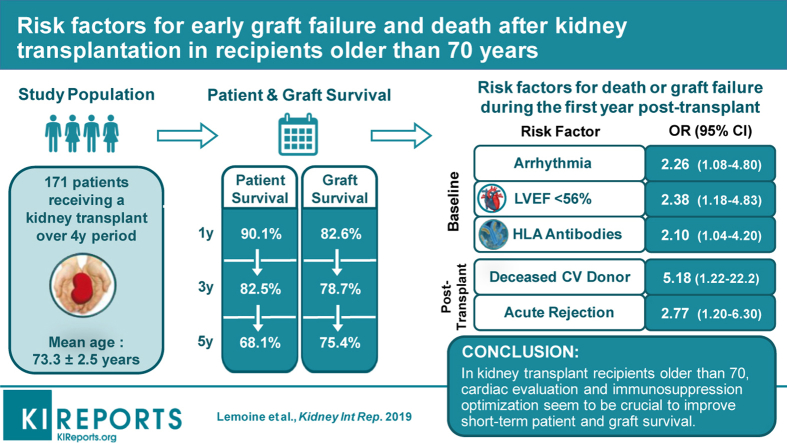
Abstract
Introduction
Although kidney transplantation carries a survival benefit compared with dialysis, mortality, especially the first year after transplantation, is high in recipients older than 70. The aim of this study was to evaluate early death and graft failure, and to determine the risk factors associated with these events in this specific population.
Methods
All patients older than 70 years who received a kidney transplant between January 2000 and December 2014 in the North-West of France were included (n = 171). Baseline characteristics and outcomes after transplantation were studied. Kaplan-Meier analysis was performed to assess patient and graft survival, and Cox regression analysis to evaluate risk factors for graft failure and patient death.
Results
The mean recipient age was 73.3 ± 2.5 years. Death-censored graft survival at 1, 3, and 5 years were 82.6%, 78.7%, and 75.4%, respectively. Patient survival at 1, 3, and 5 years was 90.1%, 82.5%, and 68.1%, respectively. One year after transplantation, 17 patients (9.9%) were dead, mainly from infectious (58.5%) or cardiovascular disease (29.4%). According to the Cox multivariate analysis, the independent risk factors for death or graft failure during the first year were arrhythmia (odds ratio [OR] 2.26; 95% confidence interval [CI] 1.08–4.8), left-ventricular ejection fraction (LVEF) under 56% (OR 2.38; 95% CI 1.18–4.83), human leucocyte antigen (HLA) antibodies (OR 2.1; 95% CI 1.04–4.2), deceased donor from cardiovascular cause (OR 5.18; 95% CI 1.22–6.3), and acute rejection (OR 2.77; 95% CI 1.2–6.3).
Conclusion
In kidney transplant recipients older than 70 years, cardiac evaluation and immunosuppression optimization seem to be crucial to improve short-term patient and graft survival.
Keywords: cardiovascular disease, elderly, graft failure, infectious disease, kidney transplantation, patient death
Graphical abstract
See Commentary on Page 640
In recent years, the number of patients older than 70 years with end-stage renal disease (ESRD) has increased both in Europe and the United States.1, 2, 3 In France, 50% of patients starting renal replacement therapy are older than 70 years,2 and more than 30% of patients with newly diagnosed ESRD are older than 70 in the United States.1
Kidney transplantation (KT) is a safe procedure improving life expectancy and quality of life in patients requiring renal replacement therapy. Therefore, the number of elderly patients receiving a kidney transplant is also growing,1, 2, 3 and, in Europe, the median age of kidney transplant recipients (KTRs) has increased by +10 years over the past 2 decades.3 Although mortality and graft loss after KT increase with the recipient’s age,4, 5 the benefits compared with dialysis have been demonstrated even in these older patients.6, 7, 8, 9 Rao and colleagues6 found that elderly KTR had a 41% lower overall risk of death compared with wait-listed patients. However, mortality is still high during the first months posttransplantation in this specific population.6, 10, 11 It can be explained by the frailty of these patients, their frequent comorbidities, including cardiovascular disease,12 and a gradual deterioration of their immune system known as “immunosenescence.”13
Therefore, despite proven benefits, these frail and comorbid patients have a reduced access to the waiting list,14 which can be explained by contraindications, but also by the reluctance of clinicians due to organ shortage and uncertain early outcomes. To date, there is a lack of studies focusing on early outcomes in KTRs older than 70 years. Indeed, most observational studies include patients older than 65 years, but their results cannot be extrapolated to recipients older than 70 considering their small proportion. In this context, it is crucial to determine predictive factors that could help clinicians to select these elderly patients for transplantation, and thereby improve their early outcomes.
The aim of this study was to evaluate patient death and graft failure during the first year following KT in recipients older than 70 years, and to determine risk factors associated with these major events.
Subjects and Methods
Study Population
We performed a multicenter retrospective study in 4 kidney transplant centers in the North-West of France. All patients older than 70 years who received a kidney transplant between January 2000 and December 2014 were included. The follow-up period was at least 1 year, and the data were retrospectively reviewed in each local medical record.
In France, the allocation system is not exactly based on the old for old Eurotransplant system. When 2 kidneys are removed, 1 of them is allocated to the local graft team. At the local level, kidney graft allocation is based on the application of a score that considers the length of registration on the waiting list and on dialysis, the number of HLA incompatibilities, the differential age between donor and recipient (difference <15 years), the distance between the sample and grafting sites, and the indicator of difficulty to access to the graft. The second kidney is allocated based on national priorities (hyperimmunized patients and children). In the absence of national priority, the allocation system is based on the previously described score. As in the old for old Eurotransplant system, old grafts are allocated to elderly recipients, and short cold ischemia time should be achieved, but a difference is that HLA compatibility is also considered.
All the research procedures were approved by the local ethics committee of Rouen, France (E2016-37).
Outcome Variables
The data recorded at baseline included recipient characteristics at the time of transplantation, such as age, sex, body mass index, cause of ESRD, comorbidities (hypertension, diabetes, cardiomyopathy, LVEF, arrhythmia, atrial fibrillation or flutter, coronary artery disease, and peripheral vascular disease), type and duration of dialysis, and panel-reactive antibodies. Hypertension was defined as a pretransplantation office or clinic blood pressure ≥140/90 mm Hg, measured in sitting position after 5 minutes of rest, or as the prescription of 1 or more antihypertensive treatment. Cardiovascular pretesting protocol consisted of systematic echocardiography, electrocardiogram, and stress test. Coronarography was necessary if stress test was not normal. LVEF was evaluated by transthoracic echocardiography. The term cardiomyopathy included ischemic, valvular, and hypertensive cardiopathies.
We also gathered information on donor (type, age, and cause of death), graft (cold ischemic time, HLA mismatches, delayed graft function, duration of initial hospitalization, perioperative complications), and immunosuppressive (IS) therapy. Delayed graft function was defined as a requirement for dialysis within the first week of transplantation.
All the adverse events occurring during the first year of transplantation were studied, such as infectious diseases, cardiovascular events, urologic complications, cancer or hemopathy, and biopsy-proven acute rejection (BPAR). BK virus reactivation was defined as a plasma BK virus level >104 copies/ml. Cytomegalovirus infection was defined as the presence of viral load >104 copies/ml or clinical symptoms of specific organ infection with a positive result on culture. Severe infection was defined as an infectious disease leading to hospitalization or patient death. Recurrent urinary tract infections were defined as at least 2 episodes of urinary tract infection during the first year of transplantation. Acute humoral and cellular rejections were defined according to Banff classification.15
At 3 and 12 months, serum creatinine level, IS therapy, and drug concentrations were also studied. The estimated glomerular filtration rate was calculated based on the Modification of Diet in Renal Disease formula.
Main outcomes during the first year of transplantation were graft failure and patient death. Graft failure was defined as the need for renal replacement therapy for more than 3 months. Information on the cause of these events also was collected.
Finally, we also studied patient and graft survival of the entire cohort at the end of the follow-up period, in December 2015.
Statistical Analysis
Quantitative variables were expressed as mean ± SD, whereas qualitative variables were expressed in numbers and percentages. The Kaplan-Meier method was used to assess patient and graft survival. Cox regression analysis was used to evaluate the potential risk factors for graft failure or patient death during the first year of KT. We chose to perform 2 different Cox regression analyses. The first included the baseline characteristics, which can be evaluated by the clinician before the inscription on the waiting list. The second included the posttransplantation data, which are important to monitor during follow-up. The proportional hazard assumption was tested visually by the aspect of the Kaplan-Meier curve to estimate if there was severe violation of the hypothesis. A receiver operating characteristic curve was used to calculate the area under the receiver operating characteristic curve to identify the optimal cutoff value for LVEF to predict risk of death or graft failure in KTRs. All factors with P < 0.1 in the univariate analysis were included in the multivariate model. A P < 0.05 was considered statistically significant.
Results
Baseline Characteristics
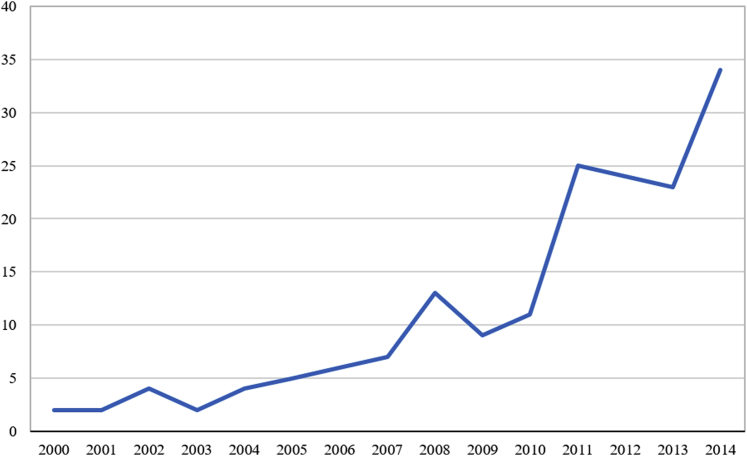
From January 2000 through December 2014, 171 patients were included. The mean follow-up was 3.5 ± 3.1 years. KT in elderly patients increased dramatically over the study period, from 2 cases annually in 2000, to 34 cases in 2014 (Figure 1).
Figure 1.
Number of annual kidney transplantations in patients older than 70 during the study period.
The baseline characteristics of the study population are described in Table 1. The mean age of recipients was 73.3 ± 2.5 years (range, 70–82 years) and most of them were men (67.8%). A total of 146 patients (85.8%) needed dialysis before the KT and the mean time on dialysis was 2.7 ± 2.5 years (range, 0–14 years). Diabetes (16.4%, n = 28) and hypertension (15.8%, n = 27) were the main causes of ESRD besides unknown causes (19.9%, n = 34).
Table 1.
Baseline characteristics of the KT recipients older than 70 years (n = 171)
| Recipient | All | Death <1 yr (n = 17) | |
|---|---|---|---|
| Age, yr (mean ± SD) | 73.3 ± 2.5 | 74.0 ± 2.6 | |
| Range, yr | 70.0–82.4 | 70.0–78.5 | |
| Male, n (%) | 116 (67.8) | 12 (70.6) | |
| BMI, kg/m2 (mean ± SD) | 25.5 ± 3.4 | 23.8 ± 1.9 | |
| Primary renal disease, n (%) | |||
| Diabetes | 28 (16.4) | 2 (11.8) | |
| Vascular | 27 (15.8) | 7 (41.2) | |
| Interstitial | 19 (11.1) | 2 (11.8) | |
| Glomerular | 25 (14.6) | 1 (5.9) | |
| Polycystic | 23 (13.5) | 2 (11.8) | |
| Unknown | 34 (19.9) | 2 (11.8) | |
| Mode of dialysis, n (%) | |||
| Preemptive transplant | 24 (14) | 0 (0) | |
| Hemodialysis | 123 (72.8) | 15 (88.2) | |
| Peritoneal dialysis | 22 (13) | 2 (11.8) | |
| Time on dialysis before transplant, yr (mean ± SD) | 2.7 ± 2.5 | 3.9 ± 2.8 | |
| Waiting time, yr (mean ± SD) | 0.97 ± 0.97 | 1.2 ± 0.9 | |
| HLA antibodies, n (%) | 38 (22.2) | 6 (35.3) | |
| Comorbidities, n (%) | |||
| Hypertension | 159 (95.2) | 16 (94.1) | |
| Diabetes | 40 (23.9) | 3 (17.6) | |
| Coronary artery disease | 35 (20.9) | 6 (35.3) | |
| Cardiomyopathy | 72 (43.1) | 10 (58.8) | |
| Peripheral vascular disease | 24 (14.1) | 3 (17.6) | |
| Arrhythmia | 28 (16.8) | 5 (29.4) | |
| Plasma albumin (g/L) | 39.7 ± 4.6 | 40 ± 0.5 | |
| Donor | |||
| Deceased donor, n (%) | 168 (98.2) | 17 (100) | |
| Deceased from cardiovascular cause | 121 (72.9) | 15 (88.2) | |
| Age, yr (mean ± SD) | 70.1 ± 10.5 | 70 ± 7.5 | |
| Transplantation characteristics | |||
| Cold ischemic time, h (mean ± SD) | 17.8 ± 5 | 17.3 ± 2.6 | |
| Delayed graft function, n (%) | 48 (30.6) | 3 (17.6) | |
| Initial hospitalization duration | 22.3 ± 22 | 31.1 ± 32 | |
| Range, d | 3–173 | 4–140 |
BMI, body mass index; HLA, human leucocyte antigen; KT, kidney transplant.
In our population, most of the donors were deceased (98.2%, n = 168), mainly from cardiovascular cause (72.9%, n = 121). Their mean age was 70.1 ± 10.5 years (range, 17–88 years) and 156 donors (91.2%) were expanded criteria donors (ECDs).
After the KT, the initial hospitalization duration was 22.3 ± 22 days (range, 3–173 days) and one-third of the recipients presented a delayed graft function. For 12 recipients (7%), dialysis could not be stopped after the KT. All the patients received an induction therapy by interleukin-2 receptor antagonist, 20 mg at day 0 and day 4 (81.5%, n = 137) or thymoglobulin, 250 to 300 mg spread over 4 to 5 days (18.5%, n = 31). In the 4 centers, the choice of the induction treatment was based on panel-reactive antibody. Most of the patients received calcineurin inhibitors (tacrolimus trough level 8–12 ng/dl or cyclosporine trough level 200–300 ng/ml), mycophenolate mofetil (2 g daily), and corticosteroids (10 mg daily) as initial IS therapy. IS regimen during the first year of transplantation is detailed in Table 2.
Table 2.
Detailed immunosuppressive regimen during the first year of transplantation
| Induction, n (%) | |
| IL-2 receptor antagonist | 137 (81.5) |
| Thymoglobulin | 31 (18.5) |
| Initial immunosuppressive therapy, n (%) | |
| Corticosteroids | 166 (100) |
| Tacrolimus | 98 (59) |
| Cyclosporine | 68 (41) |
| Mycophenolate mofetil | 165 (99.4) |
| First month, n (%) or mean ± SD | |
| Tacrolimus | 97 (58.4) |
| Trough level (ng/ml) | 8.8 ± 3 |
| Cyclosporine | 66 (39.8) |
| Trough level (ng/ml) | 222 ± 99 |
| Corticosteroids | 166 (100) |
| Dose (mg/d) | 21.3 ± 12.2 |
| Mycophenolate mofetil | 165 (99.4) |
| Dose (mg/d) | 2027 ± 480 |
| Azathioprine | 1 (0.6) |
| mTOR inhibitor | 4 (2.4) |
| Trough level (ng/ml) | 5 ± 2.6 |
| Belatacept | 3 (1.8) |
| Third month, n (%) or mean ± SD | |
| Tacrolimus | 78 (52.7) |
| Trough level | 8.1 ± 2.8 |
| Cyclosporine | 57 (39) |
| Trough level | 152 ± 76 |
| Corticosteroids | 135 (91.2) |
| Dose | 10 ± 7.8 |
| Mycophenolate mofetil | 131 (89.1) |
| Dose | 1597 ± 607 |
| Azathioprine | 1 (0.7) |
| mTOR inhibitor | 7 (4.8) |
| Trough level | 8.6 ± 3.3 |
| Belatacept | 5 (3.4) |
| Twelfth month, n (%) or mean ± SD | |
| Tacrolimus | 70 (53) |
| Trough level | 7 ± 3.4 |
| Cyclosporine | 50 (37.9) |
| Trough level | 121 ± 44 |
| Corticosteroids | 88 (65.7) |
| Dose | 6.1 ± 5.9 |
| Mycophenolate mofetil | 107 (82.3) |
| Dose | 1350 ± 599 |
| Azathioprine | 4 (3.1) |
| mTOR inhibitor | 8 (6) |
| Trough level | 8.4 ± 6.2 |
| Belatacept | 4 (3) |
IL, interleukin; mTOR, mammalian target of rapamycin.
Adverse Events
During the first year of transplantation, the average duration of hospitalization was 49 ± 45 days (range, 8–357 days). Table 3 shows the adverse events during this period. The most common side effects were infectious diseases (83.2%, n = 134), and 96 patients (60%) had 1, or more, severe infection. Sixty-seven patients (42.9%) experienced a cytomegalovirus infection and 16 (10.3%) a BK virus reactivation. Recurrent urinary tract infections (18.7%, n = 29) and pneumonitis (19.6%, n = 31) were frequent in this geriatric population. Seventy-three patients (45.1%) suffered from a cardiovascular event, mainly acute decompensated heart failure (13.5%), thrombosis (12.9%), and new-onset atrial fibrillation (11.1%).
Table 3.
Adverse events during the first year of transplantation, n (%)
| Days of hospitalization, mean ± SD | 49 ± 45 |
| Range, d | 8–357 |
| BK virus | 16 (10.3) |
| CMV | 67 (42.9) |
| Infection | 134 (83.2) |
| Severe infection | 96 (60) |
| Bacterial infection | 112 (69.6) |
| Recurrent urinary tract infections | 29 (18.7) |
| Pneumonitis | 31 (19.6) |
| Viral infection | 21 (13.3) |
| Fungal infection | 16 (10.1) |
| Parasitic infection | 6 (3.9) |
| Cardiovascular event | 73 (45.1) |
| ADHF | 23 (13.5) |
| Recurrent ADHF | 10 (5.8) |
| Deep vein thrombosis/Pulmonary embolism | 22 (12.9) |
| Arrhythmia | 19 (11.1) |
| Myocardial ischemia | 10 (5.8) |
| Cerebrovascular event | 3 (1.8) |
| Urologic complication | 94 (56.9) |
| Lymphocele | 19 (11.1) |
| Hematoma | 19 (11.1) |
| Acute urinary retention | 18 (10.5) |
| Ureteral stenosis | 18 (10.5) |
| Transplant renal artery stenosis | 9 (5.3) |
| Skin tumor | 11 (7.1) |
| Solid tumor | 5 (3.2) |
| Hemopathy/Lymphoma | 6 (3.8) |
| TCMR | 27 (17.1) |
| Time to TCMR, d (mean ± SD) | 124 ± 104 |
| ABMR | 8 (5.2) |
| Time to ABMR, d (mean ± SD) | 171 ± 128 |
| Death | 17 (9.9) |
| Cause of death | |
| Infection | 10 (58.8) |
| Cardiovascular disease | 5 (29.4) |
| Graft loss | 40 (23.4) |
| Death-censored graft loss | 29 (16.9) |
| Cause of graft loss | |
| Death | 11 (28.2) |
| Rejection | 10 (25.6) |
| Vascular | 7 (17.9) |
ABMR, antibody-mediated rejection; ADHF, acute decompensated heart failure; CMV, cytomegalovirus; TCMR, T-cell mediated rejection.
Thirty-three patients (20.9%) had a BPAR during the first year of graft, among them more than one-half occurred during the first 3 months. Twenty-seven (17.1%) were T-cell–mediated rejection and 8 (5.2%) were antibody-mediated rejection. T-cell–mediated rejections were treated by steroid therapy (i.v. solumedrol 500 mg daily for 3 days, followed by oral prednisone 1 mg/kg per day with progressive decrease) whereas antibody-mediated rejections were treated according to the different centers by steroids, rituximab, plasma exchange, and/or Ig. Among the recipients with BPAR, 11 (33.3%) experienced death-censored graft failure and 2 (6%) died during the first year of KT. Among the patients with graft failure, 2 died during the first year of transplantation, a few weeks after the graft loss. Three deaths were due to infectious disease and 1 to cardiovascular event.
Patient and Graft Survival
At 3 and 12 months, mean estimated glomerular filtration rate was 37.5 ± 15 ml/min per 1.73 m2 and 40.9 ± 15.7 ml/min per 1.73 m2, respectively.
At the end of the first year, 17 patients (9.9%) were dead, mostly from infectious diseases (58.5%, n = 10) and cardiovascular events (29.4%, n = 5). Among them, 6 patients (3.5%) died during the first 3 months of KT. Three of the 10 patients who died from an infectious disease had received a treatment for acute rejection. Characteristics of patients who died during the first year are described in Table 1. The first cause of graft loss was death with functioning graft (28.2%, n = 11). Death-censored graft loss occurred in 29 patients (16.9%), and the 2 main causes were rejection (25.6%, n = 10) and vascular event (17.9%, n = 7).
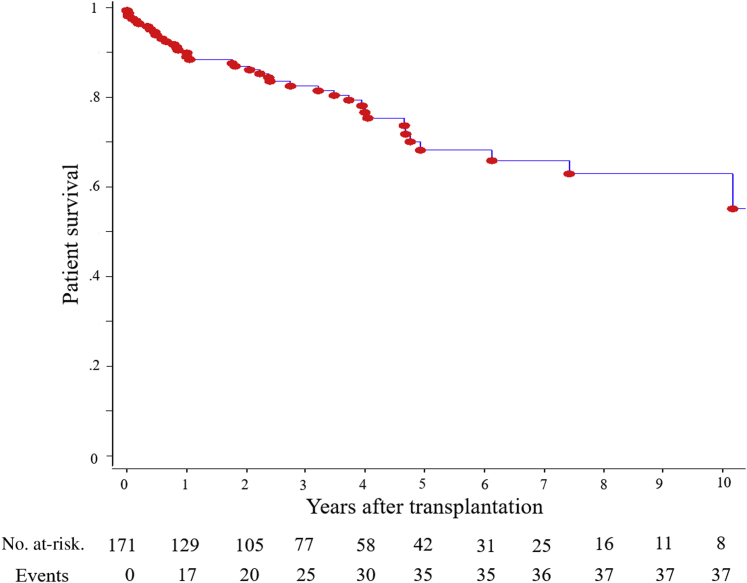
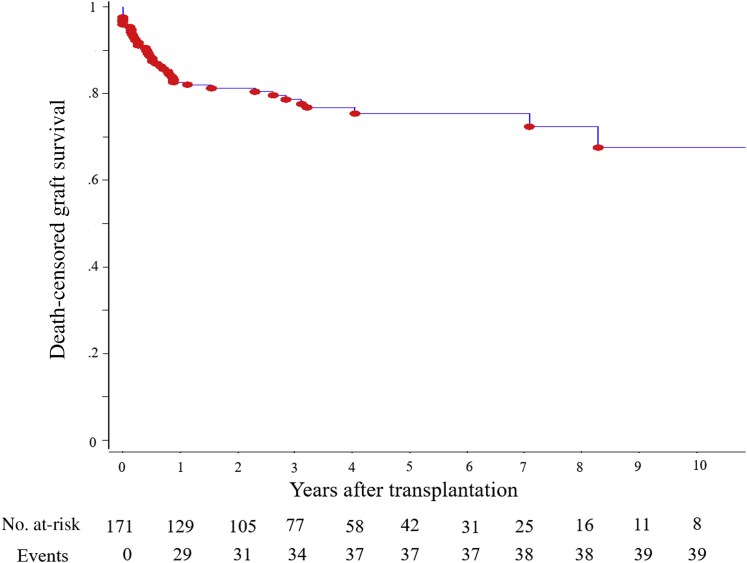
Figure 2 shows the recipient survival during the follow-up. Patient survival at 1, 3, and 5 years was 90.1%, 82.5%, and 68.1%, respectively. Infection was the most common cause of death (47.5%, n = 19), followed by cardiovascular disease (25%, n = 10) and cancer (15%, n = 6). During the study period, 72 patients (42.1%) experienced graft loss. The main cause of graft loss was death (45.8%, n = 33) (Table 3). Figure 3 shows the death-censored graft survival, which was 82.6%, 78.7%, and 75.4% at 1, 3, and 5 years, respectively. The most common reasons for death-censored graft loss were rejection episodes (22.2%, n = 16) and vascular events, including artery or vein thrombosis (9.7%, n = 7) (Table 4).
Figure 2.
Patient survival rates following kidney transplantation in recipients older than 70 years.
Figure 3.
Death-censored graft survival rates following kidney transplantation in recipients older than 70 years.
Table 4.
Patient and graft survival of the KTR older than 70 years
| Death, n (%) | 40 (23.4) |
| Time to death, yr (mean ± SD) | 2.9 ± 3.3 |
| Death with functioning graft, n (%) | 33 (19.3) |
| Cause of death, n (%) | |
| Infection | 19 (47.5) |
| Cardiovascular | 10 (25) |
| Malignancy | 6 (15) |
| Graft loss, n (%) | 72 (42.1) |
| Time to graft loss, yr (mean ± SD) | 2.2 ± 2.9 |
| Death-censored graft loss, n (%) | 39 (22.8) |
| Cause of graft loss, n (%) | |
| Death | 33 (45.8) |
| Acute rejection | 10 (13.8) |
| Chronic rejection | 6 (8.3) |
| Vascular | 7 (9.7) |
| Primary failure | 3 (4.2) |
| Chronic dysfunction | 6 (8.3) |
| Infection | 3 (4.2) |
| Recurrent nephropathy | 2 (2.8) |
| Urologic | 1 (1.4) |
| Malignancy, n (%) | 53 (33.1) |
| Time to malignancy, yr (mean ± SD) | 2.7 ± 2.1 |
| Follow-up, yr (mean ± SD) | 3.5 ± 3.1 |
Analysis of Risk Factors
The receiver operating characteristic curve determined that the optimal cutoff value of LVEF was 56%. The area under the receiver operating characteristic curve was 0.601 (P = 0.071), with a sensitivity of 34% and a specificity of 85%.
To determine the risk factors for patient death or graft failure during the first year of KT, we performed a Cox regression analysis. Univariate and multivariate analyses on baseline characteristics revealed that HLA antibodies (hazard ratio [HR] 2.1; 95% CI 1.04–4.2), arrhythmia (HR 2.26, 95% CI 1.08–4.8), and LVEF ≤ 56% (HR 2.38, 95% CI 1.18–4.83) were independent risk factors (Table 5). Among the posttransplantation characteristics, Cox multivariate analysis showed that donor deceased from cardiovascular cause (HR 5.18; 95% CI 1.2–22.2) and acute rejection (HR 2.77; 95% CI 1.2–6.3) were independent risk factors (Table 6). Delayed graft function, donor age, or IS therapy were not independently associated with patient death or graft failure.
Table 5.
Risk factors for patient death or graft loss during the first year of transplantation: baseline characteristics
| Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| P | HR | 95% CI | P | |
| Body mass index | 0.2 | |||
| Hypertension | 0.9 | |||
| Diabetes | 0.4 | |||
| Coronary artery disease | 0.4 | |||
| Antiaggregants | 0.2 | |||
| Waiting time | 0.5 | |||
| Duration of dialysis | 0.2 | |||
| HLA antibodies | 0.07 | 2.1 | 1.04–4.2 | 0.04 |
| Arrhythmia | 0.02 | 2.26 | 1.08–4.8 | 0.03 |
| LVEF ≤ 56% | 0.02 | 2.38 | 1.18–4.83 | 0.02 |
CI, confidence interval; HLA, human leucocyte antigen; HR, hazard ratio; LVEF, left-ventricular ejection fraction.
Table 6.
Risk factors for patient death or graft loss during the first year of transplantation: posttransplantation characteristics
| Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| P | HR | 95% CI | P | |
| Cold ischemic time | 0.8 | |||
| HLA mismatch | 0.6 | |||
| CMV mismatch | 0.3 | |||
| Induction | 0.7 | |||
| Donor age | 0.09 | 0.99 | 0.95–1.04 | 0.7 |
| Deceased donor from cardiovascular cause | 0.07 | 5.18 | 1.2–22.2 | 0.03 |
| Delayed graft function | 0.05 | 0.71 | 0.3–1.7 | 0.4 |
| Initial hospitalization | < 0.001 | 1.01 | 0.99–1.02 | 0.2 |
| Acute rejection | < 0.001 | 2.77 | 1.2–6.3 | 0.01 |
CI, confidence interval; CMV, cytomegalovirus; HLA, human leucocyte antigen; HR, hazard ratio.
Discussion
Many studies demonstrate that KT decreases the long-term mortality rate of recipients compared with patients on the waiting list, even among the older patients. However, these studies also show that the risk of death of the elderly KTR remains higher than in wait-listed patients during at least 3 months, leading to a time to equal survival of more than 1 year.6, 16 The present study specifically focused on the risk factors for patient death and graft loss during the first year of transplantation.
With the aging of the ESRD population, the number of elderly kidney transplant candidates is growing. In this study, we observed a significant and progressive increase in the number of patients transplanted over time, from 2000 to 2014. In our population of KTRs older than 70 years, we found an acceptable patient survival. In their multicenter study, Rao et al.6 reported a 4-year survival of 66% among 2438 KTRs older than 70. Three years later, Heldal et al.10 described a patient survival of 89%, 74%, and 64% at 1, 3, and 5 years, respectively, among 117 patients receiving a KTR after 70 years between 2000 and 2007. The mortality is slightly lower in our population, despite a lower rate of living donors. This can be explained by the study period; indeed, most of the recipients included in our study were transplanted between 2007 and 2014. Recently, Legeai et al.17 reported a 3-year mortality of 17.6% in 877 French transplant recipients older than 70 years and 17.5% in 342 wait-listed patients of the same age. Most of our patients are part of this cohort, and our results are similar for transplanted patients. The absence of difference between the 2 groups may be explained by the first 3 months of high risk of death in KTRs,17 which highlights the interest to find risk factors for early death in these elderly patients. Death-censored graft survival is also acceptable in our population. Rao et al.6 found that death-censored graft survival was 90.4% and 85.2% at 1 and 3 years, respectively, and these better results can be explained by the high rate of ECDs in our study population. Patient death is the first cause of graft loss in this analysis, which is also found in several studies.6, 18, 19 Indeed, the elderly recipients have a shorter life expectancy and die frequently with a functioning graft.
Our investigations revealed that HLA antibodies were independent risk factors associated with graft failure or patient death during the first year of KT, which has already been reported.12, 18, 20 Among the posttransplantation characteristics, donor deceased from cardiovascular cause was also an independent risk factor. Most of the donors were ECDs in our study, and this result highlights the fact that ECDs are a heterogeneous pool of donors, which must be distinguished to predict graft outcomes. The type of donor has frequently been considered, and most studies report a lower mortality rate in a context of living donor, even with older living donors.6, 21, 22, 23, 24 In our population, the rate of living donors is low, and we hypothesize that the 1-year patient and graft survival would have been better with a higher incidence of living donors. Given the shortage of graft and the good outcomes of KTRs from living donors, the clinicians must promote this type of donor, especially in these elderly KT candidates. When it is not possible, the “old for old” allocation and the use of an ECD kidney seem to be good alternatives.19, 25, 26, 27
In this study, arrhythmia and an LVEF ≤56% were independent risk factors for early death or graft failure. Arrhythmia has already been described as a risk factor in other studies on younger recipients.23, 28 Lentine et al.29 also reported that new-onset atrial fibrillation after KT was a risk factor for death or graft loss. Arrhythmia is frequently associated with congestive heart failure, and we can hypothesize that it leads to an increased risk of cardiovascular events in patients with a suboptimal renal function due to a suboptimal kidney graft. To our knowledge, the LVEF has not yet been reported as predictive, but several studies found an increased risk of death or graft loss in patients with congestive heart failure.12, 23, 28, 30 However, the LVEF has been assessed by transthoracic echocardiography in our population, and an important limitation is its reproducibility between patients, which is lower than with invasive procedures.31 The American Heart Association and the American College of Cardiology Foundation nonetheless recommend the assessment of left-ventricular function by echocardiography in potential KT candidates (Class IIa; Level of Evidence B).32 Furthermore, the cardiovascular events were frequent in our population and were the second cause of death. This highlights the crucial role of a suitable assessment of cardiovascular status before transplantation in geriatric recipients, which is challenging especially owing to the prevalence of the disease in this population and the extended waiting periods between initial evaluation and transplantation. Patients with ESRD have frequent cardiovascular comorbidities, and the clinicians need reproducible and functional cardiac explorations to predict the cardiovascular outcomes of these elderly recipients. Today, there is no recommendation for cardiac screening before the KT in elderly candidates, and more evidence is required, ideally from randomized clinical trials. Moreover, IS drugs, in particular steroids and calcineurin inhibitors, further aggravate the cardiovascular risk after KT, and we can hypothesize that the older recipients with a high cardiovascular risk may benefit from a calcineurin inhibitor–free IS regimen, using mammalian target of rapamycin inhibitors or belatacept.
The first cause of death in our study was infectious disease, which is coherent with other reports in this old population.21 Likewise, Meier-Kriesche et al.33 described an exponentially increased risk of infectious death in older transplant recipients. These last years, a new concept is emerging, known as immunosenescence.34 With aging, the immune system is changing, resulting in defects in both innate and adaptive immunity.35 Considering this, elderly patients receiving an IS therapy are at high risk of infectious disease, and Trouillhet et al.36 reported a higher rate of bacterial infections in recipients older than 65 years. Furthermore, the cytomegalovirus infections are higher in our population than in younger KTRs.37 This highlights the question of a prolonged cytomegalovirus prophylaxis in these elderly recipients. Conversely, it seems that immunosenescence leads to a decreased risk of acute rejection episodes in the elderly recipients,5, 38 but, on the other hand, the higher donor age is associated with increasing rejection rates.39 Many studies have already reported that BPAR was a risk factor for graft failure.12, 20, 40 In our study, we used a composite endpoint and BPAR was an independent risk factor for early death or graft failure. Even though rejection episodes would be less common in elderly recipients, they could lead to more deleterious effects in these patients41 and the prognosis after rejection was poor in our population. Indeed, rejections and rejection therapy in the elderly are associated with the risk of over-immunosuppression, aggravation of comorbidities, and graft loss.42 The latter point may be due to the older age of donors, associated with increased preexisting tubulo-interstitial lesions, sensitivity to ischemia/reperfusion, and a higher immunogenicity.13 Given the balance between infectious diseases and BPAR, IS therapy is challenging, and clinical trials evaluating the safety and efficacy of IS regimens in those older than 70 are lacking.43 Indeed, published transplantation guidelines make no specific recommendation for older KTRs.44 In 2009, Badowski et al.40 reported an improved graft and patient survival with reduction of IS treatment among 189 patients older than 60 years. Two years later, Gill et al.45 concluded that thymoglobulin was preferable in the older high-risk recipients with a high-risk donor but the mean recipient age was 65 years. Interestingly, in our study, we found that the IS therapy was not a risk factor for early patient death or graft loss. Considering that the acute rejection episodes during the first year of transplantation are risk factors for early patient death or graft failure, we propose not to decrease the initial IS therapy in this population, to limit the real danger, which is the risk of early acute rejection. Today, it is not possible to conclude which IS regimen is the most adapted in these particular patients, and prospective comparative trials including geriatric recipients are warranted to improve the support in this population.
In this study, there is a high risk of malignancies. In the United States, Kasiske et al.46 reported a cumulative incidence of skin cancer of 3.3% at 12 months and 7.5% at 36 months, and 2.3% and 7.4% for nonskin malignancies, among patients of all ages. It is less than in our population, but this study also showed that the age at transplantation was a risk factor for malignancies after transplantation (relative risk 4.93 for nonskin malignancies and 27.1 for skin malignancies in patients older than 65 years). In France, cumulative incidence of all cause of malignancies was reported to reach 7.9% in KTRs.2 Unfortunately, in our study, we do not have a control population, so we cannot compare with a younger age group.
Moreover, in this study, there is a high rate of solid tumors within the first year of transplantation, and we may suppose that some tumors may be present before transplantation. In our population, mean waiting time is not far from a year, and pretransplantation investigations may be 1 or 2 years old when the patient receives a graft. This highlights the need for adequate pretransplant cancer screening, but another question is the repetition of this screening during time on the waiting list.
This study has some limitations. First, and even though it was a multicenter study, the number of subjects was limited. This is partly because KT in patients older than 70 is not as frequent as KT in younger patients, because these patients are not referred to the transplantation centers. Although the multicenter design of our study increases the external validity of our results, the relatively small number of patients included is a potential limit that should be considered. Second, no control population was studied, because the aim of the study was not to prove the benefit of KT in elderly patients, but to determine the risk factors for early death and graft failure to help clinicians who need to determine which elderly patient would benefit from transplantation and which would not. The ultimate goal is to improve the outcomes of recipients older than 70 after KT, but a large-scale study is needed to extrapolate our results to all elderly KTRs. Third, another limitation is the absence of quality-of-life or frailty data. Indeed, improving quality of life after KT in this elderly population is crucial, but there are very few good studies in this field.47 Frailty is thought to estimate physiologic reserves and has been defined by Fried et al.48 as a clinical syndrome in which 3 or more of the following criteria were present: unintentional weight loss, self-reported exhaustion, weakness, slow walking speed, and low physical activity. Frailty is frequent in dialysis patients,49 and its prevalence increases with the age of patients with end-stage renal failure.50 In KT, several studies have shown that frailty was an independent risk factor for perioperative complications51 or early hospitalization,52 and a 2015 study reported a risk of death after transplantation 2.17 times higher in frail recipients.53 It therefore appears crucial to identify frail candidates. Finally, a strength of this study lies in the precision of the recorded data, which are not extracted from a database, but have been systematically collected in detail in each patient’s medical records. Furthermore, we decided to focus on the early posttransplantation outcomes, studying the 1-year patient and graft survival, which has not yet been reported in this elderly population.
In conclusion, this study shows that KT in patients older than 70 years is a safe procedure if the recipients are carefully selected. The clinicians should consider the cardiovascular risk of these patients before registration on the waiting list, especially in recipients with low LVEF or arrhythmia. Then, the risk of acute rejection must be considered because of its severe consequences. Owing to the potential clinical implications, these results must be confirmed by large-scale multicenter studies.
Disclosure
All the authors declared no competing interests.
References
- 1.Saran R., Li Y., Robinson B. US Renal Data System. 2015 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2016;67(3 Suppl 1) doi: 10.1053/j.ajkd.2015.12.014. Svii, S1–S305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.REIN-Rapport annuel. 2013. http://www.agence-biomedecine.fr/IMG/pdf/rapport_ rein2013.pdf Available at:
- 3.Eurotransplant Annual Report. 2015, Chapter 5: Kidney:donation, waiting lists and transplants. https://www.eurotransplant.org/cms/mediaobject.php?file=AR_ET_20153.pdf Available at:
- 4.Knoll G.A. Kidney transplantation in the older adult. Am J Kidney Dis. 2013;61:790–797. doi: 10.1053/j.ajkd.2012.08.049. [DOI] [PubMed] [Google Scholar]
- 5.Dempster N.J., Ceresa C.D.L., Aitken E. Outcomes following renal transplantation in older people: a retrospective cohort study. BMC Geriatr. 2013;13:79. doi: 10.1186/1471-2318-13-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rao P.S., Merion R.M., Ashby V.B. Renal transplantation in elderly patients older than 70 years of age: results from the Scientific Registry of Transplant Recipients. Transplantation. 2007;83:1069–1074. doi: 10.1097/01.tp.0000259621.56861.31. [DOI] [PubMed] [Google Scholar]
- 7.Humar A., Denny R., Matas A.J. Graft and quality of life outcomes in older recipients of a kidney transplant. Exp Clin Transplant. 2003;1:69–72. [PubMed] [Google Scholar]
- 8.Jassal S.V., Krahn M.D., Naglie G. Kidney transplantation in the elderly: a decision analysis. J Am Soc Nephrol. 2003;14:187–196. doi: 10.1097/01.asn.0000042166.70351.57. [DOI] [PubMed] [Google Scholar]
- 9.Pérez-Sáez M.J., Arcos E., Comas J. Survival benefit from kidney transplantation using kidneys from deceased donors over 75 years - a time dependent analysis. Am J Transplant. 2016;16:2724–2733. doi: 10.1111/ajt.13800. [DOI] [PubMed] [Google Scholar]
- 10.Heldal K., Hartmann A., Grootendorst D.C. Benefit of kidney transplantation beyond 70 years of age. Nephrol Dial Transplant. 2010;25:1680–1687. doi: 10.1093/ndt/gfp681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heldal K., Hartmann A., Leivestad T. Renal transplantation is also an option for patients over 70. Tidsskr Nor Lægeforen. 2011;131:2004–2007. doi: 10.4045/tidsskr.10.1391. [DOI] [PubMed] [Google Scholar]
- 12.Faravardeh A., Eickhoff M., Jackson S. Predictors of graft failure and death in elderly kidney transplant recipients. Transplantation. 2013;96:1089–1096. doi: 10.1097/TP.0b013e3182a688e5. [DOI] [PubMed] [Google Scholar]
- 13.Musso C.G., Giordani M.C., Imperiali N. Aging kidney transplantation. Rev Invest Clin. 2016;68:68–74. [PubMed] [Google Scholar]
- 14.Knoll G.A. Is kidney transplantation for everyone? The example of the older dialysis patient. Clin J Am Soc Nephrol. 2009;4:2040–2044. doi: 10.2215/CJN.04210609. [DOI] [PubMed] [Google Scholar]
- 15.Haas M., Sis B., Racusen L.C. 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14:272–283. doi: 10.1111/ajt.12590. [DOI] [PubMed] [Google Scholar]
- 16.Wolfe R.A., Ashby V.B., Milford E.L. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 17.Legeai C., Andrianasolo R.M., Moranne O. Benefits of kidney transplantation for a national cohort of patients aged 70 years and older starting renal replacement therapy. Am J Transplant. 2018;18:2695–2707. doi: 10.1111/ajt.15110. [DOI] [PubMed] [Google Scholar]
- 18.Cho H., Yu H., Shin E. Risk factors for graft failure and death following geriatric renal transplantation. PLoS One. 2016;11:e0153410. doi: 10.1371/journal.pone.0153410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frei U., Noeldeke J., Machold-Fabrizii V. Prospective age-matching in elderly kidney transplant recipients: a 5-year analysis of the Eurotransplant Senior Program. Am J Transplant. 2008;8:50–57. doi: 10.1111/j.1600-6143.2007.02014.x. [DOI] [PubMed] [Google Scholar]
- 20.Heldal K., Hartmann A., Leivestad T. Clinical outcomes in elderly kidney transplant recipients are related to acute rejection episodes rather than pretransplant comorbidity. Transplantation. 2009;87:1045–1051. doi: 10.1097/TP.0b013e31819cdddd. [DOI] [PubMed] [Google Scholar]
- 21.Karim A., Farrugia D., Cheshire J. Recipient age and risk for mortality after kidney transplantation in England. Transplantation. 2014;97:832–838. doi: 10.1097/01.TP.0000438026.03958.7b. [DOI] [PubMed] [Google Scholar]
- 22.Englum B.R., Schechter M.A., Irish W.D. Outcomes in kidney transplant recipients from older living donors. Transplantation. 2015;99:309–315. doi: 10.1097/TP.0000000000000607. [DOI] [PubMed] [Google Scholar]
- 23.Meier-Kriesche H.U., Ojo A.O., Cibrik D.M. Relationship of recipient age and development of chronic allograft failure. Transplantation. 2000;70:306–310. doi: 10.1097/00007890-200007270-00012. [DOI] [PubMed] [Google Scholar]
- 24.Gill J., Bunnapradist S., Danovitch G.M. Outcomes of kidney transplantation from older living donors to older recipients. Am J Kidney Dis. 2008;52:541–552. doi: 10.1053/j.ajkd.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 25.Ma M.K.M., Lim W.H., Craig J.C. Mortality among younger and older recipients of kidney transplants from expanded criteria donors compared with standard criteria donors. Clin J Am Soc Nephrol. 2016;11:128–136. doi: 10.2215/CJN.03760415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lloveras J., Arcos E., Comas J. A paired survival analysis comparing hemodialysis and kidney transplantation from deceased elderly donors older than 65 years. Transplantation. 2015;99:991–996. doi: 10.1097/TP.0000000000000474. [DOI] [PubMed] [Google Scholar]
- 27.Mezrich J.D., Pirsch J.D., Fernandez L.A. Differential outcomes of expanded-criteria donor renal allografts according to recipient age. Clin J Am Soc Nephrol. 2012;7:1163–1171. doi: 10.2215/CJN.00150112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weinhandl E.D., Snyder J.J., Israni A.K. Effect of comorbidity adjustment on CMS criteria for kidney transplant center performance. Am J Transplant. 2009;9:506–516. doi: 10.1111/j.1600-6143.2008.02527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lentine K.L., Schnitzler M.A., Abbott K.C. Incidence, predictors, and associated outcomes of atrial fibrillation after kidney transplantation. Clin J Am Soc Nephrol. 2006;1:288–296. doi: 10.2215/CJN.00920805. [DOI] [PubMed] [Google Scholar]
- 30.Machnicki G., Pinsky B., Takemoto S. Predictive ability of pretransplant comorbidities to predict long-term graft loss and death. Am J Transplant. 2009;9:494–505. doi: 10.1111/j.1600-6143.2008.02486.x. [DOI] [PubMed] [Google Scholar]
- 31.Van Royen N., Jaffe C.C., Krumholz H.M. Comparison and reproducibility of visual echocardiographic and quantitative radionuclide left ventricular ejection fractions. Am J Cardiol. 1996;77:843–850. doi: 10.1016/s0002-9149(97)89179-5. [DOI] [PubMed] [Google Scholar]
- 32.Lentine K.L., Costa S.P., Weir M.R. Cardiac disease evaluation and management among kidney and liver transplantation candidates: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2012;60:434–480. doi: 10.1016/j.jacc.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 33.Meier-Kriesche H.U., Ojo A.O., Hanson J.A. Exponentially increased risk of infectious death in older renal transplant recipients. Kidney Int. 2001;59:1539–1543. doi: 10.1046/j.1523-1755.2001.0590041539.x. [DOI] [PubMed] [Google Scholar]
- 34.Martins P.N.A., Pratschke J., Pascher A. Age and immune response in organ transplantation. Transplantation. 2005;79:127–132. doi: 10.1097/01.tp.0000146258.79425.04. [DOI] [PubMed] [Google Scholar]
- 35.McKay D., Jameson J. Kidney transplantation and the ageing immune system. Nat Rev Nephrol. 2012;8:700–708. doi: 10.1038/nrneph.2012.242. [DOI] [PubMed] [Google Scholar]
- 36.Trouillhet I., Benito N., Cervera C. Influence of age in renal transplant infections: cases and controls study. Transplantation. 2005;80:989–992. doi: 10.1097/01.tp.0000173822.05877.d7. [DOI] [PubMed] [Google Scholar]
- 37.Ekberg H., Tedesco-Silva H., Demirbas A. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007;357:2562–2575. doi: 10.1056/NEJMoa067411. [DOI] [PubMed] [Google Scholar]
- 38.Meier-Kriesche H.U., Ojo A., Hanson J. Increased immunosuppressive vulnerability in elderly renal transplant recipients. Transplantation. 2000;69:885–889. doi: 10.1097/00007890-200003150-00037. [DOI] [PubMed] [Google Scholar]
- 39.Tullius S.G., Milford E. Kidney allocation and the aging immune response. N Engl J Med. 2011;364:1369–1370. doi: 10.1056/NEJMc1103007. [DOI] [PubMed] [Google Scholar]
- 40.Badowski M., Gurk-Turner C., Cangro C. The impact of reduced immunosuppression on graft outcomes in elderly renal transplant recipients. Clin Transplant. 2009;23:930–937. doi: 10.1111/j.1399-0012.2009.01028.x. [DOI] [PubMed] [Google Scholar]
- 41.Meier-Kriesche H.U., Srinivas T.R., Kaplan B. Interaction between acute rejection and recipient age on long-term renal allograft survival. Transplant Proc. 2001;33:3425–3426. doi: 10.1016/s0041-1345(01)02477-0. [DOI] [PubMed] [Google Scholar]
- 42.Lehner L.J., Staeck O., Halleck F. Need for optimized immunosuppression in elderly kidney transplant recipients. Transplant Rev. 2015;29:237–239. doi: 10.1016/j.trre.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 43.Blosser C.D., Huverserian A., Bloom R.D. Age, exclusion criteria, and generalizability of randomized trials enrolling kidney transplant recipients. Transplantation. 2011;91:858–863. doi: 10.1097/TP.0b013e31820f42d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.KDIGO Clinical Practice Guideline for the Care of Kidney Transplant Recipients. http://www.kdigo.org/clinical_practice_guidelines/pdf/TxpGL_publVersion.pdf Available at:
- 45.Gill J., Sampaio M., Gill J.S. Induction immunosuppressive therapy in the elderly kidney transplant recipient in the United States. Clin J Am Soc Nephrol. 2011;6:1168–1178. doi: 10.2215/CJN.07540810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kasiske B.L., Snyder J.J., Gilbertson D.T., Wang C. Cancer after kidney transplantation in the United States. Am J Transplant. 2004;4:905–913. doi: 10.1111/j.1600-6143.2004.00450.x. [DOI] [PubMed] [Google Scholar]
- 47.Segall L., Nistor I., Pascual J. Criteria for and appropriateness of renal transplantation in elderly patients with end-stage renal disease: a literature review and position statement on behalf of the European Renal Association-European Dialysis and Transplant Association Descartes Working Group and European Renal Best Practice. Transplantation. 2016;100:e55–e65. doi: 10.1097/TP.0000000000001367. [DOI] [PubMed] [Google Scholar]
- 48.Fried L.P., Tangen C.M., Walston J. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 49.McAdams-DeMarco M.A., Law A., Salter M.L. Frailty as a Novel Predictor of Mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc. 2013;61:896–901. doi: 10.1111/jgs.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johansen K.L., Chertow G.M., Jin C., Kutner N.G. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18:2960–2967. doi: 10.1681/ASN.2007020221. [DOI] [PubMed] [Google Scholar]
- 51.Makary M.A., Segev D.L., Pronovost P.J. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901–908. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 52.McAdams-DeMarco M.A., Law A., Salter M.L. Frailty and early hospital readmission after kidney transplantation: frailty and readmission after KT. Am J Transplant. 2013;13:2091–2095. doi: 10.1111/ajt.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McAdams-DeMarco M.A., Law A., King E. Frailty and mortality in kidney transplant recipients:frailty and mortality. Am J Transplant. 2015;15:149–154. doi: 10.1111/ajt.12992. [DOI] [PMC free article] [PubMed] [Google Scholar]