See Clinical Research on Page 667
Snake bite poisoning is listed as one of the underreported causes of tropical diseases by the World Health Organization. Accurate estimates of snake bite–related global morbidity and mortality are not available. As per a recent annual estimate, approximately 81,000 to 138,000 deaths occur from approximately 1.8 million to 2.7 million snake envenomings throughout the world.1 Although it is a global problem of worrisome enormity, most of the deaths occur in Africa, Southeast Asia, and South Asia. Rural-dwelling low-income populations are particularly vulnerable to a snake bite. Acute kidney injury (AKI) can result, predominantly due to envenoming by hematotoxic vipers (Russel’s viper, Echis carinatus, and the hump-nosed pit viper). Envenoming from a sea-snake bite contributes significantly to AKI in certain countries, such as Sri Lanka.
Snake venom is a complex mixture of multiple enzymes and proteins. The venom is evolutionarily designed in such a way that its components afflict multiple organ systems and tissues of the victim instantaneously, resulting in incapacitation and death.
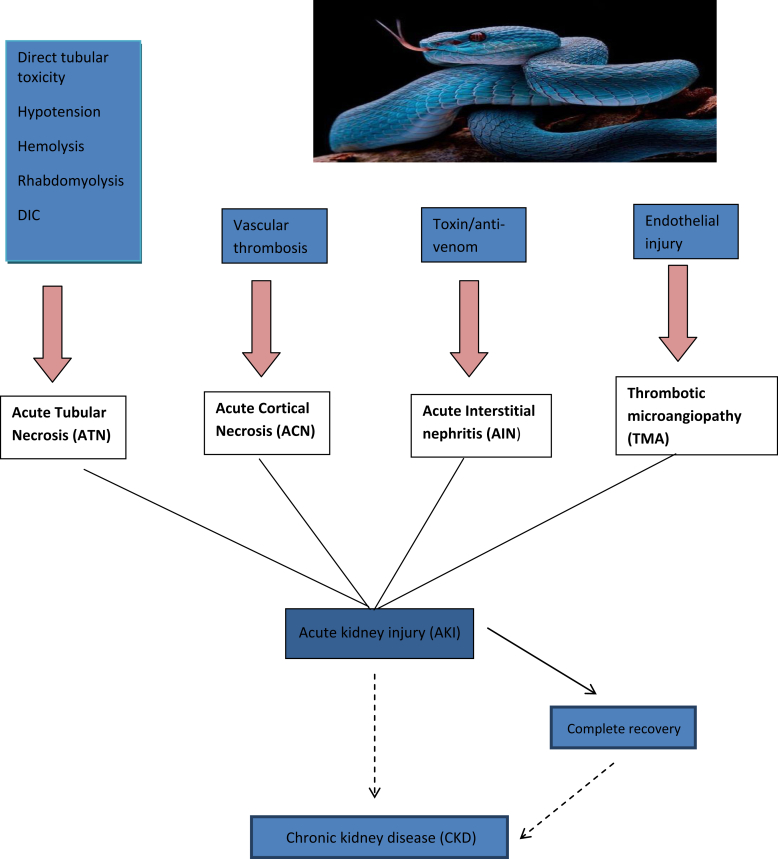
AKI following snake bite envenoming is multifactorial, including direct nephrotoxicity of venom, coagulopathy, hypotension, disseminated intravascular coagulation, intravascular hemolysis, thrombotic microangiopathy, “capillary leak syndrome,” rhabdomyolysis, complement activation, and secondary sepsis (Figure 1).2 Although the predominant renal pathology is acute tubular necrosis, snake envenoming is a major cause for acute renal cortical necrosis of varying severity. Occlusive thrombosis of lobar and sublobar arteries ensues in renal cortical necrosis. In a kidney biopsy series of 44 patients with AKI following a snake bite, acute tubular necrosis was the predominant pathology in 73% and acute cortical necrosis was observed in 27% of patients.3 In a study of 26 patients of biopsy-proven myoglobin pigment–induced AKI, snake envenoming was the cause in 10 (38%) patients.4 Other less common renal pathologies increasingly reported in recent times include acute interstitial nephritis and thrombotic microangiopathy. In a study of 196 patients with AKI following snake bite poisoning, acute interstitial nephritis was noted in 20 (23.5%) of the 86 kidney biopsies.5 It is hypothesized that hypersensitivity to venom or other immunological phenomena may be involved in the pathogenesis of acute interstitial nephritis. Thrombotic microangiopathy is postulated to be due to endothelial injury inflicted by certain constituents of snake venom. Some components of the venom possibly act as Von Willebrand factor activators and initiate thrombotic microangiopathy.6
Figure 1.
Acute kidney injury following snake bite envenoming is multifactorial. DIC, disseminated intravascular coagulation.
The immediate outcome of AKI following a snake bite includes death, nonrecovery (direct progression to end-stage renal disease), partial recovery (dialysis-independent state), and complete recovery. Nonrecovery is often due to diffuse cortical necrosis and thrombotic microangiopathy, whereas patchy cortical necrosis usually is associated with partial recovery of renal function.
There is a growing body of evidence that AKI of any cause, after an apparent recovery, can slip into the conundrum of “maladaptive repair” and result in chronic kidney disease (CKD). This is all the more relevant in AKI due to a snake bite. From the limited available literature, it is evident that AKI following a snake bite may be particularly vulnerable for progression to CKD. In a descriptive study of 54 patients with AKI following a snake bite, Herath et al.7 observed progression to CKD in 20 (37%) patients at 1 year. Serum creatinine at 2 months after AKI and length of duration of renal replacement therapy were predictors of CKD progression.7 Waikhom et al.,8 in a prospective study of 60 patients with dialysis-requiring AKI due to snake bite, documented CKD progression in 25 (41%) patients at a mean follow-up of 45 months. Such a higher rate and rapid CKD progression are unusual in acute tubular necrosis of other causes. Concurrent presence of other pathologies, most likely patchy cortical necrosis, could be the plausible explanation for this worrisome phenomenon. The addition of every new patient with CKD is problematic in low- and middle-income countries, which are already overburdened with patients with CKD. Efforts to mitigate the menace of AKI and subsequent CKD following snake bite poisoning should be multipronged: (i) sensitize the at-risk population and improve their health-seeking behavior; (ii) ensure widespread availability of anti–snake venom in the affected regions; (iii) devise appropriate treatment protocols with emphasis on the adequacy of anti–snake venom dosing; (iv) minimize “bite-to-needle” interval (time lag between snake bite and administration of anti–snake venom); (v) ensure early identification and appropriate management of AKI; (vi) provide regular follow-up and optimal care of patients with partial renal recovery; and (vii) provide periodic follow-up to patients who have had apparent clinical recovery from AKI to look for evidence for renal dysfunction.
Many urinary and serum biomarkers have been studied and validated for early recognition of AKI in at-risk situations, differentiation between prerenal AKI and acute tubular necrosis, and for prediction of severity of AKI, but there are very few studies on utility of biomarkers in assessing long-term outcomes following AKI. In the SAPPHIRE validation study, the product of urinary levels of tissue inhibitor of metalloproteinase–2 and insulin-like growth factor binding protein-7 at admission was found to be predictive of a composite of mortality or renal replacement therapy at 9 months in patients who developed AKI.9
Jaswanth et al.,10 in a study published in this issue, analyzed urinary beta-2 microglobulin as a potential marker of persistent tubular dysfunction after clinical recovery from AKI following snake bite poisoning. This study assumes importance as being the first on this pertinent issue. Forty-two patients who sustained AKI due to a snake bite were followed for 6 months. Of them, 6 (14.3%) patients progressed to CKD. Urinary beta 2 microglobulin levels were measured at 3 timelines after discharge and they were compared with those of healthy controls. In a substantial proportion of patients, urinary beta 2 microglobulin levels were significantly higher compared with controls (in 70.7%, 48.8%, and 51.2% of patients at 2 weeks, 3 months, and 6 months, respectively). Similar trends were observed even after excluding patients who developed CKD.10 The observation that the levels remained elevated as compared with controls in approximately one-half of the study population even at 6 months after clinical recovery from AKI assumes greater significance. This subset of patients, in all likelihood, would develop CKD and they must be diligently followed.
It would, however, be prudent to exercise caution in drawing any solid inferences from this study in view of the shortcomings, such as the smaller study population, higher attrition rate, and shorter duration of follow-up of patients. Also, a point to ponder is the appropriateness of the choice of biomarker. Beta 2 microglobulin is filtered at the glomerulus, reabsorbed, and catabolized by renal tubules. Hence, it may serve as a marker of tubular injury and dysfunction. But it may not be a suitable marker to assess renal functional status in situations in which there is a varying degree of cortical necrosis in addition to acute tubular necrosis. In such instances, an additional marker, such as “uromodulin,” which represents intact renal mass, would be valuable. In patchy cortical necrosis, reduction in renal mass may be masked in the initial stages by hyperfiltration of surviving nephrons with preservation of glomerular filtration rate. Uromodulin has been shown to be a robust marker of intact renal mass. Plasma level of uromodulin inversely correlates with renal mass.11
There are many unresolved challenges in the clinical scenario of AKI following poisoning from a snake bite: (i) the development of kits to measure plasma levels of individual venom components; (ii) the manufacture of antivenom that is effective against relatively infrequent species, such as the hump-nosed pit viper; (iii) ascertaining the role of steroids in snake bite–induced acute interstitial nephritis and the role of plasmapheresis in thrombotic microangiopathy triggered by a snake bite; and (iv) multicentric studies involving a large number of patients to identify determinants and predictors of long-term renal dysfunction
It is time to give a clarion call to the international medical fraternity to pay more attention to this underreported tropical, sinister disease.
Disclosure
The author declared no competing interests.
References
- 1.World Health Organization Seventy-First World Health Assembly, Report by Director General, Global Snakebite Burden. https://www.who.int/snakebites/en/ Available at:
- 2.Sitprija V. Snakebite nephropathy. Nephrology. 2006;11:442–448. doi: 10.1111/j.1440-1797.2006.00599.x. [DOI] [PubMed] [Google Scholar]
- 3.Chugh K.S. Snake-bite-induced acute renal failure in India. Kidney Int. 1989;35:891–907. doi: 10.1038/ki.1989.70. [DOI] [PubMed] [Google Scholar]
- 4.Sakthirajan R., Dhanapriya J., Varghese A. Clinical profile and outcome of pigment-induced nephropathy. Clin Kidney J. 2018;11:348–352. doi: 10.1093/ckj/sfx121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dineshkumar T., Dhanapriya J., Murugananth S. Snake envenomation-induced acute interstitial nephritis. Journal of Integrative Nephrology and Andrology. 2018;5:14–17. [Google Scholar]
- 6.Dineshkumar T., Dhanapriya J., Sakthirajan R. Thrombotic microangiopathy due to Viperidae bite: two case reports. Indian J Nephrol. 2017;27:161–164. doi: 10.4103/0971-4065.196936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herath H.M.N.J., Wazil A.W.M., Abeysekara D.T.D.J. Chronic kidney disease in snake envenomed patients with acute kidney injury in Sri Lanka: a descriptive study. Postgrad Med J. 2012;88:138. doi: 10.1136/postgradmedj-2011-130225. [DOI] [PubMed] [Google Scholar]
- 8.Waikhom R., Sircar D., Patil K. Long-term renal outcome of snake bite and acute kidney injury: a single-center experience. Ren Fail. 2012;34:271–274. doi: 10.3109/0886022X.2011.647297. [DOI] [PubMed] [Google Scholar]
- 9.Koyner J.L., Shaw A., Chawla L.S. Tissue inhibitor metalloproteinase-2 (TIMP-2) – IGF-Binding Protein -7 (IGFBP7) levels are associated with adverse long term outcomes in patients with AKI. J Am Soc Nephrol. 2015;26:1747–1754. doi: 10.1681/ASN.2014060556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaswanth C., Priyamvada P.S., Zachariah B. Short-term changes in urine beta 2 microglobulin following recovery of acute kidney injury resulting from snake envenomation. Kidney Int Rep. 2019;4:667–673. doi: 10.1016/j.ekir.2019.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steubl D., Block M., Herbst V. Plasma uromodulin correlates with kidney function and identifies early stages in chronic kidney disease patients. Medicine (Baltimore) 2016;95:e3011. doi: 10.1097/MD.0000000000003011. [DOI] [PMC free article] [PubMed] [Google Scholar]