of cryptococcosis due to Cryptococcus gattii. Clin Infect Dis. 2013; 57: 543–551. [DOI] [PubMed] [Google Scholar]
- 30. Ulett KB, Cockburn JW, Jeffree R, Woods ML. Cerebral cryptococcoma mimicking glioblastoma. BMJ Case Reports. 2017; doi:10.1136/bcr-2016-218824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Prideaux B, Stoeckli M. Mass spectrometry imaging for drug distribution studies. J Proteomics. 2012; 75: 4999–5013. [DOI] [PubMed] [Google Scholar]
- 32. Caprioli RM, Farmer TB, Gile J. Molecular imaging of biological samples: localization of pPeptides and proteins using MALDI-TOF MS. Anal Chem. 1997; 69: 4751–4760. [DOI] [PubMed] [Google Scholar]
- 33. Stoeckli M, Chaurand P, Hallahan DE, Caprioli RM. Imaging mass spectrometry: a new technology for the analysis of protein expression in mammalian tissues. Nat Med. 2001; 7: 493–496. [DOI] [PubMed] [Google Scholar]
- 34. Trim PJ, Francese S, Clench MR. Imaging mass spectrometry for the assessment of drugs and metabolites in tissue. Bioanalysis. 2009; 1: 309–319. [DOI] [PubMed] [Google Scholar]
- 35. Prideaux B, Via LE, Zimmerman MD et al. . The association between sterilizing activity and drug distribution into tuberculosis lesions. Nat Med. 2015; 21: 1223–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marko-Varga G, Fehniger TE, Rezeli M, Dome B, Laurell T, Vegvari A. Drug localization in different lung cancer phenotypes by MALDI mass spectrometry imaging. Journal of proteomics. 2011; 74: 982–992. [DOI] [PubMed] [Google Scholar]
- 37. Norris JL, Caprioli RM. Analysis of tissue specimens by matrix-assisted laser desorption/ionization imaging mass spectrometry in biological and clinical research. Chem Rev. 2013; 113: 2309–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Castellino S, Groseclose MR, Wagner D. MALDI imaging mass spectrometry: bridging biology and chemistry in drug development. Bioanalysis. 2011; 3: 2427–2441. [DOI] [PubMed] [Google Scholar]
- 39. Aichler M, Walch A. MALDI Imaging mass spectrometry: current frontiers and perspectives in pathology research and practice. Lab Invest. 2015; 95: 422–431. [DOI] [PubMed] [Google Scholar]
- 40. Schwartz SA, Reyzer ML, Caprioli RM. Direct tissue analysis using matrix-assisted laser desorption/ionization mass spectrometry: practical aspects of sample preparation. J Mass Spectrom. 2003; 38: 699–708. [DOI] [PubMed] [Google Scholar]
- 41. Jones EA, Deininger S-O, Hogendoorn PCW, Deelder AM, McDonnell LA. Imaging mass spectrometry statistical analysis. J Proteomics. 2012; 75: 4962–4989. [DOI] [PubMed] [Google Scholar]
- 42. Schwamborn K, Krieg RC, Reska M, Jakse G, Knuechel R, Wellmann A. Identifying prostate carcinoma by MALDI-Imaging. Int J Mol Med. 2007; 20: 155–159. [PubMed] [Google Scholar]
- 43. Hu J, Liu F, Ju H. MALDI-MS Patterning of caspase activities and its application in the assessment of drug resistance. Angew Chem Int Ed Engl. 2016; 55: 6667–6670. [DOI] [PubMed] [Google Scholar]
- 44. Lee RFS, Theiner S, Meibom A, Koellensperger G, Keppler BK, Dyson PJ. Application of imaging mass spectrometry approaches to facilitate metal-based anticancer drug research. Metallomics. 2017; 9: 365–381. [DOI] [PubMed] [Google Scholar]
- 45. Theiner S, Van Malderen SJM, Van Acker T et al. . Fast high-resolution laser ablation-inductively coupled plasma mass spectrometry imaging of the distribution of platinum-based anticancer compounds in multicellular tumor spheroids. Anal Chem. 2017; 89: 12641–12645. [DOI] [PubMed] [Google Scholar]
- 46. Phelan VV, Fang J, Dorrestein PC. Mass spectrometry analysis of Pseudomonas aeruginosa treated with azithromycin. J Am Soc Mass Spectrom. 2015; 26: 873–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Prideaux B, ElNaggar MS, Zimmerman M, Wiseman JM, Li X, Dartois V. Mass spectrometry imaging of levofloxacin distribution in TB-infected pulmonary lesions by MALDI-MSI and continuous liquid microjunction surface sampling. Int J Mass Spectrom. 2015; 377: 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Prentice BM, Chumbley CW, Caprioli RM. Absolute quantification of rifampicin by MALDI imaging mass spectrometry using multiple TOF/TOF events in a single laser shot. J Am Soc Mass Spectrom. 2017; 28: 136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Aikawa H, Hayashi M, Ryu S et al. . Visualizing spatial distribution of alectinib in murine brain using quantitative mass spectrometry imaging. Sci Rep. 2016; 6: 23749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Emmert-Buck MR, Bonner RF, Smith PD et al. . Laser capture microdissection. Science. 1996; 274: 998–1001. [DOI] [PubMed] [Google Scholar]
- 51. Bonner RF, Emmert-Buck M, Cole K et al. . Laser capture microdissection: molecular analysis of tissue. Science. 1997; 278: 1481–1483. [DOI] [PubMed] [Google Scholar]
- 52. Cahill JF, Kertesz V, Van Berkel GJ. Laser dissection sampling modes for direct mass spectral analysis. Rapid Commun Mass Spectrom. 2016; 30: 611–619. [DOI] [PubMed] [Google Scholar]
- 53. Zhao Y, Prideaux B, Nagasaki Y et al. . Unraveling drug penetration of echinocandin antifungals at the site of infection in an intra-abdominal abscess model. Antimicrob Agents Chemother. 2017; 61: e01009–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zimmerman M, Lestner J, Prideaux B et al. . Ethambutol Partitioning in Tuberculous Pulmonary Lesions Explains Its Clinical Efficacy. Antimicrob Agents Chemother. 2017; 61: e00924–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Paterson PJ, Seaton S, Prentice HG, Kibbler CC. Treatment failure in invasive aspergillosis: susceptibility of deep tissue isolates following treatment with amphotericin B. J Antimicrob Chemother. 2003; 52: 873–876. [DOI] [PubMed] [Google Scholar]
- 56. Vergidis P, Clancy CJ, Shields RK et al. . Intra-abdominal candidiasis: the importance of early source control and antifungal treatment. PloS One. 2016; 11: e0153247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bassetti M, Peghin M, Carnelutti A et al. . Clinical characteristics and predictors of mortality in cirrhotic patients with candidemia and intra-abdominal candidiasis: a multicenter study. Intensive Care Med. 2017; 43: 509–518. [DOI] [PubMed] [Google Scholar]
- 58. Arendrup MC, Perlin DS. Echinocandin resistance: an emerging clinical problem? Curr Opin Infect Dis. 2014; 27: 484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Perlin DS. Echinocandin resistance, susceptibility testing and prophylaxis: implications for patient management. Drugs. 2014; 74: 1573–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shields RK, Nguyen MH, Press EG, Clancy CJ. Abdominal candidiasis is a hidden reservoir of echinocandin resistance. Antimicrob Agents Chemother. 2014; 58: 7601–7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Urb M, Snarr BD, Wojewodka G et al. . Evolution of the immune response to chronic airway colonization with Aspergillus fumigatus hyphae. Infect Immun. 2015; 83: 3590–3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Takazono T, Sheppard DC. Aspergillus in chronic lung disease: modeling what goes on in the airways. Med Mycol. 2017; 55: 39–47. [DOI] [PubMed] [Google Scholar]
- 63. Bernhard W, Hoffmann S, Dombrowsky H et al. . Phosphatidylcholine molecular species in lung surfactant: composition in relation to respiratory rate and lung development. Am J Respir Cell Mol Biol. 2001; 25: 725–731. [DOI] [PubMed] [Google Scholar]
- 64. Hall Z, Ament Z, Wilson CH et al. . Myc expression drives aberrant lipid metabolism in lung cancer. Cancer Res. 2016; 76: 4608–4618. [DOI] [PubMed] [Google Scholar]
- 65. Campoli P, Al Abdallah Q, Robitaille R et al. . Concentration of antifungal agents within host cell membranes: a new paradigm governing the efficacy of prophylaxis. Antimicrob Agents Chemother. 2011; 55: 5732–5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Campoli P, Perlin DS, Kristof AS, White TC, Filler SG, Sheppard DC. Pharmacokinetics of posaconazole within epithelial cells and fungi: insights into potential mechanisms of action during treatment and prophylaxis. J Infect Dis. 2013; 208: 1717–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Farowski F, Cornely OA, Vehreschild JJ et al. . Intracellular concentrations of posaconazole in different compartments of peripheral blood. Antimicrob Agents Chemother. 2010; 54: 2928–2931. [DOI] [PMC free article] [PubMed] [Google Scholar]