Abstract
Objectives:
To investigate the cord blood levels of adipokine and to assess their association with the fetal insulin resistance and fetal outcomes in newborns of gestational diabetic women (GDM).
Methods:
This cross-sectional study was performed in 40 GDM women and 40 healthy pregnant women (HPW) in the Department of Obstetrics and Gynecology at Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER) hospital in Puducherry, India, during the period from May 2016 to December 2017. Cord blood samples were collected at delivery from GDM and HPW groups. Cord plasma biochemical parameters such as insulin, C-peptide, adiponectin, leptin, resistin, and visfatin concentrations were measured. Leptin/adiponectin ratio (L/A), homeostasis model assessment of insulin resistance (HOMA2-IR), insulin sensitivity (HOMA2-%S) and beta cell function (HOMA2-%B) were calculated. The pregnancy outcomes such as birth weight (BW), Ponderal index and Apgar scores of the baby were measured.
Results:
The BW and Ponderal index of the baby were found to be significantly higher in GDM newborns compared to HPW newborns. Cord plasma insulin, C-peptide, HOMA2 –IR, visfatin, leptin, and L/A ratio were significantly higher whereas adiponectin level was lower in GDM compared to HPW. A significant positive correlation was observed between L/A ratio and fetal HOMA2-IR.
Conclusion:
Altered adipokine levels with increased L/A ratio was observed among the new-borns of Indian gestational diabetic mothers. There was an association between increased L/A ratio, insulin resistance and increased Ponderal index among the new-borns.
Gestational diabetes mellitus (GDM) is characterized by any degree of maternal glucose intolerance developed or first detected during pregnancy.1,2 The prevalence of GDM among Indian pregnant women is around 17% in comparison with other populations which is around 4%.3-5 Gestational diabetes mellitus is associated with adverse maternal and fetal complications such as shoulder dystocia, respiratory distress, neonatal hypoglycemia, hyperbilirubinemia, and macrosomia.6 Furthermore, GDM mothers and their offspring are at an increased risk of developing type 2 diabetes mellitus (T2DM), metabolic syndrome (MetS) and cardiovascular complications later in their life.7-9 Children of Indian GDM mothers are associated with obesity, insulin resistance and cardiovascular complications.10 The prevalence of T2DM has significantly increased in India showing as high as 13%.11 This indicates that the increased cases of GDM may account for the higher prevalence of T2DM in India. However, the underlying mechanisms of transmission of metabolic diseases to offspring in GDM are unknown. The intrauterine environment of GDM women can have adverse effects on their newborns. Maternal hyperglycemia translates into fetal hyperglycemia and hyperinsulinemia which lead to excess fat deposition in adipose tissue of the fetus resulting in adiposity.12 However, maternal hyperglycemia causes excessive oxidative stress in growing fetus resulting in congenital anomalies and fetal priming for future metabolic diseases in GDM newborns.13 A recent study in Chinese GDM newborns has shown higher fetal insulin resistance and is correlated with maternal insulin resistance.14 However, so far no studies were carried out in Indian GDM women regarding the level of fetal insulin resistance in their newborns. Adipokines are secreted by adipose tissue with paracrine and endocrine properties. Adipokines such as leptin, adiponectin, resistin, and visfatin are involved in body weight regulation, insulin resistance, lipid metabolism, and adiposity. Leptin and adiponectin are involved in the pathogenesis of metabolic diseases such as obesity, T2DM, and GDM. Studies have shown that increased maternal leptin and decreased adiponectin levels in GDM women are involved in the development of insulin resistance.15,16 Recent studies have shown that there is altered maternal visfatin and resistin levels in GDM women but their role in the pathogenesis of GDM remains unclear.17,18 However, the role of adipokines in the development of insulin resistance in newborns of GDM mothers is not yet fully explored. There are only limited studies reported on the levels of these adipokines in the newborns of GDM women with conflicting results.19 Furthermore, there are no studies carried out among the newborns of GDM women from India on the relationship between adipokine levels and the pathogenesis of GDM. That is quite significant considering India as the diabetic capital of the world. No studies have been carried out so far on the levels of adipokine in the cord blood of Indian GDM newborns.
Thus, we hypothesized that there is an altered adipokine level in newborns of GDM women. Further, this could be involved in the development of fetal insulin resistance and fetal adiposity. The present study aimed to investigate the cord blood levels of adipokine and to assess their relationship with insulin resistance, beta cell function, and fetal outcomes in newborns of Indian pregnant women with GDM.
Methods
This was a cross-sectional study carried out in the Department of Biochemistry and Obstetrics & Gynecology, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER) Hospital, Puducherry, India after obtaining approval from JIPMER Scientific Advisory Committee (Approval no: JSAC 32/6/2016) and the Institute Ethics Committee (Approval no: JIP/IEC/2016/25/825). The study was conducted between May 2016 and Dec 2017 on a South Indian Tamil population recruited from the Department of Obstetrics and Gynecology according to the ethical guidelines of Indian Council of Medical Research for Biomedical research on human participants.
A total of 80 primi pregnant women were recruited for this cross-sectional study. All consecutive GDM patients on insulin therapy were recruited for this study. Women with GDM were diagnosed based on the International Association of Diabetes and Pregnancy Study Groups criteria.20 Primi gravidae pregnant women with GDM on insulin therapy (n=40) and healthy pregnant women (HPW) without any complications (n=40) in the age group of 18-30 years were included in the present study. We calculated the sample size using the Open Epi program 9 (Open Source Epidemiology statistics for Public Health, version 3.03). The calculation was based on a confidence interval of 95%, power as 90 and ratio of sample size as 1. Women with gestational diabetes mellitus on medical nutrition therapy, hypothyroidism, pre-existing glucose intolerance, pregnancy-induced hypertension, polycystic ovarian syndrome, major vascular complications, known infections in current pregnancy and autoimmune disorders were excluded from the study.
Data and blood sample collection
Five milliliters of umbilical cord blood was collected immediately after delivery from all study subjects. Plasma was separated by centrifugation at 3500 rpm for 10 min and stored immediately at -40°C until further analysis. Maternal characteristics such as age, pre-pregnancy body mass index (BMI) and maternal weight gain were recorded. Fetal outcomes such as gestational age at the time of delivery, birth weight, and length were documented. Apgar scores were also noted soon after delivery at one minute and at 5 minutes. Newborns appearance, pulse, grimace response, activity (muscle tone) and respiratory rate have been considered for Apgar score and was recorded on a scale of 0 to 2. Ponderal Index (PI) was calculated by the formula: PI= birth weight (g)/birth length (3 cm) x 100.
Measurement of biochemical parameters
Biochemical parameters such as cord plasma glucose and lipid profile were estimated using enzymatic kits with Olympus AU400 fully automated Clinical Chemistry Analyser (Beckman Coulter, USA). Cord plasma insulin (Diasource Immunoassays S.A, Belgium, with sensitivity 0.17 µIU/ml, intra-assay CV 4.8-6.0% and inter-assay CV 8.1-9.0%) C-peptide (Sigma diagnostics, Livonia, USA, with assay sensitivity 0.2 ng/ml), leptin (DBC Inc, Canada, with sensitivity 0.50 ng/ml, intra-assay CV 3.7 - 5% and inter-assay CV 5.8%), adiponectin (Assaypro, St. Charles, MO, with sensitivity 0.3 ng/ml, intra-assay CV 4.4% and inter-assay CV 9.9%), resistin (Raybiotech, Suite 100 Norcross, GA, with sensitivity 2pg/ml, intra-assay CV <10% and inter-assay CV <12%) and visfatin (Raybiotech, Suite 100 Norcross, GA, with sensitivity 0.778 ng/ml, intra-assay CV <10% and inter-assay CV <15%) were measured by above mentioned ELISA kits.
Assessment of insulin resistance, insulin sensitivity, and beta cell function
A computer-based programme called HOMA calculator version 2.2.2 (University of Oxford) was used to calculate the homeostasis model assessment of insulin resistance (HOMA2-IR), insulin sensitivity (HOMA2-%S) and beta cell function (HOMA2-%B) from the values of cord plasma glucose and insulin levels.21,22
Statistical analysis
The data were analyzed using the Statistical Package of Social Service (Version 19, SPSS Inc., Chicago, IL, USA). Descriptive data were shown as mean ± standard deviation (SD). Kolmogorov-Smirnov test was used to see the normal distribution of parameters studied. Comparison between the means of 2 groups was analyzed by unpaired Student’s t-test. The differences in percentages of categorical variables were carried out by using a Chi-square test. Pearson’s correlation was applied to assess the correlation between anthropometric and biochemical parameters. A p-value less than 0.05 was considered as statistically significant.
Results
Maternal and neonatal characteristics
Table 1 shows maternal and neonatal characteristics of all pregnant women involved in this study. There was no significant difference found in the mean age, pre-pregnancy BMI, Apgar scores between the groups. However, birth weight (2.87 ± 0.44 versus 3.18 ± 0.46) and PI of the babies (2.71 ± 0.37 versus 3.04 ± 0.47) were significantly higher (p<0.05) among GDM newborns in comparison with HPW newborns.
Table 1.
Maternal and neonatal characteristics of the study groups.
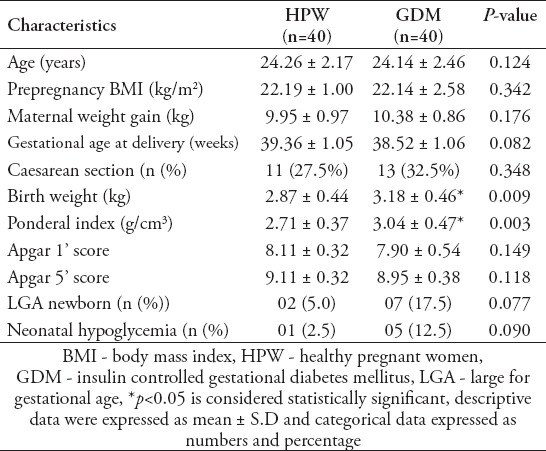
Fetal insulin resistance, insulin sensitivity and beta cell function of HPW and GDM newborns
Table 2 presents parameters of fetal HOMA2-IR, HOMA2-%S and HOMA2-%B of HPW and GDM newborns. No significant differences were noted for cord plasma glucose levels among HPW and GDM newborns. Cord plasma insulin, C-peptide, and HOMA2-IR levels were significantly higher (p<0.05) whereas HOMA-%S level was significantly lower (p<0.05) in GDM newborns when compared with HPW newborns.
Table 2.
Fetal insulin resistance, insulin sensitivity and beta cell function of healthy pregnant and GDM newborns.
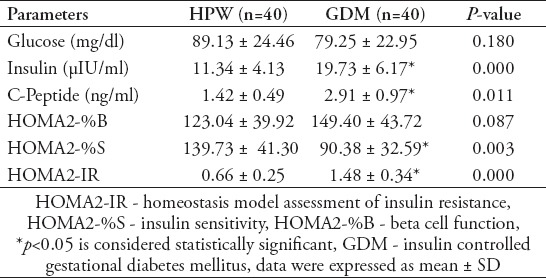
Cord plasma lipid profile and adipokine levels of HPW and GDM newborns
Table 3 shows the cord plasma lipid profile and adipokine levels of healthy pregnant and GDM newborns. No significant changes were noted for cord plasma total cholesterol, LDL cholesterol, HDL cholesterol and resistin levels among HPW and GDM newborns. Cord plasma TG, VLDL cholesterol and adiponectin levels were significantly lower (p<0.05) whereas cord serum leptin, L/A ratio and visfatin levels were significantly higher (p<0.05) in GDM newborns when compared with HPW newborns.
Table 3.
Cord plasma lipid profile and adipokine levels of healthy pregnant and GDM newborns.
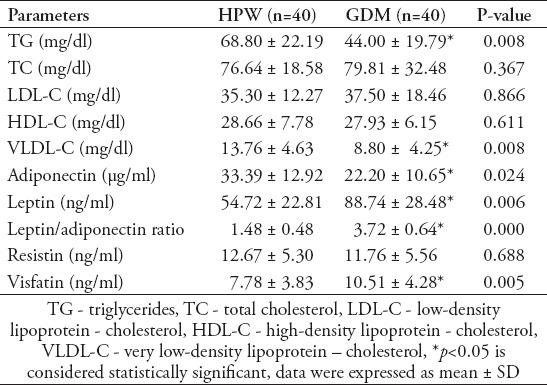
Correlations between fetal insulin resistance and various metabolic parameters in newborns of GDM women
In the GDM newborns, PI of babies was positively correlated to cord blood insulin, C-peptide, HOMA-IR, leptin and L/A ratio and negatively correlated to HOMA-%S (Table 4). In the GDM newborns L/A ratio was positively correlated with HOMA- IR levels (r=0.438, p<0.05) (Figure 1) and negatively correlated with HOMA-%S (r= -0.438, p<0.05). No correlations were found between visfatin and fetal insulin resistance. However, there was a negative correlation between visfatin levels with total cholesterol (r= -0.335), LDL cholesterol (r= -0.414), and TG levels (r= -0.465), (p<0.05) in GDM newborns.
Table 4.
Correlations between the Ponderal index and fetal metabolic parameters in newborns of GDM group.
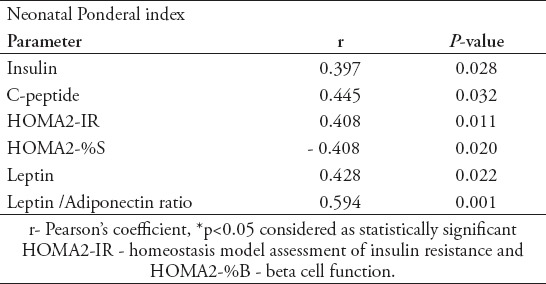
Figure 1.
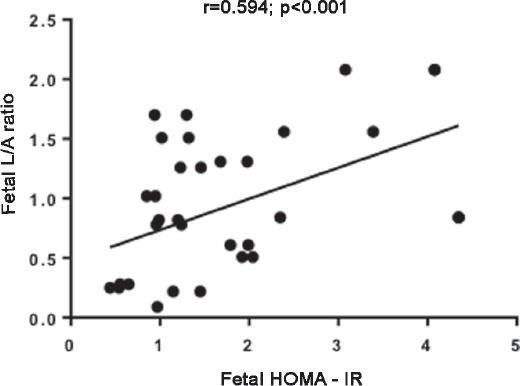
Correlation of fetal HOMA - IR with fetal L/A ratio. L/A ratio - leptin / adiponectin ratio, Pearson correlation test, p<0.001.
Discussion
The most significant findings of this study were higher fetal insulin resistance and lower insulin sensitivity among the newborns of Indian GDM pregnant women when compared to HPW newborns. Further, increased cord plasma leptin, L/A ratio, visfatin levels and PI were observed in GDM newborns. A decreased cord plasma adiponectin was observed in GDM newborns and the L/A ratio positively correlated with fetal insulin resistance. These observations suggest that newborns of Indian GDM mother with higher insulin resistance and altered adipokine levels are associated with fetal adiposity.
The rapid increase in the prevalence of T2DM and metabolic syndrome in the general population has become a worldwide problem in all countries. India is the diabetic capital of the world with 72 million diabetic patients and this is expected to double by 2025. The prevalence of T2DM among Indians is 13% and GDM is 17%.3,11 Besides the lifestyle, GDM has an impact on the development of T2DM and obesity. Children of Indian GDM mothers are associated with adiposity, insulin resistance and cardiovascular complications.10 It is clear that the offspring of GDM women are more prone to develop T2DM and obesity later in their adult life. However, the underlying mechanism of fetal priming of metabolic diseases in GDM newborns is unknown. Recent studies reveal that adverse intrauterine milieu such as hyperglycemia and heightened oxidative stress can affect the fetal growth and are involved in fetal programming for future disease.23
Adipokines such as leptin, adiponectin, resistin, and visfatin are secreted from maternal adipose tissue and placenta. They are involved in the regulation of body weight, insulin resistance, lipid metabolism, adipogenesis, and inflammation. Several studies have shown the altered levels of adipokine and its association with insulin resistance in pregnant women with GDM.24-26 Adiponectin is an anti-inflammatory adipokine involved in glucose and lipid metabolism and enhances insulin sensitivity through increased fatty acid oxidation and inhibition of hepatic glucose production. It has been shown that adiponectin improves insulin resistance and reduces fasting glucose level through activating AMP-kinase and PPAR-a.27 A decreased level of adiponectin was reported in GDM women28,29 and is an independent negative predictor of gestational diabetes mellitus.30 Leptin is a pro-inflammatory adipokine involved in body weight regulation and glucose metabolism by regulating food intake. Moreover, leptin suppresses the secretion of insulin from pancreatic b cells.31 Studies reported that homozygous mutations in the leptin or leptin receptor (OB-R) genes creates obese, insulin resistant and diabetic mice.32,33 Increase34 or unaltered leptin levels26 were reported in GDM women. Leptin levels are positively associated with increased body weight, insulin resistance, and inflammation. Leptin/adiponectin ratio (L/A ratio) is a potential parameter used to evaluate insulin resistance in patients with metabolic syndrome, T2DM and in GDM.35,36 Although leptin or adiponectin levels are independently associated with risk for the development of MetS and T2DM, the risk association is stronger with L/A ratio.37,38 Maternal visfatin and resistin levels were reported in GDM with conflicting results.24,25 Visfatin has insulin-mimetic effects by binding to the insulin receptor. It is associated with markers of obesity, insulin resistance, and metabolic syndrome.39 The role of resistin in the pathogenesis of GDM is unknown. This suggests that adipokines play a major role in the pathogenesis of GDM by modulating the insulin secretion, insulin sensitivity, and insulin resistance. However, only limited studies reported the cord blood levels of adipokine in newborns of GDM women and its association with the fetal insulin resistance, beta cell function, and fetal adiposity. The present study aimed to investigate the cord blood levels of adipokine and to assess their relationship with insulin resistance, beta cell function and fetal outcomes in newborns of Indian pregnant women with GDM.
In this study, we found significantly higher levels of cord plasma insulin, C-peptide, and HOMA2-IR and lower levels of HOMA2-%S in GDM newborns. These findings suggest that newborns of Indian GDM mother and more prone to develop insulin resistance. These observations are in agreement with previous reports from other populations.14,40,41 There was no impairment in the beta cell function among the newborns of GDM mothers as supported by other studies.14,41 These results reveal that the newborns of GDM mothers are at increased of insulin resistance than beta cell dysfunction.
In our study, we found an increased cord plasma leptin and decreased adiponectin levels in newborns of GDM when compared to HPW newborns. Similar results have been reported with newborns of GDM mothers with higher leptin concentrations42,43 and decreased adiponectin levels.44 Cord plasma leptin levels were reported to be positively correlated with fetal adiposity.45 In our study, we found a positive correlation of leptin levels with a PI of the babies. We also found an increased L/A ratio in the GDM newborns and it had a strong positive correlation with fetal HOMA2-IR. These observations suggest that altered levels of leptin and adiponectin affect the fetal insulin resistance which favors fetal adiposity in newborns of GDM mothers.
The levels of fetal resistin were reported to be either not altered46 or elevated47 in GDM newborns as compared to HPW newborns. We found no differences in fetal resistin levels between GDM and HPW groups. Further studies are needed to explain the role of resistin in fetal metabolism. Decreased cord plasma visfatin levels have been reported in GDM.47 However, we found that visfatin was significantly increased in GDM newborns when compared to HPW newborns. This finding is supported by the fact that the circulating visfatin concentrations have been shown to increase in parallel with glucose administration.48 No correlations were found between visfatin and insulin resistance. However, it showed a negative correlation with total cholesterol, LDL-cholesterol and TG levels among the GDM newborns. In the GDM group, we found a decreased TG and VLDL-cholesterol levels in the cord blood. Similar results were reported in other studies.49,50 Studies have shown that cord blood TG levels were negatively correlated with birth weight, resulting in significantly higher TG levels in SGA newborns compared with AGA or LGA infants of GDM pregnancy.51 This shows that visfatin plays a role in fetal lipid metabolism and adiposity.
Study limitations
We recruited only primi pregnant women to avoid the impact of metabolic changes caused by previous pregnancies. We excluded GDM mothers with co-morbidities such as hypothyroidism and hypertension, and so forth which may influence our results. The main limitation of the present study is the smaller sample size. Further GDM mothers were on insulin therapy would have influenced our results.
In conclusion, altered adipokine levels with increased L/A ratio was observed among the newborns of Indian gestational diabetic mothers. There was an association between increased L/A ratio, insulin resistance, and increased PI among the newborns.
We recommend future studies with a larger sample size to clarify the exact role of these adipokines in the development of fetal insulin resistance and fetal adiposity. Further studies are needed to use these adipokines as a predictor of future development of T2DM and other metabolic diseases in GDM offsprings.
Acknowledgment
We are grateful to Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry, India for providing research grant for Ph.D. scholars (Grant sanction order No. JIP/Res/ Intra-PhD/Phs1/01/2016-17, dated 09 Sep 2016). Also we thank the Department of Biotechnology (DBT), India for providing financial support for the conduct of this study.
Footnotes
References
- 1.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26:S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Gestational diabetes mellitus. Diabetes Care. 2004;27:S88–S90. doi: 10.2337/diacare.27.2007.s88. [DOI] [PubMed] [Google Scholar]
- 3.Seshiah V, Balaji V, Balaji MS, Sanjeevi CB, Green A. Gestational diabetes mellitus in India. J Assoc Physicians India. 2004;52:707–711. [PubMed] [Google Scholar]
- 4.Yue DK, Molyneaux LM, Ross GP, Constantino MI, Child AG, Turtle JR. Why does ethnicity affect prevalence of gestational diabetes?The underwater volcano theory. Diabet Med. 1996;13:748–752. doi: 10.1002/(SICI)1096-9136(199608)13:8<748::AID-DIA164>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 5.Beischer NA, Wein P, Sheedy MT, Steffen B. Identification and treatment of women with hyperglycaemia diagnosed during pregnancy can significantly reduce perinatal mortality rates. Aust N Z J Obstet Gynaecol. 1996;36:239–247. doi: 10.1111/j.1479-828x.1996.tb02703.x. [DOI] [PubMed] [Google Scholar]
- 6.Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, et al. HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 7.Metzger BE. Long-term outcomes in mothers diagnosed with gestational diabetes mellitus and their offspring. Clin Obstet Gynecol. 2007;50:972–979. doi: 10.1097/GRF.0b013e31815a61d6. [DOI] [PubMed] [Google Scholar]
- 8.Catalano PM, Kirwan JP, Haugel-de Mouzon S, King J. Gestational diabetes and insulin resistance:role in short- and long-term implications for mother and fetus. J Nutr. 2003;133:S1674–S1683. doi: 10.1093/jn/133.5.1674S. [DOI] [PubMed] [Google Scholar]
- 9.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes:a systematic review and meta-analysis. Lancet. 2009;373:1773–1779. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 10.Krishnaveni GV, Veena SR, Hill JC, Kehoe S, Karat SC, Fall CH. Intrauterine exposure to maternal diabetes is associated with higher adiposity and insulin resistance and clustering of cardiovascular risk markers in Indian children. Diabetes Care. 2010;33:402–404. doi: 10.2337/dc09-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anjana RM, Deepa M, Pradeepa R, Mahanta J, Narain K, Das HK, et al. Prevalence of diabetes and prediabetes in 15 states of India:results from the ICMR-INDIAB population-based cross-sectional study. Lancet Diabetes Endocrinol. 2017;5:585–596. doi: 10.1016/S2213-8587(17)30174-2. [DOI] [PubMed] [Google Scholar]
- 12.Pedersen J. Weight and length at birth of infants of diabetic mothers. Acta Endocrinol (Copenh) 1954;16:330–342. doi: 10.1530/acta.0.0160330. [DOI] [PubMed] [Google Scholar]
- 13.Eriksson UJ. Congenital anomalies in diabetic pregnancy. Semin Fetal Neonatal Med. 2009;14:85–93. doi: 10.1016/j.siny.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Wang Q, Huang R, Yu B, Cao F, Wang H, Zhang M, et al. Higher fetal insulin resistance in Chinese pregnant women with gestational diabetes mellitus and correlation with maternal insulin resistance. PloS One. 2013;8:e59845. doi: 10.1371/journal.pone.0059845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cseh K, Baranyi E, Melczer Z, Csákány GM, Speer G, Kovács M, et al. The pathophysiological influence of leptin and the tumor necrosis factor system on maternal insulin resistance:negative correlation with anthropometric parameters of neonates in gestational diabetes. Gynecol Endocrinol. 2002;16:453–460. [PubMed] [Google Scholar]
- 16.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 17.Liang Z, Wu Y, Xu J, Fang Q, Chen D. Correlations of serum visfatin and metabolisms of glucose and lipid in women with gestational diabetes mellitus. J Diabetes Investig. 2016;7:247–252. doi: 10.1111/jdi.12385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bao W, Baecker A, Song Y, Kiely M, Liu S, Zhang C. Adipokine levels during the first or early second trimester of pregnancy and subsequent risk of gestational diabetes mellitus:A systematic review. Metabolism. 2015;64:756–764. doi: 10.1016/j.metabol.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li LJ, Rifas-Shiman SL, Aris IM, Young JG, Mantzoros C, Hivert MF, et al. Associations of maternal and cord blood adipokines with offspring adiposity in Project Viva:is there an interaction with child age? Int J Obes. 2018;42:608–617. doi: 10.1038/ijo.2017.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinert LS. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy:comment to the International Association of Diabetes and Pregnancy Study Groups Consensus Panel. Diabetes Care. 2010;33:e97. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar H, Mishra M, Bajpai S, Pokhria D, Arya AK, Singh RK, et al. Correlation of insulin resistance, beta cell function and insulin sensitivity with serum sFas and sFasL in newly diagnosed type 2 diabetes. Acta Diabetol. 2013;50:511–518. doi: 10.1007/s00592-011-0307-8. [DOI] [PubMed] [Google Scholar]
- 22.Mojiminiyi OA, Abdella NA. Effect of homeostasis model assessment computational method on the definition and associations of insulin resistance. Clin Chem Lab Med. 2010;48:1629–1634. doi: 10.1515/CCLM.2010.303. [DOI] [PubMed] [Google Scholar]
- 23.Rodríguez-Rodríguez P, Ramiro-Cortijo D, Reyes-Hernández CG, López de Pablo AL, González MC, Arribas SM. Implication of Oxidative Stress in Fetal Programming of Cardiovascular Disease. Front Physiol [Internet] 2018. [[cited 2019 Jan 10]]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5974054 . [DOI] [PMC free article] [PubMed]
- 24.Fasshauer M, Blüher M, Stumvoll M. Adipokines in gestational diabetes. Lancet Diabetes Endocrinol. 2014;2:488–499. doi: 10.1016/S2213-8587(13)70176-1. [DOI] [PubMed] [Google Scholar]
- 25.Bellos I, Fitrou G, Pergialiotis V, Perrea DN, Daskalakis G. Serum levels of adipokines in gestational diabetes:a systematic review. J Endocrinol Invest. 2018 doi: 10.1007/s40618-018-0973-2. [DOI] [PubMed] [Google Scholar]
- 26.Xu J, Zhao YH, Chen YP, Yuan XL, Wang J, Zhu H, et al. Maternal circulating concentrations of tumor necrosis factor-alpha, leptin, and adiponectin in gestational diabetes mellitus:a systematic review and meta-analysis. ScientificWorldJournal. 2014;2014:926932. doi: 10.1155/2014/926932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsuchida A, Yamauchi T, Kadowaki T. Nuclear receptors as targets for drug development:molecular mechanisms for regulation of obesity and insulin resistance by peroxisome proliferator-activated receptor gamma, CREB-binding protein, and adiponectin. J Pharmacol Sci. 2005;97:164–170. doi: 10.1254/jphs.fmj04008x2. [DOI] [PubMed] [Google Scholar]
- 28.Doruk M, Uğur M, Oruç AS, Demirel N, Yildiz Y. Serum adiponectin in gestational diabetes and its relation to pregnancy outcome. J Obstet Gynaecol. 2014;34:471–475. doi: 10.3109/01443615.2014.902430. [DOI] [PubMed] [Google Scholar]
- 29.Bhograj A, Suryanarayana KM, Nayak A, Murthy NS, Dharmalingam M, Kalra P. Serum adiponectin levels in gestational diabetes mellitus. Indian J Endocrinol Metab. 2016;20:752–755. doi: 10.4103/2230-8210.192909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iliodromiti S, Sassarini J, Kelsey TW, Lindsay RS, Sattar N, Nelson SM. Accuracy of circulating adiponectin for predicting gestational diabetes:a systematic review and meta-analysis. Diabetologia. 2016;59:692–699. doi: 10.1007/s00125-015-3855-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kieffer TJ, Heller RS, Leech CA, Holz GG, Habener JF. Leptin suppression of insulin secretion by the activation of ATP-sensitive K+channels in pancreatic beta-cells. Diabetes. 1997;46:1087–1093. doi: 10.2337/diab.46.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 33.Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 34.Yang M, Peng S, Li W, Wan Z, Fan L, Du Y. Relationships between plasma leptin levels, leptin G2548A, leptin receptor Gln223Arg polymorphisms and gestational diabetes mellitus in Chinese population. Sci Rep. 2016;6:23948. doi: 10.1038/srep23948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inoue M, Maehata E, Yano M, Taniyama M, Suzuki S. Correlation between the adiponectin-leptin ratio and parameters of insulin resistance in patients with type 2 diabetes. Metabolism. 2005;54:281–286. doi: 10.1016/j.metabol.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Finucane FM, Luan J, Wareham NJ, Sharp SJ, O'Rahilly S, Balkau B, et al. Correlation of the leptin:adiponectin ratio with measures of insulin resistance in non-diabetic individuals. Diabetologia. 2009;52:2345–2349. doi: 10.1007/s00125-009-1508-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rueda-Clausen CF, Lahera V, Calderón J, Bolivar IC, Castillo VR, Gutiérrez M, et al. The presence of abdominal obesity is associated with changes in vascular function independently of other cardiovascular risk factors. Int J Cardiol. 2010;139:32–41. doi: 10.1016/j.ijcard.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Oda N, Imamura S, Fujita T, Uchida Y, Inagaki K, Kakizawa H, et al. The ratio of leptin to adiponectin can be used as an index of insulin resistance. Metabolism. 2008;57:268–273. doi: 10.1016/j.metabol.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 39.Chang YH, Chang DM, Lin KC, Shin SJ, Lee YJ. Visfatin in overweight/obesity, type 2 diabetes mellitus, insulin resistance, metabolic syndrome and cardiovascular diseases:a meta-analysis and systemic review. Diabetes Metab Res Rev. 2011;27:515–527. doi: 10.1002/dmrr.1201. [DOI] [PubMed] [Google Scholar]
- 40.Ortega-Senovilla H, Schaefer-Graf U, Meitzner K, Abou-Dakn M, Graf K, Kintscher U, et al. Gestational diabetes mellitus causes changes in the concentrations of adipocyte fatty acid-binding protein and other adipocytokines in cord blood. Diabetes Care. 2011;34:2061–2066. doi: 10.2337/dc11-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo ZC, Delvin E, Fraser WD, Audibert F, Deal CI, Julien P, et al. Maternal glucose tolerance in pregnancy affects fetal insulin sensitivity. Diabetes Care. 2010;33:2055–2061. doi: 10.2337/dc10-0819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lepercq J, Cauzac M, Lahlou N, Timsit J, Girard J, Auwerx J, et al. Overexpression of placental leptin in diabetic pregnancy:a critical role for insulin. Diabetes. 1998;47:847–850. doi: 10.2337/diabetes.47.5.847. [DOI] [PubMed] [Google Scholar]
- 43.Gross GA, Solenberger T, Philpott T, Holcomb WL, Landt M. Plasma leptin concentrations in newborns of diabetic and nondiabetic mothers. Am J Perinatol. 1998;15:243–247. doi: 10.1055/s-2007-993935. [DOI] [PubMed] [Google Scholar]
- 44.Cortelazzi D, Corbetta S, Ronzoni S, Pelle F, Marconi A, Cozzi V, et al. Maternal and foetal resistin and adiponectin concentrations in normal and complicated pregnancies. Clin Endocrinol (Oxf) 2007;66:447–453. doi: 10.1111/j.1365-2265.2007.02761.x. [DOI] [PubMed] [Google Scholar]
- 45.Chen H, Xu L, Zhu W, Wu Y, Xu M, Wang Z. Impact of cord blood adiponectin and leptin levels and maternal obesity on birth weight of infants born to women with gestational diabetes mellitus. J Reprod Med. 2017;62:179–183. [PubMed] [Google Scholar]
- 46.Vitoratos N, Dimitrakaki A, Vlahos NF, Gregoriou O, Panoulis K, Christopoulos P, et al. Maternal and umbilical resistin levels do not correlate with infant birth weight either in normal pregnancies and or in pregnancies complicated with gestational diabetes. J Matern-Fetal Neonatal Med. 2010;23:1019–1023. doi: 10.3109/14767050903551459. [DOI] [PubMed] [Google Scholar]
- 47.Oncul M, Tuten A, Erman H, Gelisgen R, Benian A, Uzun H. Maternal and cord blood apelin, resistin and visfatin levels in gestational diabetes mellitus. Minerva Med. 2013;104:527–535. [PubMed] [Google Scholar]
- 48.Haider DG, Schaller G, Kapiotis S, Maier C, Luger A, Wolzt M. The release of the adipocytokine visfatin is regulated by glucose and insulin. Diabetologia. 2006;49:1909–1914. doi: 10.1007/s00125-006-0303-7. [DOI] [PubMed] [Google Scholar]
- 49.Sobki SH, Al-Senaidy AM, Al-Shammari TA, Inam SS, Al-Gwiser AA, Bukhari SA. Impact of gestational diabetes on lipid profiling and indices of oxidative stress in maternal and cord plasma. Saudi Med J. 2004;25:876–880. [PubMed] [Google Scholar]
- 50.Couch SC, Philipson EH, Bendel RB, Wijendran V, Lammi-Keefe CJ. Maternal and cord plasma lipid and lipoprotein concentrations in women with and without gestational diabetes mellitus. Predictors of birth weight? J Reprod Med. 1998;43:816–822. [PubMed] [Google Scholar]
- 51.Schaefer-Graf UM, Graf K, Kulbacka I, Kjos SL, Dudenhausen J, Vetter K, et al. Maternal lipids as strong determinants of fetal environment and growth in pregnancies with gestational diabetes mellitus. Diabetes Care. 2008;31:1858–1863. doi: 10.2337/dc08-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]


