Abstract
The disease San Joaquin Valley Fever (coccidioidomycosis) is caused by the inhalation of Coccidioides arthroconidia. In vivo, arthroconidia transform into pathogenic structures termed spherules. Exposure to the host milieu triggers spherule development; however, the molecular mechanisms responsible for the morphological shift are not well characterized. This study compared the morphogenesis of five strains of both species of Coccidioides in two media types to improve the in vitro model of dimorphism that can be easily reproduced, and is amenable to tissue culture. We also sought to establish a modern record of the morphological switch among commonly used lab strains through a detailed account of growth under various conditions. Spherules from five strains were grown in standard (Converse) and experimental media (RPMI-sph). Strain behavior was quantified by median spherule size and spherule concentration, beginning 3 days after inoculation and followed for 10 days of growth. There were significant differences observed among Coccidioides immitis and C. posadasii strains, as well as differences between the in vitro systems.
Keywords: Coccidioides, valley fever, coccidioidomycosis, BSL3, spherules
Introduction
Certain arid and semi-arid regions of the Americas are natural reservoirs for the fungal pathogens Coccidioides immitis and C. posadasii, the causative agents of coccidioidomycosis, a respiratory infection often referred to as valley fever.1,2 These pathogenic fungi are responsible for many community acquired pneumonia cases in the highly endemic regions of North America.3 Disease manifestation is variable, ranging from asymptomatic to severe acute or chronic pneumonia. In 1–5% of cases dissemination to other body sites occurs, and is fatal without treatment in meningitis cases.3–5 Variability in disease manifestation makes both diagnosis and treatment a challenge; in 2007–2008 during an enhanced surveillance study, half of Arizona patients surveyed reported at least one emergency room visit, and a median of two physician visits (range 1–63, interquartile range [IQR] 1–3) for a correct diagnosis.6
The organism is able to infect a wide range of mammals, including humans, dogs, and various wildlife, with unpredictable disease outcomes. The causes of variation in clinical manifestations are unknown; however, host response, pathogen genetics, and degree of exposure all play some role.1,4,7,8 The molecular mechanisms that initiate the parasitic lifecycle, as well as a full understanding of pathogenesis and pathogenicity factors, remain elusive. In the saprobic phase, both species cycle between filamentous mycelia and 2–3 μm diameter barrel-shaped asexual arthroconidia.1,9,10 In the environment air-dispersed arthroconidia can germinate into mycelia upon returning to the soil. Alternatively, these mature arthroconidia may be inhaled by a susceptible host. In vivo, a parasitic life cycle is initiated when inhaled arthroconidia undergo a unique morphological shift within the host lung environment. Arthroconidia swell into large multinucleated spherules that contain potentially hundreds of endospores. Approximately 5 days after the initial infection, 1–2 μm diameter endospores are released when the mature spherule ruptures. The cycle continues, and fungal burden may increase exponentially, as each endospore can develop into a new spherule.7,8,11
The molecular mechanisms that initiate the morphological switch from a saprobic to a parasitic phase are not understood. Transcripts involved in response to oxidative stress (catalases and 4-Hydroxyphenylpyruvate dioxygenase [HPPD]), pH regulation (ureases), cell wall biosynthesis (alpha (1,3) glucan synthase, amylases, and SOWgp) are observed in spherules, relative to mycelia, and may represent a specific response to the host environment.12,13
Unraveling the factors that enhance morphogenesis, and thus affect pathogenesis, will involve both in vivo and in vitro models to study the organism.11,12,14 To date there are few publications detailing the complex methodology for culturing this organism, the most recent published in 1988.10,15–17
Converse media, a complex chemically defined salt/glucose media, is widely used for growing the parasitic phase of Coccidioides.10 In the context of the mammalian respiratory system, the composition of Converse media poses two distinct problems for in vitro studies of host-pathogen interactions. First, 8 out of the 11 ingredients in Converse media are salts, which are not available in the same concentrations in vivo. Second, the highly saline environment is toxic to host immune cells. Host-pathogen interaction studies are of interest as it has been shown that Coccidioides may secrete soluble factors that inhibit host response, prevent phagosome lysosome fusion, and develop into spherules after phagocytosis.18–20
A previous study used a supplemented Roswell Park Memorial Institute (RPMI) media (RPMI-sph) to produce continuous cultures of spherules.15 The components of the supplemented media are similar to commonly used cell culture media, namely, RPMI-1640 and fetal bovine serum (FBS), which would be amenable to the study of host-pathogen interactions. Thus, to advance a media supporting both in vitro spherule production and growth of host immune cells, we compared spherule development in both Coccidioides species grown in supplemented RPMI-sph media and Converse media under different conditions.
This study provides a detailed methodology for spherule development in five strains of Coccidioides in two in vitro systems and tests the utility of a risk-group two (RG2) strain of Coccidioides. Several obstacles hinder the progress of Coccidioides research, two of which we sought to address. Our specific objectives were to (1) investigate the adequacy of a previously published growth media that simulates growth conditions encountered by fungus in the host environment;15(2) identify characteristics of an RG2 strain that could be used as an alternative model for risk-group three (RG3) wild-type Coccidioides, which must be worked with in a biosafety level 3 (BSL3) laboratory.21 To assess the utility of an RG2-strain in modeling fungal growth and development, we chose the Δcts2/Δard1/Δcts3 strain, which was derived from the clinical isolate C735 of C. posadasii, originally created for use as a vaccine candidate. This strain lacks two chitinase genes that prevent endospore development and spherule rupture, effectively halting the parasitic lifecycle.21In vitro, it produces mycelia and arthroconidia, the latter converting to nonendosporulating spherules. We compared the development of the Δcts2/Δard1/Δcts3 RG2 strain to wild-type RG3 strains to assess similarities and differences in vitro.
Materials
Biosafety
Risk group three (RG3)
All work with active RG3 cultures was conducted in a biosafety level three facility (BSL3) following recommendations outlined in Biosafety in Microbiological and Biomedical Laboratories.22 Risk assessments of standard operating procedures (SOP), which included hazard mitigation specific to the agents, laboratory procedures, and personnel training were approved by the Institutional Biosafety Committee (IBC) at Northern Arizona University prior to conducting this work.
RG3 personnel protective equipment (PPE)
Personnel were required to wear coverall suits (Tyvek, Dupont, Santa Barbara, California, USA) over designated laboratory scrubs, long boot covers (Tyvek, USA) over designated laboratory shoes, primary extended cuff nitrile gloves (Kimberly-Clark, Owensboro, Kentucky, USA), secondary nitrile or latex gloves (Kimberly-Clark, USA) and a powered air purifying respirator (3 M Versaflo, Dekalb, Illinois, USA) to work within the BSL3 facility.
RG3 containment, inactivation and decontamination
Work involving live RG3 organism was conducted within a Class II, Type B2 biosafety cabinet (Baker, Sanford, Maine, USA), with the exception of steps involving centrifugation in which case, cultures were placed in a sealed rotor prior to being removed from the cabinet. Inactivation was confirmed by plating 10% of the sample on appropriate media using appropriate incubation conditions (see media and growth conditions) prior to removing the samples from the BSL3 facility. Contact with fresh 10% bleach for 2 minutes was used to decontaminate all items removed from the biosafety cabinet.23
Risk group two (RG2)
All work with active risk group two organism was conducted in a biosafety level two (BSL2) laboratory, following standard guidelines outlined in Biosafety in Microbiological and Biomedical Laboratories. In general, SOPs for the BSL2 work closely mirrored BSL3 work in terms of containment and decontamination.
RG2 personnel protective equipment (PPE)
Personnel working with active RG2 organism were required to wear disposable forward facing gowns (Health Products Express, Boston, Massachusetts, USA), primary extended cuff nitrile gloves, secondary nitrile or latex gloves (Kimberly-Clark, USA).
Strains
The four wild-type RG3 strains utilized were: C. posadasii isolates C735 (ATCC 96335) and Silveira (ATCC 28868), C. immitis isolate RMSCC 2006, and C. immitis hybrid strain RS. The RG2 C. posadasii strain was derived from the clinical isolate C735 and was obtained through Biodefense and Emerging Infections (BEI, Thousand Oaks, California, USA) repository, (NIAID, NIH: Coccidioides posadasii, Δcts2/Δard1/Δcts3, NR-166).21 The murine alveolar macrophage cell line MH-S ATCC CRL-2019 was obtained from American Type Culture Collection (ATCC, Manassas, Virginia, USA).
Media and growth conditions
Saprobic growth conditions
Mycelia
Fungal glycerol stocks were pipetted into a 250 ml vented baffle flask containing 50 ml of 2 × glucose yeast extract (2 × GYE; 2% glucose (VWR, USA), 1% yeast extract (Difco, Detroit, Michigan, USA) on a shaking incubator (GeneMate Orbital Shaker Mini, Stockton, California, USA) at 30°C for 48 hours.
Arthroconidia
Thirty milliliters of 2 × GYE agar using the same formulation described above plus 1.5% bacteriological agar (Difco, USA) was poured onto a 125 ml vented suspension flasks (VWR, Batavia, Illinois, USA). One milliliter of mycelia or arthroconidia was inoculated into individual cell culture flasks, and cells were spread in the entire surface of the agar media with the help of a cell-scraper. Hygromycin (Sigma-Aldrich, St. Louis, Missouri, USA) 75 μg/ml was included in all solid media of the Δcts2/Δard1/Δcts3 strain for selection purposes. Arthroconidia was harvested from the flasks through the addition of 30 ml of sterile 1 × PBS (Gibco, Gaithersburg, Maryland, USA) into each flask and lightly scraping the media surface with a cell scraper (GeneMate, USA). To remove additional hyphal fragments and debris, the cell suspension was filtered through sterile miracloth filtration funnels or 100 μm filters (Falcon, Austin, Texas, USA) assembled into a 50 ml conical tube. To rinse the arthroconidia, the suspensions were spun down at 9000 × g for 5 minutes, the PBS was removed, and fresh 30 ml of PBS was added and vortexed vigorously for 1 minute (centrifugation and suspension steps were repeated twice). Arthroconidia were counted on a hemocytometer and stored at 4°C. To check viability, 100 μl serial dilutions of the counted cell suspension were plated on 2 × GYE and grown at room temperature for 5 days to obtain colony forming unit counts.
Parasitic growth conditions
Chemically defined modified Converse media (ammonium acetate 0.016 M (Sigma-Aldrich, USA), KH2PO4 anhydrous 0.0037 M (Amresco, Cleveland, Ohio, USA), K2HPO4 anhydrous 0.003 M (Amresco, USA), MgSO4*7H2O 0.0016 M (Amresco, USA), ZnSO4*7H2O 1.25 × 10−5 M (Fisher Scientific, Waltham, Massachusetts, USA), NaCl 2.4 × 10−4 M (Fisher Scientific, USA), CaCl2*2H2O 2.04 × 10−5 M (Amresco, USA), NaHCO3 0.1.43 × 10−4 M (Sigma-Aldrich, USA), 0.05% Tamol® (Dupont, USA, purchased from Northeastern Laboratories, USA), 4% glucose (Amresco, USA), 0.5% N-Z amine (Sigma-Aldrich, USA) was made as previously reported and stored at room temperature (see Table 1).10 Converse media should be made fresh from stocks and shaken well before use (due to occasional salt precipitation). The experimental supplemented RPMI media termed RPMI-sph was prepared as previously reported.15 Briefly, 10.2 g/l of powdered RPMI-1640 (Gibco, USA), 10% FBS (Gibco, USA) and 0.08% Tamol® (Dupont, USA, purchased from Northeastern Laboratories, USA) were added to autoclaved reverse osmosis (RO) water and filter sterilized using 0.2 μm Millipore filtration system and stored at 4°C. Media in flasks were inoculated with equivalent amounts of arthroconidia to a final concentration of 106 cells/ml. Cultures were grown in vented baffled flasks (VWR, USA) on a shaking incubator (GeneMate Orbital Shaker Mini, USA) at 90 rpm. The four RG3 strains were grown at one condition: 10% CO2, 39°C, at ambient oxygen (Thermo Forma Series II water jacketed CO2/O2 incubator, USA). The Δcts2/Δard1/Δcts3 (RG2) strain was grown at the following two conditions: 10% CO2, 39°C, ambient oxygen; and 10% CO2, 39°C, 1% O2 (Thermo Forma Series II water jacketed CO2/O2 incubator, USA). The oxygen sensor was calibrated prior to incubation; oxygen levels were maintained by influx of nitrogen gas monitored by the incubator. The MHS cell culture media was prepared according to the ATCC product sheet: 10.2 g/l of powdered RPMI-1640 (Gibco, USA) supplemented with 10% FBS (Gibco, USA), 1% penicillin-streptomycin (Gibco, USA), 0.1 mM nonessential amino acids, 25 mM HEPES (Gibco, USA), and 0.2% sodium bicarbonate (Gibco, USA). Media was filter-sterilized using 0.2 μm filter (Millipore, Kankakee, Illinois, USA). Arthroconidia generally transitioned to spherules at the 3 day timepoint (first measurement), and endosporulation likely occurred on day 7 for all RG3 strains with endospores arresting growth due to depletion of nutrient in media; however, this was not directly measured.
Table 1.
Detailed protocol for stocks solutions and 1 L of modified Converse Media.
| Reagenta | mw | g/l | Molarity | Volume/literb |
|---|---|---|---|---|
| Ammonium acetate | 77.08 | 123.0 | 1.596 M | 10 ml |
| KH2PO4anhydrous* | 136.09 | 51.0 | 0.37 M | 10 ml |
| K2HPO4anhydrous* | 174.18 | 52.3 | 0.3 M | 10 ml |
| MgSO4*7H2O | 246.5 | 39.5 | 0.16 M | 10 ml |
| ZnSO4*7H2O | 287.56 | 0.72 | 2.5 mM | 5 ml |
| NaCl | 58.44 | 1.4 | 24 mM | 10 ml |
| CaCl2*2H2O | 147.02 | 0.6 | 4.08 mM | 5 ml |
| NaHCO3 | 84.01 | 1.2 | 14.3 mM | 10 ml |
| Tamol** | NA | 50.0 g | 50 g/l | 10 ml |
| Glucose*** | 180.86 | 40.0 | 40 g/l | 100 ml |
| N-Z amine*** | NA | 5.0 | 5 g/l | 10 ml |
*May use hydrated forms but adjust weight.
**Omit from medium for agar plates.
***Filter sterilize and add after autoclaving.
aPrepare each stock solution in 1 liter of bottled water (DDI and clean glassware) and number as shown above. The stocks may be kept for 1 year at 4°C.
bMeasure 820 mL DDI water into container for agar, 810 mL for liquid. Pipette individual reagents into the flask, swirling to mix between each addition. pH to 6.5. Autoclave for 30 min. For agar, add 18 g/l purified agar. When cool, add sterile filtered glucose and N-Z amine (if making solid Converse plates eliminate N-Z amine).
MH-S growth conditions
Freshly thawed 1 ml aliquots of MH-S cells were combined with 9 ml of prewarmed RPMI cell culture media and spun at 1400 rpm for 5 minutes. Media was removed and replaced with fresh RPMI cell culture media that was 37°C. Culture was placed in vented treated cell culture flasks (Corning, Corning, New York, USA) and incubated at 37°C, 5% CO2 for 48 hours.
Quantification and microscopy
A 1 ml sample of each RG3 strain (Silveira, C735, 2006 and RS) was removed from the cultures on day 3, 4, 5, 6, 7, and 10 in order to progressively observe spherule quantity and size. Cells were fixed for 48 hours using 900 μl of cells and 100 μl of 10% formalin. Counting was done using a hemocytometer and compound light microscope using a 40 × objective lens. Spherule diameters were obtained by placing 20 μl of sample on a glass slide. To prevent spherule rupture, autoclaved horsehair was used to support the weight of the coverslip. Cells presenting a characteristic large round spherule structure were included in measurements and counts, whereas atypical cells (e.g., endospores, clustering cells, hyphae, and conidia) were not included in counts so as to not over estimate the number of true spherules (Fig. S1). Measurements were made using Invitrogen EVOS FL auto cell imaging system (Thermoscientific, USA) using a 40 × objective lens. For the RG2 strain, a 1 ml sample was removed from the cultures on day 3, 5, 6, 7, and 10 to progressively observe changes in spherule size and quantity (day 4 was lost due to technical issues). Spherules were not fixed in formalin but were quantified using the same methodology mentioned above. Slide preparation and diameter measurements were done in the same manner as the RG3 strains. However, hyphal fragments were filtered out of cultures using 100 μm filters (Falcon, USA) assembled into a 50 ml conical tube. In addition to passing the culture through the filter, a vigorous rinse using 1 × PBS was used to dislodge spherules that stuck to the hyphae. To simplify the comparison between the RG2 and RG3 strains, the results of both media types were combined for both quantification and diameter. To assess the impact of oxygen concentration on the number of converted spherules, cultures were grown at 39°C, 10% CO2, and either ambient or 1% oxygen for 144 hours. Due to the less restrictive nature of the BSL2 lab setting, replicates of the cultures were lyophilized in order to obtain dry weight of each sample. Baffled flask contents were transferred to 50 ml conical tubes and spun down at max speed for 15 minutes. Culture media was removed; samples were washed with 1 × PBS, spun down at 12,000 × g’s for 15 minutes, and the 1 × PBS was removed. The samples were stored at −80°C for a minimum of 30 minutes and then lyophilized (Labconco, Kansas City, Missouri, USA) until the pellet appeared completely desiccated.
The morphology of fixed RG3 and RG2 and nonfixed RG2 spherules were also investigated by scanning electron microscope (Zeiss Supra 40-VP FESEM, USA). Cells were diluted 5 times in 1 × PBS and passed through a 0.4 μm filter apparatus in order to retain the cells on a solid surface. The samples were allowed to settle on the filter paper for 60 minutes and submerged in 2.5% glutaraldehyde (GA; Electron Microscopy Sciences, Hatfield, Pennsylvania, USA) prepared using 1 × PBS for 30 minutes and then rinsed 3 times for 2 minutes with 1 × PBS. The filters were then saturated with 1% osmium tetroxide (OsO4 Electron Microscopy Sciences, USA) in sterile deionized (DI) H2O for 40 minutes, then rinsed once for 2 minutes in 1 × PBS, and then washed twice for 2 minutes in sterile DI H2O. The samples were dehydrated using a graded ethanol series; 30% EtOH for 5 minutes, 50% for 5 minutes, 70% for 5 minutes, 95% for 5 minutes, and 3 times in 100% for 10 minutes. The filters were submerged in a 50/50 solution of 100% EtOH and hexamethyldisilazane (HMDS; Ted Pella, Redding, California, USA) for 10 minutes and then submerged in 100% HMDS twice for 10 minutes. Samples were allowed to air dry and then lightly glued to a sample stub. Stubs were sputter coated (Denton Vacuum Desk II, gold palladium target, Denton Vacuum, Moorestown, New Jersey, USA) for 30 seconds.
Growth of murine cell line in experimental RPMI and chemically defined media
Macrophages were grown for 48 hours in RPMI cell culture media, under ATCC product sheet recommendations. Cells were detached from treated cell culture flasks, quantified, and spun down at 1400 rpm to remove the media. The cell pellets were resuspended in 10 ml of Converse media, RPMI-sph media, and RPMI cell culture media respectively in triplicate. Cultures were incubated at 5% CO2 and 37°C for 48 hours. Cells were detached from the flask and stained with Trypan blue (Gibco, La Jolla, California, USA) and counted using a hemocytometer to determine viability.
Statistical analyses
All statistical analyses were performed using Prism (GraphPad, USA). The mean spherule diameter was compared on each day sampled using an unpaired Mann–Whitney two-tailed t test. A P-value of less than 0.01 was considered statistically significant. Comparison of RG2 spherule concentration and dry culture weight in hypoxic and normoxic conditions was done using a 2-way analysis of variance (ANOVA), a P-value of 0.01 was considered statistically significant. Comparison of the media on RG2 dry culture weight in hypoxic and normoxic conditions was done using an unpaired Mann–Whitney two-tailed t test. A P-value of less than 0.01 was considered statistically significant.
Results
Media effects on density and size for different wild-type RG3 strains
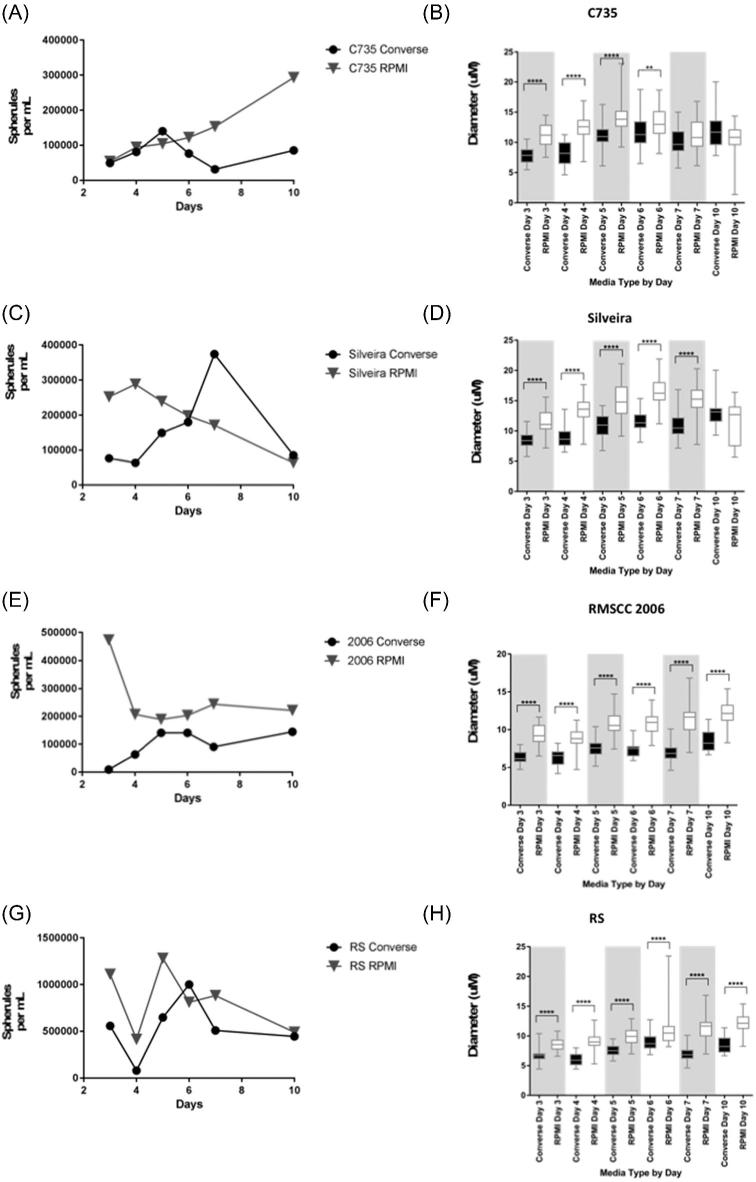
C735 cultures had very similar cell densities in the different medias (Fig. 1A) on days 3 (Converse 4.95 × 104, RPMI-sph 5.40 × 104) and 4 (Converse 8.10 × 104, RPMI-sph 9.45 × 104) but different densities by day 10 (Converse 8.55 × 104, RPMI-sph 2.93 × 105). Cultures grown in RPMI-sph media had steadily increasing growth peaking on day 10 (2.93 × 105), whereas Converse media cultures had distinct peaks at day 5 (1.40 × 105) and day 10 (8.55 × 104). C735 cultures produced significantly larger spherules in RPMI-sph media than Converse media on days 3, 4, and 5 (P < .0001). Significance declined on day 6 (P < .01) and was not significant on days 7 and 10. The smallest mean diameter for this strain was observed on day 3 in Converse media (7.383 μm, standard deviation [SD] 1.306) while the largest mean diameter was observed on day 5 in RPMI-sph media (13.91 μm, SD 2.778; Fig. 1B). Variation in spherule diameter peaked in RPMI-sph media on day 7 (SD = 2.73) and in Converse media on day 10 (SD = 3.063).
Figure 1.
Measurements of wild-type RG3 strains tested in Converse and RPMI-sph media over 10 days for (A) spherule density (spherules/mL) of C. posadasii C735 (B) spherule diameter (μm) for C. posadasii C735 (C) spherule density (spherules/ml) of C. posadasii Silveira (D) spherule diameter (μm) for C. posadasii Silveira (E) spherule density (spherules/ml) of C. immitis RMSCC2006 (F) spherule diameter (μm) for C. immitis RMSCC2006 (G) spherule density (spherules/ml) of C. immitis RS (H) spherule diameter (μm) for C. immitis RS. ****(P < .0001), **(P < .01).
Silveira cultures had very different daily cell densities patterns (Fig. 1C). RPMI-sph culture concentration peaked on day 4 (2.88 × 105) and was lowest on day 10 (6.30 × 104). Converse media cultures peaked on day 7 (3.74 × 105) and were lowest on day 4 (6.30 × 104). Silveira cultures produced significantly larger spherules in RPMI-sph media than Converse media (Fig. 1D; P < .0001), on all days, except day 10. The smallest mean diameter (8.544 μm, SD 1.431) occurred on day 3, in Converse media. The largest mean diameter was observed on day 6 in RPMI-sph, (16.34 μm, SD 2.580). When observed under the microscope, Silveira cultures had highly variable spherule diameters within each time point (range SD 1.665–3.612), this was especially prevalent in RPMI-sph cultures.
For RMSCC 2006, RPMI-sph cultures had a generally increasing trend in the concentration of cells (Fig. 1E) on each day sampled, except for day 3. The largest difference in cell concentrations was observed on day 3 (Converse 9.00 × 103, RPMI-sph 4.73 × 105). RMSCC 2006 cultures produced significantly larger diameter spherules in RPMI-sph media than Converse media on all days sampled (Fig. 1F; P < .0001). The smallest mean diameter (6.361 μm, SD 0.08919) was observed in Converse media on day 3, while the largest mean spherule diameter (12.14 μm, SD 0.58) was observed in RPMI-sph on day 10. It was observed that spherule diameters were very similar for each day, with low standard deviation values (SD range 0.8919–1.941).
RS cultures had different cell concentrations for the two media types on each day sampled but followed similar patterns of growth and concentration (Fig. 1G). RS grown in Converse media peaked in cell concentration on day 6 (1.00 × 106) and was lowest on day 4 (8.10 × 104). RPMI-sph cultures had the highest density on day 7 (8.82 × 105) and the lowest on day 4 (4.14 × 104). RS cultures produced significantly larger spherules in RPMI-sph media than Converse media on all days sampled (Fig. 1H; P < .001). The smallest mean spherule diameter (6.012 μm, SD 1.137) was observed in Converse media on day 4, and the largest mean spherule diameter (12.14 μm, SD 1.581) was in RMPI media on day 10. RS spherules had low standard deviation values for the duration of the experiment (SD range 0.9839–2.744), suggesting synchronous growth.
Spherule density and diameter of the RG2-strain, Δcts2/Δard1/Δcts3
Converse media cultures resulted in slightly higher cell counts than in RPMI-sph media on each day sampled (Fig. 2A). In RPMI-sph media the amount of spherules/ml present was highest on day 3 (9.9 × 104) and day 10 (9.9 × 104). In Converse media cultures the number of spherules present per milliliter of media was highest on day 5 (1.85 × 105) and day 7 (1.85 × 105). Significantly larger spherules in Converse media than RPMI-sph media were produced on all days tested (Fig. 2B; P > .0001). The smallest mean diameter was observed on day 3 in RPMI-sph media (6.16 μm, SD 1.332), and the largest mean diameter was observed on day 6 in Converse media (17.49 μm, SD 1.943).
Figure 2.
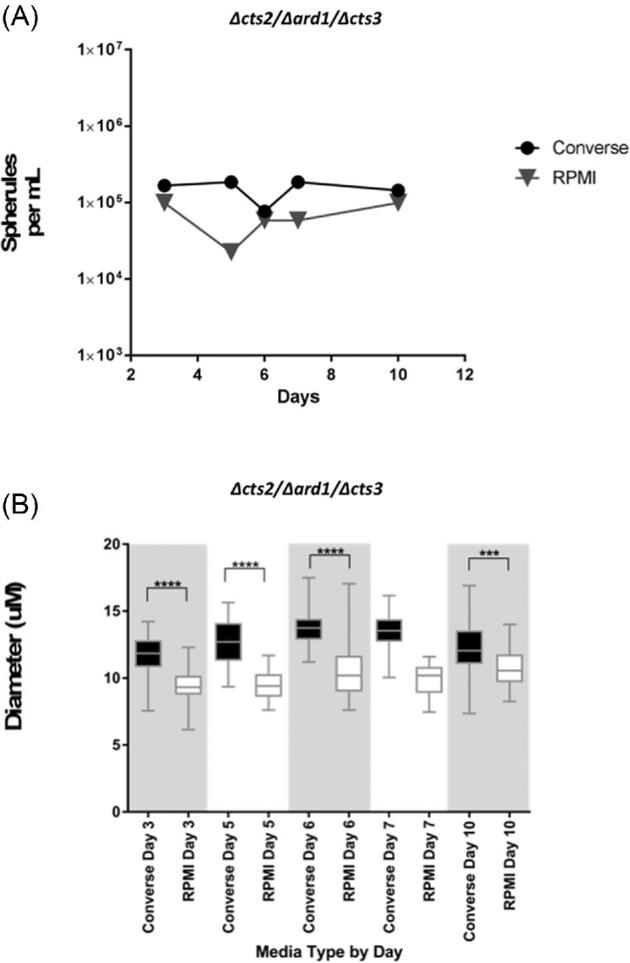
Spherule production of Δcts2/Δard1/Δcts3 RG2 strain in Converse and RPMI-sph media tested over 10 days. (A) Number of spherules per ml and (B) diameter (μm) of spherules in each tested media. ****(P < .0001), ***(P < .001).
Effect of oxygen concentration on Δcts2/Δard1/Δcts3 RG2 growth
Cultures grown in Converse media or RPMI-sph in reduced oxygen (1%) produced more spherules/ml (1.4 × 105, 7.17 × 104) than in ambient oxygen (4.0 × 104, 4.33 × 104). The interaction of the media was not significant, but there was a significant interaction between oxygen concentrations on spherule density (P = .0138; Fig. 3A).
Figure 3.
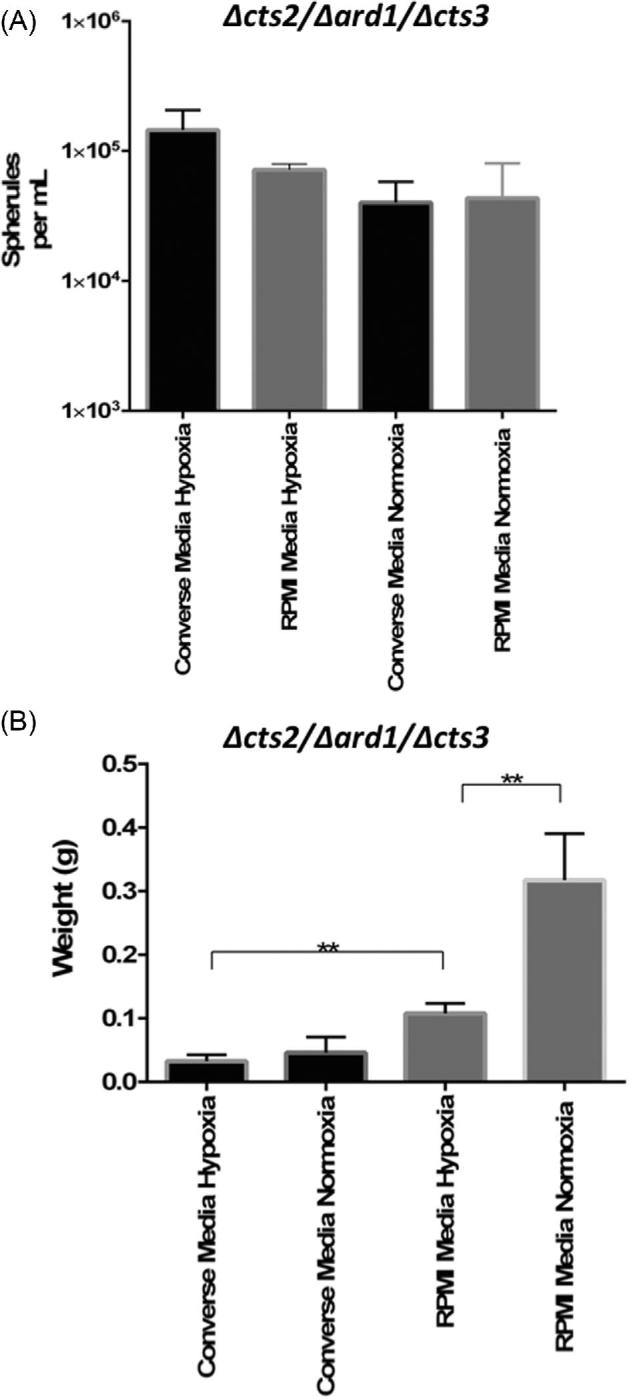
The spherule conversion of Δcts2/Δard1/Δcts3 RG2 strain in Converse and RPMI-sph media in hypoxia (1% O2) or normoxia (ambient O2). Samples were collected on day 6 and quantified (A) number of spherules per ml and (B) dry biomass weight. **(P < .01).
The effect of the experimental conditions on total culture growth was measured by recording the dry weight of each sample. The analysis revealed the highest percentage of variation was due to the media (52.28%, P < .0001) followed by the interaction of the oxygen concentration (21.67%, P < .0001). Cultures grown in the supplemented RPMI-sph media yielded a significantly higher overall weight (0.1075 g) in comparison to Converse media (0.03258 g) in reduced oxygen (Fig. 3B; P < .01). In ambient oxygen, RPMI-sph cultures also had a significantly higher (P < .0001) dry weight (0.3171 g) than Converse media cultures (0.04587 g).
Δcts2/Δard1/Δcts3 RG2 vs. C735 parental RG3 strain
No statistical significance was found when comparing the daily cell densities of each strain (Fig. 4A). We observed that the RG2 strain Δcts2/Δard1/Δcts3 produced significantly larger spherules on day 3 (10.62 μm) and day 7 (11.80 μm) compared to the parental C735 strain (9.489 μm and 10.64 μm, respectively; P < 0001). C735 produced significantly larger spherules on day 5 (P < .001, cts2/Δard1/Δcts3 11.11 μm, C735 12.53 μm). There was not a significant difference on day 6 or day 10 (Fig. 4B).
Figure 4.
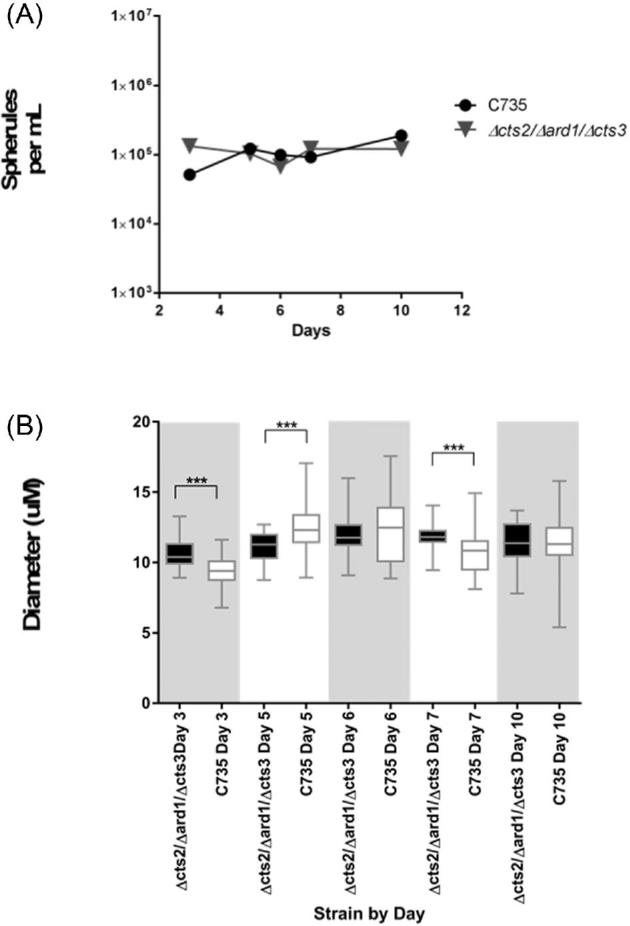
The results of both media types were combined when comparing (A) spherule density per ml and (B) spherule diameter (μm) of spherules for wild-type parental strain C735 and the RG2 strain Δcts2/Δard1/Δcts3. ***(P < .001).
MH-S cell viability
To assess the ability of a mammalian cell line to survive in the media of interest, a murine macrophage cell line (MH-S) was grown in either standard RPMI media as recommended by ATCC, RPMI-sph, or Converse media. Viability of the cells was determined at inoculation and after 48 hours. In standard RPMI media, cells had a 2% decrease in viability, in RPMI-sph media there was a 3% decrease, whereas all cells grown in Converse media did not survive after 48 hours (data not shown).
Scanning electron microscopy
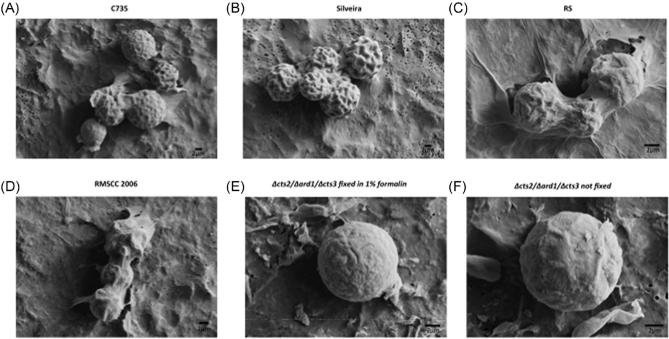
The outer surface of the five strains had a corrugated texture not visible using a light microscope (Fig. 5). The corrugation can be described as unsymmetrical ridges and troughs, which made up the exterior of the spherule. Between the strains there was observed variation among the height of ridges and depth of troughs, but it was not quantified in any manner, as C735, RS and RG2 strain were assessed on day 5, and Silveira day 3. The RG2 strain Δcts2/Δard1/Δcts3 had a similar morphology, regardless of formalin fixation, but the formation of ridges and troughs was much less pronounced. Previous SEM of in vitro spherules looked at structures after 48 hours, and little evidence of these ridged structures were observed.24 Because the RG2 strain likely represents an immature spherule, we believe that the structures are not artifacts of processing but rather indicative of formation of crosswalls and endospore compartments. Additionally, the two C. immitis strains appear to have greater extracellular fibrous structures, possibly glycoproteins or polysaccharides, but this remains to be investigated.
Figure 5.
Scanning electron microscopy of spherules from (A) C735, (B) Silveira, (C) RS, (D) RMSCC2006, (E) Δcts2/Δard1/Δcts3 fixed in 1% formalin, and (F) Δcts2/Δard1/Δcts3 not fixed. The bars located on the lower right-hand corner of each picture indicate 2 μm scale. Cells were collected on day 5 (C735, RS, RMSCC2006 and Δcts2/Δard1/Δcts3) or day 3 (Silveira).
Discussion
Various techniques have been used to understand the critical and unique parasitic lifecycle of Coccidioides spp., including antigen detection, targeted gene deletion, transcriptome, and proteome analysis. An in vitro spherule culture is often crucial for such experiments. The dimorphic life cycle of Coccidioides species creates culturing challenges. Mycelia are easily cultured in many standard solid or liquid fungal growth media, but spherules can be more difficult to establish and have prompted many publications.8,10,15–17,25 When investigating the mechanisms critical for morphogenesis, propagation, and maintenance of the parasitic lifecycle, exposure to the mammalian respiratory system likely provides necessary cues that initiate and maintain the morphological switch.12 Converse media has been routinely used for obtaining spherules, but it is unlikely that the components reflect what is available to Coccidioides in vivo given the toxicity to mammalian cells, which also limits host pathogen interaction studies. In contrast, the components of the RPMI-sph media (amino acids, vitamins, and serum) can sustain mammalian derived cells.26–29 The previous publication of the RPMI-sph formulation used the media to produce a continuous culture of spherules by daily filtration of spherules from hyphal fragments, achieving 100% spherules by day 6. While continuous culture was not the focus of this paper, we did observe peak in diameter and concentration around this time period. Thus, we suggest that day 5 to 6 is an optimal growth endpoint for mature spherules for most strains.
In this article, we have evaluated the performance of a more novel media formulation in comparison to the extensively used Converse media to establish a contemporary record of variation in lab strain behavior. The conversion of five strains of Coccidioides arthroconidia to pathogenic structures in vitro, in two media types; a supplemented RPMI-sph media and chemically defined Converse media, was examined. The data herein presented suggest that RPMI-sph media is a reliable and easy-to-use method to grow spherules (Fig. 1–4). The experimental media is compatible with host cells, allowing for direct host-pathogen interaction studies, in addition to providing the pathogen with a nutrition source that is more similar to an in vivo system.
Quantification of cell density and size were used to determine if RPMI-sph media alters the behavior of Coccidioides in vitro compared to the commonly used Converse formulation. Not all of the arthroconidia convert to spherules, and in fact many remain as conidia or develop into hyphae, which is reflected in the reported spherule counts. In supplemental Figure 1 we provide an image of each RG3 strain at each time point in both media types. We chose to measure and count cells that exhibit the distinct spherule morphology so that our comparisons among strains and media were consistent and comparable. For example, in Figure S1, panel K and L show unconverted arthroconidia and mycelia present in the C735 cultures. Additionally, Figure S1, panel N shows RMCC 2006 spherules clumped together, which is a common observation and can be problematic for quantification.
Overall, both spherule density and size were higher in RPMI-sph compared to Converse media in all RG3 strains tested (Fig. 1). C. posadasii strains C735 and Silveira had a greater diversity of spherule sizes (higher standard deviation values) when compared to C. immitis strains RMSCC 2006 and RS. This difference was first noticed during microscopic observation of the cultures, and confirmed with statistical analysis (Fig. 1). In addition to the morphological differences between strains, there were statistically significant differences observed within each strain when comparing the two media types. The nutrients available in RPMI-sph media could account for the overall difference in terms of the quantity and size of spherules (Fig. 1). The largest spherules were observed on day 6 when the Silveira strain was grown in RPMI-sph media (∼20 μm), and the spherule sizes were consistent with sizes reported elsewhere.13,15,30 Interestingly, C735 had a noticeable amount of “debris” in the culture that was not observed with the other strains, which appeared to be hyphal fragments, unconverted arthroconidia, and cellular lysates (data not shown). The presence of the debris was not problematic during the experiments but rather a notable phenotypic variation.
In addition to characterizing the behavior of four wild-type strains grown in RPMI-sph media, we provide information on the RG2 strain Δcts2/Δard1/Δcts3, in an effort to encourage the research community to utilize the attenuated strain for training purposes, to develop specific standard operating procedures (SOPs) and generate preliminary data for this important fungal pathogen. Researchers who commonly use a BSL3 facility are familiar with the expense of working in a high containment lab. In addition to requiring appropriately trained personnel in costly PPE, experimental progress is slowed due to the necessary high level of containment.22,31 Development and optimization of new protocols can require repeated nonexperimental usage of the BSL3 facility. Therefore, using an attenuated strain enables researchers to obtain preliminary data or develop SOPs in a BSL2 facility, reducing the amount of nonexperimental time in high containment, and reducing overall cost.
We have used the RG2 strain to optimize culturing conditions, as well as to improve protocol execution. The strain has proved to be particularly useful for this purpose, allowing for a seamless transition to our high containment lab with negligible troubleshooting. The behavior of the attenuated strain was similar to the parent C735 (Fig. 4) and is useful as a model system. However, there were noticeable differences between the two strains. The RG2 strain produced more spherules with larger diameters in Converse media than in RPMI-sph media, in all conditions, whereas the opposite was observed in the parent strain and all other wild-type RG3 strains (Fig. 1–2). In both media types, the derivative strain of C735 produced more mycelia than its parent, likely due to chitin defects caused by the deletion of cts2 and cts3, and consequently the impaired ability to produce mature endosporulating spherules.21 Mycelial growth in the supplemented RPMI-sph media was enhanced, which could be due to altered nutrition. Interestingly, mycelial growth decreased under hypoxic conditions, in both media types (Fig. 3B). In both 2-way ANOVA analyses, the effect of decreased oxygen was the most significant interaction (rather than media) in terms of spherule density and culture weight. The increase in spherule morphogenesis, and a decrease in overall weight, together suggest that reduced oxygen may inhibit mycelia growth, while promoting the development of spherules via mechanisms that should be further explored (Fig. 3). Hyphae and unconverted conidia are often a nuisance when culturing spherules. Based on these observations, decreasing the oxygen concentration in concert with increasing CO2 might increase spherule conversion rates.
Previous research has shown that Coccidioides arthroconidia are readily engulfed by phagocytic cells such as macrophages, and that the spherules may start to develop within the phagosome.19,20 Most published host pathogen interaction studies report using Converse media to grow spherules, which are then rinsed and occasionally formalin fixed before exposure to mammalian cells.18–20,32 The effect of harvesting and rinsing is unknown, but certainly secreted proteins or other structures could be disturbed by this process. The RPMI-sph media decreased the viability of MH-S cells by 1%, whereas these cells were not viable in Converse media. Thus, the RPMI-sph media can provide an environment where fungal morphogenesis could occur in the presence of immune cells, allowing for more tractability in the design of pathogen-host interaction studies. Many formulations of RPMI are available (such as without phenol red), and these were not all tested with the current study. It is possible that even greater refinement of protocols can be attained by exploring alternative media. The RG2 strain provides the needed starting point for these future studies.
In addition to utilizing microscopy to quantify spherule size and concentration between the two media types, a scanning electron microscope was used for close observation of the outer surface of the spherule structure (Fig. 5). The most intriguing observation was the wrinkled exterior of all of the spherule structures. The depth of the invaginations varied between strains, with Silveira having the most pronounced ridges. To rule out the possibility of formalin creating the texture, a sample of the RG2 strain was prepared without formalin. The texture was less pronounced but persisted (Fig. 5). Although the phenotypic difference could be due to chitin reduction, these preliminary findings suggest the surface invagination may be part of the organic structure of Coccidioides spherule outer wall and to our knowledge has not been previously reported.
In summary, all five strains converted to spherules in both Converse media and RPMI-sph media. The four RG3 strains had few hyphae present in any of the cultures, regardless of the media; overall, cultures grown in RPMI-sph media had higher spherule conversion rates and larger diameters for the RG3 strains. When utilizing the RG2 strain, Converse media resulted in a higher yield of spherules with fewer hyphae. However, if direct host-pathogen interactions are of interest, RPMI-sph is a media that supports both mammalian cells and spherule development. Additionally, implementing hypoxic conditions during incubation can reduce hyphal growth, but this was only thoroughly tested in the RG2 strain. In summary, we characterized the morphological transition in two media types for multiple RG3 strains of Coccidioides, and the data suggest that given the same environment, strains exhibit variation in growth. This differing strain behavior highlights an important consideration: the phenotypic diversity among strains and between species of Coccidioides should be taken into account for future studies.
Supplementary Material
Acknowledgments
Thank you to Paul Keim and the Pathogen and Microbiome Institute (NAU) staff for access and support of the Biosafety Level Three facility, Aubrey Funke (NAU) for assistance with preparing and obtaining scanning electron microscope images, and to Kaitlyn Parra (NAU) for assisting with measurement data. Table 1 details the steps to prepare the stock reagents, and 1 L of Converse media and was developed by Rosemary Hayden. Funding to support this work was provided to BMB by ABRC 14–082975 and NIH/NIAID K22-A1104801.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
References
- 1. Pappagianis D. Coccidioidomycosis. In: Laboratory Diagnosis of Infectious Diseases. New York, NY: Springer, 1988: 600–623. [Google Scholar]
- 2. Barker BM, Jewell KA, Kroken S, Orbach MJ. The population biology of Coccidioides: epidemiologic implications for disease outbreaks. Ann N Y Acad Sci. 2007; 1111: 147–163. [DOI] [PubMed] [Google Scholar]
- 3. Valdivia L, Nix D, Wright M et al.. Coccidioidomycosis as a common cause of community-acquired pneumonia. Emerg Infect Dis. 2006; 2: 958–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown J, Benedict K, Park BJ, Thompson GR, III. Coccidioidomycosis: epidemiology. Clin Epidemiol. 2013; 5: 185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Foley CG, Tsang CA, Christ C AS. Impact of disseminated coccidioidomycosis in Arizona. In: 2007–2008. 55th Annu. Coccidioidomycosis Study Group Meet. 2011. [Google Scholar]
- 6. Tsang CA, Anderson SM, Imholte SB et al.. Enhanced surveillance of coccidioidomycosis, Arizona, USA, 2007–2008. Emerg Infect Dis. 2010; 16: 1738–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cole GT, Hung C-Y. The parasitic cell wall of Coccidioides immitis. Med Mycol. 2001; 39: 31–40. [PubMed] [Google Scholar]
- 8. Cole GT, Sun SH. Arthroconidium-Spherule-Endospore Transformation in Coccidioides immitis. In: Fungal Dimorphism. Boston, MA: Springer US, 1985: 281–333. [Google Scholar]
- 9. Lewis ERG, David VR, Doyle AL et al.. Differences in host innate responses among Coccidioides isolates in a murine model of pulmonary coccidioidomycosis. Eukaryot Cell. 2015; 14: 1043–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Converse JL, Besemer AR. Nutrition of the parasitic phase of Coccidioides Immitis in a chemically defined liquid medium. J Bacteriol. 1959; 78: 231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Drutz DJ, Huppert M. Coccidioidomycosis: Factors affecting the host-parasite interaction. J Infect Dis. 1983; 147: 372–390. [DOI] [PubMed] [Google Scholar]
- 12. Whiston E, Zhang Wise H, Sharpton TJ, Jui G, Cole GT, Taylor JW. Comparative transcriptomics of the saprobic and parasitic growth phases in Coccidioides spp. PLoS One. 2012; 7: e41034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Viriyakosol S, Singhania A, Fierer J, Goldberg J, Kirkland TN, Woelk CH. Gene expression in human fungal pathogen Coccidioides immitis changes as arthroconidia differentiate into spherules and mature. BMC Microbiol. 2013; 13: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kirkland TN. A few shared up-regulated genes may influence conidia to yeast transformation in dimorphic fungal pathogens. Med Mycol. 2016; 54: 648–653. [DOI] [PubMed] [Google Scholar]
- 15. Petkus AF, Baum LL, Ellis RB, Stern M, Danley DL. Pure spherules of Coccidioides immitis in continuous culture. J Clin Microbiol. 1985; 22: 165–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ampel NM, Wieden MA. Discrepancy between growth of Coccidioides immitis in bacterial blood culture media and a radiometric growth index. Diagn Microbiol Infect Dis. 1988; 9: 7–10. [DOI] [PubMed] [Google Scholar]
- 17. Newcomer N, Newcomer VD. The embryonated egg as a culture medium for the animal phase of Coccidioides immitis. J Infect Dis. 1952; 90: 258–266. [DOI] [PubMed] [Google Scholar]
- 18. Gonzalez A, Hung CY, Cole GT. Coccidioides releases a soluble factor that suppresses nitric oxide production by murine primary macrophages. Microb Pathog. 2011; 50: 100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beaman L, Holmberg CA. In vitro response of alveolar macrophages to infection with Coccidioides immitis. Infect Immun. 1980; 28: 594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beaman L, Benjamini E, Papagianis D. Role of lymphocytes in macrophage-induced killing of Coccidioides immitis in vitro. Infect Immun. 1981; 34: 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xue J, Chen X, Selby D, Hung CY, Yu JJ, Cole GT. A genetically engineered live attenuated vaccine of Coccidioides posadasii protects BALB/c mice against coccidioidomycosis. Infect Immun. 2009; 77: 3196–3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. CDC editors : Casey Chosewood L., Deborah E. Wilson. Biosafety in microbiological and biomedical laboratories. Biosaf Microbiol Biomed Lab. Washington, D.C.: U.S. Dept. of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institutes of Health, 2009. Print. [Google Scholar]
- 23. Vogler AJ, Nottingham R, Parise KL, Keim P, Barker BM. Effective disinfectants for coccidioides immitis and c. posadasii. Appl Biosaf. 2015; 20: 154–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huppert M, Sun SH, Harrison JL. Morphogenesis throughout saprobic and parasitic cycles of Coccidioides immitis. Mycopathologia. 1982; 78: 107–122. [DOI] [PubMed] [Google Scholar]
- 25. Lubarsky R, Plunkett OA. In vitro production of the spherule phase of Coccidioides immitis. J Bacteriol. 1955; 70: 182–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Furth R, Raeburn JA, van Zwet TL. Characteristics of human mononuclear phagocytes. Blood. 1979; 54: 485–500. [PubMed] [Google Scholar]
- 27. Hou FF, Boyce J, Zhang Y, Owen WFO Jr.. Phenotypic and functional characteristics of macrophage-like cells differentiated in pro-inflammatory cytokine-containing cultures. Immunol Cell Biol. 2000; 78: 205–213. [DOI] [PubMed] [Google Scholar]
- 28. Tang L, Zhang Z, Zheng J, Sheng J, Liu K. Phenotypic and functional characteristics of dendritic cells derived from human peripheral blood monocytes. J Zhejiang Univ Sci B. 2005; 6: 1176–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Petkus AF, Baum LL. Natural killer cell inhibition of young spherules and endospores of Coccidioides immitis. J Immunol. 1987; 139: 3107–3111. [PubMed] [Google Scholar]
- 30. Lee C-Y, Thompson GR, Hastey CJ et al.. Coccidioides Endospores and spherules draw strong chemotactic, adhesive, and phagocytic responses by individual human neutrophils. PLoS One. 2015; 10: e0129522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Anonymous The approved list of biological agents advisory committee on dangerous pathogens. HSE Books Health and Safety Executive Committee. [Google Scholar]
- 32. Slagle DC, Cox RA, Kuruganti U. Induction of tumor necrosis factor alpha by spherules of Coccidioides immitis. Infect Immun. 1989; 57: 1916–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.