Abstract
Although microRNAs have been validated to play prominent roles in the occurrence and development of human bladder cancer (BC), alterations and function of many microRNAs (miRNAs) in bladder cancer invasion are not fully explored yet. miR-146b was reported to be a tumor suppressor or oncomiRNA in various types of cancer. However, its accurate expression, function, and mechanism in bladder cancer remain unclear. Here we discovered that miR-146b was frequently upregulated in bladder cancer tissues compared with adjacent non-cancerous tissues. Inhibition of miR-146b resulted in a significant inhibitory effect on the invasion of bladder cancer cells by reducing mmp2 mRNA transcription and protein expression. We further demonstrated that knockdown of miR-146b attenuated ETS2 expression, which was the transcription factor of matrix metalloproteinase (MMP)2. Moreover, mechanistic studies revealed that miR-146b inhibition stabilized ARE/poly(U)-binding/degradation factor 1 (auf1) mRNA by directly binding to its mRNA 3′ UTR, further reduced ets2 mRNA stability, and finally inhibited mmp2 transcription and attenuated bladder cancer invasion abilities. The identification of the miR-146b/AUF1/ETS2/MMP2 mechanism for promoting bladder cancer invasion provides significant insights into understanding the nature of bladder cancer metastasis. Targeting the pathway described here may be a novel approach for inhibiting invasion and metastasis of bladder cancer.
Keywords: miR-146b, human bladder cancer invasion, MMP2, AUF1, ETS2
Introduction
Bladder cancer is one of the most common urinary malignancies worldwide, characterized by a high rate of recurrence and no targeted therapy method.1 In China, approximately 80,500 cases of bladder cancer are diagnosed with roughly 32,900 cancer-related deaths in 2015.2 Approximately 70% of cases are diagnosed as non-muscle-invasive bladder cancer (NMIBC), whereas the remaining 30% of cases are classified as muscle-invasive bladder cancer (MIBC). The standard treatment for MIBC is radical cystectomy, which provides only a 5-year survival rate of 50%.3 Thus, a better understanding of the targets is urgently needed to explore the molecular mechanisms involved in the invasion ability of MIBC.
MicroRNAs (miRNAs) are short non-coding RNA molecules that usually repress gene expression by binding to the 3′ UTR of their target mRNAs.4 Increasing evidence indicates that miRNAs play important roles in the formation of bladder cancer.5, 6 The expression of miR-146b has been found in almost all human organs, and the dysregulation of miR-146b has been reported in a variety of human malignancies.7 A recent study reported that overexpression of miR-146b in breast cancer cells downregulated epidermal growth factor receptor expression, inhibited invasion and migration in vitro, and suppressed experimental lung metastasis in vivo,8 suggesting that miR-146b serves as a tumor suppressor. However, some studies reported that miR-146b might act as an oncogene to promote proliferation, migration, and invasion of colorectal and prostate cancers.9, 10 Despite the fact that surging studies of the biogenesis and mechanisms of miR-146b were involved in the pathogenesis of multiple tumors, the accurate expression level and mechanistic function of miR-146b in bladder cancer remain unclear.
AU-rich element (ARE)/poly(U)-binding/degradation factor 1 (AUF1), also known as heterogeneous nuclear ribonucleoprotein D, is a family of four isoforms (37, 40, 42, and 45 kDa), which result from alterative splicing of a single pre-mRNA.11 AUF1 forms direct complexes with a variety of AU-rich conserved elements in the 3′ UTR of many transcripts.11, 12 AUF1 is predominantly associated with mRNA-destabilizing activity.13 Furthermore, several lines of evidence support a role of AUF1 in the initiation and progression of cancer.14 Indeed, high AUF1 levels were detected in numerous malignancies, including cancers of the breast, skin, thyroid, and liver.14, 15 However, many potential roles of deregulated AUF1 expression or activity in tumor initiation and progression remain unresolved. In addition, little is known about the mechanisms that control AUF1 expression in many cellular contexts, including those that decrease AUF1 levels during carcinogenesis.
In our research, we demonstrated that miR-146b might be a potential clinical biomarker in bladder cancer. The roles of miR146b on bladder cancer invasion may be through regulation of the AUF1/ETS2/matrix metalloproteinase (MMP)2-signaling pathway. These results might give us great insights into the molecular mechanism of miR-146b-regulated invasion, and miR-146b may be a potential candidate for application in the treatment of bladder cancer.
Results
miR-146b Is Highly Expressed in Bladder Cancer Cell Lines and Tissues
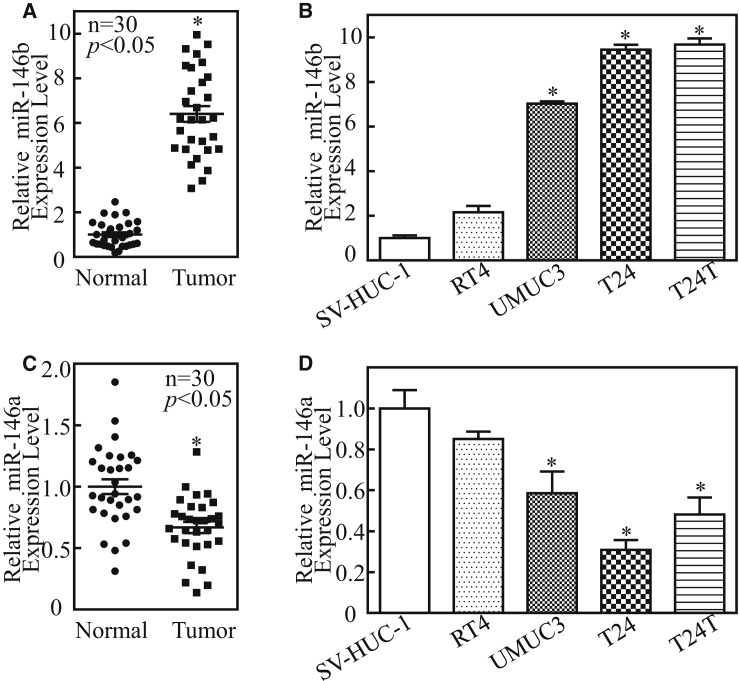
To understand the clinical interrelation of the expression level of miR-146b in the bladder cancer tissues, we analyzed 30 pairs of bladder cancer tissue samples and non-tumor tissues from patients by the method of real-time PCR. The results showed that the expression of miR-146b was significantly upregulated in the bladder cancer tumor tissues as compared with the paired adjacent non-tumor bladder tissues (Figure 1A). The levels of miR-146b were also assessed in human bladder cancer cell lines (RT4, UMUC3, T24, and T24T) and normal urothelial cell lines (SV-HUC-1). As shown in Figure 1B, a similar expression trend of miR-146b was observed in bladder cancer cell lines in comparison to normal urothelial cell lines. miR-146b is a member of the miR-146 family and shares its seed and targets with miR-146a; we next detected the expression level of miR-146a in the bladder cancer tissues and cells. The results showed that the expression of miR-146a was downregulated in the bladder cancer tumor tissues and bladder cancer cells (Figures 1C and 1D). Taken together, the above results indicate that miR-146b upregulation has the potential to serve as a new prognostic biomarker for clinical bladder cancer patients.
Figure 1.
miR-146b Was Overexpressed in Human Bladder Cancer Tissues and Cell Lines
(A and C) Total RNA was extracted from human bladder cancer tissues (tumor) and the paired adjacent normal tissues (normal) of 30 patients and then subjected to real-time PCR analyses to determine miR-146b (A) and miR-146a (C) expression levels. Data represent mean ± SD (*p < 0.05). (B and D) miR-146b (B) and miR-146a (D) levels in human bladder cancer cell lines (RT4, UMUC3, T24, and T24T) were determined and compared with two normal urothelial cell lines (SV-HUC-1) by real-time PCR. miR-146b and miR-146a expression were normalized to U6 expression; error bars represent the mean ± SD, and the asterisk (*) indicates a significant change relative to the control group (p < 0.05).
miR-146b Overexpression Was Essential for Human Bladder Cancer Invasion and Anchorage-Independent Growth
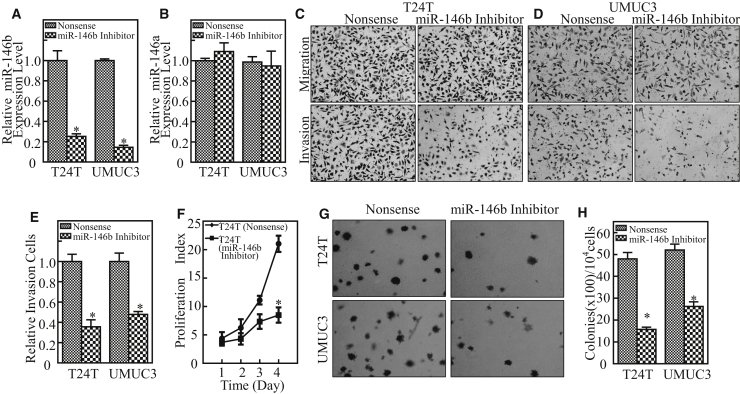
To identify the biological function of miR-146b in human bladder cancer, miR-146b inhibitor was transfected into T24T and UMUC3 cells. As shown in Figure 2A, the stable transfectants T24T (miR-146b inhibitor) and UMUC3 (miR-146b inhibitor) and their corresponding nonsense control transfectants were established and identified by real-time PCR. The inhibition of miR-146b showed no impact on the expression of miR-146a in T24T and UMUC3 cells (Figure 2B), excluding the cross-reactivity of miR-146a with miR-146b. The results from invasion assays indicated that the inhibition of miR-146b led to a dramatic decrease of bladder cancer cell invasion ability (Figures 2C–2E). Moreover, inhibition of miR-146b dramatically impaired the monolayer growth of T24T and UMUC3 cells, and it inhibited bladder cancer cell anchorage-independent growth (Figures 2F–2H). Taken together, these results demonstrate a novel positive regulatory effect of miR-146b on human bladder cancer cell invasion and anchorage-independent growth.
Figure 2.
miR-146b Overexpression Was Crucial for Human Bladder Cancer Cell Invasion and Anchorage-Independent Growth
(A) miR-146b inhibitor and its nonsense control plasmids were stably transfected into T24T and UMUC3 cells, and stable transfectants were identified by real-time PCR. The error bars represent the mean ± SD. Student’s t test was used to determine the p value, and the asterisk (*) indicates a significant decrease relative to nonsense control cells (*p < 0.05). (B) miR-146a expression was dectected in the indicated cells. The error bars represent the mean ± SD. Student’s t test was used to determine the p value. (C–E) The invasion abilities of T24T (miR-146b inhibitor) (C), UMUC3 (miR-146b inhibitor) (D), and their nonsense transfectants were determined by using BD BioCoat Matrigel Invasion Chamber applied with the matrigel (E). The error bars represent mean ± SD from three independent experiments. Student’s t test was utilized to determine the p value, and the asterisk (*) indicates a significant decrease as compared with nonsense control transfectants, *p < 0.05. (F) T24T transfectants as indicated were plated in 96-well plates at a density of 5,000 cells/well. The cell culture medium was then replaced with 0.1% FBS DMEM-F12 (1:1) and cultured for 12 h. The cells were replaced with normal medium and cultured for another 1, 2, 3, or 4 days. Subsequently, ATP activity assay was performed using the protocol described in the Materials and Methods. The asterisk (*) indicates a significant decrease from nonsense control. (G) The indicated cells were subjected to anchorage-independent soft agar assay using the protocol described in the Materials and Methods. Representative images of colonies of indicated cells were photographed under an Olympus DP71. (H) The number of colonies was counted with more than 32 cells of each colony, and the results were presented as colonies per 104 cells; the error bars show mean ± SD from three independent experiments. The asterisk (*) indicates a significant decrease in comparison to those of nonsense control transfectants (**p < 0.05).
MMP2 Was a miR-146b Downstream Effector for Bladder Cancer Cell Invasion
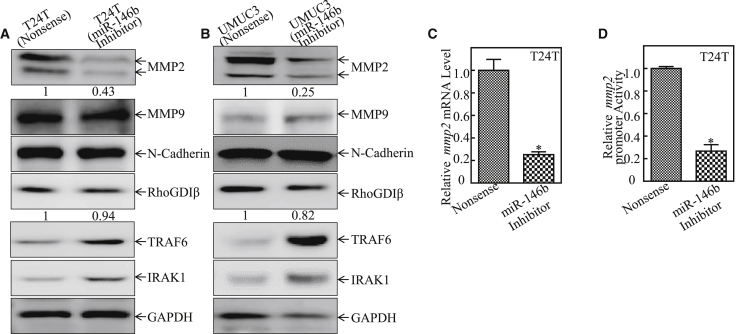
To elucidate the mechanism underlying the miR-146b regulation of bladder cancer invasion, the expression levels of MMP2, MMP9, N-Cadherin, and RhoGDIβ were determined in miR-146b inhibition transfectants in comparison to their nonsense transfectants. As shown in Figures 3A and 3B, a deficiency of miR-146b resulted in the upregulation of TRAF6 and IRAK1 expression (known targets of miR-146b) and a dramatic attenuation of abundance of MMP2 protein in both T24T and UMUC3 cells, while there was no consistent impact on the expression of MMP9, N-Cadherin, and RhoGDIβ under the same experimental conditions in both cells.
Figure 3.
miR-146b Overexpression Promoted mmp2 mRNA Transcription in Human Bladder Cancer Cells
(A and B) The indicated cell extracts were subjected to western blot to determine the protein expressions of MMP2, MMP9, N-Cadherin, and RhoGDIβ. TRAF6, IRAK1, and GAPDH were used as protein loading controls. (C) T24T (miR-146b inhibitor) and T24T (nonsense) cells were cultured in 6-well plates until cell density reached 70%–80%. Following synchronization for 12 h, the medium was then replaced with 5% FBS DMEM-Ham’s F12 for another 12 h, and the cells were extracted for total RNA with Trizol reagent. Real-time PCR was used to determine mmp2 mRNA expression, and β-Actin was used as an internal control. Data represent mean ± SD (*p < 0.05). (D) Human mmp2 promoter-driven luciferase reporter was used to evaluate its promoter transcription activity in the indicated transfectants. The results were normalized by internal TK activity. The error bars represent the mean ± SD. Student’s t test was used to determine the p value, and the asterisk (*) indicates a significant decrease relative to nonsense control cells (*p < 0.05).
MMP2 possesses the function of enzymatic activity and plays a vital role in mediating the degradation of type IV collagen, which is a major structural component of the basement membrane of tissues.16 Our recent study revealed that MMP2 overexpression is crucial for human bladder cancer invasion,17 whereas the inhibition of MMP2 expression by the anti-cancer agent isorhapontigenin (ISO) dramatically attenuated both bladder cancer invasion in vitro and highly invasive bladder cancer formation in vivo.18 Given the critical role of MMP2 in bladder cancer invasion, the upregulation of MMP2 by miR-146b may play a major role in its promotion of bladder cancer invasion.
To investigate the mechanisms underlying the miR-146b upregulation of MMP2 protein, we first examined its mRNA levels. The results showed that mmp2 mRNA was markedly decreased in miR-146b inhibition transfectants (Figure 3C). Further, human mmp2 promoter luciferase reporter was then transfected into T24T (miR-146b inhibitor) and T24T (nonsense) cells. The results showed that the inhibition of miR-146b decreased mmp2 promoter-driven reporter transcription activity in T24T cells (Figure 3D).
miR-146b Increased mmp2 Transcription through Elevating ETS2 Protein Expression
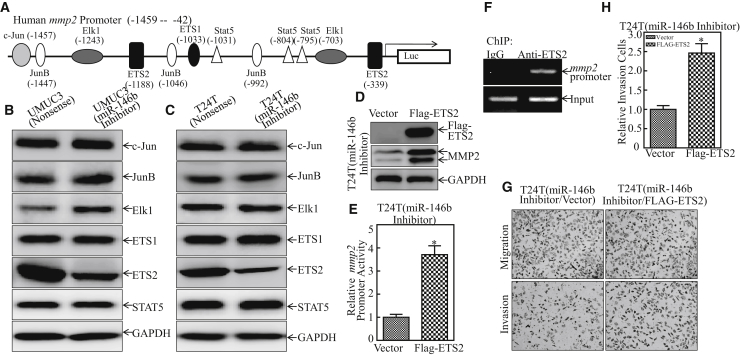
The above results demonstrate that miR-146b increased mmp2 mRNA transcription in human bladder cancer and that MMP2 is crucial for miR-146b-induced human bladder cancer invasion. To investigate the mechanisms underlying the miR-146b-increased mmp2 transcription, we bioinformatically analyzed the potential transcriptional factors that could bind to the −1,459 to −42 region of the mmp2 promoter (Figure 4A). Then we evaluated the expression of these transcription factors in both miR-146b-knockdown cells and their scramble nonsense transfectants. The results showed that only ETS2 exhibited low expression, while others showed no significant differences by the inhibition of miR-146b (Figures 4B and 4C), suggesting that ETS2 might be the effector for regulating MMP2 expression.
Figure 4.
ETS2 is a miR-146b-Regulated Transcription Factor Mediating MMP2 Upregulation in Human Bladder Cancer Cells
(A) Potential transcription factor-binding sites in the mmp2 promoter region (–1,459 to −42) were analyzed by using the TRANSFAC 8.3 engine online. (B and C) The indicated cell extracts were subjected to western blot for determination of the expression of c-Jun, JunB, Elk1, ETS2, and STAT5. GAPDH was used as a protein loading control. The results represented one of three independent experiments. (D) The overexpressed FLAG-ETS2 plasmid was stably transfected into T24T (miR-146b inhibitor) cells, and the cell extracts were then subjected to western blot for the determination of FLAG, ETS2, and MMP2 expressions, and GAPDH was used as a protein loading control. (E) The mmp2 promoter-driven luciferase reporter together with the TK reporter was transfected into the indicated cells. Luciferase activity of each transfectant was evaluated and the error bars show mean ± SD from three independent experiments. The asterisk (*) indicates a significant increase as compared with nonsense transfectant (p < 0.05). (F) ChIP assay was carried out using anti-ETS2 antibody to detect the interaction of ETS2 with the mmp2 promoter. (G and H) The invasion abilities of T24T (miR-146b inhibitor/vector) and T24T (miR-146b inhibitor/FLAG-ETS2) cells were determined, as described in the Materials and Methods. The error bars represent mean ± SD from three independent experiments. Student’s t test was utilized to determine the p value. The asterisk (*) indicates a significant increase in comparison to T24T (miR-146b inhibitor/vector) (*p < 0.05) (H).
To further test the role of ETS2, the plasmid of FLAG-ETS2 was stably transfected into T24T (miR-146b inhibitor) cells (Figure 4D). The results showed that overexpression of ETS2 remarkably increased MMP2 protein expression (Figure 4D) and promoted mmp2 promoter-driven luciferase reporter transcriptional activity (Figure 4E). Moreover, the chromatin immunoprecipitation (ChIP) assay was carried out by using anti-ETS2 antibody, and the results showed that ETS2 could directly bind to the mmp2 promoter, as shown in Figure 4F, demonstrating that ETS2 does act as a transcription factor of the mmp2 promoter. Furthermore, the invasion ability of T24T (miR-146b inhibitor/FLAG-ETS2) was elevated as compared to T24T (miR-146b inhibitor/vector) cells (Figures 4G and 4H). Collectively, our results strongly demonstrate that ETS2 is the critical factor mediating miR-146b promotion of bladder cancer invasion through directly binding to the mmp2 promoter, thereby promoting MMP2 expression.
miR-146b Promoted ETS2 Protein Expression through Elevating Its mRNA Stability
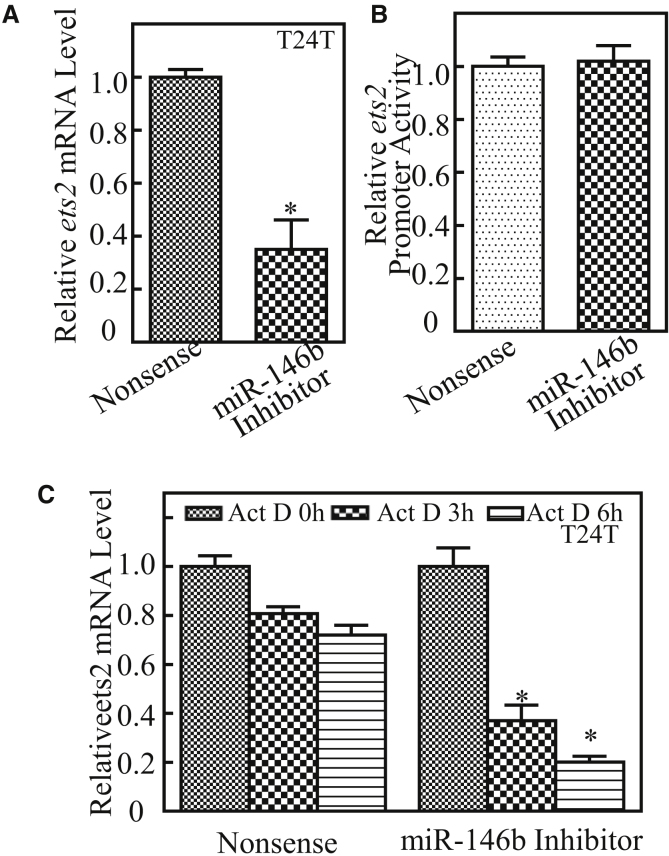
To investigate the mechanisms underlying miR-146b’s upregulation of ETS2 protein, we first examined its mRNA levels by real-time PCR. Consistent with protein abundance in T24T (miR-146b inhibitor) and T24T (nonsense), ets2 mRNA levels were profoundly impaired in miR-146b inhibition transfectants (Figure 5A). The ets2 promoter-driven luciferase reporter and PGL3 basic luciferase reporter were next transfected into T24T (miR-146b inhibitor) cells, and the stable transfectants were then used to assess the potential miR-146b upregulation of ets2 mRNA transcription. The results showed that ets2 promoter transcriptional activity was comparable between T24T (miR-146b inhibitor) and T24T (nonsense) transfectants (Figure 5B), excluding the possibility that miR-146b upregulation of ets2 mRNA transcription. Therefore, we explored the possibility that miR-146b stabilizes ets2 mRNA. Upon inhibition of new mRNA transcription with Actinomycin D (Act D), ets2 mRNA degradation rates in T24T (miR-146b inhibitor) were much faster than in T24T (nonsense) cells (Figure 5C). Collectively, our results reveal that miR-146b overexpression plays an essential role in maintaining ets2 mRNA stability.
Figure 5.
miR-146b Overexpression Promoted ETS2 Expression by Stabilizing Its mRNA
(A) T24T (miR-146b inhibitor) and T24T (nonsense) cells were cultured in 6-well plates until cell density reached 70%–80%. Following synchronization for 12 h, the medium was then replaced with 5% FBS DMEM-Ham’s F12 for another 12 h, and the cells were extracted for total RNA with Trizol reagent. Real-time PCR was used to determine ets2 mRNA expression, and β-Actin was used as an internal control. Data represent mean ± SD (*p < 0.05). (B) Human ets2 promoter-driven luciferase reporter was used to evaluate its promoter transcription activity in the indicated transfectants. The results were normalized by internal TK activity. (C) T24T (miR-146b inhibitor) and T24T (nonsense) cells were seeded into 6-well plates. After synchronization, the indicated cells were treated with Actinomycin D (Act D) for the indicated time points. Total RNA was then isolated and subjected to real-time PCR analysis for mRNA levels of ets2, and β-Actin was used as an internal control. The error bars represent mean ± SD from three independent experiments. Student’s t test was utilized to determine the p value. The asterisk (*) indicates a significant decrease in comparison to T24T (nonsense) cells (*p < 0.05).
miR-146b Stabilized ets2 mRNA through Attenuating AUF1 Protein Expression
AUF1, one of the best-characterized ARE-binding proteins (AUBPs), is able to bind to many ARE-mRNAs and assemble other factors necessary in the recruitment of the mRNA degradation machinery.19 HuR, a ubiquitously expressed member of the Hu family of RNA-binding proteins, related to Drosophila ELAV, selectively binds AREs and stabilizes ARE-containing mRNAs when overexpressed in cultured cells,20 while NCL is a protein, primarily in the nucleus, that has four RNA-binding domains and can stabilize mRNA.21 To determine the upstream regulators mediating ets2 mRNA stabilization by miR-146b, AUF1, HuR, and NCL were tested and compared in T24T (miR-146b inhibitor) and T24T (nonsense) cells.
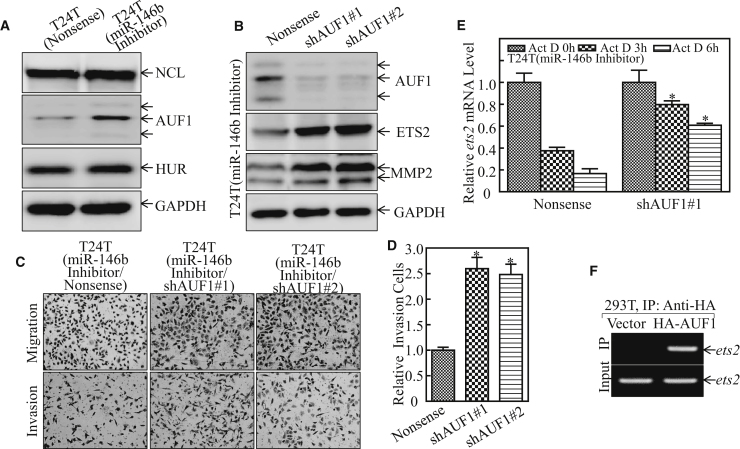
As the data show in Figure 6A, while HuR and NCL proteins were comparable between T24T (miR-146b inhibitor) and T24T (nonsense) cells, AUF1 was elevated in miR-146b inhibition cells. These results exclude the participation of HuR and NCL in the miR-146b stabilization of ets2 mRNA. Thus, we knocked down AUF1 in T24T (miR-146 inhibitor) cells by using short hairpin RNAs (shRNAs) specifically targeting human AUF1 and the stable transfectants; T24T (miR-146 inhibitor/shAUF1#1), T24T (miR-146 inhibitor/shAUF1#2), and its scramble transfectant T24T (miR-146 inhibitor/nonsense) were established and identified (Figure 6B). As expected, AUF1 knockdown in T24T (miR-146 inhibitor) cells led to increased expression of ETS2 and MMP2 protein (Figure 6B), and it restored ets2 mRNA stability as well as bladder cancer cell invasion (Figures 6C–6E), suggesting that AUF1 might be a mediator linking miR-146b to ETS2 and associated with bladder cancer invasion. To determine whether AUF1 is able to directly bind to ets2 mRNA, RNA-IP assay was performed in HEK293T cells that expressed hemagglutinin (HA)-AUF1. The results showed that AUF1 did bind to ets2 mRNA (Figure 6F). Taken together, our results demonstrate that miR-146 inhibits AUF1 expression, which results in less AUF1 binding to ets2 mRNA, consequently leading to ets2 mRNA stabilization, in turn promoting mmp2 mRNA transcription and protein expression, and finally enhancing bladder cancer invasion.
Figure 6.
AUF1 Was a miR-146b Downstream Effector Responsible for the Destabilization of ets2 mRNA via Direct Binding
(A) The indicated cell extracts were subjected to western blot for the determination of NCL, HuR, and AUF1 protein expression levels. (B) AUF1-knockdown constructs were stably transfected into T24T (miR-146b inhibitor) cells. The indicated cell extracts were subjected to western blot for the determination of AUF1, ETS2, and MMP2 protein expression levels. The stable transfectants were used to determine their invasion abilities in comparison to nonsense control transfectants, as described in the Materials and Methods. (C and D) The images were photographed under an Olympus DP71, the number of the cells was counted by the software Image J, and the invasion rate was normalized with the insert control according to the manufacturer’s instructions (C). The results are presented with the mean ± SD from triplicate. Student’s t test was utilized to determine the p value; the double asterisk (**) indicates a significant decrease in comparison to scramble vector transfectants (**p < 0.05), while the single asterisk (*) indicates a significant increase in comparison to T24T (miR-146b inhibitor/nonsense) transfectants (*p < 0.05) (D). (E) Ets2 mRNA stability was evaluated by real-time PCR in the presence of Act D in T24T (miR-146b inhibitor/shAUF1) cells and its nonsense control. The results are presented with the mean ± SD from triplicate. Student’s t test was utilized to determine the p value (*p < 0.05). (F) The HA-AUF1 construct was transfected into 293T cells and HA-AUF1 protein was pulled down with anti-HA beads. The mRNAs bound to AUF1 protein were used to carry out RT-PCR for the determination of ets2 mRNA expression.
miR-146b Promoted auf1 mRNA Degradation by Directly Targeting Its 3′ UTR
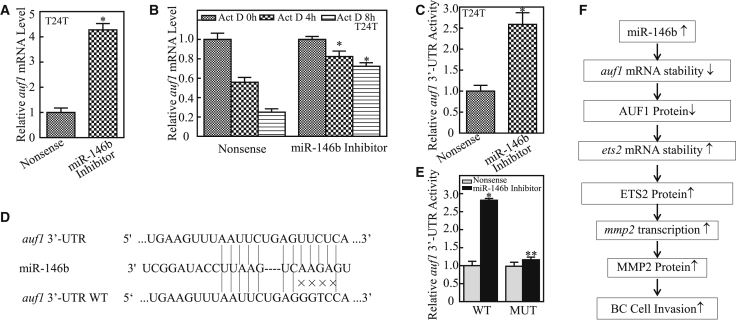
To elucidate how miR-146 inhibits AUF1 protein expression, we assessed the effect of miR-146 on auf1 mRNA abundance in T24T cells. As shown in Figure 7A, auf1 mRNA’s level was elevated in miR-146b inhibition transfectants as compared with its scramble nonsense transfectants. Therefore, we explored the possibility that miR-146 destabilizes auf1 mRNA. Upon the inhibition of new mRNA transcription with Act D, auf1 mRNA degradation rates in T24T (nonsense) were much faster than in T24T (miR-146b inhibitor) cells (Figure 7B), consistent with the observation of miR-146 destabilization of auf1 mRNA. miRNAs play biological roles by modulating target gene expression via their binding to the 3′ UTR of target genes to cause mRNA stability alteration or protein translation suppression.22 Therefore, we next evaluated the auf1 mRNA 3′ UTR activity between T24T (nonsense) and T24T (miR-146b inhibitor) cells. The results showed that auf1 mRNA 3′ UTR activity in T24T (miR-146b inhibitor) cells was significantly higher than that observed in T24T (nonsense) cells (Figure 7C), revealing that miRNAs might be involved in this regulation. To test this notion, a bioinformatics search for putative miRNAs that could potentially target the 3′ UTR of auf1 mRNA was performed using TargetScan (version [v.]7.0; http://www.targetscan.org/vert_72/).23 Surprisingly, the results indicated that there were multiple putative miRNA-binding sites in the 3′ UTR of auf1 mRNA, including binding sites for miR-146b (Figure 7D).
Figure 7.
miR-146b Was Responsible for the Destabilization of auf1 mRNA via Direct Binding to Its 3′ UTR
(A) T24T (miR-146b inhibitor) and T24T (nonsense) cells were cultured in 6-well plates until cell density reached 70%–80%. Following synchronization for 12 h, the medium was then replaced with 5% FBS DMEM-Ham’s F12 for another 12 h, and the cells were extracted for total RNA with Trizol reagent. Real-time PCR was used to determine auf1 mRNA expression, and β-Actin was used as an internal control. Data represent mean ± SD (*p < 0.05). (B) T24T (miR-146b inhibitor) and T24T (nonsense) cells were seeded into 6-well plates. After synchronization, the indicated cells were treated with Act D for the indicated time points. Total RNA was then isolated and subjected to real-time PCR analysis for mRNA levels of auf1, and β-Actin was used as an internal control. The error bars represent mean ± SD from three independent experiments. Student’s t test was utilized to determine the p value. The asterisk (*) indicates a significant increase in comparison to T24T (nonsense) cells (*p < 0.05). (C) The pMIR-AUF1 3′ UTR reporter was transiently transfected into the indicated cells, and luciferase activity of each transfectant was evaluated. The luciferase activity was presented as a relative to nonsense transfectant with normalization by using pRL-TK as an internal control. The error bars show mean ± SD from three independent experiments. The asterisk (*) indicates a significant increase in T24T (miR-146b inhibitor) in comparison to nonsense transfectant (p < 0.05). (D) The predicted miR-146b-binding site existed in the 3′ UTR of auf1 mRNA, and its mutants (MUTs) were generated in the binding site. (E) The pMIR-AUF1 3′ UTR reporters (WT and MUT) were co-transfected with pRL-TK into the indicated cells. At 24 h post-transfection, the transfectants were extracted for determination of the luciferase activity, and TK was used as the internal control. Luciferase activity of each transfectant was evaluated, and the results were presented as relative AUF1 3′ UTR activity. The error bars show mean ± SD from three independent experiments. The double asterisk (**) indicates a significant inhibition of 3′ UTR activity in mutant transfectant in comparison to the mutant of WT AUF1 3′ UTR luciferase reporter transfectant (p < 0.05). (F) The proposed mechanisms underlying miR-146b overexpression in the promotion of human bladder cancer cell invasion. miR-146b overexpression destabilizes auf1 mRNA, which further elevates ets2 mRNA stability, in turn promoting the transcription of MMP2 and protein expression, and finally it increases the invasion ability of human bladder cancer cells.
To test whether miR-146b is able to bind to the 3′ UTR of auf1 mRNA directly for stabilization of auf1 mRNA, the mutation of the miR-146b-binding site in auf1 mRNA 3′ UTR luciferase reporter was constructed, as indicated in Figure 7D. Auf1 mRNA 3′ UTR luciferase reporter or auf1 mRNA 3′ UTR luciferase reporter with the mutation of miR-146b-binding sites was transiently co-transfected with pRL-TK into T24T (miR-146b inhibitor) and T24T (nonsense) cells. The results showed that auf1 mRNA 3′ UTR luciferase activity was significantly increased in T24T (miR-146b inhibitor) cells as compared to T24T (nonsense) cells, whereas the miR-146b-binding site mutation in auf1 3′ UTR luciferase reporter attenuated the responses of T24T cells to miR-146b inhibition (Figure 7E). These results strongly demonstrate that the direct binding of miR-146b to the 3′ UTR of auf1 mRNA is crucial for miR-146b stabilization of auf1 mRNA. Taken together, our results demonstrate that miR-146b overexpression decreases auf1 mRNA expression by directly binding to its 3′ UTR, therefore stabilizing ets2 mRNA, in turn increasing mmp2 mRNA transcription and protein expression, and finally promoting bladder cancer invasion, as diagrammed in Figure 7F.
Discussion
Invasive bladder cancer has been a persistent challenge for scientists and urologists for the past decades due to its poor prognosis, even when applying current therapeutic strategies.24 A better understanding of the molecular mechanisms involved in the invasion ability of bladder cancer could contribute to the discovery of therapeutic targets, which is urgently needed to help the increasing number of bladder cancer patients.
Accumulating evidence from preclinical and clinical studies has shown that miRNAs play in the development of human malignancies has been evidenced.25 Although miRNAs exert their functions through sequence-specific binding to the mRNA of their target genes, it has been revealed that miRNAs also have cell type-specific signatures on target mRNA expression and are stage specific during cancer progression.18 Dysregulation of miR-146b has been documented in a variety of human malignancies, including gastric cancer,26 thyroid carcinoma,27 osteosarcoma,28 and glioma.29 The role of miR-146b as an oncogene was first identified in papillary thyroid carcinoma, which showed that the overexpression of miR-146b is associated with the aggressiveness of papillary thyroid carcinoma.30 miR-146b, as a downstream regulator of BCR-ABL1, promotes the leukemic transformation of hematopoietic cells by targeting several important genes (NUMB, NOTCH2, BRCA1, etc.) to affect cell proliferation and genome instability.31 However, conflicting results that support the role of miR-146b as a tumor suppressor have also been reported. miR-146b is significantly dysregulated in human glioblastoma tissues. miR-146b overexpression inhibits glioma cell migration and invasion by targeting MMP16.32 In the current study, miR-146b is shown to be upregulated in highly invasive bladder cancer cells, in comparison to human normal bladder urothelial cells. The upregulation of miR-146b is also observed in human invasive bladder cancers in comparison to their adjacent normal bladder tissues. Inhibition of miR-146b significantly inhibited bladder cancer invasion. Moreover, we define MMP2 as the miR-146b downstream effector responsible for its mediation of bladder cancer invasion. Our results provide evidence of a first-time link between miR-146b overexpression and bladder cancer invasion, which offers new insight into the therapeutic targeting of miR-146b for advanced bladder cancers.
As a family of proteolytic enzymes, the MMPs degrade multiple components of the extracellular matrix. A variety of clinical and experimental evidence has implicated that MMPs play a critical role in tumor neoangiogenesis, invasion, and metastasis, and therefore they represent ideal pharmacologic targets for cancer therapy.33, 34 Among them, MMP2 hydrolyzes type IV collagen primarily, which is the major structural component of the basement membrane.33, 35 Multiple studies have verified that MMP2 is critical for mediating bladder cancer invasion,36 but the maintenance of MMP2 abundance has not been well studied. Based on our results here, miR-146b overexpression significantly increases MMP2 protein level, which is known to regulate bladder cancer cell invasion. Interestingly, the inhibition of miR-146b greatly impaired mmp2 mRNA transcription in bladder cancer cells, finally inhibiting bladder cancer invasion ability. To the best of our knowledge, this is the first study of a cross-talk between miR-146b and the regulation of mmp2 mRNA transcription.
The ETS family of transcription factors has the conserved primary sequence of their DNA-binding domains that binds to a purine-rich GGA(A/T) core sequence.37, 38 ETS proteins play crucial roles in cell proliferation, differentiation, development, stem cell development, transformation, and angiogenesis, and they participate in the malignancy of tumor cells by activating the transcription of several cancer-related genes, such as proteases and angiogenesis-related genes.39 Binding sites for the ETS family are found in promoters of the MMP family,40 and the regulation of matrix-degrading proteases by ETS factors in tumor invasion and metastasis is well established.41 In this study, we found that miR-146b overexpression promoted ETS2 protein expression. The results of our ChIP assay showed that ETS2 could directly bind to the mmp2 promoter and increase MMP2 protein expression, evidence demonstrating the oncogenic role of ETS2 in bladder cancer. However, a tumor suppressor function of ETS2 has previously been reported through the activation of the miR-196b/FOXO1/p27-signaling pathway in human bladder cancer cells.42 These results reveal that the prognosis value of ETS2 depends on its downstream targets in the specific signaling axis of bladder cancer cells.
AUF1, one of the best-characterized RNA-binding proteins, has been reported to decrease the stability of some target transcripts.43 AUF1 might elicit anti-tumorigenic roles as a destabilizing agent for the mRNAs encoding the anti-apoptotic protein Bcl-2 and the proliferative protein cyclinD1,44, 45 and it can also suppress the expression of pro-inflammatory factors (e.g., interleukin [IL]-6, granulocyte-macrophage colony stimulating factor [GM-CSF], iNOS, and COX-2) by binding to the mRNAs that encode them, repressing their production, and thereby suppressing a pro-transformation state.46 The current study provides strong evidence from this study showing that miR-146b overexpression downregulates AUF1 expression. Mechanistic studies reveal that AUF1 is responsible for destabilizing ets2 mRNA by directly binding to it. AUF1 serves as a miR-146b downstream effector for the promotion of bladder cancer invasion. Such tumor suppressor properties of AUF1 in human bladder cancers are also in agreement with our recent findings that ATG7/autophagy-mediated AUF1 protein degradation expression promotes the bladder cancer invasion abilities,47 suggesting that the elevation of AUF1 might be of therapeutic benefit for human bladder cancer patients.
AUF1 expression could be regulated at multiple levels, including mRNA transcription, mRNA stability, and protein translation and degradation. miRNAs, a class of small (21- to 23-nt) non-protein-coding RNAs, could regulate either mRNA stability or protein translation.48 Recently, Al-Khalaf and Aboussekhra12 demonstrated that the tumor suppressor functions of miR-146b are mediated through the repression of the oncogenic potentials of AUF1 in osteosarcoma cells. In the current study, miR-146b overexpression reduced auf1 mRNA stability and protein expression. We also show that the AUF1 3′ UTR luciferase reporter activity is remarkably increased in miR-146b inhibition transfectant and such elevation can be completely impaired if the miR-146b-binding site on AUF1 3′ UTR luciferase reporter is mutated. We conclude that miR-146b exerts its oncogenic role in human bladder cancer cells through its directly inhibiting AUF1 expression.
In summary, the present study defines that miR-146b is overexpressed in both human bladder cancer tissues and cell lines. Our studies have revealed a new AUF1/ETS2/MMP2 pathway that is responsible for the oncogenic role of miR-146b in bladder cancer invasion. miR-146b overexpression destabilizes auf1 mRNA via directly binding to its 3′ UTR, further elevates ets2 mRNA stability followed by promoting mmp2 mRNA transcription and protein expression, and finally promotes the invasion ability of human bladder cancer cells. Our new findings raise the potential for developing a miR-146b-based specific therapeutic strategy for the treatment of human bladder cancer patients.
Materials and Methods
Plasmids, Antibodies, and Reagents
The miR-146b inhibitor and its nonsense control were purchased from System Biosciences (pLKO.1-miRZip146b) (Palo Alto, CA, USA). The plasmids were transfected into bladder cancer cells by using PolyJet DNA In Vitro Transfection Reagent (SignaGen Laboratories, Gaithersburg, MD, USA), according to the manufacturer’s instructions, and stable transfectants were selected with puromycin (0.2–0.3 mg/mL) for 3 or 4 weeks to get the stable miR-146 inhibition bladder cancer cells. The plasmid of the human mmp2 promoter- (from −1,459 to −42) driven luciferase reporter was constructed with Xho I and Hind III, using genomic DNA purified from UMUC3 cells based on the NCBI database (GenBank: NC_000016). The human AUF1 3′ UTR was cloned into the pMIR-reporter luciferase vector through the Xho I and Sac I sites. The AUF1 3′ UTR point mutation was amplified from the wild-type (WT) template by overlap PCR using the following primers: forward, 5′-CCCTCTGAAGTTTAAGGTCGAGTTCTCATTAAAA-3′; reverse, 5′-TTTTAATGAGAACTCGACCTTAAACTTCAGAGGG-3′. The HA-tagged AUF1 constitutively expressed plasmid was obtained from Addgene (Cambridge, MA, USA). The constructs of shRNA specifically targeting AUF1 (shAUF1) were purchased from GeneCopoeia (Rockville, MD, USA). All plasmids were prepared by the Plasmid Preparation/Extraction Maxi Kit from QIAGEN (Valencia, CA, USA). The plasmid of FLAG-ETS2 was kindly gifted by Dr. Wen-chang Lin from the Institute of Biomedical Sciences, Academic Sinica, Nankang, Taipei 115, Taiwan, China.49 The antibodies specific against N-Cadherin, c-Jun, Elk1, ETS1, STAT5, GAPDH, and HuR were bought from Cell Signaling Technology (Beverly, MA, USA). The antibody specific against AUF1 was purchased from Aviva Systems Biology (San Diego, CA, USA). Antibodies against MMP2, MMP9, JunB, ETS2, NCL, and RhoGDIβ were bought from Santa Cruz Biotechnology (Dallas, TX, USA). Act D was bought from Calbiochem (Billerica, MA, USA).
Cell Culture and Transfection
The human bladder cancer cell lines RT4, T24, T24T, and UMUC3 and the normal urinary epithelial cell lines SV-HUC-1 were used in the study. All cancer cell lines were generously provided by Dr. Haishan Huang (Wenzhou Medical University, Zhejiang, China), and they were subjected to DNA tests and authenticated before use for the studies. UMUC3 cells were maintained at 37°C in a 5% CO2 incubator in DMEM (Gibco, Grand Island, NY, USA), supplemented with 10% fetal bovine serum (FBS) (Gibco, Grand Island, NY, USA). T24 and T24T cells were cultured with a 1:1 mixture of DMEM-Ham’s F12 medium (Gibco, Grand Island, NY, USA), supplemented with 5% FBS. RT4 cells were cultured with 1640 (Gibco, Grand Island, NY, USA), supplemented with 10% FBS. SV-HUC-1 cells were cultured with F-12K medium supplemented with 10% FBS. Stable transfections were performed with constructs using PolyJet DNA In Vitro Transfection Reagent (SignaGen Laboratories, Gaithersburg, MD, USA), according to the manufacturer’s instructions, and stable transfectants were selected with puromycin (0.2–0.3 mg/mL) or hygromycin B (200–400 mg/mL) for 3 or 4 weeks according to the different antibiotic resistance plasmids transfected.42, 50
Western Blot Analysis
Bladder cancer cells were seeded in six-well plates and cultured in normal culture medium until 70%–80% confluence. The whole-cell extracts were then prepared, as described in the previous studies.51, 52 Cell extracts were subjected to western blot analysis, as previously described.53 The images were acquired by scanning with the phosphor imager (Typhoon FLA 7000, GE Healthcare).
Real-Time qPCR
First, we extracted the RNA from the cultured cells and tissues using TRIzol reagent (Invitrogen, USA).
Reverse transcriptase was used to produce the first-strand cDNA (Aidlab, China), according to the manufacturer’s instructions. miRNA Real-time PCR Assay kit (Aidlab, China) was used to detect the expression level of miR-146b. Furthermore, U6 was chosen to be the internal control. The primers used in this study were as follows: miR-146b (forward 5′-TGACCCATCCTGGGCCTCAA-3′, reverse 5′-CCAGTGGGCAAGATGTGGGCC-3′), U6 (forward 5′-CTCGCTTCGGCAGCACA-3′, reverse 5′-AACGCTTCACGAATTTGCGT-3′), human MMP2 (forward 5′-CAAGTGGGACAAGAACCAGA-3′, reverse 5′-CCAAAGTTGATCATGTC-3′), human AUF1 (forward 5′-AAATTGAATGGGAAGGTGAT-3′, reverse 5′-GAACCCACGCCTCTTATTG-3′), human ETS2 (forward 5′-AGCGTCACCTACTGCTCTGTCA-3′, reverse 5′-CCGTTGCACATCCAGCAA-3′), and human GAPDH (forward 5′-GATGATCTTGAGGCTGTTGTC-3′, reverse 5′-CAGGGCTGCTTTTAACTCTG-3′).
Luciferase Assay
For the determination of mmp2 promoter-driven luciferase activity and ets2 promoter-driven luciferase activity, the indicated cells were each transiently co-transfected with pRL-TK, together with the related promoter-driven luciferase reporter. At 24 h after transfection, luciferase activity was determined using the Luciferase Assay System Kit (Promega, Madison, WI, USA). For the determination of auf1 mRNA 3′ UTR activity, T24T (nonsense) and T24T (miR-146b inhibitor) cells were transiently transfected with pRL-TK together with auf1 mRNA 3′ UTR luciferase reporter. At 24 h after transfection, luciferase activity was determined using the Luciferase Assay System Kit (Promega, Madison, WI, USA). The results were normalized by internal TK signal. All experiments were done in triplicate and the results expressed as mean ± SE.
RNA-IP Assay
RNA immunoprecipitation was performed as previously described.42 The RNAs in the buffer were extracted by TriZol reagent. Semiquantitative RT-PCR was performed to detect the ETS2 presented in the immune complex.
ChIP Assay
ChIP assay was carried out as described previously by using reagents that were purchased from Millipore Technologies (Billerica, MA, USA).54 Briefly, the indicated genomic DNA and the proteins were cross-linked with 1% formaldehyde (Protocol, 245-684). The cross-linked cells were pelleted, resuspended in lysis buffer, and sonicated to generate 200- 500-bp chromatin DNA fragments. After centrifugation, the supernatant fractions were diluted 10-fold and then incubated with anti-ETS2 antibody (Santa Cruz Biotechnology, sc-351) or the control rabbit immunoglobulin G (IgG) (Santa Cruz Biotechnology, sc-2027) overnight at 4°C, respectively. The immune complex was captured by protein G-agarose (Santa Cruz Biotechnology, C1014) saturated with salmon sperm DNA (Upstate Biotechnology, 0606031838), then eluted with the elution buffer. DNA-protein cross-linking was reversed by heating overnight at 65°C. DNA was purified and subjected to PCR analysis. To specifically amplify the region containing the putative responsive elements on the human mmp2 promoter, PCR was performed with the following pair of primers: 5′-CCTAGGCTGGTCCTTACTG-3′ and 5′-GCCCAGAGATGAAAAACAGC-3′. The PCR products were separated on 2% agarose gels and stained with ethidium bromide; the images were then scanned with a UV light.
Cell Migration and Invasion Assay
Control inserts without matrigel and permeable support for a 24-well plate with 8.0-μm transparent polyethylene terephthalate (PET) membrane were purchased from Corning (Corning, NY, USA), and the invasion kit was purchased from BD Biosciences (Bedford, MA, USA). The invasion assay was performed in normal cell culture serum according to the manufacturer’s instructions. After incubation for 24 h, the cells both on the inside and outside of the chamber were fixed with 3.7% formalin for 2 min, washed twice, then transferred to 100% methanol for 20 min, washed twice again, and then finally the cells were stained by Giemsa (1:20 diluted with PBS) at room temperature for 15 min in the dark. They were again washed twice, then the non-invaded cells were scraped off with a cotton swab (PBS wetted) 4 times. The images were taken under an Olympus DP71 microscope (Olympus America, Center Valley, PA, USA), and the number of the cells in each image was counted by the software Image J. The invasion rate was normalized with the insert control according to the manufacturer’s instructions.
Cell Proliferation Analysis
Cell viability was determined by utilizing the CellTiter-Glo Luminescent Cell Viability Assay Kit (Promega, Madison, WI), according to the manufacturer’s instructions. Briefly, cells were plated in 96-well plates at a density of 5,000 cells/well and allowed to adhere overnight. The cell culture medium was replaced with 0.1% FBS DMEM or 0.1% FBS DMEM-F12 (1:1) and cultured for 12 h; then it was replaced with normal medium and cultured for another 1, 2, 3, or 4 days, and then 50 μL CellTiter-Glo assay reagent was added to each well. The contents were mixed on an orbital shaker for 2 min to induce cell lysis, and then they were incubated at room temperature for 10 min to stabilize the luminescent signal. Results were read on a microplate luminometer LB 96V (Berthold, Bad Wildbad, Germany). Cell viability (%) was defined as the relative absorbance of treated samples versus that of the untreated control. All experiments were performed in 96-well plates for each experiment and repeated at least three times.
Anchorage-Independent Growth Assay
Anchorage-independent growth in soft agar (soft agar assay) was performed as described in our earlier study.42 Briefly, the 1 × 104 cells in 10% FBS Basal Medium Eagle (BME) containing 0.33% soft agar were seeded over the basal layer containing 0.5% agar containing 10% FBS BME in each well of 6-well plates. The plates were incubated in a 5% CO2 incubator at 37°C for 3 weeks. Colonies were captured under a microscope and only colonies with over 32 cells were counted. The results were presented as mean ± SD obtained from three independent experiments.
Clinical Specimens
In all, 30 pairs of bladder cancer tissues and matched adjacent non-tumor tissues from the patients were obtained between 2015 and 2018 from the Beilun People’s Hospital and HwaMei Hospital, University of Chinese Academy of Sciences, Ningbo, Zhejiang, China. The patients’ clinical characteristics are listed in Table S1. The current study was approved by the Ethics Review Board of Ningbo University, Ningbo, China. The tissue samples were snap frozen in liquid nitrogen at the time of surgery, RNA was extracted, and synthesized cDNA was stored at −80°C.
Statistical Analysis
Statistical analysis was performed using Prism 5.0 Software (GraphPad, San Diego, CA, USA). All data are presented as the means of triplicate assays ± SD. Student’s t test was employed to determine the significance of differences between various groups. The differences were considered significant at p < 0.05.
Author Contributions
X.Z. and Y.L. conceived and designed the study. C.X., L.R., and J.Z. detected the cells’ biological function, conducted the RT-PCR assays, carried out the western blot assays and luciferase reporter assays, and performed the statistical analysis. J.W. and Y.L. provided the human bladder cancer tissues specimens. J.Z. and Y.L. drafted the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
We thank Dr. Wen-chang Lin from the Institute of Biomedical Sciences, Academic Sinica, Nankang, Taipei 115, Taiwan, China, for the generous gifts of the plasmid of FLAG-ETS2. This work was supported by the Ningbo Social Development Major Project (2017C510009) and the foundation from the Health and Family Planning Commission of Zhejiang province (2018KY747).
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtn.2019.04.007.
Contributor Information
Junlan Zhu, Email: junlanzhu@163.com.
Yang Li, Email: li_yangvip@126.com.
Xingguo Zhang, Email: xgzhang666@163.com.
Supplemental Information
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J. Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Chen W., Zheng R., Baade P.D., Zhang S., Zeng H., Bray F., Jemal A., Yu X.Q., He J. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Witjes J.A., Compérat E., Cowan N.C., De Santis M., Gakis G., Lebret T., Ribal M.J., Van der Heijden A.G., Sherif A., European Association of Urology EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur. Urol. 2014;65:778–792. doi: 10.1016/j.eururo.2013.11.046. [DOI] [PubMed] [Google Scholar]
- 4.Lewis B.P., Burge C.B., Bartel D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 5.Xu M., Li J., Wang X., Meng S., Shen J., Wang S., Xu X., Xie B., Liu B., Xie L. MiR-22 suppresses epithelial-mesenchymal transition in bladder cancer by inhibiting Snail and MAPK1/Slug/vimentin feedback loop. Cell Death Dis. 2018;9:209. doi: 10.1038/s41419-017-0206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guancial E.A., Bellmunt J., Yeh S., Rosenberg J.E., Berman D.M. The evolving understanding of microRNA in bladder cancer. Urol. Oncol. 2014;32 doi: 10.1016/j.urolonc.2013.04.014. 41.e31-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu S.D., Chu C.H., Tsou A.P., Chen S.J., Chen H.C., Hsu P.W., Wong Y.H., Chen Y.H., Chen G.H., Huang H.D. miRNAMap 2.0: genomic maps of microRNAs in metazoan genomes. Nucleic Acids Res. 2008;36:D165–D169. doi: 10.1093/nar/gkm1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hurst D.R., Edmonds M.D., Scott G.K., Benz C.C., Vaidya K.S., Welch D.R. Breast cancer metastasis suppressor 1 up-regulates miR-146, which suppresses breast cancer metastasis. Cancer Res. 2009;69:1279–1283. doi: 10.1158/0008-5472.CAN-08-3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu Y., Wu G., Yan W., Zhan H., Sun P. miR-146b-5p regulates cell growth, invasion, and metabolism by targeting PDHB in colorectal cancer. Am. J. Cancer Res. 2017;7:1136–1150. [PMC free article] [PubMed] [Google Scholar]
- 10.Gao S., Zhao Z., Wu R., Wu L., Tian X., Zhang Z. MiR-146b inhibits autophagy in prostate cancer by targeting the PTEN/Akt/mTOR signaling pathway. Aging (Albany N.Y.) 2018;10:2113–2121. doi: 10.18632/aging.101534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White E.J., Brewer G., Wilson G.M. Post-transcriptional control of gene expression by AUF1: mechanisms, physiological targets, and regulation. Biochim. Biophys. Acta. 2013;1829:680–688. doi: 10.1016/j.bbagrm.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Khalaf H.H., Aboussekhra A. MicroRNA-141 and microRNA-146b-5p inhibit the prometastatic mesenchymal characteristics through the RNA-binding protein AUF1 targeting the transcription factor ZEB1 and the protein kinase AKT. J. Biol. Chem. 2014;289:31433–31447. doi: 10.1074/jbc.M114.593004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lal A., Mazan-Mamczarz K., Kawai T., Yang X., Martindale J.L., Gorospe M. Concurrent versus individual binding of HuR and AUF1 to common labile target mRNAs. EMBO J. 2004;23:3092–3102. doi: 10.1038/sj.emboj.7600305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zucconi B.E., Wilson G.M. Modulation of neoplastic gene regulatory pathways by the RNA-binding factor AUF1. Front. Biosci. 2011;16:2307–2325. doi: 10.2741/3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdelmohsen K., Tominaga-Yamanaka K., Srikantan S., Yoon J.H., Kang M.J., Gorospe M. RNA-binding protein AUF1 represses Dicer expression. Nucleic Acids Res. 2012;40:11531–11544. doi: 10.1093/nar/gks930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mook O.R., Frederiks W.M., Van Noorden C.J. The role of gelatinases in colorectal cancer progression and metastasis. Biochim. Biophys. Acta. 2004;1705:69–89. doi: 10.1016/j.bbcan.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Jin H., Yu Y., Hu Y., Lu C., Li J., Gu J., Zhang L., Huang H., Zhang D., Wu X.R. Divergent behaviors and underlying mechanisms of cell migration and invasion in non-metastatic T24 and its metastatic derivative T24T bladder cancer cell lines. Oncotarget. 2015;6:522–536. doi: 10.18632/oncotarget.2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang G., Wu A.D., Huang C., Gu J., Zhang L., Huang H., Liao X., Li J., Zhang D., Zeng X. Isorhapontigenin (ISO) Inhibits Invasive Bladder Cancer Formation In Vivo and Human Bladder Cancer Invasion In Vitro by Targeting STAT1/FOXO1 Axis. Cancer Prev. Res. (Phila.) 2016;9:567–580. doi: 10.1158/1940-6207.CAPR-15-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gratacós F.M., Brewer G. The role of AUF1 in regulated mRNA decay. Wiley Interdiscip. Rev. RNA. 2010;1:457–473. doi: 10.1002/wrna.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brennan C.M., Steitz J.A. HuR and mRNA stability. Cell. Mol. Life Sci. 2001;58:266–277. doi: 10.1007/PL00000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdelmohsen K., Gorospe M. RNA-binding protein nucleolin in disease. RNA Biol. 2012;9:799–808. doi: 10.4161/rna.19718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu T., Chong Y., Lu S., Wang R., Qin H., Silva J., Kitamura E., Chang C.S., Hawthorn L., Cowell J.K. miR-339 Promotes Development of Stem Cell Leukemia/Lymphoma Syndrome via Downregulation of the BCL2L11 and BAX Proapoptotic Genes. Cancer Res. 2018;78:3522–3531. doi: 10.1158/0008-5472.CAN-17-4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis B.P., Shih I.H., Jones-Rhoades M.W., Bartel D.P., Burge C.B. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 24.Chou R., Selph S.S., Buckley D.I., Gustafson K.S., Griffin J.C., Grusing S.E., Gore J.L. Treatment of muscle-invasive bladder cancer: A systematic review. Cancer. 2016;122:842–851. doi: 10.1002/cncr.29843. [DOI] [PubMed] [Google Scholar]
- 25.Chi Y., Zhou D. MicroRNAs in colorectal carcinoma–from pathogenesis to therapy. J. Exp. Clin. Cancer Res. 2016;35:43. doi: 10.1186/s13046-016-0320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoon S.O., Kim E.K., Lee M., Jung W.Y., Lee H., Kang Y., Jang Y.J., Hong S.W., Choi S.H., Yang W.I. NOVA1 inhibition by miR-146b-5p in the remnant tissue microenvironment defines occult residual disease after gastric cancer removal. Oncotarget. 2016;7:2475–2495. doi: 10.18632/oncotarget.6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deng X., Wu B., Xiao K., Kang J., Xie J., Zhang X., Fan Y. MiR-146b-5p promotes metastasis and induces epithelial-mesenchymal transition in thyroid cancer by targeting ZNRF3. Cell. Physiol. Biochem. 2015;35:71–82. doi: 10.1159/000369676. [DOI] [PubMed] [Google Scholar]
- 28.Xu E., Zhao J., Ma J., Wang C., Zhang C., Jiang H., Cheng J., Gao R., Zhou X. miR-146b-5p promotes invasion and metastasis contributing to chemoresistance in osteosarcoma by targeting zinc and ring finger 3. Oncol. Rep. 2016;35:275–283. doi: 10.3892/or.2015.4393. [DOI] [PubMed] [Google Scholar]
- 29.Liu J., Xu J., Li H., Sun C., Yu L., Li Y., Shi C., Zhou X., Bian X., Ping Y. miR-146b-5p functions as a tumor suppressor by targeting TRAF6 and predicts the prognosis of human gliomas. Oncotarget. 2015;6:29129–29142. doi: 10.18632/oncotarget.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geraldo M.V., Fuziwara C.S., Friguglieti C.U., Costa R.B., Kulcsar M.A., Yamashita A.S., Kimura E.T. MicroRNAs miR-146-5p and let-7f as prognostic tools for aggressive papillary thyroid carcinoma: a case report. Arq. Bras. Endocrinol. Metabol. 2012;56:552–557. doi: 10.1590/s0004-27302012000800015. [DOI] [PubMed] [Google Scholar]
- 31.Zhang H.M., Li Q., Zhu X., Liu W., Hu H., Liu T., Cheng F., You Y., Zhong Z., Zou P. miR-146b-5p within BCR-ABL1-Positive Microvesicles Promotes Leukemic Transformation of Hematopoietic Cells. Cancer Res. 2016;76:2901–2911. doi: 10.1158/0008-5472.CAN-15-2120. [DOI] [PubMed] [Google Scholar]
- 32.Xia H., Qi Y., Ng S.S., Chen X., Li D., Chen S., Ge R., Jiang S., Li G., Chen Y. microRNA-146b inhibits glioma cell migration and invasion by targeting MMPs. Brain Res. 2009;1269:158–165. doi: 10.1016/j.brainres.2009.02.037. [DOI] [PubMed] [Google Scholar]
- 33.Winer A., Adams S., Mignatti P. Matrix Metalloproteinase Inhibitors in Cancer Therapy: Turning Past Failures Into Future Successes. Mol. Cancer Ther. 2018;17:1147–1155. doi: 10.1158/1535-7163.MCT-17-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stamenkovic I. Matrix metalloproteinases in tumor invasion and metastasis. Semin. Cancer Biol. 2000;10:415–433. doi: 10.1006/scbi.2000.0379. [DOI] [PubMed] [Google Scholar]
- 35.Tao L., Li Z., Lin L., Lei Y., Hongyuan Y., Hongwei J., Yang L., Chuize K. MMP1, 2, 3, 7, and 9 gene polymorphisms and urinary cancer risk: a meta-analysis. Genet. Test. Mol. Biomarkers. 2015;19:548–555. doi: 10.1089/gtmb.2015.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng M., Wang J., Zhang D., Jin H., Li J., Wu X.R., Huang C. PHLPP2 stabilization by p27 mediates its inhibition of bladder cancer invasion by promoting autophagic degradation of MMP2 protein. Oncogene. 2018;37:5735–5748. doi: 10.1038/s41388-018-0374-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oikawa T., Yamada T. Molecular biology of the Ets family of transcription factors. Gene. 2003;303:11–34. doi: 10.1016/s0378-1119(02)01156-3. [DOI] [PubMed] [Google Scholar]
- 38.Yordy J.S., Muise-Helmericks R.C. Signal transduction and the Ets family of transcription factors. Oncogene. 2000;19:6503–6513. doi: 10.1038/sj.onc.1204036. [DOI] [PubMed] [Google Scholar]
- 39.Fry E.A., Inoue K. Aberrant expression of ETS1 and ETS2 proteins in cancer. Cancer Rep. Rev. 2018 doi: 10.15761/CRR.1000151. Published online April 23, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh S., Barrett J., Sakata K., Tozer R.G., Singh G. ETS proteins and MMPs: partners in invasion and metastasis. Curr. Drug Targets. 2002;3:359–367. doi: 10.2174/1389450023347489. [DOI] [PubMed] [Google Scholar]
- 41.Trojanowska M. Ets factors and regulation of the extracellular matrix. Oncogene. 2000;19:6464–6471. doi: 10.1038/sj.onc.1204043. [DOI] [PubMed] [Google Scholar]
- 42.Zhu J., Li Y., Tian Z., Hua X., Gu J., Li J., Liu C., Jin H., Wang Y., Jiang G. ATG7 Overexpression Is Crucial for Tumorigenic Growth of Bladder Cancer In Vitro and In Vivo by Targeting the ETS2/miRNA196b/FOXO1/p27 Axis. Mol. Ther. Nucleic Acids. 2017;7:299–313. doi: 10.1016/j.omtn.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raineri I., Wegmueller D., Gross B., Certa U., Moroni C. Roles of AUF1 isoforms, HuR and BRF1 in ARE-dependent mRNA turnover studied by RNA interference. Nucleic Acids Res. 2004;32:1279–1288. doi: 10.1093/nar/gkh282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lapucci A., Donnini M., Papucci L., Witort E., Tempestini A., Bevilacqua A., Nicolin A., Brewer G., Schiavone N., Capaccioli S. AUF1 Is a bcl-2 A + U-rich element-binding protein involved in bcl-2 mRNA destabilization during apoptosis. J. Biol. Chem. 2002;277:16139–16146. doi: 10.1074/jbc.M201377200. [DOI] [PubMed] [Google Scholar]
- 45.Lin S., Wang W., Wilson G.M., Yang X., Brewer G., Holbrook N.J., Gorospe M. Down-regulation of cyclin D1 expression by prostaglandin A(2) is mediated by enhanced cyclin D1 mRNA turnover. Mol. Cell. Biol. 2000;20:7903–7913. doi: 10.1128/mcb.20.21.7903-7913.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marx J. Cancer research. Inflammation and cancer: the link grows stronger. Science. 2004;306:966–968. doi: 10.1126/science.306.5698.966. [DOI] [PubMed] [Google Scholar]
- 47.Zhu J., Tian Z., Li Y., Hua X., Zhang D., Li J., Jin H., Xu J., Chen W., Niu B. ATG7 Promotes Bladder Cancer Invasion via Autophagy-Mediated Increased ARHGDIB mRNA Stability. Adv. Sci. 2019;6:1801927. doi: 10.1002/advs.201801927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hicks R.M. Effect of promoters on incidence of bladder cancer in experimental animal models. Environ. Health Perspect. 1983;50:37–49. doi: 10.1289/ehp.835037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liao Y.L., Hu L.Y., Tsai K.W., Wu C.W., Chan W.C., Li S.C., Lai C.H., Ho M.R., Fang W.L., Huang K.H., Lin W.C. Transcriptional regulation of miR-196b by ETS2 in gastric cancer cells. Carcinogenesis. 2012;33:760–769. doi: 10.1093/carcin/bgs023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu J., Li Y., Chen C., Ma J., Sun W., Tian Z., Li J., Xu J., Liu C.S., Zhang D. NF-κB p65 Overexpression Promotes Bladder Cancer Cell Migration via FBW7-Mediated Degradation of RhoGDIα Protein. Neoplasia. 2017;19:672–683. doi: 10.1016/j.neo.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu J., Zhang J., Huang H., Li J., Yu Y., Jin H., Li Y., Deng X., Gao J., Zhao Q., Huang C. Crucial role of c-Jun phosphorylation at Ser63/73 mediated by PHLPP protein degradation in the cheliensisin a inhibition of cell transformation. Cancer Prev. Res. (Phila.) 2014;7:1270–1281. doi: 10.1158/1940-6207.CAPR-14-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu J., Huang G., Hua X., Li Y., Yan H., Che X., Tian Z., Liufu H., Huang C., Li J. CD44s is a crucial ATG7 downstream regulator for stem-like property, invasion, and lung metastasis of human bladder cancer (BC) cells. Oncogene. 2019 doi: 10.1038/s41388-018-0664-7. Published online January 11, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu J., Li Y., Luo Y., Xu J., Liufu H., Tian Z., Huang C., Li J., Huang C. A Feedback Loop Formed by ATG7/Autophagy, FOXO3a/miR-145 and PD-L1 Regulates Stem-Like Properties and Invasion in Human Bladder Cancer. Cancers (Basel) 2019;11:E349. doi: 10.3390/cancers11030349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang H., Zhu J., Li Y., Zhang L., Gu J., Xie Q., Jin H., Che X., Li J., Huang C. Upregulation of SQSTM1/p62 contributes to nickel-induced malignant transformation of human bronchial epithelial cells. Autophagy. 2016;12:1687–1703. doi: 10.1080/15548627.2016.1196313. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.