Introduction
Rhabdomyolysis is a clinical syndrome characterized by injury to skeletal muscle cells causing leakage of muscle-cell contents into the circulation leading to myoglobinuria. Acute kidney injury is a complication of severe rhabdomyolysis. There are many reported causes of rhabdomyolysis.1 Rhabdomyolysis secondary to genetic defects is commonly overlooked. Diagnostic delay is common, hence a high index of suspicion is required for diagnosis due to its rarity. After diagnosis, further episodes of rhabdomyolysis, renal injury, and other complications can be prevented. Here, we report a case of an adolescent female patient presenting with rhabdomyolysis secondary to McArdle disease, a genetic disease causing defective glycogenolysis leading to acute kidney injury.
Case Presentation
A 16-year-old girl presented to our outpatient clinic with complaints of abdominal discomfort, nausea and vomiting, and decreased urine output for a duration of 1 week. History of 1-episode brownish urine 2 days before admission was present. She gave history of doing 17 squats during an exercise session in school, following which she developed swelling of bilateral thighs and also developed the previously described symptoms gradually. There was long-term history of easy fatigability that worsened after exertion. There was no history of arthritis, skin rashes, or photosensitivity. On examination, vitals were stable. General and systemic examination was normal. Investigations showed serum creatinine: 7.2 mg/dl, urine examination showed trace proteinuria with myoglobin positivity. No active urinary sediments were present. Serum creatine phosphokinase (CPK) levels: 2970 IU/l. Complements were normal. Antinuclear antibody test and extractable nuclear antigens profile test were negative.
Two sessions of hemodialysis were done following which the patient improved symptomatically. She became dialysis independent. Within 1 week, serum creatinine reduced to normal range. CPK level reduced to 443 U/l.
CPK level done 1 week after discharge raised to 4395 U/l. Serum creatinine was normal. Table 1 shows the relevant laboratory investigations done. Table 2 shows CPK levels, serum creatine values, and urine myoglobin results during inpatient and 3-month follow-up.
Table 1.
Laboratory values
| Test name | Result | Normal range |
|---|---|---|
| Hemoglobin (g/dl) | 10.5 | 12.0–15.0 |
| Total counts (cells/cumm) | 16,500 | 4000–11,000 |
| Platelet count (lakhs/cumm) | 2.33 | 1.5–4.5 |
| Blood urea nitrogen (mg/dl) | 78 | 7.9–20.1 |
| Creatinine (mg/dl) | 7.2 | 0.60–1.10 |
| Sodium (mmol/l) | 134 | 136–146 |
| Potassium (mmol/l) | 5.1 | 3.5–5.1 |
| Chloride (mmol/l) | 103 | 101–109 |
| Bicarbonate (mmol/l) | 19 | 21–31 |
| CPK (U/l) | 2697 | <140 |
| Urine myoglobin | Positive | Negative |
| Urine color | Straw yellow/clear | |
| Urine specific gravity | 1.015 | 1.001–1.035 |
| Urine glucose | Negative | Negative |
| Urine protein | Trace | Negative |
| Urine bilirubin | Negative | Negative |
| Urine ketone | Negative | Negative |
| Urine urobilinogen (Eu/dl) | 0.2 | 0.2–1.0 |
| Urine sediment | NIL | |
| Urine pus cells (cells/hpf) | 2–3 | <5 cells |
| Urine RBC (cells/hpf) | NIL | 0–2 |
| Urine epithelial cells (cells/hpf) | 4–6 | 0–4 |
| Urine casts (cells/hpf) | NIL | NIL |
| Urine crystals (cells/hpf) | NIL | NIL |
| Antinuclear antibody | Negative | Negative |
| Extractable nuclear antigens | Negative | Negative |
| C3 (mg/dl) | 119.80 | 90–180 |
| C4 (mg/dl) | 29 | 10–40 |
| ASO titre (IU/ml) | 800 | 0–200 |
| Urine PCR | 1.90 | <0.3 |
| Uric acid (mg/dl) | 10.8 | 2.6–6.0 |
| Phosphorus (mg/dl) | 4.9 | 2.5–4.5 |
| Calcium (mg/dl) | 8.5 | 8.8–10.6 |
| Serum cholesterol (mg/dl) | 138 | <200 |
| HIV | Nonreactive | Nonreactive |
| HCV | Nonreactive | Nonreactive |
| HBV | Nonreactive | Nonreactive |
| Blood culture | Negative | Negative |
| Urine culture | Negative | Negative |
| Peripheral smear | Microcytic hypochromic anemia with neutrophilic leukocytosis | |
| Blood grouping and typing | B positive |
HBV, hepatitis B virus; HCV, hepatitis C virus; HPF, high-power field; PCR, polymerase chain reaction; RBC, red blood cell
Table 2.
CPK levels, serum creatine values, and urine myoglobin results during inpatient and 3-month follow-up
| Day no. | CPK (U/l) | Creatinine (mg/dl) | Hemodialysis | Myoglobin urine |
|---|---|---|---|---|
| 01 | 2697 | 7.2 | 1st Session | Positive |
| 02 | 2971 | 3.8 | ||
| 03 | 2435 | 5.2 | 2nd Session | |
| 04 | 1324 | 2.5 | ||
| 08 | 443 | 1.8 | ||
| 15 | 4329 | 0.8 | Negative | |
| 60 | 2697 | 0.5 | Negative | |
| 90 | 3933 | 0.6 | Negative |
As autoimmune diseases were ruled out using various serological investigations, nonischemic forearm stress test was done to rule out metabolic myopathy.
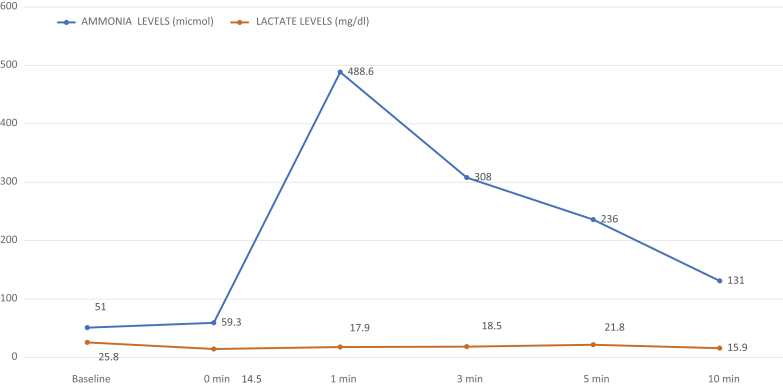
Forearm stress test was positive, which showed no increase in blood lactate level with a normal increase in ammonia. Table 3 shows plasma lactate and ammonia levels during forearm stress test. Figure 1 is a graph showing results of the forearm stress test.
Table 3.
Plasma lactate and ammonia levels during forearm stress test
| Fore arm stress test | Baseline | 0 min | 1 min | 3 min | 5 min | 10 min |
|---|---|---|---|---|---|---|
| Ammonia levels, μmol | 51 | 59.3 | 488.6 | 308 | 236 | 131 |
| Lactate levels, mg/dl | 25.8 | 14.5 | 17.9 | 18.5 | 21.8 | 15.9 |
Figure 1.
Forearm stress test showing no increase in blood lactate production.
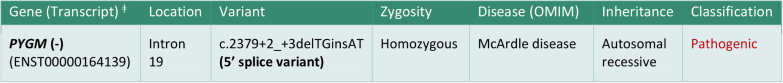
The patient was not willing for muscle biopsy. Hence, we went ahead with genetic testing. The method used for genetic analysis was targeted gene sequencing, confirmed by Sanger sequencing. Genetic study showed homozygous 5ʹ splice site indel variation in intron 19 of the PYGM gene that affects the invariant GT donor splice site of exon 19 (c.2379+2_+3delTGinsAT; ENST00000164139). Figure 2 shows the genetic study test result. Hence, the patient was diagnosed with McArdle disease, rhabdomyolysis, and acute kidney injury. The parents had a nonconsanguineous marriage. Genetic analysis could not be tested for parents and a younger sibling (sister) of the patient because of logistic reasons. However, they have been advised to undergo genetic study for providing genetic counseling to the family.
Figure 2.
Genetic analysis report showing pathogenic homozygous splice mutation in the PYGM gene. ‡Gene transcript is the exact nucleotide sequence and position of a particular instance of said gene in an individual.
The patient was advised to avoid acute heavy exercise, ensure adequate hydration, high carbohydrate diet, and was started on levocarnitine tablets and creatine powder supplementation. After 1 month of follow-up, the patient was asymptomatic but CPK was still high. Renal function test (RFT) was normal with good urine output.
Discussion
Rhabdomyolysis is a clinical syndrome characterized by breakdown of skeletal muscle and the release of intracellular contents into the circulation. These cell contents include enzymes such as creatine phosphokinase, glutamic oxalacetic transaminase, lactate dehydrogenase, aldolase, the heme pigment myoglobin, electrolytes such as potassium and phosphates, and purines. The mechanism of renal toxicity in rhabdomyolysis is intrarenal vasoconstriction, direct tubule injury, and tubular obstruction.2 There are several reported causes of rhabdomyolysis. They are trauma, exertion, muscle hypoxia, genetic defects, infections, body temperature changes, metabolic and electrolyte disorders, drugs and toxins, and idiopathic causes.1 The common causes of rhabdomyolysis in the adolescent age group are viral myositis, trauma, connective tissue disorders, exercise, and drug overdose.
Genetic disorders presenting with rhabdomyolysis are rare and they usually manifest during childhood (Table 4). History of recurrent episodes of rhabdomyolysis and family history of recurrent episodes precipitated by mild exertion or starvation should raise the suspicion of genetically determined metabolic myopathy. Inherited genetic disorders that can cause rhabdomyolysis are metabolic myopathies or enzyme deficiencies (disorders of carbohydrate or lipid metabolism) and myopathies.3 Our patient was diagnosed with McArdle disease or glycogen storage disease type 5, which is a type of metabolic myopathy (Table 4). Metabolic myopathies are divided into 3 diverse groups. They are disorders of glycogen metabolism, lipid metabolism defects, and mitochondrial disorders. McArdle disease is the most common disorder of glycogen metabolism caused by a homozygous mutation in the PYGM gene, leading to partial or complete absence of the myophosphorylase enzyme (Table 4). From a clinical standpoint, metabolic myopathies are viewed as static or dynamic disorders. McArdle disease is a dynamic disorder that exhibits symptoms and signs related to exercise, such as cramps, myalgias, exercise intolerance, and myoglobinuria. The myophosphorylase enzyme is involved in the breakdown of glycogen to glucose for the use in muscle. It removes 1,4 glycosyl residues from outer branches of glycogen and adds inorganic phosphate to form glucose-1 phosphate.4
Table 4.
Teaching points
|
|
|
|
|
Early diagnosis is necessary to facilitate learning the life skill required to manage the condition and to prevent rhabdomyolysis. There are 5 features of McArdle disease necessary for the diagnosis (Table 4). First, exercise intolerance, present with episodes of muscle pain and tachycardia at the beginning of any physical activity and during strenuous activity, isometric muscle contraction, and resistance training. All skeletal muscles are involved. Second, muscle contractures present with severe rigidity and associated pain in the muscles. The patient might report severe cramps. Muscle contractures can affect any muscle. Third, second-wind phenomenon, which occurs after 8 to 10 minutes of aerobic activity when the symptoms of exercise tolerance disappear and the patient can exercise more freely. The second wind can be identified with functional exercise testing with cardiac monitoring such as the 12-minute walk test cycle ergometry. Fourth, patients present with episodes of rhabdomyolysis/myoglobinuria. Severe muscle contracture followed by muscle swelling and pain with flu-like symptoms is seen. Discoloration of urine can be present, which is described as tea, red wine, or coca cola–colored urine. When patients develop severe episodes, they may develop acute kidney injury. CPK level can be markedly elevated (40,000–250,000 IU/l). Fifth, additional investigations to confirm the diagnosis. Baseline CPK is usually raised to 10 to 15 times the normal value. Serum uric acid levels are frequently elevated. Nonischemic forearm exercise test shows no significant rise in lactate. DNA analysis for initial screening of common mutations in northern Europeans (p.Arg50X and p.Gly205Ser) followed by next tier full PYGM sequencing. Muscle biopsy is rarely required to rule out vacuolar myopathy, subsarcolemmal glycogen deposition, and absent muscle glycogen phosphorylase activity (Table 4).5
Our patient had features of exertion intolerance, recurrent episodes of rhabdomyolysis and myoglobinuria, and elevated renal parameters requiring 2 cycles of hemodialysis. The patient’s mean serum CPK level during inpatient and follow-up was 2603 IU/l. Serum uric acid levels were elevated. Nonischemic forearm stress test was classically positive, as there was no significant rise in lactate (Figure 1). Muscle biopsy, even though rarely required, could not be done. DNA analysis for common mutations showed a pathogenic homozygous 5-splice variant mutation in the PYGM gene. This confirms the diagnosis of McArdle disease causing rhabdomyolysis and acute kidney injury. Genetic sequencing of the parents and siblings is important to ascertain phase information and carrier status, respectively, for genetic counseling purposes (Table 4). Compound heterozygous variants are well-known pathogenic variants in the PYGM gene. But in our case, even though parents had a nonconsanguineous marriage, the patient has a homozygous, autosomal recessive pathogenic variant that is quite rare. This probably can be due to inbreeding within the same communities, as the custom is common in south India, which is influenced by geographic and sociocultural factors.
Molecular Gene Analysis
DNA testing was done using targeted gene sequencing confirmed by the Sanger sequencing method. DNA was extracted from the peripheral blood and targeted gene capture was performed using a custom capture kit. The libraries were then sequenced to mean >80 to 100X coverage on illumine sequencing platform. The sequences detected were aligned to the human reference genome (GRCh37/hg19) using the BWA program6, 7 and analyzed using Picard and GATK version 3.68, 9 to identify variants relevant to the clinical indication. We followed the GATK best practices framework for the identification of variants in the sample. Gene annotation of the variants was performed using the VEP programS1 against the Ensembl release 87 human gene model.S2 Clinically relevant mutations were annotated using published variants in the literature and a set of disease databases: ClinVar, OMIM, GWAS, HGMD, and SwissVar.S3–S7 Common variants were filtered based on allele frequency in 1000 Genome Phase 3, ExAC, EVS, dbSNP147, 1000 Japanese Genome, and the internal Indian population database.S8–S12 Nonsynonymous variants effect was calculated using multiple algorithms, such as PolyPhen-2, SIFT, MutationTaster2, Mutation Assessor, and LRT. Validation of the variants was done by Sanger sequencing to rule out false positives.
Sugie et al.S13 performed genetic analysis on 11 Japanese patients with McArdle disease and identified a single-codon deletion at codon 708/709 in exon 17 as a predominant mutation in the Japanese population, which is different from the most common mutations seen in US or UK patients (CGA to TGA at codon 49 in exon 1, p.R49X now called as p.R50X). Bruno et al.S14 in 2006 performed genetic analysis on 68 Italian patients with McArdle disease and identified 30 different mutations in the PYGM gene with 19 novel mutations. The homozygous 5-splice pathogenic mutation at intron 19 identified in our patient was first reported in this study.S14 Vieitez et al.S15 performed molecular analysis on 123 European patients with McArdle disease and identified 20 novel mutations and identified no genotype-phenotype correlation and relation of gender effect on the phenotype. Until the present, 147 pathogenic mutations and 39 polymorphisms have been reported in the PYGM gene. Missense mutation accounts for 50%, with exons 1 and 17 being mutational hotspots in the PYGM gene. Mutation c.148C>T (commonly known as p. R50X) is the most common mutation in most of the study populations.S16
At present, no effective gene therapy is available to treat myophosphorylase deficiency/McArdle disease in humans.S17 The most beneficial intervention is a combination of aerobic conditioning and ensuring that sufficient blood glucose is constantly available to the patients’ working muscles during the daytime (Table 4). This is achieved by taking a diet with a high proportion (65%) of complex carbohydrates (such as those found in vegetables, fruits, cereals, bread, pasta, and rice) and a low proportion (20%) of fat. In addition, the ingestion of simple carbohydrates (20–40 g of glucose or fructose in adults and 20 g in children) approximately 5 minutes before engaging in strenuous exercise, can be helpful. Patients should take advantage of the second-wind phenomenon with a low-intensity, 5- to 15-minute warm-up period. Very intense exercises are strongly discouraged in these patients. A Cochrane review published in 2010 found minimal benefit with low-dose carnitine (60 mg/kg per day). Ramipril (2.5 mg/d), can be effective only for patients with polymorphism known as the D/D ACE phenotype.S18 According to small clinical reports, vitamin B6 supplementation 50 mg/d to 90 mg/d seems to reduce exercise intolerance and enhance performance.
In patients with rhabdomyolysis and acute kidney injury, based on the severity of renal failure, treatment modality can be either conservative or invasive. Conservative approach includes fluid resuscitation with initial bolus of 10 to 20 ml/kg or 1 l i.v. isotonic saline and 1 l glucose 5% with 100 mmol bicarbonate. This initial bolus is followed by maintenance fluid of 3 to 6 l or more than 10 l/d to maintain urine output of 1 to 2 ml/kg per hour. Urine alkalinization to maintain pH of urine higher than 6.5 with 200 to 300 mEq of bicarbonate i.v. daily or 30 to 44 mEq/l of isotonic saline at an infusion rate of 100 ml/h. Diuretic agent attracts the fluid from interstitial space and so may reduce muscular swelling. Mannitol 20% i.v. 100-ml bolus followed by maintenance dose of 10 ml/h i.v. can be given. In severe cases of rhabdomyolysis and acute kidney injury, patients should be treated with intermittent hemodialysis or continuous renal replacement therapy based on the hemodynamic stability of the patient. High permeability dialyzer membranes can be beneficial.S19
Conclusion
Patients with rhabdomyolysis and acute kidney injury following exercise should be evaluated for genetic defects. A high index of suspicion is required because of its rarity. Early diagnosis is necessary to restrict vigorous physical activity and to prevent rhabdomyolysis and other fatal complications. Rhabdomyolysis, when treated early and aggressively, has excellent prognosis for the recovery of full renal function. Simple measures of lifestyle and dietary changes are effective.
Disclosure
All the authors declared no competing interests.
Acknowledgments
Funding was provided by Sri Ramachandra Institute of Higher Education and Research, Chennai, India 600116.
Footnotes
Supplementary References.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
References
- 1.Bosch X., Poch E., Grau J.M. Rhabdomyolysis and acute kidney injury. N Engl J Med. 2009;361:62–72. doi: 10.1056/NEJMra0801327. [DOI] [PubMed] [Google Scholar]
- 2.Sauret J.M., Marinides G., Wang G.K. Rhabdomyolysis. Am Fam Physician. 2002;65:907–912. [PubMed] [Google Scholar]
- 3.Brumback R.A., Feeback D.L., Leech R.W. Rhabdomyolysis in childhood. A primer on normal muscle function and selected metabolic myopathies characterized by disordered energy production. Pediatr Clin North Am. 1992;39:821–858. doi: 10.1016/s0031-3955(16)38377-8. [DOI] [PubMed] [Google Scholar]
- 4.Tobon A. Metabolic myopathies. Continuum (Minneap Minn) 2013;19:1571–1597. doi: 10.1212/01.CON.0000440660.41675.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scalco R.S., Morrow J.M., Booth S. Misdiagnosis is an important factor for the diagnostic delay in McArdle disease. Neuromuscul Disord. 2017;27:852–855. doi: 10.1016/j.nmd.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Li H., Durbin R. Fast and accurate long-read alignment with burrows-wheeler transform. Bioinformatics. 2010;26:589–595. doi: 10.1093/bioinformatics/btp698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer L.R., Zweig A.S., Hinrichs A.S. The UCSC genome browser database: extensions and updates 2013. Nucleic Acids Res. 2012;41(Database issue):D64–D69. doi: 10.1093/nar/gks1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKenna A., Hanna M., Banks E. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H., Handsaker B., Wysoker A. The Sequence Alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.