Abstract
Background/Aims
The aromatase inhibitors (AIs) are well known anti-hormonal therapy in endocrine-responsive breast cancer patients. It can lead to dyslipidemia and be the risk factor of cardiovascular disease due to low estrogen level. However, some recent studies comparing AIs with placebo have shown controversial results. The aim of this study was to investigate lipid profiles, measurement of carotid intima-media thickness (IMT) and the presence of plaque among endocrine-responsive breast cancer treated with AIs compared to ones that were not treated with AIs.
Methods
A total of 85 postmenopausal women, who underwent breast cancer surgery during the age of 50 to 64 without history of statin use were included. There were 42 patients who were treated with AIs over 1 year (group 1) and 43 patients without AIs use (group 2). Serum total cholesterol, high density lipoprotein cholesterol, triglycerides, fasting blood glucose, carotid IMT, and presence of plaque were assessed.
Results
The baseline characteristics were similar between two groups and there was no significant difference in carotid IMT irrespective of AIs administration. However, ultrasonographic evaluation of carotid artery revealed that the presence of plaque in AI users was significantly higher than in non-AI users (66.7% vs. 41.9%, p = 0.02; odds ratio, 4.21 in adjusted model; p = 0.01). History of diabetes was also the significant risk factor for the plaque formation.
Conclusions
There was no significant difference in lipid profile itself between two groups, but more importantly the presence of the plaque was much higher indicating possible detrimental effect of AI on cardiovascular system.
Keywords: Breast neoplasms, Aromatase inhibitors, Dyslipidemias, Cardiovascular diseases, Carotid plaque
INTRODUCTION
The aromatase inhibitors (AIs) are well known anti-hormonal therapy in endocrine-responsive breast cancer patients as it inhibits the synthesis of estrogen. Postmenopausal women with endocrine-responsive breast cancer in the adjuvant or metastatic settings are usually treated with AIs according to recent guidelines [1]. The AIs are used for at least 5 years in adjuvant treatment, so the long-term safety of AIs is important concern to patients who are taking AIs.
Estrogen has atheroprotective effects by maintaining normal serum lipid profiles. Estrogen also alters the coagulation and fibrinolytic system, antioxidant system, and the production of vasoactive molecules [2]. And estrogen decline occurring after the menopause can influence the development of cardiovascular disease (CVD). Thus, the incidence of atherosclerotic disease increases in postmenopausal women [3].
As a result of AIs use, serum estrogen (including estrone, estradiol, and estrone sulfate) level was decreased over 90% in postmenopausal women with advanced breast cancer [4]. Due to absolute deficiency in estrogen level, it can also lead to dyslipidemia [5]. And drugs which alter lipid profiles could increase the risk of CVD by vascular atherosclerosis [6]. In addition, atherosclerosis is one of the most important cause of morbidity and mortality even in patients with cancer [7]. For these reasons, the evaluation of the risk of CVD is important for breast cancer patients even more with the ones who are taking AIs.
There have been lots of controversies on the effect of AIs to lipid profile or CVD risk. In the previous study with postmenopausal women aged 46 to 68 years, comparing the level of lipid profile before and after the treatment of AIs (n = 20), a significant increase in total cholesterol (p = 0.05) and low density lipoprotein cholesterol (LDL-C; p < 0.01) was noticed [8]. In a BIG (Breast International Group) 1-98 trial (n = 4,922) with postmenopausal women with the median age of 61 years, comparing to tamoxifen treatment group, patients on AIs experienced more frequent low-grade hypercholesterolemia (50.6% vs. 24.6%, p < 0.001) [9]. Meanwhile, in the MA.17 trial with postmenopausal women with the median age of 63 years, Wasan et al. [10] reported that there was no significant differences in lipid profile between the treatment group (AIs [n = 181] vs. placebo [n = 161]) in a randomized placebo-controlled trial with letrozole for 5 years in postmenopausal women with breast cancer. However, all these patients already received 5 years treatment with tamoxifen prior to enrollment.
Several clinical data including MA.17 trial and ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial, use of AIs showed no difference in risk of cardiovascular morbidity or mortality between AIs and tamoxifen treatment groups [11,12]. Meanwhile, in recent largescale studies from adjuvant trials have raised concerns that long-term use of AIs do increase the risk of cardiovascular events although odds ratio (OR) were different among subgroup of AIs [13,14]. In a meta-analysis of several randomized trials, the incidence of high grade cardiovascular events were increased in AIs users comparing with the patients who were receiving tamoxifen (relative risk, 1.31; 95% confidence interval [CI], 1.07 to 1.60; p = 0.007; total n = 19,818) [13]. In a systematic review of 30,023 patients, longer duration of AIs use was associated with increased CVD development (OR, 1.26; 95% CI, 1.10 to 1.43; p < 0.001) [14]. This potential AIs toxicity on CVD risk might be one of the reasons of lack of overall survival benefit despite improvements in disease free survival in AIs treatment group [14].
Long-term risk of atherosclerosis and CVD due to cancer treatment may become an important consideration in the era of improved survival of breast cancer patients. It remains uncertain whether AI use may account for potential detrimental effects on the process of atherosclerosis when used for longer duration [15]. Carotid ultrasonography, including the measurement of carotid intima-media thickness (IMT) and carotid plaque, has been known to be a reliable marker of early atherosclerosis and cardiovascular risk. Thus, in this study, we investigated lipid profiles, actual measurement of carotid IMT and presence of plaque among endocrine-responsive breast cancer treated with AIs compared with non-AI treated women.
METHODS
Subjects
We conducted a retrospective, cross-sectional study by using data retrieved from the electronic registry of a tertiary-level, university-affiliated single institution (Severance Hospital, Yonsei University College of Medicine, Seoul, Korea). Eighty-five postmenopausal breast cancer subjects (age 50 to 64) who underwent surgery from January 2002 to October 2012 were included in this study. There were 42 patients who were treated with AIs. And there were 43 patients without any endocrine therapy. Considering anti-atherosclerotic effect of tamoxifen [16], patients who were treated with tamoxifen as the first line therapy was excluded. Also, we excluded patients who had history of statin therapy over 3 months prior to enrollment, since medications like statins may alter lipid profile and IMT.
This study was approved by the Institutional Review Board (IRB) of Severance Hospital, Yonsei University (No. 4-2018-1049) and obtaining informed consent form has been waivered by IRB.
Methods
Height, weight, and body mass index (BMI) were measured and the medical history of patients was taken. Serum total cholesterol, high density lipoprotein cholesterol (HDL-C), triglycerides, fasting insulin, and fasting blood glucose were assessed before the ultrasound evaluation.
To evaluate insulin sensitivity or resistance, the quantitative insulin-sensitivity check index (QUICKI) [17] and the homeostasis model assessment of insulin resistance (HOMA-IR) were calculated [18]. In calculation of QUICKI and HOMA-IR, patients who were receiving insulin treatment or have history of diabetes before the operation were excluded.
B-mode ultrasonography of the carotid artery was performed using an ultrasound machine (LOGIQ9, GE Medical Systems, Milwaukee, WI, USA) with a 10-MHz linear transducer. The internal carotid artery, the carotid bulb, and the extracranial common carotid artery (CCA) in the neck were examined bilaterally. The IMT was measured as the distance between the blood-intima and media-adventitia interface. The mean IMT of the CCA was measured over a segment of the CCA that was 1cm long, located approximately 2 cm below the carotidartery bulb [19].
The greatest thickness of IMT in the CCA was measured and defined as maximum IMT. The carotid plaque was defined as a focal structure encroaching into the arterial lumen or a focal thickening > 50% of the surrounding wall [20]. All measurements were performed by the same qualified observer who was blinded the actual status of the patient.
Statistics
All statistical analyses were performed with SPSS version 20.0 (IBM Co., Armonk, NY, USA). All values are reported as mean ± SD with parametric data, median (interquartile range) with non-parametric data, and real numbers of subjects with the percentage in parentheses. The normality of the distribution of continuous variables was examined using Shapiro-Wilk test. Between-group differences of the average were compared using the unpaired t test for parametric data, and the Mann-Whitney U test was used for non-parametric data. Between-group differences of numbers and percentages were compared using a chi-square test. Multivariate logistic regression analysis was used with adjustment for variables with significant associations at the level of p < 0.05 in univariate models and variables which were known as risk factors of carotid plaque formation including dyslipidemia, concurrent chemotherapy, and radiotherapy. For all tests, p < 0.05 was considered statistically significant.
RESULTS
Clinical characteristics of study population
The clinical characteristics of patients are given in Table 1. There were no significant differences in the age at the time of surgery and ultrasonography, BMI, fasting blood glucose, QUICKI, HOMA-IR, and lipid profile between two groups. History of diabetes, TNM (tumor, nodes and metastasis) stage and elapsed time to IMT after surgery were also similar in each group. More patients conducted either neoadjuvant or adjuvant chemotherapy in non-AI group (33/43, 76.7%) than in AI group (21/42, 50.0%; p = 0.010) (Table 1). Patients who received radiotherapy after the surgery showed no significant differences between two groups.
Table 1.
Baseline characteristics of the study population
| Characteristic | AI user (n = 42) | Non-AI user (n = 43) | p value |
|---|---|---|---|
| Duration of AIs use, mon | 34.3 ± 16.9 | - | - |
| Age at the time of operation, yra | 56.5 (8) | 54.0 (7) | 0.143 |
| Age at the time of ultrasonography, yra | 60.0 (8) | 57.0 (7) | 0.066 |
| Elapsed time to IMT after operation, mona | 33.0 (20) | 30.0 (40) | 0.187 |
| History of DM | 8 (19.0) | 11 (25.6) | 0.470 |
| Duration of DM, yra | 9.8 (11.0) | 6.6 (2.0) | 0.477 |
| History of HTN | 13 (31.0) | 9 (21.4) | 0.457 |
| Use of antiplateletes | 6 (14.3) | 4 (9.5) | 0.736 |
| Use of statin | 20 (60.6) | 24 (70.6) | 0.547 |
| Smoking | 0 | 1 (2.4) | 0.991 |
| TNM stage (≥ 2a) | 22 (52.4) | 18 (41.9) | 0.331 |
| BMI, kg/m2 | 24.1 ± 2.4 | 24.1 ± 2.6 | 0.999 |
| FBS, mg/dLa | 97.5 (16) | 99.0 (15) | 0.812 |
| Total cholesterol, mg/dL | 206.1 ± 42.1 | 216.7 ± 39.5 | 0.233 |
| HDL-C, mg/dL | 53.7 ± 13.4 | 53.4 ± 10.3 | 0.902 |
| Triglycerides, mg/dLa | 123.0 (77) | 129.0 (74) | 0.916 |
| LDL-C, mg/dL | 122.5 ± 37.6 | 129.5 ± 41.0 | 0.415 |
| QUICKIa | 0.35 ± 0.01 | 0.35 ± 0.01 | 0.735 |
| HOMA-IRa | 1.89 (2.12) | 1.75 (1.67) | 0.872 |
| Chemotherapy | 21 (50.0) | 33 (76.7) | 0.010 |
| Radiotherapy | 24 (57.1) | 22 (51.2) | 0.580 |
| Chemotherapy plus radiotherapy | 12 (28.6) | 18 (41.9) | 0.200 |
Values are presented as mean ± SD or number (%).
AI, aromatase inhibitor; IMT, intima-media thickness; DM, diabetes mellitus, HTN, hypertension; TMN, tumor, nodes and metastasis; BMI, body mass index; FBS, fasting blood sugar; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; QUICKI, quantitative insulin-sensitivity check index; HOMA-IR, homeostasis model assessment of insulin resistance.
Median (interquartile range) with non-parametric data. Differences between groups of patients were analyzed by the chisquare test, independent t test, and Mann-Whitney U test.
Measurement of IMT and carotid plaque
Even though there was increased tendency (median value of mean IMT 0.65 mm in AIs use group vs. 0.63 mm in non-AI use group, maximum IMT 0.74 mm in AIs vs. 0.72 mm in non-AIs), there was no significant difference in carotid IMT irrespective of AIs use (Fig. 1). However, ultrasonographic evaluation of carotid artery revealed that the presence of plaque in AI group was significantly higher than in non-AI group (66.7 % vs. 41.9 %, p = 0.022) (Fig. 2).
Figure 1.
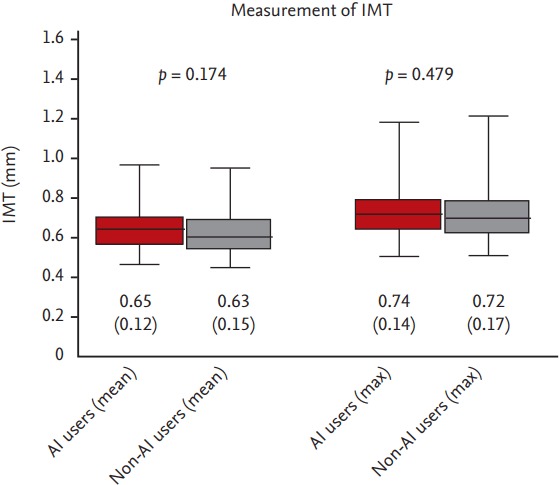
Intima-media thickness (IMT) difference between two groups was compared by Mann-Whitney U test. Data are the median (interquartile range) of the non-parametric data and real numbers of subjects with the percentage in parentheses. AI, aromatase inhibitor.
Figure 2.
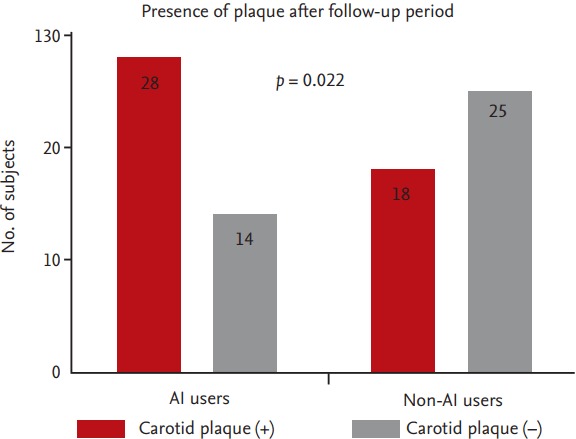
Differences in presence of carotid plaque between two groups were compared by chi-square test. AI, aromatase inhibitor.
Independent risk factors of plaque presence
Univariate and multivariate logistic regression was performed for all 85 patients. In univariate analysis, age at the time of ultrasonography (OR, 1.15; p = 0.012), the use of AIs (OR, 2.78; p = 0.023), and the history of diabetes (OR, 4.23; p = 0.019) increased the risk of carotid plaque presence significantly. In multivariate analysis, the use of AIs was an independent predictor of the presence of plaque in carotid ultrasonography (OR, 4.21; p = 0.010). History of diabetes was also a strong risk factor for the plaque (OR, 6.69; p = 0.011). The age at the time of ultrasonography was also related to the plaque presence (OR, 1.12; p = 0.048). However, BMI, lipid profile, stage at the time of primary breast cancer, history of chemotherapy, and radiotherapy did not increase the risk of plaque formation (Table 2).
Table 2.
Risk factors of plaque formation
| Variable | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age at the time of ultrasonography, yr | 1.15 (1.03–1.29) | 0.012a | 1.12 (1.00–1.26) | 0.048a |
| BMI | 1.13 (0.94–1.35) | 0.191 | ||
| FBS | 1.03 (0.99–1.06) | 0.114 | ||
| Total cholesterol | 1.00 (0.99–1.01) | 0.570 | 1.01 (1.00–1.03) | 0.056 |
| HDL-C | 1.00 (0.97–1.04) | 0.816 | ||
| Triglyceride | 1.00 (0.99–1.01) | 0.955 | ||
| LDL-C | 1.00 (0.99–1.01) | 0.724 | ||
| TNM stage (≥ 2a) | 1.07 (0.46–2.52) | 0.878 | ||
| Chemotherapy | 1.17 (0.48–2.84) | 0.726 | ||
| Radiotherapy | 1.02 (0.43–2.40) | 0.963 | ||
| CTx plus RTx | 1.17 (0.48–2.87) | 0.728 | 1.78 (0.60–5.24) | 0.297 |
| AIs use | 2.78 (1.15–6.17) | 0.023a | 4.21 (1.42–12.5) | 0.010a |
| History of DM | 4.23 (1.27–14.1) | 0.019a | 6.69 (1.54–29.0) | 0.011a |
Multivariate analysis was performed using multiple logistic regression. Variables with significant associations at the level of p < 0.05 in univariate models and variables which were known as risk factors of carotid plaque formation including dyslipidemia, concurrent chemotherapy, and radiotherapy were included in adjustment.
OR, odds ratio; CI, confidence interval; BMI, body mass index; FBS, fasting blood sugar; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol, TNM, tumor, nodes and metastasis; CTx, chemotherapy; RTx, radiotherapy; AI, aromatase inhibitor; DM, diabetes mellitus.
p values less than 0.05.
The history of diabetes was the strongest risk factor for plaque formation, and this factor can mimic the effect of AIs use. So, we also conducted multivariate analysis after excluding the patients with diabetes. And still, AIs was one of the strong risk factors for carotid plaque presence (n = 66; OR, 3.55; p = 0.031) (Supplementary Table 1).
DISCUSSION
AIs are widely used in patients with endocrine-responsive breast cancer to reduce the risk of recurrence [21]. AIs inhibit the synthesis of estrogen and are recommended over 5 years [21]. It causes several side effects including hot flushes, musculoskeletal disorder, low bone mass, and dyslipidemia [22].
In our study, there was no significant difference between two groups regarding lipid profile (total cholesterol, HDL-C, LDL-C, and triglycerides). And there was no significant difference in median and maximum IMT between two groups. Surprisingly, the use of AIs was associated with an increased risk of carotid plaque and this association was independent of age, cholesterol, history of chemotherapy, radiotherapy, or diabetes (adjusted OR, 4.21; 95% CI, 1.42 to 12.5). In a national cohort study of 375 women, age-adjusted OR of smoking in presence of carotid plaque was 3.7 (95% CI, 1.9 to 7.2) [23]. This shows that the use of AIs has similar strong associations as smoking with the presence of carotid plaque.
Several studies have raised concerns that long-term use of AIs may increase the risk of cardiovascular events [14,24]. In a recent meta-analysis with eight eligible RTCs (34,070 patients), both monotherapy with AI and sequenced therapy (tamoxifen switched to an AI) were associated with significantly higher risk of cardiovascular events (OR, 1.20 and 1.15; p = 0.030, p = 0.003, respectively), furthermore, sequenced therapy was found to have surprisingly increased risk of thromboembolic events (OR, 1.89; p = 0.005) [24]. There also was a recent population-based observational study with 74 women from heterogeneous population who received either AIs or tamoxifen and subsequently underwent cardiac angiography and the use of AIs was associated with a significant hazard for cardiovascular event (hazard ratio, 3.23; 95% CI, 1.26 to 8.25; p = 0.01) [25].
Thus, risk identification and early diagnosis of asymptomatic CVD is important for patients in endocrineresponsive breast cancer. For this reason, the measurement of carotid IMT has been widely recommended for the assessment of subclinical atherosclerosis in the asymptomatic individuals with an intermediate risk of CVD and the CCA is known to be the most reproducible segment for the measurement [26,27]. In previous studies, carotid maximum IMT has been used in the screening asymptomatic type 2 diabetic patients with severe CVD [20]. Recently, ultrasonic tissue characterization of carotid plaque has been also used in improving the prediction of coronary artery disease (CAD) events [28]. Furthermore several studies have shown that the presence of carotid plaque is better than that of carotid IMT for predicting future cardiovascular events [20,29,30].
Carotid artery IMT and plaque are both the markers of systemic atherosclerosis and predictors of CVD development [31]. However, IMT and carotid plaque showed different patterns of cardiovascular complication reflecting different biological aspects of atherogenesis. IMT on CCA was strongly related to increased risk for stroke, whereas carotid plaque was more directly related to ischemic heart disease [23], On the other words, the presence of carotid plaque, rather than the thickness of IMT, was shown to be a more powerful predictor of CVD development [23,32]. And the presence of carotid plaques was confirmed as a reliable positive predictive factor for CAD, although a statistically significant but weak correlation between IMT of the CCA and severity of CAD was found [33].
Choi et al. [34] reported that selective estrogen receptor modulation (raloxifene treatment in this study) reduced arterial plaque volume and enhanced mechanical stability of vascular calcification. In this study, analysis for plaque revealed reductions in inflammatory materials like cyclooxygenase-2 (COX-2), matrix metalloproteinase-1 (MMP-1), monocyte chemoattractant protein-1 (MCP-1), and less macrophage infiltration with upregulation of estrogen receptor α (ERα). In other animal studies raloxifene not only improved endothelial dysfunction by normalizing endothelial nitric oxide synthase 3 (eNOS) expression but also reduced the size of artherosclerotic lesion [35]. And endothelial ERα is known to be a major mediator in atheroprotection [36]. Base on above findings, we can speculate that the use of AIs, which induce extreme estrogen deprivation, can increase plaque formation by the inflammatory effects in vascular wall without altering serum cholesterol level by absolute deficient estrogen.
In this study, age and history of diabetes were also related to formation of carotid plaque. Rundek et al. [37] reported that number of carotid plaques was increased with age and history of diabetes. Cardiovascular effects of adjuvant systemic chemotherapy and radiotherapy are still controversial. Zambetti et al. [38] reported that adjuvant chemotherapy did not lead to cardiac sequelae. Indeed, there are several reports that radiotherapy with or without adjuvant chemotherapy are associated with an increased risk of CVD [39]. In this study, both systemic chemotherapy and radiotherapy did not increase the risk of carotid plaque formation statistically.
There are some limitations in our study, first, it was a retrospective study and the number of subjects in each group was relatively small. Second, IMT was assessed only once after treatment with AI. Thus, we conducted multivariate analysis with well-known risk factors for carotid plaque formation. Although smoking is one the strongest risk factors, it was not included in our assessment as there was only one participant in our study, who had history of smoking or currently smoked. Third, the elapsed time to carotid ultrasonography after surgery was relatively short in this population (mean 35.4 months) to confirm any detrimental effects of AIs to IMT. Increased IMT means the deposition of extracellular matrix material and the smooth muscle cell proliferation resulted from a chronic adaptive process [37]. Ando et al. [40] reported that the IMT on CCA increased only 0.06 mm/10 years with presence of plaque in the bulbs (PLQ-BLB) and 0.04 mm/10 years without PLQ-BLB. And in the healthy, and normolipidemic subjects, the estimated yearly increase in IMT was only 0.007 mm in women [41]. Based on above evidence, it would have been impossible to note the difference in IMT after such a short elapsed time unless the participants already had IMT at baseline. Also, carotid IMT, compared to carotid plaque, is less influenced by environmental factors [42]. Forth, in our study, only the presence of plaque was measured. For further evaluation and exact prediction of CAD, plaque area [32], and volume [43] can be used, although these methods can be more expensive, complex, and time-consuming. However, they were not available in our study because they were not part of our standard carotid ultrasonography. Lastly, there could be a selection bias. Again, we conducted multivariate analysis in which still led AI use as one of the strongest risk factors of carotid plaque formation.
We conclude that AIs use indeed affected endocrineresponsive breast cancer patients by forming more carotid plaque regardless of lipid profiles itself. Close monitoring of these patients on cardiovascular events would be important. And further prospective, long term studies comparing AIs with placebo will be needed to understand the long-term safety of AIs treatment.
KEY MESSAGE
1. Use of aromatase inhibitor in endocrine-responsive breast cancer may promote formation of carotid plaque regardless of lipid profile itself.
2. Close surveillance is warranted on those patients taking aromatase inhibitor especially with history of diabetes to intervene possible cardiovascular events.
Footnotes
No potential conflict of interest relevant to this article was reported.
Supplementary Materials
For patients without diabetes mellitus history (n = 66)
REFERENCES
- 1.Goldhirsch A, Glick JH, Gelber RD, et al. Meeting highlights: international expert consensus on the primary therapy of early breast cancer 2005. Ann Oncol. 2005;16:1569–1583. doi: 10.1093/annonc/mdi326. [DOI] [PubMed] [Google Scholar]
- 2.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340:1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 3.Barrett-Connor E. Sex differences in coronary heart disease: why are women so superior? The 1995 Ancel Keys Lecture. Circulation. 1997;95:252–264. doi: 10.1161/01.cir.95.1.252. [DOI] [PubMed] [Google Scholar]
- 4.Geisler J, King N, Anker G, et al. In vivo inhibition of aromatization by exemestane, a novel irreversible aromatase inhibitor, in postmenopausal breast cancer patients. Clin Cancer Res. 1998;4:2089–2093. [PubMed] [Google Scholar]
- 5.Chlebowski RT, Anderson GL, Geller M, Col N. Coronary heart disease and stroke with aromatase inhibitor, tamoxifen, and menopausal hormone therapy use. Clin Breast Cancer. 2006;6 Suppl 2:S58–S64. doi: 10.3816/cbc.2006.s.005. [DOI] [PubMed] [Google Scholar]
- 6.Kannel WB, Wilson PW. Comparison of risk profiles for cardiovascular events: implications for prevention. Adv Intern Med. 1997;42:39–66. [PubMed] [Google Scholar]
- 7.Boring CC, Squires TS, Tong T, Montgomery S. Cancer statistics, 1994. CA Cancer J Clin. 1994;44:7–26. doi: 10.3322/canjclin.44.1.7. [DOI] [PubMed] [Google Scholar]
- 8.Elisaf MS, Bairaktari ET, Nicolaides C, et al. Effect of letrozole on the lipid profile in postmenopausal women with breast cancer. Eur J Cancer. 2001;37:1510–1513. doi: 10.1016/s0959-8049(01)00155-1. [DOI] [PubMed] [Google Scholar]
- 9.Coates AS, Keshaviah A, Thurlimann B, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrineresponsive early breast cancer: update of study BIG 1-98. J Clin Oncol. 2007;25:486–492. doi: 10.1200/JCO.2006.08.8617. [DOI] [PubMed] [Google Scholar]
- 10.Wasan KM, Goss PE, Pritchard PH, et al. The influence of letrozole on serum lipid concentrations in postmenopausal women with primary breast cancer who have completed 5 years of adjuvant tamoxifen (NCIC CTG MA.17L) Ann Oncol. 2005;16:707–715. doi: 10.1093/annonc/mdi158. [DOI] [PubMed] [Google Scholar]
- 11.Goss PE, Ingle JN, Martino S, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst. 2005;97:1262–1271. doi: 10.1093/jnci/dji250. [DOI] [PubMed] [Google Scholar]
- 12.Arimidex, Tamoxifen, Alone or in Combination (ATAC) Trialists’ Group. Forbes JF, Cuzick J, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9:45–53. doi: 10.1016/S1470-2045(07)70385-6. [DOI] [PubMed] [Google Scholar]
- 13.Cuppone F, Bria E, Verma S, et al. Do adjuvant aromatase inhibitors increase the cardiovascular risk in postmenopausal women with early breast cancer? Meta-analysis of randomized trials. Cancer. 2008;112:260–267. doi: 10.1002/cncr.23171. [DOI] [PubMed] [Google Scholar]
- 14.Amir E, Seruga B, Niraula S, Carlsson L, Ocana A. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst. 2011;103:1299–1309. doi: 10.1093/jnci/djr242. [DOI] [PubMed] [Google Scholar]
- 15.Lintermans A, Neven P. Safety of aromatase inhibitor therapy in breast cancer. Expert Opin Drug Saf. 2015;14:1201–1211. doi: 10.1517/14740338.2015.1053458. [DOI] [PubMed] [Google Scholar]
- 16.Herrington DM, Reboussin DM, Brosnihan KB, et al. Effects of estrogen replacement on the progression of coronary-artery atherosclerosis. N Engl J Med. 2000;343:522–529. doi: 10.1056/NEJM200008243430801. [DOI] [PubMed] [Google Scholar]
- 17.Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 19.Polak JF, O’Leary DH, Kronmal RA, et al. Sonographic evaluation of carotid artery atherosclerosis in the elderly: relationship of disease severity to stroke and transient ischemic attack. Radiology. 1993;188:363–370. doi: 10.1148/radiology.188.2.8327679. [DOI] [PubMed] [Google Scholar]
- 20.Irie Y, Katakami N, Kaneto H, et al. The utility of carotid ultrasonography in identifying severe coronary artery disease in asymptomatic type 2 diabetic patients without history of coronary artery disease. Diabetes Care. 2013;36:1327–1334. doi: 10.2337/dc12-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winer EP, Hudis C, Burstein HJ, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report 2004. J Clin Oncol. 2005;23:619–629. doi: 10.1200/JCO.2005.09.121. [DOI] [PubMed] [Google Scholar]
- 22.Buzdar AU. Data from the arimidex, tamoxifen, alone or in combination (ATAC) trial: implications for use of aromatase inhibitors in 2003. Clin Cancer Res. 2004;10:355S–361S. doi: 10.1158/1078-0432.ccr-031203. [DOI] [PubMed] [Google Scholar]
- 23.Ebrahim S, Papacosta O, Whincup P, et al. Carotid plaque, intima media thickness, cardiovascular risk factors, and prevalent cardiovascular disease in men and women: the British Regional Heart Study. Stroke. 1999;30:841–850. doi: 10.1161/01.str.30.4.841. [DOI] [PubMed] [Google Scholar]
- 24.Aydiner A. Meta-analysis of breast cancer outcome and toxicity in adjuvant trials of aromatase inhibitors in postmenopausal women. Breast. 2013;22:121–129. doi: 10.1016/j.breast.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 25.Seruga B, Zadnik V, Kuhar CG, et al. Association of aromatase inhibitors with coronary heart disease in women with early breast cancer. Cancer Invest. 2014;32:99–104. doi: 10.3109/07357907.2014.880452. [DOI] [PubMed] [Google Scholar]
- 26.Kanters SD, Algra A, van Leeuwen MS, Banga JD. Reproducibility of in vivo carotid intima-media thickness measurements: a review. Stroke. 1997;28:665–671. doi: 10.1161/01.str.28.3.665. [DOI] [PubMed] [Google Scholar]
- 27.Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:e50–e103. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Katakami N, Takahara M, Kaneto H, et al. Ultrasonic tissue characterization of carotid plaque improves the prediction of cardiovascular events in diabetic patients: a pilot study. Diabetes Care. 2012;35:2640–2646. doi: 10.2337/dc12-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma AM, Gupta A, Kumar PK, et al. A review on carotid ultrasound atherosclerotic tissue characterization and stroke risk stratification in machine learning framework. Curr Atheroscler Rep. 2015;17:55. doi: 10.1007/s11883-015-0529-2. [DOI] [PubMed] [Google Scholar]
- 30.Vigili de Kreutzenberg S, Fadini GP, Guzzinati S, et al. Carotid plaque calcification predicts future cardiovascular events in type 2 diabetes. Diabetes Care. 2015;38:1937–1944. doi: 10.2337/dc15-0327. [DOI] [PubMed] [Google Scholar]
- 31.Chambless LE, Folsom AR, Clegg LX, et al. Carotid wall thickness is predictive of incident clinical stroke: the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol. 2000;151:478–487. doi: 10.1093/oxfordjournals.aje.a010233. [DOI] [PubMed] [Google Scholar]
- 32.Spence JD, Eliasziw M, DiCicco M, Hackam DG, Galil R, Lohmann T. Carotid plaque area: a tool for targeting and evaluating vascular preventive therapy. Stroke. 2002;33:2916–2922. doi: 10.1161/01.str.0000042207.16156.b9. [DOI] [PubMed] [Google Scholar]
- 33.Holaj R, Spacil J, Petrasek J, Malik J, Aschermann M, Haas T. Relation of the thickness of the intima and media of the common carotid artery, atherosclerotic plaque in the carotids and manifestations of atherosclerosis in the vessels of the lower extremity in comparison to coronary atherosclerosis. Cas Lek Cesk. 1998;137:716–720. [PubMed] [Google Scholar]
- 34.Choi BG, Vilahur G, Zafar MU, et al. Selective estrogen receptor modulation influences atherosclerotic plaque composition in a rabbit menopause model. Atherosclerosis. 2008;201:76–84. doi: 10.1016/j.atherosclerosis.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamas AZ, Caliman IF, Dalpiaz PL, et al. Comparative effects of estrogen, raloxifene and tamoxifen on endothelial dysfunction, inflammatory markers and oxidative stress in ovariectomized rats. Life Sci. 2015;124:101–109. doi: 10.1016/j.lfs.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Billon-Gales A, Fontaine C, Douin-Echinard V, et al. Endothelial estrogen receptor-alpha plays a crucial role in the atheroprotective action of 17beta-estradiol in low-density lipoprotein receptor-deficient mice. Circulation. 2009;120:2567–2576. doi: 10.1161/CIRCULATIONAHA.109.898445. [DOI] [PubMed] [Google Scholar]
- 37.Rundek T, Arif H, Boden-Albala B, Elkind MS, Paik MC, Sacco RL. Carotid plaque, a subclinical precursor of vascular events: the Northern Manhattan Study. Neurology. 2008;70:1200–1207. doi: 10.1212/01.wnl.0000303969.63165.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zambetti M, Moliterni A, Materazzo C, et al. Long-term cardiac sequelae in operable breast cancer patients given adjuvant chemotherapy with or without doxorubicin and breast irradiation. J Clin Oncol. 2001;19:37–43. doi: 10.1200/JCO.2001.19.1.37. [DOI] [PubMed] [Google Scholar]
- 39.Hooning MJ, Botma A, Aleman BM, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst. 2007;99:365–375. doi: 10.1093/jnci/djk064. [DOI] [PubMed] [Google Scholar]
- 40.Ando F, Takekuma K, Niino N, Shimokata H. Ultrasonic evaluation of common carotid intima-media thickness (IMT): influence of local plaque on the relationship between IMT and age. J Epidemiol. 2000;10:S10–S17. doi: 10.2188/jea.10.1sup_10. [DOI] [PubMed] [Google Scholar]
- 41.Junyent M, Gilabert R, Nunez I, et al. Carotid ultrasound in the assessment of preclinical atherosclerosis: distribution of intima-media thickness values and plaque frequency in a Spanish community cohort. Med Clin (Barc) 2005;125:770–774. doi: 10.1016/s0025-7753(05)72186-2. [DOI] [PubMed] [Google Scholar]
- 42.Moskau S, Golla A, Grothe C, Boes M, Pohl C, Klockgether T. Heritability of carotid artery atherosclerotic lesions: an ultrasound study in 154 families. Stroke. 2005;36:5–8. doi: 10.1161/01.STR.0000149936.33498.83. [DOI] [PubMed] [Google Scholar]
- 43.Spence JD. Ultrasound measurement of carotid plaque as a surrogate outcome for coronary artery disease. Am J Cardiol. 2002;89:10B–15B. doi: 10.1016/s0002-9149(01)02327-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For patients without diabetes mellitus history (n = 66)


