Highlights
-
•
Dosimetric parameters for the heart and lung are associated with overall survival in esophageal cancer patients.
-
•
Heart and lung doses were associated with cardiac and pulmonary complications.
-
•
Patients with cardiac and pulmonary complications are strongly correlated with survival outcomes.
-
•
Dosimetric relationship with clinical outcomes are predictive for surgical and non-surgical patients.
Abstract
Purpose
To analyze associations between heart and lung dose and overall survival (OS) in patients with esophageal cancer who received concurrent chemo-radiotherapy (CCRT) with or without surgery.
Patients and methods
Patients received intensity-modulated radiation therapy (median dose 50.4 Gy) from 2004 through 2016. Cutoff points for continuous variables were calculated using the method of Contal and O’Quigley. Kaplan-Meier method with log-rank tests was used to calculate survival. OS was analyzed with both univariate and multivariable Cox models.
Results
In all, 560 patients were analyzed; median follow-up time was 29.3 months, and 5-year OS rate was 41.7%. Heart V30 >45% and mean lung dose (MLD) >10 Gy were found to be independently associated with worse survival after adjustment for other clinical and dosimetric factors (P < 0.05). Heart and lung doses were also found to be risk factors for radiation-induced cardiac and pulmonary complications (P < 0.05): 8.5% of patients with heart V30 ≤45% had cardiac complications vs. 15% for V30 >45% (P = 0.046); 18.8% of patients with MLD ≤10 Gy had pulmonary complications vs. 27% for MLD >10 Gy (P = 0.020). Having cardiac complications was associated with worse survival (5-year OS rates 27.6% with vs. 43.2% without, P = 0.012), and having pulmonary complications was associated with worse survival as well (5-year OS rates 23.1% with vs. 47.4% without, P < 0.001).
Conclusion
Both heart and lung doses independently predicted worse OS in patients with esophageal cancer, even after adjustment for other clinical and dosimetric factors, and were also risk factors for radiation-induced complications. Both irradiated heart and lung doses should be minimized as a whole.
1. Introduction
Radiation therapy is a critical component in the care of patients with esophageal cancer (EC). The prognosis for patients with localized EC has improved significantly with the advent of preoperative concurrent chemoradiotherapy (CCRT), which confers 5-year overall survival (OS) rates of up to 47% [1], [2], and definitive CCRT has been accepted as standard therapy for patients with inoperable disease or those who cannot tolerate surgery [3], [4], [5]. Despite improved outcomes with the use of modern-day radiation techniques such as intensity-modulated radiation therapy (IMRT) and proton therapy [6], [7], cardiac injury and mortality are common after radiotherapy for EC [6], [8]. Radiation-induced cardiac complications (RICC) are linked with higher heart doses [9], [10], and heart dose was recently shown to independently predict inferior OS in patients with lung cancer [9], [11]. However, little evidence is available on potential associations between irradiated heart dose and survival in patients with EC.
Radiation pneumonitis is common in lung cancer but is much less so in EC because the radiation dose is lower than those used for lung cancer [12]. Some studies have reported lung dose to be an independent predictor of survival in patients with lung cancer [11], [13], but whether it affects survival in patients with EC is unknown. Here we assessed associations between heart and lung doses and OS in a large group of patients with EC receiving CCRT with or without surgery during the modern era.
2. Patients and methods
2.1. Patients
In this institutional review board approved study, we retrospectively reviewed a prospectively maintained database of patients with EC in the Department of Radiation Oncology at The University of Texas MD Anderson Cancer Center to find consecutive patients who underwent CCRT with or without surgery from 2004 through 2016. Clinical disease staging was based on the 7th edition of the American Joint Committee on Cancer Staging Manual. Patients with hematogenous metastasis, those who had received 3-dimensional (3D) conformal radiation therapy or proton therapy, and those who received radiation doses >50.4 Gy or <45 Gy were ineligible.
2.2. Therapy
All patients received CCRT with or without surgery; chemotherapy consisted of a fluoropyrimidine (intravenous or oral) and either a platinum compound or a taxane. Some patients underwent induction chemotherapy before CCRT. At 1 month after CCRT, the multidisciplinary team evaluated treatment responses in light of patients’ performance status, comorbid conditions, and preferences and decided at that time to proceed with surgery or observation.
2.3. Contouring and dosimetric analysis
Radiation therapy was planned with 4D CT simulation. Plans for all patients must have met the dose-volume constraints for organs at risk (OARs) according to the MD Anderson Cancer Center Thoracic Radiation Oncology guidelines, which are consistent with the National Comprehensive Cancer Network 2017 Guidelines for EC. All patients were treated with IMRT to a median dose of 50.4 Gy (range, 45–50.4 Gy) given in 1.8-Gy fractions. Critical structures (spinal cord, heart, and total lungs) were contoured by one investigator on the average-intensity phase image of the 4D CT, and the contours were independently reviewed for accuracy and consistency by a second investigator. Dose–volume histogram data were extracted from Pinnacle or Eclipse treatment planning systems in 0.01-Gy increments, and the following dosimetric variables were computed: mean heart dose (MHD), mean lung dose (MLD), heart V5-50 (in 5-Gy increments), and lung V5-50 (also in 5-Gy increments).
2.4. Follow-up
Follow-up generally involved a first visit at 1 month after treatment and then 3–4 months thereafter for the first 2 years, followed by every 5–6 months from years 3–5, and annually thereafter. At each visit, CT or PET/CT scans and blood tests obtained. Esophagogastroduodenoscopy/endoscopic ultrasonography (EGD/EUS) with biopsy was done every 6 months for the first 18 months and then once a year thereafter. Follow-up for survival, RICC, and radiation-induced pulmonary complications (RIPC) was done through our institution’s Tumor Registry, electronic medical records, and the Social Security Database.
2.5. Statistical analysis
The endpoint for analysis was OS, and was calculated from the date of initial treatment to death or last follow-up. The Kaplan-Meier method was used to estimate survival, with log-rank tests used to compare curves. Cut-off points for the PTV, patient age, and lung and heart doses were determined by the method of Contal and O’Quigley [14], which was conducted by Stata version 14. Univariate analysis (UVA) and multivariable analysis (MVA) were done with Cox proportional hazards models. Covariates with P < 0.1 in the UVA were entered into the MVA models. A stepwise forward approach (likelihood ratio) was used to obtain the final MVA model. Chi-square test was used for categorical variables. Spearman's rank correlation coefficient was used to test correlations between variables. Statistical significance was defined as P < 0.05. Statistical analyses were done with IBM SPSS 23.0 (IBM, Chicago, IL).
3. Results
3.1. Baseline patient and treatment characteristics
Characteristics of the 560 patients analyzed are shown in Supplementary Table S1. The median follow-up interval was 29.3 months (range, 1.4–137.4 months). The median patient age was 62 years (range, 20–86 years). Most patients (63.4%) had stage III/IV disease, and 54.6% underwent surgery. There was no any correlation of concurrent chemotherapy regimen with heart and lung complications. Moreover, there was no any association with induction chemotherapy and heart and lung complications.
3.2. Univariate and multivariable models of overall survival
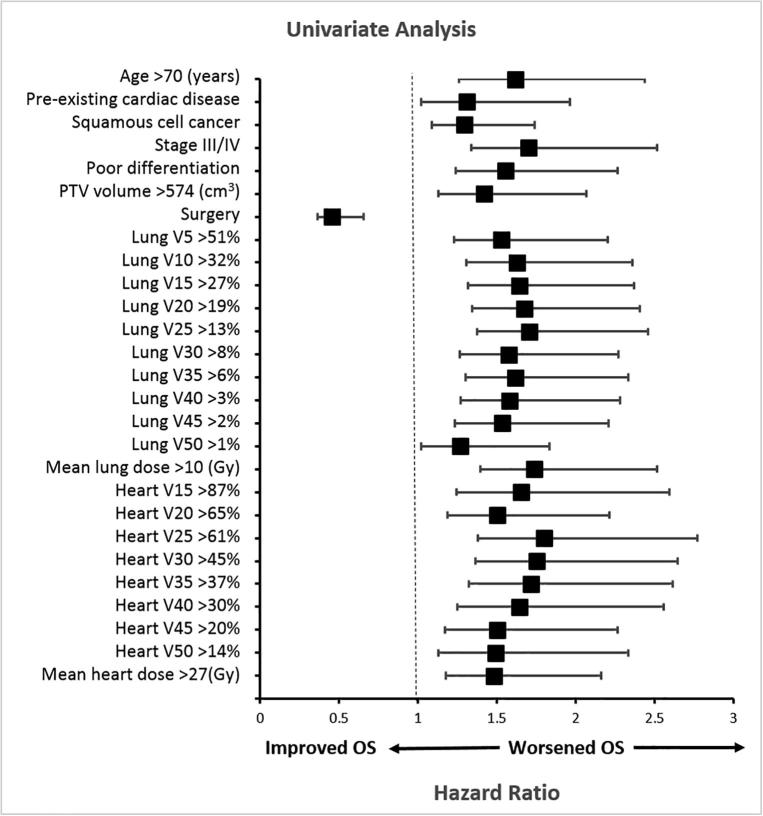
The median survival interval was 40.1 months, and the 5-year OS rate was 41.7%. Results of UVA of factors potentially associated with survival are shown in Fig. 1 and Supplementary Table S2. Clinical factors associated with inferior OS in UVA were age >70 years, pre-existing cardiac disease, squamous cell cancer, stage III/IV disease, poor differentiation, lack of surgery, and PTV >574 cm3 (all P < 0.05). Sex, performance status score, induction chemotherapy, and tumor location did not impact OS (all P > 0.05). All dosimetric variables were associated with worse OS on UVA (all P < 0.05), except for low heart doses (heart V5 and V10).
Fig. 1.
Univariate analysis of factors associated with overall survival (by forest plot representation). The x axis shows the hazard ratio. Variables with P values ≧0.05 are shown (see Supplementary Table S1 for additional variables and associated P values). Vx, volume of the organ receiving at least × Gy.
All clinical and dosimetric covariates with P < 0.1 in UVA for OS were entered into the MVA model, age >70 years (hazard ratio [HR] 1.390, 95% confidence interval [CI] 1.069–1.809, P = 0.014); stage III/IV disease (HR 1.577, 95% CI 1.225–2.028, P < 0.001); poor differentiation (HR 1.485, 95% CI 1.180–1.868, P = 0.001); lack of surgery (HR 0.479, 95% CI 0.380–0.603, P < 0.001); heart V30 >45% (HR 1.402, 95% CI 1.077–1.826, P = 0.012); and MLD >10 Gy (HR 1.355, 95% CI 1.068–1.720, P = 0.012) were all associated with worse survival. Histology, PTV, heart V5-25 or V35-50, MHD, and lung V5-50 were not associated with OS (all P > 0.05).
3.3. Heart dose variables and overall survival
Because heart V30 was the strongest dosimetric predictor of survival in our MVA models, we analyzed its potential correlations with other clinical and dosimetric variables. Correlations between heart V30 and lung dose variables were very weak (0 < r ≤ 0.3, P < 0.001), as were the correlations between heart V30 and PTV, and tumor stage (0 < r < 0.3, P < 0.001); no correlation was found between heart V30 and tumor location (P = 0.127) (Supplementary Table S3). However, strong correlations were found between heart V30 and other heart dose variables (0.3 < r < 0.9, P < 0.001) (Supplementary Table S3). To avoid confounding from internal interaction of heart dose variables, we considered heart dose variables individually in separate MVA models. Heart V15, V20, V25, V30, V35, and MHD were found to be significant independent predictors of OS (Table 1). Heart V5-10 and V40-50 were not associated with OS (all P > 0.05).
Table 1.
Patient and treatment characteristics.
| Characteristic | No. of patients (n = 560) |
% |
|---|---|---|
| Age, years | ||
| ≤70 | 114 | 20.4 |
| >70 | 446 | 79.6 |
| Sex | ||
| Male | 480 | 85.7 |
| Female | 80 | 14.3 |
| ECOG score | ||
| 0–1 | 518 | 92.5 |
| 2 | 42 | 7.5 |
| Pre-existing cardiac disease | ||
| Yes | 129 | 23.0 |
| No | 431 | 77.0 |
| Histology | ||
| Adenocarcinoma | 481 | 85.9 |
| Squamous cell cancer | 78 | 13.9 |
| Other | 1 | 0.2 |
| Stage | ||
| I + II | 205 | 36.6 |
| III + IV | 355 | 63.4 |
| Differentiation | ||
| Well/moderate | 243 | 43.4 |
| Poor | 317 | 56.6 |
| Tumor location | ||
| Upper/middle | 78 | 13.9 |
| Distal | 482 | 86.1 |
| PTV, cm3 | ||
| ≤574 | 233 | 41.6 |
| >574 | 327 | 58.4 |
| Induction chemotherapy | ||
| Yes | 201 | 35.9 |
| No | 359 | 64.1 |
| Surgery | ||
| Yes | 306 | 54.6 |
| No | 254 | 45.4 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; PTV, planning tumor volume.
3.4. Heart dose and radiation-induced cardiac complications
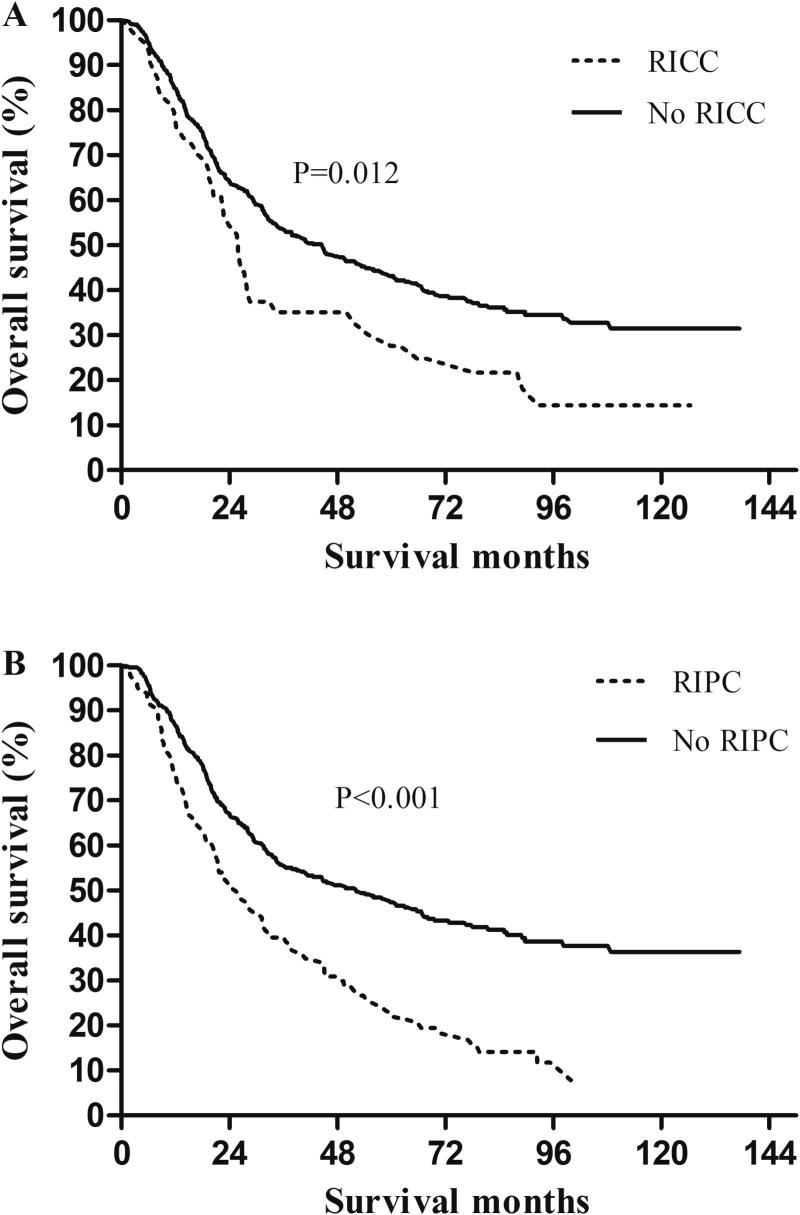
In all, 54 patients (9.6%) experienced radiation induced cardiac complications (RICC), including arrhythmia in 13 patients (2.3%), pericardial effusion in 24 (4.3%), myocardial infarction in 2 (0.4%), congestive heart failure in 5 (0.9%), coronary artery disease in 3 (0.5%), and other cardiac toxicity in 11 (2.0%). Chi-square tests showed that higher heart V30–45 and higher MHD were risk factors for RICC (all P < 0.05), however, heart V5–25 and heart V50 were not (P > 0.05). RICC rates for patients with heart V30 ≤45% vs. >45% were 8.5% vs. 15% (P = 0.046). Patients who experienced RICC had worse survival than those who did not, with corresponding 5-year OS rates of 27.6% and 43.2% (P = 0.012) (Fig. 2).
Fig. 2.
Overall survival according to (A) radiation-induced cardiac complications (RICC), and (B) radiation-induced pulmonary complications (RIPC).
3.5. Lung dose variables and overall survival
Because MLD was the strongest dosimetric predictor of survival, we analyzed its correlation with other clinical factors and dosimetric variables. Correlations between MLD and PTV, tumor location, and tumor stage were weak (0 < r < 0.3, P < 0.001) (Supplementary Table S4). However, significant correlations were found between MLD and other lung dose variables (0.3 < r < 0.9, P < 0.001) (Supplementary Table S4). To avoid confounding from internal interaction of lung dose variables, we added the lung dose variables into the MVA model separately, as we did for the heart dose variables. Lung V20, V25, and MLD were found to be significant independent predictors of OS (Table 2), but lung V5-15 and lung V30-50 were not (all P > 0.05) (Table 3).
Table 2.
Multivariate analysis models for heart dose and overall survival in esophageal cancer patients.
| Factors | Hazard ratio | 95% Confidence interval | P value | ||
|---|---|---|---|---|---|
| Model 1 (heart V15) | Age >70 years | 1.434 | 1.103 | 1.865 | 0.007 |
| Stage III/IV | 1.670 | 1.297 | 2.152 | <0.001 | |
| Poor differentiation | 1.469 | 1.168 | 1.848 | 0.001 | |
| Surgery | 0.483 | 0.383 | 0.608 | <0.001 | |
| Mean lung dose >10 Gy | 1.374 | 1.085 | 1.741 | 0.008 | |
| Heart V15 >87% | 1.485 | 1.111 | 1.985 | 0.008 | |
| Model 2 (heart V20) | Age >70 years | 1.410 | 1.084 | 1.833 | 0.010 |
| Stage III/IV | 1.629 | 1.267 | 2.094 | <0.001 | |
| Poor differentiation | 1.470 | 1.169 | 1.848 | 0.001 | |
| Surgery | 0.476 | 0.378 | 0.600 | <0.001 | |
| Mean lung dose >10 Gy | 1.374 | 1.083 | 1.743 | 0.009 | |
| Heart V20 >65% | 1.313 | 1.027 | 1.680 | 0.030 | |
| Model 3 (heart V25) | Age >70 years | 1.414 | 1.088 | 1.839 | 0.010 |
| Stage III/IV | 1.614 | 1.256 | 2.075 | <0.001 | |
| Poor differentiation | 1.475 | 1.173 | 1.855 | 0.001 | |
| Surgery | 0.489 | 0.388 | 0.616 | <0.001 | |
| Mean lung dose >10 Gy | 1.370 | 1.080 | 1.737 | 0.009 | |
| Heart V25 >61% | 1.415 | 1.074 | 1.865 | 0.014 | |
| Model 4 (heart V30) | Age >70 years | 1.390 | 1.069 | 1.809 | 0.014 |
| Stage III/IV | 1.577 | 1.225 | 2.028 | <0.001 | |
| Poor differentiation | 1.485 | 1.180 | 1.868 | 0.001 | |
| Surgery | 0.479 | 0.380 | 0.603 | <0.001 | |
| Mean lung dose >10 Gy | 1.355 | 1.068 | 1.720 | 0.012 | |
| Heart V30 >45% | 1.402 | 1.077 | 1.826 | 0.012 | |
| Model 5 (heart V35) | Age >70 years | 0.012 | 1.401 | 1.077 | 0.012 |
| Stage III/IV | 0.000 | 1.585 | 1.231 | <0.001 | |
| Poor differentiation | 0.001 | 1.476 | 1.173 | 0.001 | |
| Surgery | 0.000 | 0.480 | 0.381 | <0.001 | |
| Mean lung dose >10 Gy | 0.008 | 1.380 | 1.088 | 0.008 | |
| Heart V35 >37% | 0.037 | 1.337 | 1.018 | 0.037 | |
| Model 6 (mean heart dose) | Age >70 years | 1.423 | 1.095 | 1.848 | 0.008 |
| Stage III/IV | 1.622 | 1.262 | 2.084 | <0.001 | |
| Poor differentiation | 1.458 | 1.158 | 1.836 | 0.001 | |
| Surgery | 0.474 | 0.376 | 0.598 | <0.001 | |
| Mean lung dose >10 Gy | 1.372 | 1.080 | 1.744 | 0.010 | |
| Mean heart dose >27 Gy | 1.275 | 1.002 | 1.622 | 0.048 | |
Table 3.
Multivariate analysis models for lung dose and overall survival for esophageal cancer patients.
| Factors | HR | 95% CI |
P value | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Model 1 (lung V20) | Age >70 years | 1.381 | 1.062 | 1.798 | 0.016 |
| Stage III/IV | 1.595 | 1.240 | 2.053 | <0.001 | |
| Poor differentiation | 1.493 | 1.187 | 1.878 | 0.001 | |
| Surgery | 0.476 | 0.378 | 0.600 | <0.001 | |
| Heart V30 >45% | 1.413 | 1.083 | 1.845 | 0.011 | |
| Lung V20 >19% | 1.283 | 1.013 | 1.626 | 0.039 | |
| Model 2 (lung V25) | Age >70 years | 1.369 | 1.053 | 1.781 | 0.019 |
| Stage III/IV | 1.591 | 1.236 | 2.047 | <0.001 | |
| Poor differentiation | 1.489 | 1.184 | 1.874 | 0.001 | |
| Surgery | 0.476 | 0.378 | 0.600 | <0.001 | |
| Heart V30 >45% | 1.402 | 1.073 | 1.833 | 0.013 | |
| Lung V25 >13% | 1.298 | 1.023 | 1.647 | 0.032 | |
| Model 3 (mean lung dose) | Age >70 years | 1.390 | 1.069 | 1.809 | 0.014 |
| Stage III/IV | 1.577 | 1.225 | 2.028 | <0.001 | |
| Poor differentiation | 1.485 | 1.180 | 1.868 | 0.001 | |
| Surgery | 0.479 | 0.380 | 0.603 | <0.001 | |
| Heart V30 >45% | 1.402 | 1.077 | 1.826 | 0.012 | |
| Mean lung dose >10 Gy | 1.355 | 1.068 | 1.720 | 0.012 | |
3.6. Lung dose and radiation-induced pulmonary complications
In all, 128 patients (22.9%) experienced RIPC, including radiation pneumonitis in 60 (10.7%), radiation pulmonary fibrosis in 16 (2.9%), and pleural effusion in 84 (15%). Chi-square tests showed that all lung doses were risk factors for RIPC (all P < 0.05) except for lung V10 (P = 0.069). RIPC rates for patients with MLD ≤10 Gy vs. >10 Gy were 18.8% vs. 27% (P = 0.020). Patients who experienced RIPC had worse survival than those who did not, with corresponding 5-year OS rates of 23.1% and 47.4% (P < 0.001) (Fig. 2).
3.7. Heart V30 and mean lung dose and overall survival in surgery and non-surgery cohorts
Patients who received surgery had less treatment-related complications than those who did not have surgery (RIPC: 19.6% vs. 26.8%, P = 0.044; RICC: 7.8% vs. 11.8%, P = 0.113). One potential reason is that surgical patients tend to be younger with better performance status and less cardiac morbidities. We determined the association heart V30 and MLD between OS in surgery and non-surgery cohorts, separately. In the surgery cohort, heart V30 remained independently associated with OS after adjusting other variables (P = 0.001), while MLD trended toward significance (P = 0.060). In the non-surgery cohort, MLD remained independently associated with OS after adjusting other variables (P = 0.028), and heart V30 trended towards significance (P = 0.088).
4. Discussion
In this detailed review of clinical and dosimetric predictors of OS in a large cohort of patients undergoing modern-day treatment for EC with long follow-up, we identified both heart and lung doses as independent predictors of worse OS, even after adjustment for key clinical factors. Specifically, heart V30 and MLD were the strongest predictors of the heart and lung metrics, and they remained to be the independent predictors in both surgery and non-surgery cohorts. Cut point analysis revealed that keeping heart V30 ≤45% and MLD ≤10 Gy was associated with 6.5% and 8.2% absolute decrease in RICC and RIPC, respectively. Accordingly, the OS was improved, with the decreased RICC and RIPC.
That thoracic radiation can lead to cardiac death is not a new concept. Several studies have reported cardiac mortality in long-term survivors of breast cancer or lymphoma [15], [16], [17], [18], and mortality rates become higher with longer follow-up [16]. However, cardiac mortality among patients with EC has been under-reported for three main reasons. First, the evidence from breast cancer or Hodgkin lymphoma survivors suggests that RICC occurs long after radiotherapy [16], but given the shorter survival times for patients with EC (median OS less than 2 years [19]), the belief has been that few will live long enough to experience cardiac events. Second, priority is always given to sparing the lungs to reduce the risk of radiation pneumonitis, but sparing the heart has not been given equal prioritization. Third, identifying and coding RICC and cause of death for patients with EC has been challenging, as the RICC is often obscured by cardiac disease that is present before radiotherapy [9]. Thus, the clinical relevance of irradiated heart volumes for patients with EC has been unclear.
Survival times for localized EC have improved substantially in recent years, with the median survival interval increasing from the typical 24 months to 49.5 months in the Cross trial [1]. Extension of survival times for patients with EC correspondingly increases the importance of RICC and cardiac mortality. Numerous studies have reported heart dose as being independently associated with inferior OS in lung cancer [9], [11], [13], [20]. In one of those studies, an MVA of 251 patients with locally advanced non-small cell lung cancer (NSCLC) showed that higher heart V50 independently predicted worse survival when stratified by heart V50 <25% vs. ≥25%, with 2-year OS rates of 45.9% vs. 26.7% (P < 0.001) [13]. In RTOG 0617, both UVA and MVA models suggested that heart V5 and heart V30 predicted survival [11]. We did not find lower heart doses (e.g., V5 or V10) to be significant in the current analysis. Although heart V30 was the strongest heart variable, heart V15–25, V35, and MHD each contributed to survival when analyzed alone. Others have also identified MHD, heart V5, heart V30, and heart V50 as being important predictors of survival [11], [13], suggesting that considering heart dosimetry as a whole would be more fruitful in estimating survival rather than emphasizing a single variable. Although other investigators have not found heart dose to be linked with survival [9], [10], [21], [22], [23], others have noted associations between heart dose with cardiac events [9], [10]. In one such study of 112 patients with NSCLC from several prospective trials, heart dose was associated with RICC in both UVA and MVA, but was not associated with OS [9]. Several reasons may explain the lack of influence of heart dose on survival in these studies. First, nearly all studies enrolled patients who died any time after treatment, and the cause of death for those who died earlier was attributed to either cancer or its treatment, which may obscure any influence of heart dose on survival. Also, the follow-up intervals are typically too short to detect cardiac mortality, and the numbers of patients analyzed, particularly those with EC, are often quite small.
RICC is a late toxicity [24]. Radiotherapy for breast cancer involves exposing the heart to 1–5 Gy [15], [25], [26], [27] levels that cause mainly ischemic heart disease, which tends to occur 10 years or more after the radiation [15], [28], [29]. The dose to and the volume of heart irradiated influence the incidence, type, severity, and mortality associated with RICC [15], [30], [31]. Patients with EC are typically older, are more likely to have pre-existing cardiac disease, and receive much higher heart doses than patients with breast cancer or Hodgkin lymphoma. Thus, RICC may begin quite early and manifest in different ways, including pericardial effusion, ischemic heart disease, pericarditis, arrhythmia, or congestive heart failure [9], [10], [19], [31]. Ishikura et al., analyzing long-term toxicity among 78 patients with cancer of the thoracic esophagus, found that 16 (21%) had pericarditis and 2 (3%) had congestive heart failure [19].
Many cardiac dosimetry measures have been linked with RICC in thoracic cancer, including MHD, heart V5, V30, and V50, among others [9], [10], [13], which also supports the need to consider heart dosimetry as a whole rather than emphasize a single variable. Wang et al. reported that the 2-year competing risk–adjusted RICC rates for an MHD of 10 Gy were 4%, for MHD 10–20 Gy 7%, and for MHD >20 Gy 21% for patients with NSCLC [10]. Dess et al. reported that the 2-year cumulative incidence of grade ≥3 RICC for patients with MHD >11 Gy was 18% vs. 2% for MHD ≤11 Gy (P < 0.01) [9] and further found that higher heart V30-45 and MHD were also risk factors for RICC (P < 0.05). In the current study, patients with heart V30 >45% had a much higher RICC rate than those with heart V30 ≤45%.
Although heart dose has not always been linked directly with worse survival [9], [10], it may affect survival indirectly because it is associated with RICC, which contributes to decreased OS [9], [13], [19]. One study of 125 patients with NSCLC from 4 prospective trials showed that MHD was associated significantly with grade ≥3 RICC, which in turn was associated with decreased OS [9]. Our study showed similar results. Patients who experienced RICC had greatly inferior survival compared with those without RICC (5-year OS rates 27.6% vs. 43.2%, P = 0.012).
The association between irradiated lung dose and radiation pneumonitis is well established [32], [33] and supports our finding of an association between MLD and decreased OS among patients with EC. Because radiation pneumonitis is considered an acute toxicity that is both common and can be lethal [11], [34], [35], it is reasonable to assume that lung dose contributes to survival [13], [23], [35]. One retrospective study of 256 patients with lung cancer who underwent definitive radiation therapy reported that 3-year OS rates differed among patients with no, mild, or severe radiation pneumonitis at 33.4%, 38.2%, or 0%, and severe radiation pneumonitis was found to be the most important contributor to poor survival in MVA [35]. Thus lung dose should always be optimized and minimized in treatment planning. Some of the cutoff points for heart and lung dose were much lower in the current study than in previous reports of lung cancer and what is used in clinical practice [11,36]. Possible reasons for this include differences in endpoints (OS in the current study vs. complications in others) and interactions between lung dose and heart dose, which may result in lower doses contributing to survival.
Our study had several strengths. First, the endpoint was OS, which is relatively objective and accurate; however, this study was also a retrospective single-institution analysis. Second, to our knowledge, this is the first and largest comprehensive evaluation of clinical and dosimetric factors and survival in EC. Third, all patients were treated with IMRT, and thus the results were not confounded by the use of other radiation modalities such as proton therapy or 3D conformal radiation therapy. Fourth, the follow-up interval was long enough to evaluate potential cardiac mortality. However, attendant to any dosimetric study is the likelihood that dosimetric variables interact with each other and with some clinical variables as well, making isolated analysis of organs at risk challenging. Both heart and lung dose should be optimized and minimized during radiation treatment planning. Future studies are needed to validate our findings. We are currently investigating potential blood biomarkers of cardiac and pulmonary damage at our institution.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2019.04.016.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.van Hagen P., Hulshof M.C., van Lanschot J.J. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro J., van Lanschot J.J., Hulshof M.C. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16(9):1090–1098. doi: 10.1016/S1470-2045(15)00040-6. [DOI] [PubMed] [Google Scholar]
- 3.Markar S., Gronnier C., Duhamel A. Salvage surgery after chemoradiotherapy in the management of esophageal cancer: is it a viable therapeutic option? J Clin Oncol. 2015;33(33):3866–3873. doi: 10.1200/JCO.2014.59.9092. [DOI] [PubMed] [Google Scholar]
- 4.Marks J.L., Hofstetter W., Correa A.M. Salvage esophagectomy after failed definitive chemoradiation for esophageal adenocarcinoma. Ann Thorac Surg. 2012;94(4):1126–1132. doi: 10.1016/j.athoracsur.2012.05.106. discussion 1132–1123. [DOI] [PubMed] [Google Scholar]
- 5.Cooper J.S., Guo M.D., Herskovic A. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281(17):1623–1627. doi: 10.1001/jama.281.17.1623. [DOI] [PubMed] [Google Scholar]
- 6.Lin S.H., Wang L., Myles B. Propensity score-based comparison of long-term outcomes with 3-dimensional conformal radiotherapy vs intensity-modulated radiotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2012;84(5):1078–1085. doi: 10.1016/j.ijrobp.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin S.H., Komaki R., Liao Z. Proton beam therapy and concurrent chemotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2012;83(3):e345–351. doi: 10.1016/j.ijrobp.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gayed I.W., Liu H.H., Yusuf S.W. The prevalence of myocardial ischemia after concurrent chemoradiation therapy as detected by gated myocardial perfusion imaging in patients with esophageal cancer. J Nucl Med. 2006;47(11):1756–1762. [PubMed] [Google Scholar]
- 9.Dess R.T., Sun Y., Matuszak M.M. Cardiac events after radiation therapy: combined analysis of prospective multicenter trials for locally advanced non-small-cell lung cancer. J Clin Oncol. 2017;35(13):1395–1402. doi: 10.1200/JCO.2016.71.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang K., Eblan M.J., Deal A.M. Cardiac toxicity after radiotherapy for stage iii non-small-cell lung cancer: pooled analysis of dose-escalation trials delivering 70 to 90 Gy. J Clin Oncol. 2017;35(13):1387–1394. doi: 10.1200/JCO.2016.70.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradley J.D., Paulus R., Komaki R. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16(2):187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu C., Xi M., Komaki R. Dosimetric and clinical outcomes after volumetric modulated arc therapy for carcinoma of the thoracic esophagus. Adv Radiat Oncol. 2017;2(3):325–332. doi: 10.1016/j.adro.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Speirs C.K., DeWees T.A., Rehman S. Heart dose is an independent dosimetric predictor of overall survival in locally advanced non-small cell lung cancer. J Thorac Oncol. 2017;12(2):293–301. doi: 10.1016/j.jtho.2016.09.134. [DOI] [PubMed] [Google Scholar]
- 14.<An application of change point methods in studying the effect of age on survival in breast cancer.pdf>.
- 15.Darby S.C., Ewertz M., McGale P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 16.Darby S.C., McGale P., Taylor C.W., Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 2005;6(8):557–565. doi: 10.1016/S1470-2045(05)70251-5. [DOI] [PubMed] [Google Scholar]
- 17.Aleman B.M., van den Belt-Dusebout A.W., De Bruin M.L. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 2007;109(5):1878–1886. doi: 10.1182/blood-2006-07-034405. [DOI] [PubMed] [Google Scholar]
- 18.Hancock S.L., Donaldson S.S., Hoppe R.T. Cardiac disease following treatment of Hodgkin’s disease in children and adolescents. J Clin Oncol. 1993;11(7):1208–1215. doi: 10.1200/JCO.1993.11.7.1208. [DOI] [PubMed] [Google Scholar]
- 19.Ishikura S., Nihei K., Ohtsu A. Long-term toxicity after definitive chemoradiotherapy for squamous cell carcinoma of the thoracic esophagus. J Clin Oncol. 2003;21(14):2697–2702. doi: 10.1200/JCO.2003.03.055. [DOI] [PubMed] [Google Scholar]
- 20.Stam B., Peulen H., Guckenberger M. Dose to heart substructures is associated with non-cancer death after SBRT in stage I-II NSCLC patients. Radiother Oncol. 2017;123(3):370–375. doi: 10.1016/j.radonc.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 21.Guberina M., Eberhardt W., Stuschke M. Heart dose exposure as prognostic marker after radiotherapy for resectable stage IIIA/B non-small-cell lung cancer: secondary analysis of a randomized trial. Ann Oncol. 2017;28(5):1084–1089. doi: 10.1093/annonc/mdx069. [DOI] [PubMed] [Google Scholar]
- 22.Tembhekar A.R., Wright C.L., Daly M.E. Cardiac dose and survival after stereotactic body radiotherapy for early-stage non-small-cell lung cancer. Clin Lung Cancer. 2017;18(3):293–298. doi: 10.1016/j.cllc.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tucker S.L., Liu A., Gomez D. Impact of heart and lung dose on early survival in patients with non-small cell lung cancer treated with chemoradiation. Radiother Oncol. 2016;119(3):495–500. doi: 10.1016/j.radonc.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 24.van Nimwegen F.A., Cutter D.J., Schaapveld M. Simple method to estimate mean heart dose from Hodgkin lymphoma radiation therapy according to simulation X-rays. Int J Radiat Oncol Biol Phys. 2015;92(1):153–160. doi: 10.1016/j.ijrobp.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 25.Ares C., Khan S., Macartain A.M. Postoperative proton radiotherapy for localized and locoregional breast cancer: potential for clinically relevant improvements? Int J Radiat Oncol Biol Phys. 2010;76(3):685–697. doi: 10.1016/j.ijrobp.2009.02.062. [DOI] [PubMed] [Google Scholar]
- 26.Aznar M.C., Korreman S.S., Pedersen A.N., Persson G.F., Josipovic M., Specht L. Evaluation of dose to cardiac structures during breast irradiation. Br J Radiol. 2011;84(1004):743–746. doi: 10.1259/bjr/12497075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lohr F., El-Haddad M., Dobler B. Potential effect of robust and simple IMRT approach for left-sided breast cancer on cardiac mortality. Int J Radiat Oncol Biol Phys. 2009;74(1):73–80. doi: 10.1016/j.ijrobp.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 28.Azizova T.V., Muirhead C.R., Druzhinina M.B. Cardiovascular diseases in the cohort of workers first employed at Mayak PA in 1948–1958. Radiat Res. 2010;174(2):155–168. doi: 10.1667/RR1789.1. [DOI] [PubMed] [Google Scholar]
- 29.Carr Z.A., Land C.E., Kleinerman R.A. Coronary heart disease after radiotherapy for peptic ulcer disease. Int J Radiat Oncol Biol Phys. 2005;61(3):842–850. doi: 10.1016/j.ijrobp.2004.07.708. [DOI] [PubMed] [Google Scholar]
- 30.Gyenes G., Rutqvist L.E., Liedberg A., Fornander T. Long-term cardiac morbidity and mortality in a randomized trial of pre- and postoperative radiation therapy versus surgery alone in primary breast cancer. Radiother Oncol. 1998;48(2):185–190. doi: 10.1016/s0167-8140(98)00062-0. [DOI] [PubMed] [Google Scholar]
- 31.Sio T.T., Liang J.J., Chang K. Dosimetric correlate of cardiac-specific survival among patients undergoing coronary artery stenting after thoracic radiotherapy for cancer. Am J Clin Oncol. 2017;40(2):133–139. doi: 10.1097/COC.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 32.Marks L.B., Bentzen S.M., Deasy J.O. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S70–S76. doi: 10.1016/j.ijrobp.2009.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farr K.P., Khalil A.A., Knap M.M., Moller D.S., Grau C. Development of radiation pneumopathy and generalised radiological changes after radiotherapy are independent negative prognostic factors for survival in non-small cell lung cancer patients. Radiother Oncol. 2013;107(3):382–388. doi: 10.1016/j.radonc.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 34.Xu C., Xi M., Moreno A. Definitive chemoradiation therapy for esophageal cancer in the elderly: clinical outcomes for patients exceeding 80 years old. Int J Radiat Oncol Biol Phys. 2017;98(4):811–819. doi: 10.1016/j.ijrobp.2017.02.097. [DOI] [PubMed] [Google Scholar]
- 35.Inoue A., Kunitoh H., Sekine I., Sumi M., Tokuuye K., Saijo N. Radiation pneumonitis in lung cancer patients: a retrospective study of risk factors and the long-term prognosis. Int J Radiat Oncol Biol Phys. 2001;49(3):649–655. doi: 10.1016/s0360-3016(00)00783-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.