Abstract
Background/Aims
The Positron Emission Tomography Response Criteria in Solid Tumors (PERCIST) or European Organization for Research and Treatment of Cancer (EORTC) criteria are used to assess metabolic tumor responses. However, tumor responses have shown considerable discrepancies between the morphologic criteria (Response Evaluation Criteria in Solid Tumors [RECIST]) and metabolic criteria. We performed this pooled study to compare the RECIST and metabolic criteria in the assessment of tumor responses.
Methods
Electronic databases were searched for eligible articles with the terms “RECIST,” “PERCIST,” or “EORTC criteria.” The level of concordance in the tumor responses between the two criteria was estimated using κ statistics.
Results
A total of 216 patients were collected from eight studies comparing the RECIST and EORTC criteria. The agreement of tumor responses between the two criteria was moderate (κ = 0.447). Eighty-six patients (39.8%) showed disagreement: tumor response was upgraded in 70 patients and downgraded in 16 when adopting the EORTC criteria. The EORTC criteria significantly increased the overall response rate (53% vs. 28%, p < 0.0001). The agreement of tumor responses between the RECIST and PERCIST was deemed fair (κ = 0.389). Of 407 patients from nine studies, 181 (44.5%) showed a discrepancy: using the PERCIST, tumor response were upgraded in 151 patients and downgraded in 30. When adopting the PERCIST, the overall response rate was also significantly increased from 30% to 55% (p < 0.0001).
Conclusions
This pooled analysis demonstrates that the concordance of tumor responses between the morphologic criteria and metabolic criteria is not excellent. When adopting the metabolic criteria instead of the RECIST, overall response rates were significantly increased.
Keywords: Response evaluation criteria in solid tumors, Positron emission tomography response criteria in solid tumors, European Organization for Research and Treatment of Cancer criteria, Tumor response
INTRODUCTION
The World Health Organization guidelines [1] and the Response Evaluation Criteria in Solid Tumors (RECIST) [2,3] are the morphologic criteria commonly used to assess tumor response in clinical practice. However, these criteria depending on the size changes based on computed tomography (CT) have limitations in tumors with obscure margins, cystic lesion, or scar tissue. In particular, measuring the longest diameter of lesions on CT is not always possible in gastrointestinal tumors. Because patients treated with targeted agents were not included in the data warehouse [4] when the RECIST version 1.1 was revised [3], there has also been concern regarding the assessment of tumor responses with the RECIST in patients receiving targeted agents [5]. Molecular targeted agents tend to induce necrotic or cystic change, not tumor shrinkage, in solid tumors [6]. Therefore, morphologic response criteria may be not well suited for assessing the efficacy of targeted therapies that stabilize diseases.
[18F]-fluorodeoxyglucose ([18F]-FDG) uptake is enhanced in most malignant tumors which in turn can be measured by positron emission tomography (PET). [18F]-FDG PET has been adopted as a new method for the diagnosis and staging of solid tumors. PET is also increasingly being used to monitor tumor responses to anti-cancer therapies. It can allow the assessment of tumor response even in the absence of anatomical changes [7-9]. There are two sets of criteria using FDG PET to quantify metabolic changes to anti-cancer treatment: the criteria developed by the European Organization for Research and Treatment of Cancer (EORTC) criteria [10] and the Positron Emission Tomography Response Criteria in Solid Tumors (PERCIST) [11]. Tumor response evaluations with the PERCIST and EORTC criteria have shown almost perfect agreement and correlated well with survival [12,13]. The metabolic response criteria may provide clinicians with a more accurate assessment of therapeutic response at an earlier stage of treatment course. However, their usefulness and advantage over the morphologic criteria (RECIST) need to be further investigated.
The assessment of tumor responses between the morphologic criteria and metabolic criteria has shown considerable discrepancies in a series of studies with a small number of patients [14-27]. We performed this pooled study to compare tumor response assessment between the morphologic criteria (RECIST 1.0 and RECIST 1.1) and metabolic criteria (EORTC criteria and PERCIST) in patients with solid tumors.
METHODS
Search strategy
A computerized systematic search of the electronic databases PubMed, Embase, Scopus, and Google Scholar (up to June 2017) was carried out to find articles with the following terms in their titles, abstracts, or keywords: “RECIST,” “PERCIST,” or “‘EORTC criteria.” In addition, we checked all the references of identified relevant articles and reviews. We also used the “related articles” feature in PubMed to identify relevant articles.
Study selection criteria
Studies comparing tumor responses by using the RECIST and metabolic criteria (EORTC or PERCIST) were considered for inclusion in this pooled study. As the RECIST 1.1 showed a high concordance with the RECIST 1.0 in the assessment of tumor responses [28,29], we included both versions without distinction in the analysis. For a more accurate comparison, however, articles adopting the modified RECIST versions developed for specific types of tumors were excluded. The searched articles were screened again by reviewing the full text, and the original articles that compared tumor responses between the morphologic criteria (RECIST 1.0 or RECIST 1.1) and metabolic criteria (PERCIST or EORTC) were included in the final analysis.
Tumor response assessment
The tumor responses according to the RECIST in each study were defined as follows [2,3]: (1) complete response (CR): disappearance of all lesions; (2) partial response (PR): at least a 30% decrease in the sum of diameters of the target lesions and no new lesions; (3) progressive disease (PD): more than a 20% increase in the sum of diameters of the target lesions (and also an absolute increase of at least 5 mm in the RECIST 1.1) or the appearance of new lesions on CT (or PET in the RECIST 1.1); and (4) stable disease (SD): neither sufficient shrinkage to qualify as PR nor sufficient increase to qualify as PD.
The tumor response guidelines for the PERCIST and EORTC criteria are briefly summarized in Table 1.
Table 1.
Tumor response assessment by two metabolic criteria (EORTC criteria and PERCIST)
| EORTC | PERCIST | |
|---|---|---|
| Complete metabolic response (CMR) | Complete resolution of FDG uptake in all lesions | Complete resolution of FDG uptake in all lesions |
| Partial metabolic response (PMR) | ≥ 25% Reduction in the sum of SUVmax after more than one cycle of treatment | ≥ 30% Reduction of the SULpeak and an absolute drop of 0.8 SULpeak units |
| Progressive metabolic disease (PMD) | ≥ 25% Increase in the sum of SUVmax or appearance of new FDG-avid lesions | ≥ 30% Increase in the SULpeak of the FDG uptake and an absolute increase of 0.8 SULpeak, or appearance of FDG-avid new lesions |
| Stable metabolic disease (SMD) | Not qualify for CMR, PMR, or PMD | Not qualify for CMR, PMR, or PMD |
EORTC, European Organization Research and Treatment of Cancer; PERCIST, Positron Emission Tomography Response Criteria in Solid Tumors; FDG, fluorodeoxyglucose; SUVmax, maximum standardized uptake value; SULpeak, peak lean body mass SUV.
Statistics
The overall response rate (ORR) was defined as the percentage of patients with CR or PR (as determined by the two RECIST versions) and those with complete metabolic response (CMR) or partial metabolic response (PMR) (as determined by the PERCIST or EORTC criteria). The ORRs between the two groups were compared by using the McNemar test and p values less than 0.05 were considered significant. The level of agreement in tumor responses between the two criteria was estimated using unweighted κ statistics. The agreement was interpreted as poor (κ < 0), slight (κ = 0 to 0.20), fair (κ = 0.21 to 0.40), moderate (κ = 0.41 to 0.60), substantial (κ = 0.61 to 0.80), and almost perfect (κ > 0.80) [30].
Ethics
This study did not require approval by an ethics committee because it was a pooled analysis with systematic review of previously published studies. We performed this study according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [31].
RESULTS
Eligible studies
Fig. 1 shows the flowchart of studies. A total of 132 studies were identified according to the search strategy: 110 were excluded after screening the titles and abstracts. The remaining 22 articles comparing tumor responses according to the RECIST and metabolic criteria were potentially relevant. However, eight studies were excluded based on the inclusion criteria: three articles used the modified RECIST and five had no details of response classification [32-36].
Figure 1.
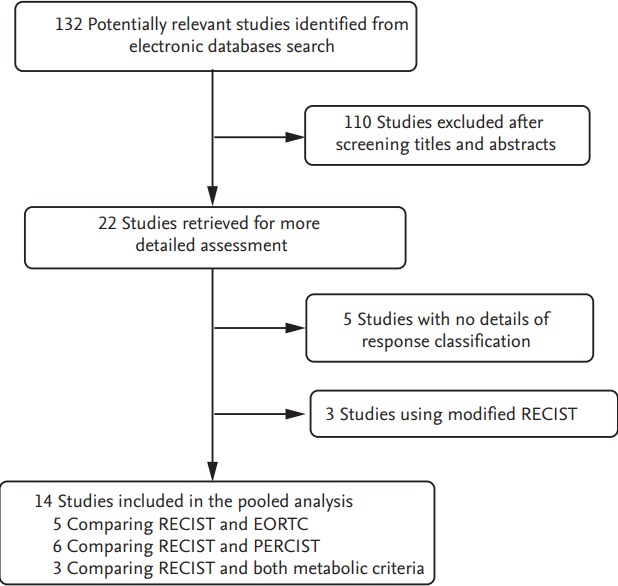
Flowchart of search process. RECIST, Response Evaluation Criteria in Solid Tumors; EORTC, European Organization Research and Treatment of Cancer; PERCIST, Positron Emission Tomography Response Criteria in Solid Tumors.
Finally, 14 studies with data comparing tumor responses by the RECIST and morphologic criteria (EORTC or PERCIST) were selected [14-27]. Five articles compared the RECIST and EORTC criteria [14,16-19] and six compared the RECIST and PERCIST [22-27]. The remaining three studies compared the RECIST and both metabolic criteria [15,20,21].
Patients’ characteristics
From the eight studies comparing the RECIST and EORTC criteria [14-21], 216 patients were included; 82 with lung cancer [19-21], 45 with colorectal cancer [14,20], 33 with head and neck cancer [18,20], 17 with malignant melanoma [17], 14 with basal cell carcinoma [15], 12 with stomach cancer [20], seven with desmoplastic small round cell tumors [16], and six with breast cancer [20] (Table 2). Eighty-nine patients (41.2%) were treated with targeted agents and 127 (58.8%) received cytotoxic chemotherapy.
Table 2.
Summary of eight studies comparing the RECIST and EORTC criteria
| Study | Tumor type | No. of pts | Treatment | Comparison | Discordant rate, % | Details of discordance |
|---|---|---|---|---|---|---|
| RECIST→ EORTC | ||||||
| Monteil et al. (2009) [14] | Colorectal cancer | 25 | Palliative chemotherapy | RECIST 1.0 vs. EORTC | 80 (20/25) | 3 PR→2 CMR, 1 PMD |
| 17 SD→3 CMR, 13 PMR, 1 PMD | ||||||
| Thacker et al. (2012) [15] | Basal cell carcinoma | 14 | Targeted agent (vismodegib) | RECIST 1.0 vs. PER- CIST | 50 (7/14) | 2 PR→1 CMR, 1 SMD |
| 4 SD→4 PMR | ||||||
| 1 PD→1 SMD | ||||||
| Magnan et al. (2013) [16] | Desmoplastic small round cell tumor | 7 | Chemotherapy | RECIST 1.0 vs. EORTC | 71.4 (5/7) | 5 SD→5 PMR |
| Adkin et al. (2014) [17] | Head & neck cancer | 27 | Targeted therapy (cetuximab) | RECIST 1.0 vs. EORTC | 51.9 (14/27) | 14 SD→9 PMR, 5 PMD |
| Zukotynski et al. (2014) [18] | Malignant melanoma | 17 | Targeted therapy (imatinib) | RECIST 1.0 vs. EORTC | 29.4 (5/17) | 1 PR→1 SMD |
| 1 SD→1 PMD | ||||||
| 3 PD→1 PMR, 2 SMD | ||||||
| Puranik et al. (2015) [19]a | Non-small cell lung cancer | 31 | Targeted therapy (gefitinib) | RECIST 1.1 vs. EORTC | 9.7 (3/31) | 3 SD→3 PMR |
| Aras et al. (2016) [20] | Colorectal cancer | 20 | Chemotherapy | RECIST 1.1 vs. EORTC | 20 (12/60) | 4 PR→3 CMR, 1 SMD |
| Lung cancer | 16 | 8 SD→7 PMR, 1 PMD | ||||
| Stomach cancer | 12 | |||||
| Head & neck cancer | 6 | |||||
| Breast cancer | 6 | |||||
| Shang et al. (2016) [21] | Non-small cell lung cancer | 35 | Chemotherapy | RECIST 1.1 vs. EORTC | 57.1 (20/35) | 16 SD→12 PMR, 4 PMD |
| 4 PD→4 SMD | ||||||
| Summary | Lung cancer | 82 | RECIST vs. EORTC | 39.8 (86/216) | 10 PR→6 CMR, 3 SMD, 1 PMD | |
| Colorectal cancer | 45 | 68 SD→3 CMR, 53 PMR, 12 PMD | ||||
| Head & neck cancer | 33 | 8 PD→1 PMR, 7 SMD | ||||
| Malignant melanoma | 17 | |||||
| Basal cell carcinoma | 14 | |||||
| Stomach cancer | 12 | |||||
| Breast cancer | 6 | |||||
| Desmoplastic small round cell tumor | 7 |
RECIST, Response Evaluation Criteria in Solid Tumors; EORTC, European Organization Research and Treatment of Cancer; pts, patients; PR, partial response; CMR, complete metabolic response; PMD, progressive metabolic disease; SD, stable disease; PMR, partial metabolic response; SMD, stable metabolic disease; PD, progressive disease.
Because the RECIST 1.1 includes positron emission tomography for the detection of new lesions, two patients with new focal fluorodeoxyglucose avid marrow lesions were reclassified as having PD.
From the nine studies comparing tumor responses by the RECIST and PERCIST, 407 patients were included [15,20-27]: 120 with colorectal cancer [20,23,27], 95 with lung cancer [20-22], 93 with breast cancer [20,25,26], 48 with esophageal cancer [24,25], 14 with basal cell carcinoma [15], 12 with stomach cancer [20], 10 with head and neck cancer [20,25], and 16 with other cancers (Table 3) [25].
Table 3.
Summary of nine studies comparing the RECIST and PERCIST
| Study | Tumor type | No. of pts | Treatment | Comparison | Discordant rate, % | Details of discordance |
|---|---|---|---|---|---|---|
| RECIST→ EORTC | ||||||
| Thacker et al. (2012) [15] | Basal cell carcinoma | 14 | Targeted agent (vismodegib) | RECIST 1.0 vs. PERCIST | 50 (7/14) | 2 PR→1 CMR, 1 SMD |
| 4 SD→4 PMR | ||||||
| 1 PD→1 SMD | ||||||
| Aras et al. (2016) [20] | Colorectal cancer | 20 | Palliative chemotherapy | RECIST 1.1 vs. PERCIST | 18.3 (11/60) | 4 PR→3 CMR, 1 SMD |
| Lung cancer | 16 | 7 SD→7 PMR | ||||
| Stomach cancer | 12 | |||||
| Head & neck cancer | 6 | |||||
| Breast cancer | 6 | |||||
| Shang et al. (2016) [21] | Non-small cell lung cancer | 35 | Chemotherapy | RECIST 1.1 vs. PERCIST | 62.9 (22/35) | 18 SD→14 PMR, 4 PMD |
| 4 PD→4 SMD | ||||||
| Ding et al. (2014) [22] | Non-small cell lung cancer | 44 | Palliative chemotherapy | RECIST 1.1 vs. PERCIST | 34.1 (15/44) | 6 PR→4 CMR, 2 SMD |
| 9 SD→1 CMR, 7 PMR, 1 PMD | ||||||
| Skougaard et al. (2014) [23] | Colorectal cancer | 61 | Palliative chemotherapy | RECIST 1.0 vs. PERCIST | 54.1 (33/61) | 1 PR→1 SMD |
| 24 SD→20 PMR, 4 PMD | ||||||
| 8 PD→4 PMR, 4 SMD | ||||||
| Yanagawa et al. (2012) [24]a | Esophageal cancer | 46 | Neoadjuvant chemotherapy | RECIST 1.1 vs. PERCIST | 56.5 (26/46) | 13 PR→13 CMR |
| 13 SD→3 CMR, 10 PMR | ||||||
| Agrawal et al. (2014) [25] | Breast cancer | 22 | Metronomic palliative hemotherapy | RECIST 1.1 vs. PERCIST | 20.6 (9/43) | 8 SD→1 CMR, 1 PMR, 6 PMD |
| PNET | 5 | 1 PD→1 CMR | ||||
| Head & neck cancer | 4 | |||||
| Sarcoma | 3 | |||||
| NHL | 2 | |||||
| Esophageal cancer | 2 | |||||
| Others | 5 | |||||
| Riedl et al. (2017) [26] | Breast cancer | 65 | Chemotherapy, targeted therapy, hormonal therapy | RECIST 1.1 vs. PERCIST | 52.3 (34/65) | 10 PR→10 CMR |
| 20 SD→6 CMR, 8 PMR, 6 PMD | ||||||
| 4 PD→3 CMR, 1 SMD | ||||||
| Bang et al. (2017) [27] | Colorectal cancer | 39 | Targeted therapy (regorafenib) | RECIST 1.1 vs. PERCIST | 61.5 (24/39) | 1 PR→1 SMD |
| 17 SD→14 PMR, 3 PMD | ||||||
| 6 PD→3 PMR, 3 SMD | ||||||
| Summary | Colorectal cancer | 120 | RECIST vs. PERCIST | 44.5 (181/407) | 37 PR→31 CMR, 6 SMD | |
| Lung cancer | 95 | 120 SD→11 CMR, 85 PMR, 24 PMD | ||||
| Breast cancer | 93 | 24 PD→4 CMR, 7 PMR, 13 SMD | ||||
| Esophageal cancer | 48 | |||||
| Basal cell carcinoma | 14 | |||||
| Stomach cancer | 12 | |||||
| Head & neck cancer | 10 | |||||
| Others | 16 |
RECIST, Response Evaluation Criteria in Solid Tumors; PERCIST, Positron Emission Tomography Response Criteria in Solid umors; pts, patients; PR, partial response; CMR, complete metabolic response; SMD, stable metabolic disease; SD, stable disease; PMR, partial metabolic response; PD, progressive disease; PMD, progressive metabolic disease; PNET, primitive neuroectodermal tumor; NHL, non-Hodgkin’s lymphoma.
Five patients were excluded from the final analysis because their disease was not classifiable according to the RECIST 1.1.
Comparison of tumor responses between the RECIST and EORTC criteria
Because the RECIST 1.1 includes PET scans for the detection of new lesions, we reclassified tumor response of two patients with new focal FDG avid marrow lesions as PD [19]. The rate of discordance in tumor responses between the RECIST (RECIST 1.0 and RECIST 1.1) and EORTC criteria varied from 9.7% in non-small cell lung cancer [19] to 80% in colorectal cancer [14] (Table 2). The agreement of tumor response between the two criteria was moderate (κ = 0.447; 95% confidence interval, 0.356 to 0.537) (Table 4). Of 216 patients, 86 (39.8%) showed discordance in the assessment of their tumor responses between the two criteria. The details of the patients showing disagreement are described in Table 2. When adopting the EORTC criteria, tumor responses were upgraded in 70 patients and downgraded in 16. The shift in tumor responses occurred most frequently in patients with SD as determined by the RECIST. Among 68 patients with SD, the tumor response in 56 patients (82.46%) was upgraded to CMR (n = 3) or PMR (n = 53) and that in 12 was downgraded to progressive metabolic disease (PMD) by using the EORTC criteria. Of 10 patients with PR, six were reclassified as showing CMR, three as having stable metabolic disease (SMD), and one as having PMD. There were eight patients with PD who were upgraded as having PMR (n = 1) or SMD (n = 7). As a result, the estimated ORRs, which were estimated in total regardless of the primary tumor sites, were significantly different between the two criteria (28.2% by the RECIST vs. 52.8% by the EORTC, p < 0.0001).
Table 4.
Comparison of tumor responses according to the RECIST and EORTC criteria
| Tumor response by the RECIST | Tumor response by the EORTC |
Total | |||
|---|---|---|---|---|---|
| CMR | PMR | SMD | PMD | ||
| CR | 5 | 0 | 0 | 0 | 5 |
| PR | 6 | 46 | 3 | 1 | 56 |
| SD | 3 | 53 | 25 | 12 | 93 |
| PD | 0 | 1 | 7 | 54 | 62 |
| Total | 14 | 100 | 35 | 67 | 216 |
The level of concordance of tumor responses between the EORTC criteria and RECIST is 0.447 (unweighted κ, 95% confidence interval, 0.356 to 0.537). The overall response rates were significantly different between the two criteria (28.2% by RECIST vs. 52.8% by EORTC, p < 0.0001).
RECIST, Response Evaluation Criteria in Solid Tumors; EORTC, European Organization Research and Treatment of Cancer; CMR, complete metabolic response; PMR, partial metabolic response; SMD, stable metabolic disease; PMD, progressive metabolic disease; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Comparison of tumor responses between the RECIST and PERCIST
The rate of disagreement in tumor responses between the RECIST and PERCIST varied from 18.3% [20] to 62.9% [25]. Of 407 patients, 181 (44.5%) showed discordance in the assessment of their tumor responses between the two criteria. The details of the patients showing discordance are summarized in Table 3. The agreement of tumor response between the two criteria was fair (κ = 0.398; 95% confidence interval, 0.323 to 0.456) (Table 5). When adopting the PERCIST, the tumor response was upgraded in 151 patients and downgraded in 30. The shift in tumor responses was also observed most frequently in patients with SD by using the RECIST. Among 120 patients with SD, the tumor response of 96 patients (80%) was upgraded to CMR (n = 11) or PMR (n = 85) and that of 24 was downgraded to PMD by adopting the PERCIST. Of 37 patients with PR, 31 were reclassified as showing CMR and six as having SMD. There were 24 patients with PD who were upgraded as showing CMR (n = 4), PMR (n = 7), and SMD (n = 13). The estimated ORRs were also significantly different between the two criteria (30.2% by the RECIST vs. 55.0% by the PERCIST, p < 0.0001).
Table 5.
Comparison of tumor responses according to the RECIST and PERCIST
| Tumor response by RECIST | Tumor response by PERCIST |
Total | |||
|---|---|---|---|---|---|
| CMR | PMR | SMD | PMD | ||
| CR | 8 | 0 | 0 | 0 | 8 |
| PR | 31 | 78 | 6 | 0 | 115 |
| SD | 11 | 85 | 60 | 24 | 180 |
| PD | 4 | 7 | 13 | 80 | 104 |
| Total | 54 | 170 | 79 | 104 | 407 |
The level of concordance of tumor responses between the RECIST and PERCIST 1.0 is 0.389 (unweighted κ, with 95% confidence interval, 0.323 to 0.456). The overall response rates were significantly different between two criteria (30.2% by RECIST vs. 55.0% by PERCIST, p < 0.0001).
RECIST, Response Evaluation Criteria in Solid Tumors; PERCIST, Positron Emission Tomography Response Criteria in Solid Tumors; CMR, complete metabolic response; PMR, partial metabolic response; SMD, stable metabolic disease; PMD, progressive metabolic disease; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
DISCUSSION
In this pooled study, we investigated the concordance between the metabolic criteria and morphologic criteria for the assessment of tumor responses in patients with solid tumors. There was a considerable discrepancy in the assessment of tumor responses between the morphologic criteria (RECIST) and metabolic criteria (EORTC or PERCIST). When adopting the EORTC criteria or PERCIST instead of the RECIST, the ORR was significantly increased, suggesting significant clinical impact of the metabolic criteria on making therapeutic decision.
In clinical practice, it is not always easy to distinguish necrotic tissue or fibrotic scar from residual tumor on CT scans [37]. In particular, with the increasing use of targeted agents, new evaluation methods are needed to accurately monitor tumor responses. [18F]-FDG PET has become a well-established method for the staging and detection of recurrence in patients with several malignancies [38]. It is also increasingly used to assess tumor responses to anti-cancer therapies [8-10]. FDG PET responses have correlated more significantly with survival than those assessed by CT [39]. However, the metabolic response criteria have shown differences compared with the morphologic criteria for the assessment of tumor responses.
In our pooled analysis of 216 patients from eight studies [14-21], the agreement of tumor responses between the RECIST and EORTC criteria was moderate (κ = 0.447). Eighty-six patients (39.8%) showed discrepancies in the assessment of tumor responses between the two criteria. Use of the EORTC criteria resulted in upgraded tumor responses in 70 patients and downgraded responses in 16. When adopting the EORTC criteria, the ORR significantly increased from 28.2% to 52.8% (p < 0.0001). The agreement of tumor responses between the RECIST and PERCIST was deemed fair (κ = 0.389). Of 407 patients from nine studies [15,20-27], 181 (44.5%) showed a disagreement in tumor responses between the two criteria. Use of the PERCIST resulted in upgraded tumor responses in 151 patients and downgraded responses in 30. When adopting the PERCIST instead of the RECIST, the ORR also significantly increased from 30.2% to 55.0% (p < 0.0001).
Early detection of the tumor response is of great value to avoid unnecessary toxicity and cost of ineffective treatments. Anatomical responses based on the size of the tumor may lag weeks or months behind metabolic response [40]. PET can detect metabolic changes after chemotherapy even when there are no or minimal morphological changes [7], which may explain the reason why tumor responses were upgraded by using the metabolic criteria in many patients who showed SD by using the RECIST in this study. In clinical practice, patients showing disease progression (PD or PMD) after anti-cancer treatment usually need a change in therapeutic approach. If the metabolic criteria had been used instead of the RECIST in this pooled study, it would have changed the treatment course in approximately 10% of the patients. This finding indicates that the clinical impact of the metabolic response criteria on making therapeutic decisions is significant.
The current pooled study has several inherent limitations. First, this study included heterogeneous patients with different types of tumors and different kinds of therapeutic agents. In addition, because of the limited number of studies, we could not compare two criteria in the subgroup with the same cancers. It is necessary to verify these results in studies with larger homogeneous patients’ cohort. Second, we included two versions of the RECIST without distinction in the analysis. Although the RECIST 1.1 has shown almost perfect agreement with the RECIST 1.0 in the assessment of tumor responses, a potential difference between the two versions might affect the results. Finally, this study could not evaluate the prognostic value of the metabolic criteria. Although the PERCIST and EORTC criteria were associated with prognosis in several studies, survival data were not enough to compare the prognostic value between the RECIST and the metabolic criteria.
In conclusion, this pooled study demonstrates that concordance in the assessment of tumor responses between the morphologic criteria (RECIST) and metabolic criteria (EORTC or PERCIST) is not excellent. When adopting the metabolic criteria instead of the RECIST, the ORR was significantly increased. The prognostic value of the metabolic criteria needs to be investigated in larger studies with homogeneous patient cohorts.
KEY MESSAGE
1. The assessment of tumor responses between the morphologic criteria and metabolic criteria has shown considerable discrepancies in studies with a small sample size.
2. The agreement of tumor responses was moderate between the morphologic (Response Evaluation Criteria in Solid Tumors [RECIST]) and metabolic criteria (European Organization for Research and Treatment of Cancer or Positron Emission Tomography Response Criteria in Solid Tumors).
3. When adopting the metabolic criteria instead of the RECIST, the overall response rate was significantly increased.
Footnotes
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 2.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 3.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 4.Bogaerts J, Ford R, Sargent D, et al. Individual patient data analysis to assess modifications to the RECIST criteria. Eur J Cancer. 2009;45:248–260. doi: 10.1016/j.ejca.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, Litiere S, de Vries EG, et al. The role of response evaluation criteria in solid tumour in anticancer treatment evaluation: results of a survey in the oncology community. Eur J Cancer. 2014;50:260–266. doi: 10.1016/j.ejca.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Shankar LK, Van den Abbeele A, Yap J, Benjamin R, Scheutze S, Fitzgerald TJ. Considerations for the use of imaging tools for phase II treatment trials in oncology. Clin Cancer Res. 2009;15:1891–1897. doi: 10.1158/1078-0432.CCR-08-2030. [DOI] [PubMed] [Google Scholar]
- 7.Krystal GW, Alesi E, Tatum JL. Early FDG/PET scanning as a pharmacodynamics marker of anti-EGFR antibody activity in colorectal cancer. Mol Cancer Ther. 2012;11:1385–1388. doi: 10.1158/1535-7163.MCT-12-0011. [DOI] [PubMed] [Google Scholar]
- 8.Skoura E, Datseris IE, Platis I, Oikonomopoulos G, Syrigos KN. Role of positron emission tomography in the early prediction of response to chemotherapy in patients with non-small-cell lung cancer. Clin Lung Cancer. 2012;13:181–187. doi: 10.1016/j.cllc.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Lee DH, Kim SK, Lee HY, et al. Early prediction of response to first-line therapy using integrated 18F-FDG PET/CT for patients with advanced/metastatic non-small cell lung cancer. J Thorac Oncol. 2009;4:816–821. doi: 10.1097/JTO.0b013e3181a99fde. [DOI] [PubMed] [Google Scholar]
- 10.Young H, Baum R, Cremerius U, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35:1773–1782. doi: 10.1016/s0959-8049(99)00229-4. [DOI] [PubMed] [Google Scholar]
- 11.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50 Suppl 1:122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JH. Comparison of the EORTC criteria and PERCIST in solid tumors: a pooled analysis and review. Oncotarget. 2016;7:58105–58110. doi: 10.18632/oncotarget.11171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skougaard K, Nielsen D, Jensen BV, Hendel HW. Comparison of EORTC criteria and PERCIST for PET/CT response evaluation of patients with metastatic colorectal cancer treated with irinotecan and cetuximab. J Nucl Med. 2013;54:1026–1031. doi: 10.2967/jnumed.112.111757. [DOI] [PubMed] [Google Scholar]
- 14.Monteil J, Mahmoudi N, Leobon S, et al. Chemotherapy response evaluation in metastatic colorectal cancer with FDG PET/CT and CT scans. Anticancer Res. 2009;29:2563–2568. [PubMed] [Google Scholar]
- 15.Thacker CA, Weiss GJ, Tibes R, et al. 18-FDG PET/CT assessment of basal cell carcinoma with vismodegib. Cancer Med. 2012;1:230–236. doi: 10.1002/cam4.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magnan H, Abramson SJ, Price AP, et al. Positron emission tomography for response assessment in desmoplastic small round cell tumor. J Pediatr Hematol Oncol. 2013;35:e190–e193. doi: 10.1097/MPH.0b013e3182707d4c. [DOI] [PubMed] [Google Scholar]
- 17.Adkins D, Ley J, Dehdashti F, et al. A prospective trial comparing FDG-PET/CT and CT to assess tumor response to cetuximab in patients with incurable squamous cell carcinoma of the head and neck. Cancer Med. 2014;3:1493–1501. doi: 10.1002/cam4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zukotynski K, Yap JT, Giobbie-Hurder A, et al. Metabolic response by FDG-PET to imatinib correlates with exon 11 KIT mutation and predicts outcome in patients with mucosal melanoma. Cancer Imaging. 2014;14:30. doi: 10.1186/s40644-014-0030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puranik AD, Purandare NC, Shah S, Agrawal A, Rangarajan V. Role of FDG PET/CT in assessing response to targeted therapy in metastatic lung cancers: morphological versus metabolic criteria. Indian J Nucl Med. 2015;30:21–25. doi: 10.4103/0972-3919.147529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aras M, Erdil TY, Dane F, et al. Comparison of WHO, RECIST 1.1, EORTC, and PERCIST criteria in the evaluation of treatment response in malignant solid tumors. Nucl Med Commun. 2016;37:9–15. doi: 10.1097/MNM.0000000000000401. [DOI] [PubMed] [Google Scholar]
- 21.Shang J, Ling X, Zhang L, et al. Comparison of RECIST, EORTC criteria and PERCIST for evaluation of early response to chemotherapy in patients with non-small-cell lung cancer. Eur J Nucl Med Mol Imaging. 2016;43:1945–1953. doi: 10.1007/s00259-016-3420-7. [DOI] [PubMed] [Google Scholar]
- 22.Ding Q, Cheng X, Yang L, et al. PET/CT evaluation of response to chemotherapy in non-small cell lung cancer: PET response criteria in solid tumors (PERCIST) versus response evaluation criteria in solid tumors (RECIST) J Thorac Dis. 2014;6:677–683. doi: 10.3978/j.issn.2072-1439.2014.05.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skougaard K, Johannesen HH, Nielsen D, et al. CT versus FDG-PET/CT response evaluation in patients with metastatic colorectal cancer treated with irinotecan and cetuximab. Cancer Med. 2014;3:1294–1301. doi: 10.1002/cam4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yanagawa M, Tatsumi M, Miyata H, et al. Evaluation of response to neoadjuvant chemotherapy for esophageal cancer: PET response criteria in solid tumors versus response evaluation criteria in solid tumors. J Nucl Med. 2012;53:872–880. doi: 10.2967/jnumed.111.098699. [DOI] [PubMed] [Google Scholar]
- 25.Agrawal A, Purandare N, Shah S, Puranik A, Banavali S, Rangarajan V. Response assessment in metronomic chemotherapy: RECIST or PERCIST? Indian J Nucl Med. 2014;29:74–80. doi: 10.4103/0972-3919.130285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riedl CC, Pinker K, Ulaner GA, et al. Comparison of FDG-PET/CT and contrast-enhanced CT for monitoring therapy response in patients with metastatic breast cancer. Eur J Nucl Med Mol Imaging. 2017;44:1428–1437. doi: 10.1007/s00259-017-3703-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bang JI, Lim Y, Paeng JC, et al. Comparison of quantitative methods on FDG PET/CT for treatment response evaluation of metastatic colorectal cancer. Nucl Med Mol Imaging. 2017;51:147–153. doi: 10.1007/s13139-016-0449-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JH, Min SJ, Jang HJ, Cho JW, Kim SH, Kim HS. Comparison of RECIST 1.0 and RECIST 1.1 in patients with metastatic cancer: a pooled analysis. J Cancer. 2015;6:387–393. doi: 10.7150/jca.11316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim JH. Comparison of the RECIST 1.0 and RECIST 1.1 in patients treated with targeted agents: a pooled analysis and review. Oncotarget. 2016;7:13680–13687. doi: 10.18632/oncotarget.7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts C. Modelling patterns of agreement for nominal scales. Stat Med. 2008;27:810–830. doi: 10.1002/sim.2945. [DOI] [PubMed] [Google Scholar]
- 31.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borksuz MF, Erselcan T, Hasbek Z, Yucel B, Turgut B. Morphologic and metabolic comparison of treatment responsiveness with 18fludeoxyglucose-positron emission tomography/computed tomography according to lung cancer type. Mol Imaging Radionucl Ther. 2016;25:63–69. doi: 10.4274/mirt.36349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ozturk H. Comparing RECIST with EORTC criteria in metastatic bladder cancer. J Cancer Res Clin Oncol. 2016;142:187–194. doi: 10.1007/s00432-015-2022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mertens LS, Fioole-Bruining A, van Rhijn BW, et al. FDG-positron emission tomography/computerized tomography for monitoring the response of pelvic lymph node metastasis to neoadjuvant chemotherapy for bladder cancer. J Urol. 2013;189:1687–1691. doi: 10.1016/j.juro.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 35.Ordu C, Selcuk NA, Akosman C, et al. Comparison of metabolic and anatomic response to chemotherapy based on PERCIST and RECIST in patients with advanced stage non-small cell lung cancer. Asian Pac J Cancer Prev. 2015;16:321–326. doi: 10.7314/apjcp.2015.16.1.321. [DOI] [PubMed] [Google Scholar]
- 36.Stefano A, Russo G, Ippolito M, et al. Evaluation of erlotinib treatment response in non-small cell lung cancer using metabolic and anatomic criteria. Q J Nucl Med Mol Imaging. 2016;60:264–273. [PubMed] [Google Scholar]
- 37.Suzuki C, Jacobsson H, Hatschek T, et al. Radiologic measurements of tumor response to treatment: practical approaches and limitations. Radiographics. 2008;28:329–344. doi: 10.1148/rg.282075068. [DOI] [PubMed] [Google Scholar]
- 38.Fletcher JW, Djulbegovic B, Soares HP, et al. Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med. 2008;49:480–508. doi: 10.2967/jnumed.107.047787. [DOI] [PubMed] [Google Scholar]
- 39.Mac Manus MP, Hicks RJ, Matthews JP, et al. Positron emission tomography is superior to computed tomography scanning for response-assessment after radical radiotherapy or chemoradiotherapy in patients with nonsmall-cell lung cancer. J Clin Oncol. 2003;21:1285–1292. doi: 10.1200/JCO.2003.07.054. [DOI] [PubMed] [Google Scholar]
- 40.Van den Abbeele AD. The lessons of GIST-PET and PET/CT: a new paradigm for imaging. Oncologist. 2008;13 Suppl 2:8–13. doi: 10.1634/theoncologist.13-S2-8. [DOI] [PubMed] [Google Scholar]


