Abstract
Introduction
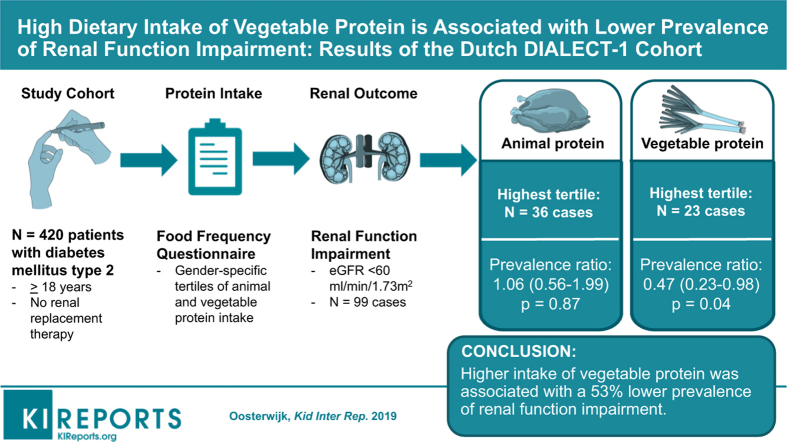
Dietary protein intake may influence development of renal function impairment in diabetes mellitus type 2 (T2DM). We assessed the association between sources of protein and prevalence of renal function impairment.
Methods
Cross-sectional analyses were performed in baseline data of 420 patients of the DIAbetes and LifEstyle Cohort Twente-1 (DIALECT-1) study. Protein intake was assessed using a Food Frequency Questionnaire, modified for accurate assessment of protein intake, including types and sources of protein. Renal function impairment was defined as estimated glomerular filtration rate (eGFR) <60 ml/min per 1.73 m2 (Chronic Kidney Disease Epidemiology Collaboration formula).
Results
Among 420 patients with T2DM, 99 renal function impairment cases were identified. Multivariate Cox proportional hazard models were used and adjusted for the main lifestyle and dietary factors. The prevalence ratios in the fully adjusted model were 1 (reference), 0.74 (95% confidence interval [CI]: 0.44–1.27; P = 0.28) and 0.47 (95% CI: 0.23–0.98; P = 0.04) according to increasing tertiles of vegetable protein intake. For animal protein intake the prevalence ratios were 1 (reference), 1.10 (95% CI: 0.64–1.88; P = 0.74) and 1.06 (95% CI: 0.56–1.99; P = 0.87) according to increasing tertiles of intake. Theoretical replacement models showed that replacing 3 energy percent from animal protein by vegetable protein lowered the prevalence ratio for the association with renal function impairment to 0.20 (95% CI: 0.06–0.63; P = 0.01).
Conclusion
In conclusion, we found that higher intake of vegetable protein was associated with a lower prevalence of renal function impairment, and theoretical replacement of animal protein with vegetable protein was inversely associated with renal function impairment among patients with T2DM.
Keywords: diabetes mellitus type 2, diet, kidney function, lifestyle
Graphical abstract
See Commentary on Page 638
T2DM affected 415 million people worldwide in 2015 and it is expected that its prevalence will rise up to 642 million people by 2040.1, 2 One major threat in these patients is the risk of diabetic kidney disease (DKD) occurring in 20% to 40% of the T2DM population,3, 4 which is 2 times higher than the prevalence rate of chronic kidney disease in the general population.5 Diabetes complications can potentially be prevented or delayed by modifying lifestyle (e.g., pursuing a healthy body weight, regular physical activity, and a healthy diet).6 Decreasing the carbohydrate intake has strong effects in lowering blood glucose and may contribute to counteract obesity,7 and therefore a low-carbohydrate diet is recommended in diabetes guidelines.6, 8 However, decreasing the dietary intake of carbohydrates naturally implies a higher proportion of other dietary macronutrients.9 This is of interest, because a high dietary intake of protein has traditionally been implicated as a factor fueling progressive impairment of renal function in chronic kidney disease.10 Therefore, within the overall strategy to delay the progression of renal function loss, the dietary counseling in patients with DKD includes the advice to limit the total protein intake to 0.8 g/kg per day.11
That the source of protein may also matter has recently been demonstrated in a subgroup of patients with T2DM included in the “Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial” (ONTARGET) study, suggesting that animal protein, in particular, may increase the risk of progressive DKD.12
We investigate the association between sources of protein and prevalence of renal function impairment in patients with T2DM. Furthermore, we performed a theoretical replacement analysis, in which we assessed the association of replacing macronutrients with both animal and vegetable sources of protein on the prevalence of renal function impairment. This analysis can provide valuable data on how to optimize the nutritional guidelines of T2DM.
Materials and Methods
Study Design
Cross-sectional analyses were performed in baseline data of the DIAbetes and LifEstyle Cohort Twente-1 (DIALECT-1) study. DIALECT-1 is an observational prospective cohort study performed in the Ziekenhuis Groep Twente Hospital (Almelo and Hengelo, The Netherlands) that investigates the effect of lifestyle and dietary habits on outcomes in patients with T2DM. Patients in DIALECT-1 were included between September 2009 and January 2016. The study was performed according to the guidelines of good clinical practice and the Declaration of Helsinki. Written informed consent was obtained from all subjects before participation. It has been approved by the local institutional review boards (METC-registration numbers NL57219.044.16 and 1009.68020) and is registered in the Netherlands Trial Register (NTR trial code 5855).
Patients
The study population consists of patients with T2DM aged ≥18 years, treated in the outpatient clinic of the Ziekenhuis Groep Twente Hospital as part of routine secondary care. Patients depending on renal replacement therapy or patients with insufficient knowledge of the Dutch language were excluded from participation.
From 1082 patients as screened by electronic patient files, 470 eligible patients were subsequently invited for a baseline visit. After the baseline visit, 20 patients were excluded because their correct diagnosis appeared to be type 1 diabetes mellitus, resulting in 450 patients eligible in DIALECT-1.13 For the current study, we excluded patients with missing data on dietary intake (n = 14) and physical activity (n = 13) and patients whose energy intake exceeded the borders of ± 3 SD of the mean energy intake (n = 3), leaving 420 patients for analysis.
Dietary Assessment
Protein intake was assessed by using a semiquantitative Food Frequency Questionnaire (FFQ). The FFQ was developed and validated at the Wageningen University in 2005. The FFQ asked the patient about dietary intake of 177 items during the past month, taking seasonal variation into account. For the present study, the FFQ was slightly modified in 2009 for accurate assessment of protein intake, including types and sources of protein. For each item, the frequency was recorded in times per day, week, or month. The number of servings was expressed in natural units (e.g., slice of bread or apple) or household measures (e.g., cup or spoon). The questionnaire was self-administered and filled out at home. The filled-in questionnaires were checked for completeness by a trained researcher, and inconsistent answers were verified with the patients. Dietary data were converted into daily nutrient intake using the Dutch Food Composition Table of 2013.14 With respect to the definition of animal and vegetable protein, the Dutch Food Composition Table distinguishes between these sources.
Additional analysis was performed within subgroups of animal and vegetable protein intake. All individual products were classified to animal or vegetable protein according to the highest content and grouped according to the Guidelines of Healthy Nutrition. We used the total protein content of the products because multiple products contain both animal and vegetable protein. The subgroups and the corresponding food items are shown in Appendix S1.
Outcome Measurement
Renal function is assessed by the eGFR, based on the Chronic Kidney Disease Epidemiology Collaboration formula,15 which is based on serum creatinine levels (μmol/l), gender, and age. We defined renal function impairment as an eGFR <60 ml/min per 1.73 m2,16 and performed additional tests with eGFR as the continuous variable. Renal function was assessed at the same time point that dietary information was collected.
Covariates
During the study visit, we collected information relevant to the medical condition and pharmacological treatment. Body weight (kg), height (cm), and waist and hip circumference (cm) were measured and the body mass index (BMI) was calculated as weight divided by the squared height (kg/m2). Information about lifestyle exposures (e.g., smoking and physical activity) was collected by a self-administered questionnaire. Physical activity was assessed by the previously validated Short Questionnaire to Assess Health enhancing physical activity questionnaire.17 An activity score is calculated based on minutes of activity per day multiplied by an intensity factor. We scored which patients meet the Dutch Healthy Exercise Norm of 30 minutes moderate intense activity a day for at least 5 days a week.18 Blood pressure was measured in a supine position by an automated device (Dinamap; GE Medical Systems, Milwaukee, WI) for 15 minutes with a 1-minute interval. The mean systolic and diastolic blood pressure of the last 3 measurements was used for analysis.
Patients were asked to collect their 24-hour urine to obtain the urinary excretion of sodium as objective measure of sodium intake, by multiplying these concentrations with the volume of the 24-hour urine collection. Patients were instructed to store the canister in a dark cool place, preferably in a refrigerator. Routine laboratory tests were performed in nonfasting venous blood, including renal function tests and HbA1c.
Statistical Analysis
All statistical analyses were performed using IBM SPSS for Windows (version 23.0; IBM Corp., Armonk, NY). Normally distributed data are presented as mean ± SD, skewed variables are presented as median [interquartile range] and dichotomous variables are presented in number (percentage). A 2-tailed P value less than 0.05 was considered statistically significant.
Total, animal, and vegetable protein intake, as well as other macronutrients in the model, were adjusted for total energy intake by the residual method.19 Therefore, we used the mean total caloric intake of our T2DM population in the regression equations. Patients were divided according to gender-specific tertiles of energy-adjusted total protein intake. Normality of data was assessed by visually inspecting the frequency histograms of each variable. Differences in characteristics between these tertiles were tested using the 1-way analysis of variance for normally distributed variables, the Kruskal-Wallis test for skewed variables, and the χ2 test for dichotomous variables.
Univariate and multivariate Cox proportional hazard models with time to event set to 1 year were used to calculate prevalence ratios for renal function impairment in each gender-specific tertile of total, animal, and vegetable protein intake.20, 21 Because of linear associations when using tertiles and to retain power, continuous variables of total, animal, and vegetable protein intake and the eGFR were used in a linear regression model to calculate regression coefficients for each incremental SD of total, animal, and vegetable protein intake on the eGFR.
Confounders were based on relevant differences in characteristics across total protein intake in the baseline table and previous literature.22, 23 Model 1 showed the crude study outcomes, and model 2 was adjusted for age. In the linear regression model, model 2 was additionally adjusted for gender. Model 3 was additionally adjusted for lifestyle variables; that is, diabetes duration (years), BMI (kg/m2), smoking status (current, former, never), physical activity (meet the Dutch Healthy Exercise Norm of 30 minutes moderate intense activity a day for at least 5 days a week), and alcohol intake (<1 unit per month, 1 unit per month – 1 unit per day, >1 unit per day). Model 4 was additionally adjusted for the energy-adjusted dietary factors like saturated fat intake (g/d), unsaturated fat intake (g/d), intake of mono- and disaccharides (g/d), intake of polysaccharides (g/d), intake of trans fatty acids (g/d), and intake of fiber (g/d). In model 4, vegetable protein was adjusted for animal protein intake, and vice versa. Urinary phosphorus excretion (mmol/24 hours) was additionally tested as confounder, to investigate potential differences in bioavailability of phosphorus in tertiles of vegetable and animal protein intake.24
To identify effect modifiers, multiple interaction terms were included in the model. In case of a significant interaction term, we performed a stratified analysis using the fully adjusted model. The use of renin angiotensin aldosterone system (RAAS) inhibitors was additionally tested as an interaction term, because it has been described that the use of RAAS inhibitors interacted with a low-protein diet to cause a short-term decrease in eGFR.25
To investigate the hypothetical prevalence of renal function impairment when replacing other macronutrients with dietary protein, several theoretical replacement models were used.23, 26 A new total energy intake was calculated based on the contribution of 7 macronutrients, represented in energy percent: vegetable protein, animal protein, saturated fatty acids, unsaturated fatty acids, mono- and disaccharides, polysaccharides, and alcohol. These energy-adjusted variables were expressed as continuous variables per 3% of energy intake in the fully adjusted model, as this best matched with the level and variation of intake of the macronutrients. To investigate the influence of replacing animal protein, vegetable protein was included in the model, together with total energy intake and mono- and disaccharides, polysaccharides, saturated fat, unsaturated fat, and alcohol (leaving out animal protein). Each nutritional variable in the model represents the difference in risk on renal function impairment when increasing its intake with 3% of energy, while keeping the other variables constant, at the expense of 3% of energy from animal protein, which was not included in the model.
Sensitivity analysis was performed in which we repeated analyses after exclusion of alcohol intake from the calculation of total energy intake.
Results
Baseline Characteristics
Baseline characteristics by gender-specific tertiles of energy-adjusted total protein intake are shown in Table 1. The total population (n = 420) included 57% male participants, mean age was 63 ± 9 years, and mean BMI was 32.9 ± 6.3 kg/m2, reflecting a predominantly obese T2DM population. Mean total protein intake was 78 ± 12 g/d, corresponding with an intake of 0.83 g/kg per day. Mean animal protein intake was 51 ± 13 g/d and mean vegetable protein intake was 28 ± 5 g/d. Vegetable protein intake across tertiles of total protein intake did not change (P = 0.23), whereas animal protein is significantly positively associated with total protein intake (P < 0.01), illustrating that the variance in total protein intake is determined by the variance in animal protein intake. Intake of unsaturated fat and carbohydrates were significantly lower in the highest tertile of protein intake compared with the lowest tertile of intake. In the highest tertile of total protein intake, patients tended to have a higher BMI, a higher total energy intake, a lower prevalence of retinopathy, and there were fewer current smokers compared with the lowest tertile of total protein intake. In the lowest tertile of total protein intake, patients had a significantly lower prevalence of albuminuria.
Table 1.
Baseline characteristics by gender-specific tertiles of energy-adjusted total protein intake of 420 patients with type 2 diabetes mellitus in the DIAbetes and LifEstyle Cohort Twente-1 population
| Total population | Tertile 1 | Tertile 2 | Tertile 3 | P | |
|---|---|---|---|---|---|
| n | 420 | 140 | 140 | 140 | |
| Age (yr) | 63 ± 9 | 63 ± 9 | 63 ± 9 | 63 ± 9 | 0.87 |
| Diabetes duration [yr] | 11 [6–18] | 11 [7–19] | 12 [7–18] | 11 [4–17] | 0.16 |
| BMI (kg/m2) | 32.9 ± 6.3 | 32.8 ± 5.9 | 32.2 ± 6.2 | 33.7 ± 6.6 | 0.11 |
| Waist-hip ratioa | 1.00 ± 0.09 | 1.00 ± 0.10 | 1.00 ± 0.09 | 1.01 ± 0.09 | 0.55 |
| Smoking, n (%) | 0.19 | ||||
| Current smoker | 65 (16) | 26 (19) | 23 (16) | 16 (11) | |
| Former smoker | 227 (54) | 67 (48) | 82 (59) | 78 (56) | |
| Never smoker | 128 (31) | 47 (34) | 35 (25) | 46 (33) | |
| Alcohol intake, n (%) | 0.85 | ||||
| Low (<1 unit/mo) | 164 (39) | 51 (36) | 60 (43) | 53 (38) | |
| Moderate (1 unit/mo – 1 unit/d) | 136 (32) | 48 (34) | 42 (30) | 46 (33) | |
| High (>1 unit/d) | 120 (29) | 41 (29) | 38 (27) | 41 (29) | |
| Physical activity – adherence to the Dutch Healthy Exercise Norm, n (%) | 249 (59) | 85 (61) | 83 (59) | 81 (58) | 0.89 |
| Systolic blood pressure (mm Hg) | 136 ± 16 | 137 ± 17 | 137 ± 16 | 135 ± 16 | 0.67 |
| Diastolic blood pressure (mm Hg) | 74 ± 10 | 75 ± 10 | 74 ± 10 | 74 ± 9 | 0.97 |
| HbA1c (mmol/mol)a | 57.3 ± 11.9 | 56.3 ± 11.2 | 57.8 ± 13.0 | 57.7 ± 11.5 | 0.52 |
| Urinary urea excretion (mmol/24 h) | 414 ± 148 | 363 ± 132 | 420 ± 142 | 457 ± 154 | <0.01 |
| Nutritional intake | |||||
| Energy (kcal) | 1898 ± 603 | 1880 ± 633 | 1851 ± 576 | 1962 ± 599 | 0.28 |
| Protein (g/d) | 78 ± 12 | 66 ± 7 | 77 ± 3 | 90 ± 8 | <0.01 |
| Animal protein (g/d) | 51 ± 13 | 40 ± 8 | 50 ± 6 | 64 ± 11 | <0.01 |
| Vegetable protein (g/d) | 28 ± 5 | 28 ± 5 | 29 ± 5 | 28 ± 6 | 0.23 |
| Carbohydrates (g/d) | 206 ± 32 | 215 ± 32 | 208 ± 26 | 197 ± 34 | <0.01 |
| Mono/disaccharides (g/d) | 93 ± 26 | 99 ± 30 | 93 ± 22 | 88 ± 25 | 0.01 |
| Polysaccharides (g/d) | 111 ± 24 | 113 ± 26 | 113 ± 23 | 107 ± 23 | 0.07 |
| Fat (g/d) | 77 ± 12 | 78 ± 11 | 77 ± 11 | 76 ± 13 | 0.51 |
| SFA (g/d) | 27 ± 6 | 27 ± 6 | 26 ± 5 | 28 ± 6 | 0.06 |
| UFA (g/d) | 43 ± 8 | 44 ± 8 | 43 ± 8 | 41 ± 8 | 0.01 |
| Transfat (g/d) | 1.71 ± 0.43 | 1.74 ± 0.42 | 1.66 ± 0.39 | 1.73 ± 0.47 | 0.29 |
| Fiber (g/d) | 21 ± 5 | 20 ± 4 | 21 ± 4 | 21 ± 5 | 0.22 |
| Estimated sodium intake (mg/d)a,b | 4250 ± 1833 | 4006 ± 1744 | 4334 ± 1687 | 4404 ± 2033 | 0.16 |
| Complications, n (%) | |||||
| Microvascular complications | 276 (66) | 88 (63) | 94 (67) | 94 (67) | 0.68 |
| Retinopathya | 102 (24) | 39 (28) | 37 (26) | 26 (19) | 0.15 |
| Neuropathy | 150 (36) | 48 (34) | 48 (34) | 54 (39) | 0.69 |
| Diabetic kidney diseasea | 169 (40) | 52 (37) | 62 (45) | 55 (39) | 0.43 |
| Albuminuriaa | 122 (29) | 30 (22) | 50 (36) | 42 (30) | 0.03 |
| eGFR <60 ml/min per 1.73 m2 | 99 (24) | 36 (26) | 33 (24) | 30 (21) | 0.70 |
| Macrovascular complications | 148 (35) | 53 (38) | 50 (36) | 45 (32) | 0.60 |
| Medication use, n (%) | |||||
| RAAS inhibitors | 281 (67) | 95 (68) | 88 (63) | 98 (70) | 0.43 |
| Insulin use | 263 (63) | 83 (59) | 92 (66) | 88 (63) | 0.54 |
| Diuretics | 218 (52) | 75 (54) | 68 (49) | 75 (54) | 0.63 |
The cutoff points for male patients were set at 71.8 g/d and 80.9 g/d and for female patients at 75.1 g/d and 83.2 g/d.
RAAS, renin angiotensin aldosterone system; SFA, saturated fatty acids; UFA, unsaturated fatty acids.
Missing values for waist-hip ratio (n = 6), HbA1c (n = 1), urinary urea excretion (n = 42), estimated sodium intake (n = 5), retinopathy (n = 2), diabetic kidney disease (n = 1), and albuminuria (n = 2).
Based on 24-hour urinary sodium excretion.
Protein Intake and Prevalence of Renal Function Impairment
Among 420 patients with T2DM in this cohort, 99 renal function impairment cases were identified, corresponding with a prevalence rate of 23.6%. The lowest number of cases was found in the highest tertile of energy-adjusted vegetable protein intake. In the multivariate model in which we adjusted for lifestyle and dietary factors, higher vegetable protein intake was significantly associated with a lower prevalence of renal function impairment. The prevalence ratio in the fully adjusted model was 1 (reference category), 0.74 (95% CI: 0.44–1.27; P = 0.28) and 0.47 (95% CI: 0.23–0.98; P = 0.04) according to increasing tertiles of vegetable protein intake (Table 2). In the highest tertile of vegetable protein intake there was a 56% lower prevalence of renal function impairment. No association with renal function impairment was found across tertiles of animal protein intake. In the second tertile, the prevalence ratio was 1.10 (95% CI: 0.64–1.88; P = 0.74) and in the third tertile 1.06 (95% CI: 0.56–1.99; P = 0.87) compared with the bottom tertile.
Table 2.
Prevalence ratios (95% confidence interval) for renal function impairment (estimated glomerular filtration rate <60 ml/min per 1.73 m2) by gender-specific tertiles of energy-adjusted total, animal, and vegetable protein intake among 420 patients with type 2 diabetes mellitus from the DIAbetes and LifEstyle Cohort Twente-1 population
| Tertile 1 | Tertile 2 | Tertile 3 | |
|---|---|---|---|
| Total protein | |||
| Mean ± SD | 66.1 ± 6.6 | 77.5 ± 2.9 | 90.2 ± 8.2 |
| Cases | 36 | 33 | 30 |
| Model 1 | 1 | 0.92 (0.57–1.47) | 0.83 (0.51–1.35) |
| Model 2 | 1 | 0.87 (0.54–1.39) | 0.79 (0.49–1.28) |
| Model 3 | 1 | 0.88 (0.54–1.41) | 0.78 (0.48–1.28) |
| Model 4 | 1 | 0.91 (0.55–1.53) | 0.84 (0.45–1.58) |
| Vegetable protein | |||
| Mean ± SD | 23.3 ± 3.1 | 28.1 ± 1.0 | 34.0 ± 4.3 |
| Cases | 40 | 36 | 23 |
| Model 1 | 1 | 0.90 (0.57–1.41) | 0.58 (0.34–0.96) |
| Model 2 | 1 | 0.82 (0.52–1.29) | 0.59 (0.35–0.99) |
| Model 3 | 1 | 0.81 (0.51–1.29) | 0.58 (0.34–0.98) |
| Model 4 | 1 | 0.74 (0.44–1.27) | 0.47 (0.23–0.98) |
| Animal protein | |||
| Mean ± SD | 38.6 ± 7.5 | 50.6 ± 3.2 | 65.1 ± 9.4 |
| Cases | 30 | 33 | 36 |
| Model 1 | 1 | 1.10 (0.67–1.80) | 1.20 (0.74–1.95) |
| Model 2 | 1 | 1.09 (0.66–1.78) | 1.12 (0.69–1.81) |
| Model 3 | 1 | 1.10 (0.67–1.80) | 1.10 (0.67–1.80) |
| Model 4 | 1 | 1.10 (0.64–1.88) | 1.06 (0.56–1.99) |
Model 1: unadjusted model
Model 2: adjusted for age (years)
Model 3: model 2 and additionally adjusted for diabetes duration (years), body mass index (kg/m2), smoking (current, former, never), physical activity (meet the Dutch Healthy Exercise Norm) and alcohol intake (<1 unit per month, 1 unit per month – 1 unit per day, >1 unit per day)
Model 4: model 3 and additionally adjusted for saturated fat intake (g/d), unsaturated fat intake (g/d), intake of mono- and disaccharides (g/d), intake of polysaccharides (g/d), intake of fiber (g/d), and intake of trans fatty acids (g/d)
Protein Intake and eGFR as Continuous Variable
Linear regression analysis with total, animal, and vegetable protein intake and eGFR as continuous variables showed almost similar results. The incremental intake of 1 SD of vegetable protein intake in the fully adjusted model was associated with a higher eGFR of 6.45 ml/min per 1.73 m2 (95% CI: 3.05–9.85; P < 0.01) (Table 3). The incremental intake of 1 SD of total and animal protein in the fully adjusted model was not significantly associated with alterations in the eGFR. For each incremental SD of total protein, the eGFR was estimated to be −0.23 (95% CI: −2.83 to 2.38; P = 0.86) lower, as well as the incremental SD of animal protein intake for which the eGFR was estimated to be −0.53 (95% CI: −3.36 to 2.30; P = 0.71) lower.
Table 3.
Regression coefficients for the estimated glomerular filtration rate by energy-adjusted total, animal, and vegetable protein intake among 420 patients with type 2 diabetes mellitus from the DIAbetes and LifEstyle Cohort Twente-1 population
| Regression coefficient per SD | |
|---|---|
| Total protein | |
| Mean ± SD | 77.9 ± 11.7 |
| Model 1 | 0.06 (−2.27 to 2.38) |
| Model 2 | 0.51 (−1.44 to 2.47) |
| Model 3 | 0.76 (−1.19 to 2.72) |
| Model 4 | −0.23 (−2.83 to 2.38) |
| Vegetable protein | |
| Mean ± SD | 28.4 ± 5.4 |
| Model 1 | 2.95 (0.65 to 5.26) |
| Model 2 | 2.59 (0.66 to 4.51) |
| Model 3 | 2.63 (0.66 to 4.60) |
| Model 4 | 6.45 (3.05 to 9.85) |
| Animal protein | |
| Mean ± SD | 51.4 ± 13.0 |
| Model 1 | −1.19 (−3.51 to 1.14) |
| Model 2 | −0.64 (−2.59 to 1.32) |
| Model 3 | −0.39 (−2.36 to 1.58) |
| Model 4 | −0.53 (−3.36 to 2.30) |
Model 1: unadjusted model
Model 2: adjusted for age (years)
Model 3: model 2 and additionally adjusted for diabetes duration (years), body mass index (kg/m2), smoking (current, former, never), physical activity (meet the Dutch Healthy Exercise Norm), and alcohol intake (<1 unit per month, 1 unit per month – 1 unit per day, >1 unit per day)
Model 4: model 3 and additionally adjusted for saturated fat intake (g/d), unsaturated fat intake (g/d), intake of mono- and disaccharides (g/d), intake of polysaccharides (g/d), intake of fiber (g/d), and intake of trans fatty acids (g/d)
Replacement of Macronutrients by Protein Sources
Theoretical replacement models for vegetable protein instead of saturated fat, unsaturated fat, mono- and disaccharides and polysaccharides showed significantly lower prevalence ratios of renal function impairment (Table 4, model 9–12). Replacing intake of 3 energy percent from animal protein by vegetable protein instead (model 13) lowered the prevalence ratio for the association with renal function impairment to 0.20 (95% CI: 0.06–0.63; P = 0.01). Replacement of total and animal protein instead of these macronutrients did not statistically significantly influence the prevalence ratio of renal function impairment (model 1–7). Replacing intake of 3 energy percent from vegetable protein by animal protein instead (model 9) increased the prevalence ratio of renal function impairment to 1.31 (95% CI: 0.94–1.83; P = 0.13).
Table 4.
Prevalence ratios (95% confidence intervals) for the association of protein intake and renal function impairment per 3 energy percent from energy-adjusted total, animal, and vegetable protein intake instead of other nutrients
| Prevalence ratio (95% confidence interval) | |
|---|---|
| Total protein | |
| Model 1: protein instead of mono and disaccharides | 0.95 (0.75–1.21) |
| Model 2: protein instead of polysaccharides | 1.01 (0.78–1.30) |
| Model 3: protein instead of SFA | 1.03 (0.75–1.42) |
| Model 4: protein instead of UFA | 0.99 (0.76–1.28) |
| Animal protein | |
| Model 4: animal protein instead of mono and disaccharides | 0.98 (0.77–1.24) |
| Model 5: animal protein instead of polysaccharides | 0.92 (0.70–1.20) |
| Model 6: animal protein instead of SFA | 1.03 (0.74–1.43) |
| Model 7: animal protein instead of UFA | 0.99 (0.76–1.27) |
| Model 8: animal protein instead of vegetable protein | 1.30 (0.93–1.81) |
| Vegetable protein | |
| Model 9: vegetable protein instead of mono and disaccharides | 0.19 (0.06–0.59) |
| Model 10: vegetable protein instead of polysaccharides | 0.25 (0.08–0.79) |
| Model 11: vegetable protein instead of SFA | 0.20 (0.06–0.66) |
| Model 12: vegetable protein instead of UFA | 0.20 (0.06–0.64) |
| Model 13: vegetable protein instead of animal protein | 0.20 (0.07–0.63) |
All models were adjusted for age (years), gender, diabetes duration (years), body mass index (kg/m2), smoking (current, former, never), physical activity (meet the Dutch Healthy Exercise Norm), alcohol intake (<1 unit per month, 1 unit per month – 1 unit per day, >1 unit per day), saturated fat intake (g/d), unsaturated fat intake (g/d), intake of mono- and disaccharides (g/d), intake of polysaccharides (g/d), intake of fiber (g/d), and intake of trans fatty acids (g/d).
SFA, saturated fatty acids; UFA, unsaturated fatty acids.
Additional Analyses
The interaction term of total protein intake and use of RAAS inhibitors in the linear regression analysis was not significantly associated with a higher eGFR of 2.67 per incremental SD of total protein intake (95% CI: −0.78 to 6.11; P = 0.13); however, the use of RAAS inhibitors itself was significantly associated with a lower eGFR of −25.36 (95% CI: −48.51 to −2.22; P = 0.03). Other variables in the fully adjusted model show no significant interaction terms.
Urinary phosphorus excretion was significantly lower in those with a reduced eGFR (23.9 ± 7.6 mmol/24 hours in patients with chronic kidney disease [CKD] versus 28.4 ± 12.4 mmol/24 hours in patients without CKD, P < 0.01). In addition, patients with CKD consume significantly less vegetable protein (27.2 ± 4.4 g/d in patients with CKD vs. 28.8 ± 5.6 g/d in patients without CKD, P < 0.01). However, there was no difference in urinary phosphorus excretion across tertiles of vegetable protein intake (28.3 ± 12.9 vs. 25.8 ± 10.1 vs. 28.0 ± 11.2 mmol/24 hours for tertiles 1, 2, and 3, respectively, P = 0.14).
In food group analyses, we found that red meat and dairy were the most important contributors to animal protein intake (Appendix S2). Red meat is associated with a higher prevalence of renal function impairment in the third tertile compared with the bottom tertile (prevalence ratio: 1.41; 95% CI: 0.83–2.41; P = 0.21), whereas dairy is not associated with renal function impairment (prevalence ratio: 0.90; 95% CI: 0.52–1.58; P = 0.72) in the third tertile compared with the first tertile (Appendix S3). In the vegetable-based food groups we see that cereals were the most important contributors, associated with a nonsignificant lower prevalence of renal function impairment (prevalence ratio: 0.67; 95% CI: 0.35–1.28; P = 0.22).
We performed sensitivity analyses in which we repeated analyses after exclusion of alcohol intake from the calculation of total energy intake. The results of these analyses remained materially unchanged compared with the primary analyses.
Discussion
In this cross-sectional study among 420 patients with long-standing T2DM, we investigated the relationship between sources of dietary protein and renal function impairment. The main finding was that vegetable protein intake was clearly associated with a lower prevalence of renal function impairment, and this association was confirmed when eGFR was analyzed as a continuous variable. Moreover, a replacement analysis shows an inverse association with renal function impairment when replacing animal protein with vegetable protein.
A beneficial association between vegetable protein intake and DKD has previously been suggested in a review that compared different dietary patterns and their effect on DKD progression.27 However, extensive differences exist between the various plant-based diets (e.g., soy-based vs. non–soy-based, vegetarian vs. vegan diets) and the total protein content could be lower in specific plant-based diets compared with conventional diets.
We are aware of one other report evaluating dietary protein sources in relation to renal function: a substudy in patients with T2DM of the landmark “Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET)”.12 The ONTARGET investigators studied prospectively the impact of dietary protein source on development of DKD. They found an almost significant inverse association between tertiles of absolute vegetable protein intake and development of DKD during 5.5 years of follow-up (odds ratio: 0.86; 95% CI: 0.74–1.01), which is in line with our findings. However, the overall protein intake in ONTARGET was lower compared with our study: 0.53 [0.34–0.82] versus 0.79 [0.58–1.07] g/kg per day for total protein and 0.09 [0.03–0.17] versus 0.28 [0.19–0.39] g/kg per day for vegetable protein. Because protein restriction of 0.8 g/kg per day is reached more often in their study, the results of ONTARGET could result from the low total protein intake.
In addition to independent replication of the ONTARGET study, the association between vegetable protein intake and lower prevalence of renal function impairment is strengthened by our theoretical replacement model. This is an isocaloric model, in which a new energy intake variable is calculated, based on all macronutrients and is held constant.28, 29 Particularly the clearly lower prevalence of renal function impairment when replacing animal protein with vegetable protein is of interest, because this beneficial replacement was also shown in a systematic review with glycemic control as study outocome.30 Shifting the focus of a lower carbohydrate content showed also a strong significant result related to the prevalence of renal function impairment when replacing carbohydrates with vegetable protein. This beneficial association was also found in a prospective study with respect to all-cause mortality in patients with T2DM.31
In comparison with other European diabetes populations, the vegetable protein intake in our cohort was more or less similar (27.5 g/d vs. 26.1 g/d), whereas the total and animal protein intake were somewhat lower in our study population (78.2 g/d vs. 94.2 g/d and 50.7 g/d vs. 59.6 g/d, respectively),31 which probably reflects regional differences in dietary habits.
Although the mechanisms behind the association between vegetable protein and lower prevalence of renal function impairment are unknown, it might be related to harmful effects related to variable bioavailability of phosphorus depending on the sources of protein. Phosphate in vegetable protein is complexed in the form of phytic acid, which is less digestible compared with phosphate from animal protein,32 and the more digestible phosphate additives are commonly used in processed foods of mainly animal origin.32, 33 In contrast to previous studies regarding source of protein and phosphaturia,24, 33 however, we found no significant difference in urinary phosphate excretion across tertiles of vegetable protein intake (P = 0.14), which may be due to variability in urinary phosphate excretion.
The strength of the DIALECT-1 cohort is that it reflects the real world of patients with T2DM and it was specifically designed to evaluate lifestyle characteristics such as dietary habits, with minimal selection bias, compared with clinical trial populations. Obviously, the cross-sectional nature of the study prevents making causal inferences about the dietary protein pattern and renal function. We cannot completely rule out reverse causation bias (i.e., that some patients had changed their diet or other lifestyle habits after developing renal function impairment), although this seems less likely because none of the patients were followed in our specific predialysis clinic. Further, we cannot distinguish between reversible hemodynamic effects of protein intake and chronic effects on development of kidney damage. The protein intake was based on self-report. Although the FFQ has some limitations, this is still the best method available in studies of this size, and the FFQ is a valid method to rank individuals according to their intake and this was confirmed by objective measurements of urinary urea excretion.
With regard to dietary protein, the current Kidney Disease: Improving Global Outcomes guideline recommends a restricted total protein intake of 0.8 g/kg per day in patients with T2DM and eGFR <30 ml/min per 1.73 m2.34 Previous research shows that each incremental 0.1 g/kg per day of total protein intake increases the risk for end-stage renal disease (hazard ratio: 1.05; 95% CI: 1.04–1.14) in patients with diabetes.35 This was not confirmed in our study, with a nonstatistically lower eGFR of −0.23 ml/min per 1.73 m2 (95% CI: −2.83 to 2.38; P = 0.86) for each incremental intake of 1 SD of total protein. According to the results from ONTARGET12 and ours, it seems that these recommendations need to be reconsidered. The data strongly suggest that it is important to differentiate between intake of animal and vegetable protein. There is no evidence for a benefit of restricting vegetable protein and, rather than focusing on protein restriction, it appears more appropriate to replace animal proteins with vegetable proteins. For T2DM in general, to maintain a low-carbohydrate diet as recommended in diabetes guidelines, a specific advice could be to focus on replacement of carbohydrates with vegetable protein. Without this specific notion, many patients will replace carbohydrates for a large part by animal protein, which seems unwarranted with the available evidence.
In the older literature, it has been described that the use of RAAS inhibitors interacted with a low-protein diet to cause a short-term decrease in eGFR.25 We did not find such an interaction, but it should be stated that our study design was not appropriate to this end. However, in patients treated with RAAS inhibitors it may be wise to consider the development of hyperkalemia when increasing vegetable protein intake.36, 37
Regarding protein-containing products, our results indicate that red meat intake, in particular, is associated with a higher prevalence of renal function impairment, although not significant. Whereas this item was not investigated in their study, the ONTARGET investigators found with respect to vegetable protein intake that frequent consumption of fruits reduced the risk of DKD (ORDKD3vs1: 0.77; 95% CI: 0.64–0.92), which is in line with our findings.
These dietary approaches can be merged together as being a plant-based diet in terms of decreasing red meat intake and increasing the intake of cereals, fruits, and nuts,38, 39, 40, 41, 42 which have previously been demonstrated to contribute to an improved glycemic control in patients with T2DM.43 In addition, other impacts of plant-based diets have been clearly demonstrated: less acid load which in turn slows CKD progression,44 a decrease in inflammatory biomarkers45 and its contribution in maintaining a metabolically balanced gut microbiota.46
From a patient perspective, it is difficult to convert from a predominantly animal protein diet to a vegan diet. Therefore, food-based guidelines or nutritional patterns would be of great value instead of nutrient-based guidelines, especially for patients with T2DM in whom conflicting advice is provided to address each macrovascular complication. A prospective analysis to investigate the longitudinal effects of both pharmacological and nutritional therapy on eGFR decline over years is needed to improve our understanding of the association between sources of protein intake and progression of renal function impairment. However, a critical appraisal in evaluating the nutritional management of protein intake is adherence to the diet, so establishing an infrastructure that allows the monitoring of adherence would be of great value.
Conclusion
In conclusion, we found that higher intake of vegetable protein was associated with a lower prevalence of renal function impairment, and theoretical replacement of animal protein with vegetable protein was inversely associated with renal function impairment among patients with T2DM.
Disclosure
JMG reports grants from Unilever, during the conduct of the study. GDL reports grants and personal fees from AstraZeneca, grants and personal fees from Sanofi, and grants from Novo Nordisk, outside the submitted work. All the other authors declared no competing interests.
Acknowledgments
The authors received funding from the Ziekenhuis Groep Twente research fund. We thank Else van den Berg, Willeke van Kampen, Sanne van Huizen, Anne Davina, Jolien Jaspers, and Manon Harmelink for their contribution to patient inclusion.
Footnotes
Appendix S1. Overview of food items included in each food category.
Appendix S2. Proportions of food groups for animal and vegetable protein.
Appendix S3. Prevalence ratios for food groups.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Overview of food items included in each food category.
Proportions of food groups for animal and vegetable protein.
Prevalence ratios for food groups.
References
- 1.World Health Organization . WHO Press; Geneva, Switzerland: 2016. Global Report on Diabetes. [Google Scholar]
- 2.International Diabetes Federation . 8th Edition. International Diabetes Federation; Brussels, Belgium: 2017. IDF Diabetes Atlas. [Google Scholar]
- 3.American Diabetes Association Microvascular complications and foot care: standards of medical care in diabetes-2018. Diabetes Care. 2018;41:S105–S118. doi: 10.2337/dc18-S010. [DOI] [PubMed] [Google Scholar]
- 4.Lea J.P., Nicholas S.B. Diabetes mellitus and hypertension: key risk factors for kidney disease. J Ntl Med Assoc. 2002;94(8 Suppl):7S–15S. [PMC free article] [PubMed] [Google Scholar]
- 5.Kazancioğlu R. Risk factors for chronic kidney disease: an update. Kidney Int Suppl. 2013;3:368–371. doi: 10.1038/kisup.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Diabetes Association Lifestyle management: standards of medical care in diabetes - 2018. Diabetes Care. 2018;41(suppl 1):S35–S50. doi: 10.2337/dc18-S004. [DOI] [PubMed] [Google Scholar]
- 7.Feinman R.D., Pogozelski W.K., Astrup A. Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base. Nutrition. 2015;31:1–13. doi: 10.1016/j.nut.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Rydén L., Grant P.J., Anker S.D. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: The Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD) Eur Heart J. 2013;34:3035–3087. doi: 10.1093/eurheartj/eht108. [DOI] [PubMed] [Google Scholar]
- 9.Westman E.C., Feinman R.D., Mavropoulos J.C. Low-carbohydrate nutrition and metabolism. Am J Clin Nutr. 2007;86:276–284. doi: 10.1093/ajcn/86.2.276. [DOI] [PubMed] [Google Scholar]
- 10.Fougue D., Laville M., Boissel J.P. Low protein diets for chronic kidney disease in non diabetic adults. Cochrane Database Syst Rev. 2009;3:CD001892. doi: 10.1002/14651858.CD001892.pub3. [DOI] [PubMed] [Google Scholar]
- 11.Ko G.J., Kalantar-Zadeh K., Goldstein-Fuchs J. Dietary approaches in the management of diabetic patients with kidney disease. Nutrients. 2017;9:824. doi: 10.3390/nu9080824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunkler D., Kohl M., Teo K.K. Dietary risk factors for incidence or progression of chronic kidney disease in individuals with type 2 diabetes in the European Union. Nephrol Dial Transplant. 2015;30(suppl 4):iv76–iv85. doi: 10.1093/ndt/gfv086. [DOI] [PubMed] [Google Scholar]
- 13.Gant C.M., Binnenmars S.H., van den Berg E. Integrated assessment of pharmacological and nutritional cardiovascular risk management: blood pressure control in the DIAbetes and LifEstyle Cohort Twente (DIALECT) Nutrients. 2017;9:709. doi: 10.3390/nu9070709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Institute for Public Health and the Environment (RIVM). Dutch Food Composition Database-NEVO online version 2013/4.0. Bilthoven, the Netherlands: RIVM; 2013.
- 15.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levey A.S., Eckardt K., Tsukamoto Y. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 17.Wendel-Vos G.C.W., Schuit A.J., Saris W.H.M. Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. J Clin Epidemiol. 2003;56:1163–1169. doi: 10.1016/s0895-4356(03)00220-8. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization . WHO Press; Geneva, Switzerland: 2010. Global recommendations on Physical Activity for Health. [PubMed] [Google Scholar]
- 19.Willet W.C., Howe G.R., Kushi L.H. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(suppl 4):1220S–1228S. doi: 10.1093/ajcn/65.4.1220S. [DOI] [PubMed] [Google Scholar]
- 20.Barros A.J.D., Hirakata V.N. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3:21. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson M.L., Myers J.E., Kriebel D. Prevalence odds ratio or prevalence ratio in the analysis of cross sectional data: what is to be done? Occup Environ Med. 1998;55:272–277. doi: 10.1136/oem.55.4.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Onyenwenyi C., Ricardo A.C. Impact of lifestyle modification on diabetic kidney disease. Curr Diab Rep. 2015;15:60. doi: 10.1007/s11892-015-0632-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunkler D., Kohl M., Heinze G. Modifiable lifestyle and social factors affect chronic kidney disease in high-risk individuals with type 2 diabetes mellitus. Kidney Int. 2015;87:487–791. doi: 10.1038/ki.2014.370. [DOI] [PubMed] [Google Scholar]
- 24.Moe S.M., Zidehsarai M.P., Chambers M.A. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:257–264. doi: 10.2215/CJN.05040610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gansevoort R.T., de Zeeuw D., de Jong P.E. Additive antiproteinuric effect of ACE inhibition and a low-protein diet in human renal disease. Nephrol Dial Transplant. 1995;10:497–504. doi: 10.1093/ndt/10.4.497. [DOI] [PubMed] [Google Scholar]
- 26.Færch K., Lau C., Tetens I. A statistical approach based on substitution of macronutrients provides additional information to models analysing single dietary factors in relation to type 2 diabetes in Danish adults: the Inter99 study. J Nutr. 2005;135:1177–1182. doi: 10.1093/jn/135.5.1177. [DOI] [PubMed] [Google Scholar]
- 27.Moorthi R.N., Vorland C.J., Hill Gallant K.M. Diet and diabetic kidney disease: plant versus animal protein. Curr Diab Rep. 2017;17:15. doi: 10.1007/s11892-017-0843-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu S, van der Schouw YT, Soedamah-Muthu SS, et al. Intake of dietary saturated fatty acids and risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition – Netherlands cohort: associations by types, sources of fatty acids and substitution by macronutrients [e-pub ahead of print]. Eur J Nutr. https://doi.org/10.1007/s00394-018-1630-4. [DOI] [PMC free article] [PubMed]
- 29.Willet W.C. Issues in analysis and presentation of dietary data. In: Willet W.C., editor. Vol 3. Oxford University Press; New York, NY: 1989. pp. 305–333. (Nutrtitional Epidemiology). [Google Scholar]
- 30.Viguiliouk E., Stewart S.E., Jayalath V.H. Effect of replacing animal protein with plant protein on glycaemic control in diabetes: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2015;7:9804–9824. doi: 10.3390/nu7125509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campmans-Kuijpers M.J., Sluijs I., Nöthlings U. Isocaloric substitution of carbohydrates with protein: the association with weight change and mortality among patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:39. doi: 10.1186/s12933-015-0202-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCarty M.F., DiNicolantonio J.J. Bioavailable dietary phosphate, a mediator of cardiovascular disease, may be decreased with plant-based diets, phosphate binders, niacin, and avoidance of phosphate additives. Nutrition. 2014;30:739–747. doi: 10.1016/j.nut.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 33.Scialla J.J., Appel L.J., Wolf M. Plant protein intake is associated with fibroblast growth factor 23 and serum bicarbonate in patients with CKD: The Chronic Renal Insufficiency Cohort Study. J Ren Nutr. 2012;22:379–388. doi: 10.1053/j.jrn.2012.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kidney Disease: Improving Global Outcomes (KDIGO) KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013;3:73–90. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 35.Metzger M., Yuan W.L., Haymann J.-P. Association of a low-protein diet with slower progression of CKD. Kidney Int Rep. 2018;3:105–114. doi: 10.1016/j.ekir.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weir M.R., Role M. Potassium homeostasis and renin-angiotensin-aldosterone system inhibitors. Clin J Am Soc Nephrol. 2010;5:531–548. doi: 10.2215/CJN.07821109. [DOI] [PubMed] [Google Scholar]
- 37.Malta D., Arcand J., Ravindran A. Adequate intake of potassium does not cause hyperkalemia in hypertensive individuals taking medications that antagonize the renin angiotensin aldosterone system. Am J Clin Nutr. 2016;104:990–994. doi: 10.3945/ajcn.115.129635. [DOI] [PubMed] [Google Scholar]
- 38.Salas-Salvadó J., Guasch-Ferré M., Lee C.H. Protective effects of the Mediterranean diet on type 2 diabetes and metabolic syndrome. J Nutr. 2016;146:920S–927S. doi: 10.3945/jn.115.218487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chauveau P., Aparicio M., Bellizzi V. Mediterranean diet as the diet of choice for patients with chronic kidney disease. Nephrol Dial Transplant. 2017;33:725–735. doi: 10.1093/ndt/gfx085. [DOI] [PubMed] [Google Scholar]
- 40.Haring B., Selvin E., Liang M. Dietary protein sources and risk for incident chronic kidney disease: results from the Atherosclerosis Risk in Communities (ARIC) Study. J Ren Nutr. 2017;24:233–242. doi: 10.1053/j.jrn.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kovesdy C.P., Kalantar-Zadeh K. DASH-ing toward improved renal outcomes: when healthy nutrition prevents incident chronic kidney disease. Nephrol Dial Transplant. 2017;32(suppl_2):ii231–ii233. doi: 10.1093/ndt/gfw247. [DOI] [PubMed] [Google Scholar]
- 42.Osté M.C.J., Gomes-Neto A.W., Corpeleijn E. Dietary Approach to Stop Hypertension (DASH) diet and risk of renal function decline and all-cause mortality in renal transplant patients. Am J Transplant. 2018;18:2523–2533. doi: 10.1111/ajt.14707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yokoyama Y., Barnard N.D., Levin S.M. Vegetarian diets and glycaemic control in diabetes: a systematic review and meta-analysis. Cardiovasc Diagn Ther. 2014;4:373–382. doi: 10.3978/j.issn.2223-3652.2014.10.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goraya N., Wesson D.E. Dietary management of chronic kidney disease: protein restriction and beyond. Curr Opin Nephrol Hypertens. 2012;21:635–640. doi: 10.1097/MNH.0b013e328357a69b. [DOI] [PubMed] [Google Scholar]
- 45.Haghighatdoost F., Bellissimo N., Totosy de Zepetnek J.O. Association of vegetarian diet with inflammatory biomarkers: a systematic review and meta-analysis of observational studies. Public Health Nutr. 2017;20:2713–2721. doi: 10.1017/S1368980017001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramezani A., Raj D.S. The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol. 2013;25:657–670. doi: 10.1681/ASN.2013080905. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Overview of food items included in each food category.
Proportions of food groups for animal and vegetable protein.
Prevalence ratios for food groups.