Abstract
Objectives
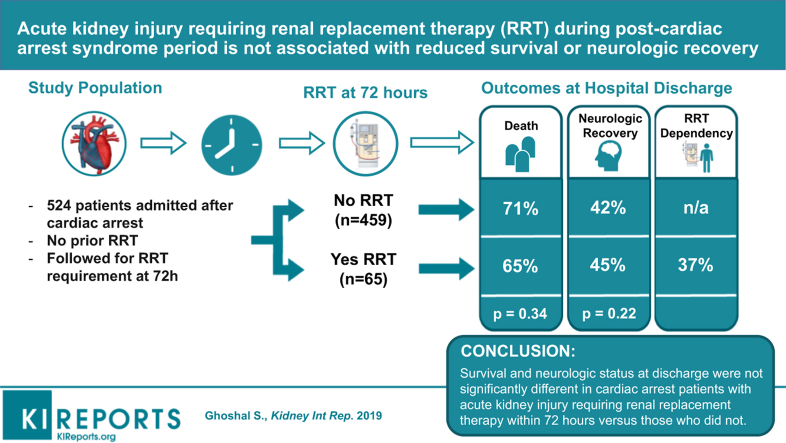
Acute kidney injury (AKI) is common after cardiac arrest (CA). Few data exist on survival and neurological outcomes measured at hospital discharge of patients with severe AKI requiring renal replacement therapy (RRT) within the first 72 hours (i.e., duration of post-CA syndrome).
Methods
Single-center, prospective, observation cohort of patients with in- or out-of-hospital CA who survived to intensive care unit admission and were considered for targeted temperature management between 2010 and 2016 were reviewed. After excluding preexisting RRT history, patients with new RRT requirements within the first 72 hours after CA were included. Primary outcome of survival and secondary outcome of good neurological recovery defined as cerebral performance category score of 1 to 2, were compared between patients with and without RRT. Within 24 hours of initiating RRT, illness severity, as measured by Sequential Organ Failure Assessment score, Acute Physiology and Chronic Health Evaluation–II, and Charlson Comorbidity Index, was compared between survivors and nonsurvivors.
Results
Of 524 patients, 65 (12.4%) had new RRT requirements within 72 hours. Survival rates and good neurological recovery at discharge were comparable between RRT and non-RRT groups (19 of 65 [29%] vs. 162 of 459 [35%], P = 0.3, and 8 of 19 [42%] vs. 73 of 162 [45%], respectively). Sixty-three percent (12 of 19) of survivors requiring RRT did not need dialysis on discharge. Among patients requiring RRT, prognostic factors, including illness severity scores and indications for RRT, did not differ between survivors and nonsurvivors.
Conclusions
Patients with severe AKI requiring RRT during the post-CA syndrome period were not associated with any significant reduction in survival or poor neurological recovery, compared with those without RRT. Among those requiring RRT, none of the known prognostic factors predicted survival.
Keywords: cardiac arrest, neurologic outcomes, renal replacement therapy
Graphical abstract
AKI is common after CA, and reported in up to 50% of patients during their hospitalization.1, 2 Up to one-third of these patients, develop severe AKI and require RRT during their post-CA hospitalization.1, 2, 3
Severe AKI after CA has been associated with increased mortality with varying relationship with neurological recovery.3 However, the literature has been limited due to variations in AKI definitions and unclear onset time of AKI after CA.
The post-CA syndrome period, defined as the first 72 hours after achieving return of spontaneous circulation, has been associated with widespread activation of inflammatory mediators. Hypoperfusion and vascular hyperpermeability may occur, leading to end-organ dysfunction, and AKI.4 In the later stages of hospitalization after CA, AKI is more likely to be secondary to sepsis and nephrotoxic agents, rather than CA-induced inflammation.5 The prognosis of patients with severe AKI requiring RRT within this post-CA syndrome period is unknown.
In this study, we aim to estimate survival and neurological recovery at hospital discharge of patients with severe AKI requiring RRT within first 72 hours of CA.
Methods
Design
We conducted a prospective cohort study of adults with return of spontaneous circulation after in-hospital or out-of-hospital CA, who were considered for targeted temperature management at Columbia University Medical Center (an academic, tertiary-care hospital) from August 2010 through December 2016. The study was approved by the Columbia University institutional review board.
Participants
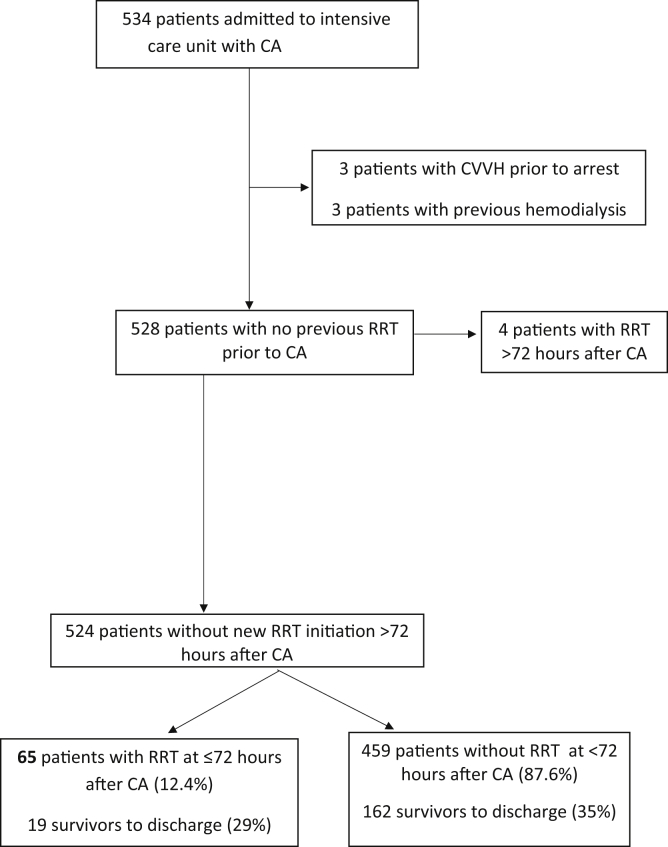
From a consecutive sample of 528 patients with CA, we included those who required RRT within 72 hours of CA as a subgroup (Supplementary Figure S1). All patients were required to survive 72 hours for study inclusion, and study follow-up began at the end of the 72-hour period. Exclusion criteria included preexisting dialysis requirements before CA. Patients who developed new RRT requirements past 72 hours of CA were excluded due to greater likelihood of multifactorial AKI from later nephrotoxic agents or nosocomial illness. Patients on veno-arterial extracorporeal membrane oxygenation or intra-aortic balloon pump were not included this study.
Targeted temperature management, based on the American Heart Association guidelines for the acute management of CA6 is described in the Supplementary Material.
Measurements
Variables were collected per Utstein guidelines.6 AKI was determined by Kidney Disease: Improving Global Outcomes criteria.7 The exposure variable of interest was whether RRT was initiated within 72 hours of CA event. Charlson Comorbidity indices were determined to assess pre-CA comorbidities.8 Serum creatinine (mg/dl) obtained by Jaffe method was recorded at the time of admission, at 48 hours, at peak value within 72 hours, and at discharge. Preexisting renal disease was defined as the available nadir value before intensive care unit admission. Illness severity within 24 hours of initiating continuous veno-venous hemofiltration was calculated by the Sequential Organ Failure Assessment score, Acute Physiology and Chronic Health Evaluation–II scores, and number of vasopressors at RRT initiation.9, 10 Two reviewers (S.G. and V.Y.) documented indications for RRT using assessments obtained from board-certified nephrologists. Indications for RRT were categorized into the following: (i) volume overload, (ii) oliguria, (iii) anuria, (iv) acidosis, (v) hyperkalemia, and (vi) uremia. Neurologic outcome was assessed at discharge by cerebral performance category score.
Statistical Analysis
Primary outcome of survival and secondary outcome of good neurological recovery, defined as cerebral performance category score of 1 to 2, were compared between patients with and without RRT. Among patients requiring RRT, illness severity scores, indications for RRT, and other patient characteristics were compared between survivors and nonsurvivors. Chi-square test and the Wilcoxon rank-sum test were used. A P value <0.05 was considered as statistically significant, and analyses were performed using STATA software (StataCorp, College Station, TX).
Results
Of 524 patients with CA included, 65 (12.4%) had new RRT requirements within 72 hours of arrest (Figure 1). Each of these patients received continuous veno-venous hemofiltration as their RRT modality, as was clinically indicated for critical illness and recent cardiac instability. Compared with the 459 patients who did not require RRT, those requiring RRT were younger, more often men, and had higher prevalence of preexisting renal disease (baseline estimated glomerular filtration rate7 <60 ml/min per 1.73 m2). The RRT group also had patients with significantly higher rates of in-hospital arrests, ventricular tachycardia/fibrillation as their initial rhythm, bystander cardiopulmonary resuscitation and defibrillation, and a slight trend toward targeted temperature management administration (Table 1).
Figure 1.
Patient selection for the prospective cohort. CA, cardiac arrest; CVVH, continuous veno-venous hemofiltration; RRT, renal replacement therapy.
Table 1.
Comparing patient characteristics and outcomes between patients with and without RRT
| Characteristics, n (%) | No RRT, n = 459 (87.6%) | RRT, n = 65 (12.4%) | P |
|---|---|---|---|
| Age, mean ± SD | 64.2 ± 17.7 | 59.8 ± 16.1 | 0.04 |
| Men | 244 (53.2) | 45 (69.2) | 0.02 |
| Pre-arrest comorbidities | |||
| Myocardial infarction | 70 (15.3) | 4 (6.2) | 0.05 |
| Heart failure | 125 (27.2) | 20 (30.8) | 0.55 |
| Hypertension | 283 (61.7) | 41 (63.1) | 0.83 |
| Renal disease | 102 (22.2) | 24 (36.9) | 0.01 |
| NIDDM | 107 (23.3) | 14 (21.5) | 0.75 |
| Liver disease | 20 (4.4) | 5 (7.7) | 0.24 |
| Cerebrovascular disease | 66 (14.4) | 4 (6.2) | 0.07 |
| PVD | 39 (8.5) | 8 (12.3) | 0.31 |
| Obesity (BMI >30 m/kg2) | 42 (9.2) | 8 (12.3) | 0.42 |
| Dementia | 64 (13.9) | 3 (4.6) | 0.04 |
| Smoking status | 0.17 | ||
| Nonsmoker | 177 (44.5) | 35 (57.4) | |
| Former smoker | 135 (33.9) | 16 (26.2) | |
| Current smoker | 86 (21.6) | 10 (16.4) | |
| Pre-arrest CPC | 0.07 | ||
| - CPC 1 | 253 (55.7) | 43 (66.2) | |
| - CPC 2 | 86 (18.9) | 15 (23.1) | |
| - CPC 3 | 108 (23.8) | 7 (10.8) | |
| - CPC 4 | 7 (1.5) | 0 (0) | |
| In-hospital arrests | 144 (31.4) | 35 (53.9) | <0.01 |
| Witnessed arrest | 352 (78.2) | 53 (81.5) | 0.54 |
| CPR provided | |||
| -No CPR | 169 (37.1) | 10 (15.4) | |
| -Yes, by a bystander | 98 (21.5) | 9 (13.9) | |
| -Yes, with medical personnel | 189 (41.5) | 46 (70.8) | |
| Defibrillation use | 148 (33.3) | 24 (38.1) | 0.45 |
| Initial rhythm | 0.11 | ||
| - VT/VF/AED Advised Shock | 93 (22.0) | 20 (34.5) | |
| - PEA | 212 (50.1) | 25 (43.1) | |
| - Asystole | 118 (27.9) | 13 (22.4) | |
| ROSC, mean ± SD | 21.6 (16.4) | 24.5 (26.6) | 0.65 |
| Temperature management | 319 (69.5) | 53 (81.5) | 0.05 |
| Survival to discharge | 162 (35.3) | 19 (29.2) | 0.34 |
| CPC for 181 survivors at discharge, n (%) | 0.22 | ||
| - CPC 1 | 32 (19.8) | 4 (21.1) | |
| - CPC 2 | 41 (25.3) | 3 (15.8) | |
| - CPC 3 | 59 (36.4) | 11 (57.9) | |
| - CPC 4 | 30 (18.5) | 1 (5.3) |
AED, automated external defibrillators; BMI, body mass index; CPC, cerebral performance score; CPR, cardiopulmonary resuscitation; NIDDM, non–insulin-dependent diabetes mellitus; PEA, pulseless electrical activity; PVD, peripheral vascular disease; ROSC, return of spontaneous circulation; RRT, renal replacement therapy; VT/VF, ventricular tachycardia/ventricular fibrillation.
P < 0.05 are in bold.
Survival and Neurological Recovery at Hospital Discharge
Survival rates to hospital discharge were comparable between RRT and non-RRT groups (19 of 65 [29%] vs. 162 of 459 [35%], P = 0.3). Rates of good neurological function at discharge among all survivors (181 of 524 [34.5%]) were also similar in the RRT and non-RRT groups (8 of 19 [42%] vs. 73 of 162 [45%], P = 0.8). Among patients requiring RRT, survivors and nonsurvivors had no significant differences in prognostic factors, including patient characteristics (Supplementary Table S1), illness severity scores (Sequential Organ Failure Assessment, Acute Physiology and Chronic Health Evaluation–II, Charlson Comorbidity Index), serum creatinine levels at any estimated time points, early RRT (defined as within 48 hours of arrest), or indications for dialysis within 24 hours of RRT initiation (Table 2). Sixty-three percent of survivors (12 of 19) in the RRT group did not require long-term dialysis on discharge.
Table 2.
Comparing indications for RRT and severity of illness between RRT survivors and nonsurvivors (n = 65)
| Characteristics, n (%) | Survivors, n = 19 (29.2%) | Nonsurvivors, n = 46 (70.8%) | P |
|---|---|---|---|
| Serum creatinine, mean ± SD | |||
| Baseline | 1.66 ± 1.02 | 1.88 ± 1.35 | 0.57 |
| 48 h after cardiac arrest | 3.97 ± 2.62 | 3.35 ± 1.60 | 0.52 |
| Peak | 4.11 ± 2.61 | 3.44 ± 1.56 | 0.41 |
| On discharge (survivors only) | 2.30 ± 1.54 | – | – |
| Illness severity score, mean ± SD | |||
| SOFA | 14.47 ± 2.17 | 14.26 ± 2.55 | 0.75 |
| APACHE-II | 32.32 ± 5.83 | 33.48 ± 5.76 | 0.44 |
| Number of pressors, mean ± SD | 2.4 ± 1.42 | 2.5 ± 0.98 | 0.81 |
| Furosemide infusion prior to RRT initiation | 3 (15.8) | 6 (13.0) | 0.71 |
| Early dialysis initiation (within 48 hours of arrest) | 13 (68.4) | 36 (78.3) | 0.40 |
| Indications for dialysis on RRT initiation | |||
| Volume overload | 7 (36.8) | 12 (26.1) | 0.39 |
| Oliguria | 5 (26.3) | 6 (13.0) | 0.28 |
| Anuria | 3 (15.8) | 17 (37.0) | 0.09 |
| Acidosis | 6 (31.6) | 17 (37.0) | 0.68 |
| Hyperkalemia | 4 (21.1) | 11 (23.9) | 1.00 |
| Uremia | 0 (0) | 1 (2.2) | 1.00 |
| Dialysis on discharge (survivors only) | 7 (36.8) | – | – |
APACHE-II, Acute Physiology and Chronic Health Evaluation–II; RRT, renal replacement therapy; SOFA, Sequential Organ Failure Assessment.
Discussion
Survival and neurological status at discharge were not significantly different in patients requiring RRT within the first 72 hours due to severe AKI, compared with those who did not need RRT. Among patients requiring RRT, 1 of 3 survive to discharge, with more than a quarter achieving good neurological recovery, and 2 of 3 of all patients with CA on RRT did not require long-term dialysis at the time of discharge. In contrast to previous literature, we did not find a difference in measured outcomes between oliguric and anuric AKI.11, 12
Our study is the first looking at the utility of RRT for severe AKI developed in the early post-CA period. The kidney is particularly susceptible to postresuscitation changes; interstitial inflammation and microvasculopathy resulting in incomplete tubular cell repair, fibrosis, and renal insufficiency.11 AKI may have an impact on neurological recovery, although clinical results vary.13, 14 In experimental modes of renal injury, AKI contributes to hippocampal inflammatory injury and altered blood-brain barrier permeability.14 There is some additional evidence that RRT may improve neurologic outcomes by removal of inflammatory mediators in the post-CA period.15, 16, 17
Further, severity of illness should not be a sole indicator to withhold RRT in the post-CA period. Among our post-CA RRT patients, survivors to discharge did not differ significantly from nonsurvivors, neither by severity of illness nor by indication for dialysis.
Our study has limitations. Although it is one of the largest studies dedicated to the utility of RRT after CA, our study size is nonetheless relatively small, and may not have enough power to detect the differences. Preexisting renal disease was defined as the available nadir value before intensive care unit admission, as outpatient creatinine values were not always available. All patients received only continuous veno-venous hemofiltration in the acute CA period, as these patients were critically ill and had had recent profound cardiac instability. Our results cannot be extrapolated to other types of RRT. Creatinine was measured nonenzymatically. We did not assess outcomes with time to intermittent hemodialysis transition later in hospitalization, nor did we assess length of stay while assessing in-hospital survival. Data regarding administration of nephrotoxins before or during CA were not collected, nor were resuscitation fluids. These factors could further refine outcomes in patients with AKI after CA.
CA is plagued with early withdrawal of life-sustaining treatments; of the 297 non-RRT patients who died, 25 patients met criteria for RRT within 72 hours but did not receive treatment, due to poor projected neurologic prognosis. This is consistent with other studies reporting only 40% of patients with CA with AKI are started on RRT because of predicted poor neurological recovery, and mortality was more than double in patients with acute renal failure without RRT.4
Long-term neurologic and renal outcomes of patients requiring RRT post-CA are still unknown, and merit further study.
Disclosure
JC reports personal fees from SAGE; grants from DANA, McDonell, SHINE, I-SPOT, ESETT, and SAGE; and other from iCE Neurosystems, outside the submitted work. All the other authors declared no competing interests.
Acknowledgments
This study was supported by the institutional funds provided to SA. SP acknowledges support of the National Institutes of Health/National Institute of Neurological Disorders and Stroke (K01ES026833). The funding sources had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Figure S1. Patient selection for the prospective renal replacement therapy (RRT) cohort after cardiac arrest (CA). CVVH, continuous veno-venous hemofiltration.
Table S1. Comparing various clinical characteristics of survivors and nonsurvivors among patients who received RRT (n = 65).
Supplemental Section 1.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Patient selection for the prospective renal replacement therapy (RRT) cohort after cardiac arrest (CA). CVVH, continuous veno-venous hemofiltration.
Comparing various clinical characteristics of survivors and nonsurvivors among patients who received RRT (n = 65).
References
- 1.Sandroni C., Dell’anna A.M., Tujjar O. Acute kidney injury after cardiac arrest: a systematic review and meta-analysis of clinical studies. Minerva Anestesiol. 2016;82:989–999. [PubMed] [Google Scholar]
- 2.Tujjar O., Mineo G., Dell’Anna A. Acute kidney injury after cardiac arrest. Crit Care. 2015;19:169. doi: 10.1186/s13054-015-0900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yanta J., Guyette F.X., Doshi A.A. Post Cardiac Arrest Service. Renal dysfunction is common following resuscitation from out-of-hospital cardiac arrest. Resuscitation. 2013;84 doi: 10.1016/j.resuscitation.2013.03.037. 137–134. [DOI] [PubMed] [Google Scholar]
- 4.Stub D., Bernard S., Duffy S.J., Kaye D.M. Post cardiac arrest syndrome: a review of therapeutic strategies. Circulation. 2011;123:1428–1435. doi: 10.1161/CIRCULATIONAHA.110.988725. [DOI] [PubMed] [Google Scholar]
- 5.Neumar R.W., Noland J.P., Adrie C. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. Circulation. 2008;118:1423–1431. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- 6.Perkins G.D., Jacobs I.G., Nadkarni V.M. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update of the Utstein Resuscitation Registry Templates for Out-of-Hospital Cardiac Arrest: a statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia); and the American Heart Association Emergency Cardiovascular Care Committee and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Resuscitation. 2015;96:328–340. doi: 10.1016/j.resuscitation.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–163. [Google Scholar]
- 8.Quan H., Li B., Couris C.M. Updating and validating the Charlson Comorbidity Index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 9.Vincent J.L., Moreno R., Takala J. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 10.Knaus W.A., Draper E.A., Wagner D.P., Zimmerman J.E. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 11.Choi H.M., Kim S.C., Kim M.G. Etiology and outcomes of anuria in acute kidney injury: a single-center study. Kidney Res Clin Pract. 2015;34:13–19. doi: 10.1016/j.krcp.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Rosa S., Antonelli M., Ronco C. Hypothermia and kidney: a focus on ischaemia-reperfusion injury. Nephrol Dial Transplant. 2017;32:241–247. doi: 10.1093/ndt/gfw038. [DOI] [PubMed] [Google Scholar]
- 13.Lu R., Kiernan M.C., Murray A.M. Kidney-brain crosstalk in the acute and chronic setting. Nat Rev Nephrol. 2015;11:707–719. doi: 10.1038/nrneph.2015.131. [DOI] [PubMed] [Google Scholar]
- 14.Hasper D., von Haehling S., Storm C. Changes in serum creatinine in the first 24 hours after cardiac arrest indicate prognosis: an observational cohort study. Crit Care. 2009;13:R168. doi: 10.1186/cc8144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chua H.R., Glassford N., Bellomo R. Acute kidney injury after cardiac arrest. Resuscitation. 2012;83:721–727. doi: 10.1016/j.resuscitation.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 16.Liu M., Liang Y., Chigurupati S. Acute kidney injury leads to inflammation and functional changes in the brain. J Am Soc Nephrol. 2008;19:1360–1370. doi: 10.1681/ASN.2007080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karnad V., Thakar B. Continuous renal replacement therapy may aid recovery after cardiac arrest. Resuscitation. 2006;68:417–419. doi: 10.1016/j.resuscitation.2005.06.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patient selection for the prospective renal replacement therapy (RRT) cohort after cardiac arrest (CA). CVVH, continuous veno-venous hemofiltration.
Comparing various clinical characteristics of survivors and nonsurvivors among patients who received RRT (n = 65).