Proteins are involved in shaping the plant phenotype in response to environmental cues. However, in order to make use of proteomic studies in modern breeding programmes, dissection of genotype×environment (G×E) effects on the relative abundance of different proteins is essential. This can be achieved through pQTL mapping but a prerequisite has been laborious analysis of alterations in the relative abundance of protein spots in very large 2-DE gel sets. New research by Rodziewicz et al. (2019) showing a streamlined process is invaluable and should allow an accelerated application of proteomic data leading to improved drought-resistant varieties.
Abiotic stresses including drought, salinity and extreme temperature are major environmental factors adversely affecting plant growth and development. The plant response is determined by genotype×environment (G×E) interactions which affect a wide range of quantitative traits from growth, development and yield characteristics to transcript, protein and metabolite abundance. Proteins are directly involved in shaping the plant phenotype with either structural or regulatory functions related to formation of the plant epigenome, transcriptome and metabolome. These functions are determined not only by the corresponding (maternal) gene sequence but also by posttranscriptional and posttranslational modifications resulting in different protein isoforms, cellular localization and interaction networks (Kosová et al., 2018). Hence studies aimed not just at a description of the proteome, but also at functional characterization with respect to phenotypic variability, are becoming increasingly important.
Recently, studies in plants have shown that qualitative or quantitative changes which some marker proteins undergo in response to stress reveal a correlation with the degree of stress tolerance. As an example, our studies on Kn-type dehydrins reveal a correlation between the relative abundance of the proteins and acquired frost tolerance expressed as the lethal temperature for 50% of the sample (LT50) in winter wheat and barley cultivars subjected to cold acclimation treatment (Kosová et al., 2008, 2013; Vítámvás et al., 2010, 2019). However, as with other quantitative traits, the quantitative changes in relative abundance of marker proteins are determined by G×E interactions which limit their application in breeding programmes. Dissection of these effects through proteomic studies aimed at pQTL mapping is essential, and a new approach from Rodziewicz et al. (2019) is an important step forward. Their effective analysis of protein abundances in very large two-dimensional (2-DE) gel sets, essential for pQTL mapping, will help drive the development of improved drought-resistant varieties.
Environment and genotype
How do different levels of environmental stress factors affect the plant? As an example, a total proteome comparison of the impacts of two levels of water deficit stress (30 and 35% soil water capacity, SWC) on crown tissues of spring barley cultivar Amulet revealed acclimation at 35% SWC but signs of damage at 30% SWC (Vítámvás et al., 2015). 35% SWC led to enhanced levels of proteins involved in key catabolic processes such as glycolysis, indicating the mobilization of energy metabolism. Stress acclimation processes reveal enhanced energy requirements due to the synthesis of a number of novel compounds. 30% SWC led to opposite patterns in some isoforms of enzymes involved in glycolysis (e.g. 2,3-bisphosphoglycerate-independent phosphoglycerate mutase-like), and increased levels of fermentation enzymes such as alcohol dehydrogenase. This indicates a shift from aerobic to less-efficient anaerobic metabolism, probably as an adaptation to avoid enhanced ROS formation.
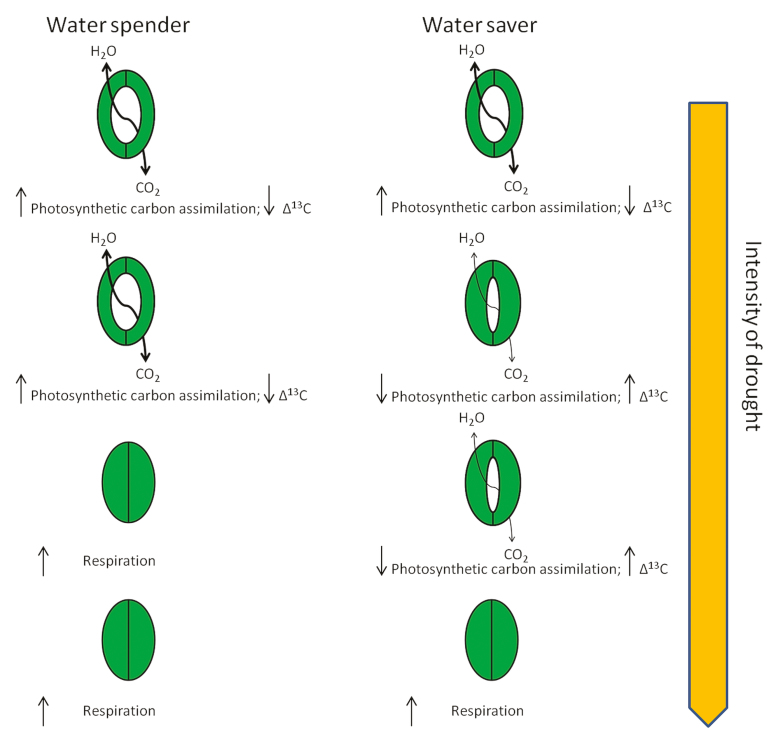
Turning to the genotype, plants can adopt various strategies to cope with water-deficit stress. For example, stomatal closure leading to a reduction of water deficit in plant tissues represents a trade-off with photosynthesis in C3 plants since it leads not only to reduced water loss but also to reduced CO2 availability for Rubisco. Accordingly two contrasting strategies can be distinguished in plants subjected to water deficit: a conservative, water-saving strategy, in which plants respond to slightly decreased water content in their cells by immediate stomatal closure thus eliminating water loss but also reducing CO2 availability to the photosynthetic apparatus; and a water-spending strategy, in which the stomata remain open until relatively severe cellular water deficit occurs. Water-spenders thus lose water but retain CO2 availability for Rubisco (Box 1; Rebetzke et al., 2002; Condon et al., 2004; Reynolds et al., 2005; Passioura, 2012). An alternative approach to resolve the trade-off between CO2 uptake and water release by stomata in C3 plants was presented by Blum (2009). Instead of a conservative water-saving strategy leading to maximized water-use efficiency (WUE), a strategy was proposed leading to maximized ‘effective use of water’ (EUW), i.e. minimizing water loss from the plant by every means except for stomatal closure.
Box 1. Contrasting strategies to cope with water-deficit stress
Two contrasting strategies by which C3 plants respond to water-deficit stress are based on stomatal openness. In ‘water spenders’ the stomata remain open during water deficit, ensuring sufficient CO2 uptake to maintain a high photosynthetic carbon assimilation rate and high Rubisco 13C discrimination rate, i.e. low Δ13C, at the expense of an enhanced rate of water loss. In contrast, ‘water savers’ reveal a conservative strategy in their response to water deficit with reduced stomatal openness in turn reducing water loss from the leaf but also CO2 uptake. This leads to a decrease in photosynthetic carbon assimilation rate and Rubisco 13C discrimination rate, i.e. high Δ13C.
Proteome composition and distinct tolerance strategies
Changes in proteome composition underlie different responses to water-deficit stress, and these reveal distinct tolerance strategies. To give an example, Urban et al. (2017) provided a comparison of responses to drought in oilseed rape cultivars Cadeli and Californium (water-savers) and Navajo and Viking (water-spenders). Results of 2D-DIGE analysis showed that in the water-saver group, proteins related to nitrogen assimilation, ATP biosynthesis and redox homeostasis increased under stress. In the water-spender category, under the same conditions, proteins involved in carbohydrate/energy metabolism, photosynthesis and rRNA processing increased (together with other ‘stress-related proteins’).
In another example, Ford et al. (2011) compared three Australian wheat cultivars—Kukri, Excalibur and RAC875—exposed to cyclic drought conditions. During the initial stages of drought stress, while Excalibur lacked significant changes in proteome composition, RAC875 showed significant changes in the relative abundance of different proteins. After more prolonged stress, all three genotypes showed an increase in the abundance of proteins with chaperone (COR410) and ROS-scavenging capacity (CAT, Cu/Zn-SOD and Mn-SOD), as well as decreases in proteins involved in the aerobic processes of energy metabolism such as photosynthesis and the Calvin cycle. The cultivars also showed different protein patterns following rewatering. Less-tolerant Kukri showed significant decreases in Calvin cycle enzymes as well as proteins involved in photosynthesis and photorespiration, and increases in proteins involved in stress responses, redox homeostasis, and protein biosynthesis (translation) and folding. More-tolerant RAC875 showed decreases in proteins involved in the Calvin cycle and photorespiration, as well as several photosystem components (e.g. photosystem I subunit VII), and increases in proteins involved in amino acid metabolism, glycolysis, gluconeogenesis, and protein translation, degradation and folding. Tolerant Excalibur showed no large decreases in Calvin cycle enzymes but increases in eight out of 12 glycolysis or gluconeogenesis enzymes, and mixed patterns in proteins belonging to the photosynthetic machinery or involved in translation and stress responses. It is evident that different cultivars, although all relatively drought tolerant, employ different strategies to cope with stress. These can be distinguished and further analysed at proteome level.
Rollins et al. (2013) compared two barley genotypes originating from arid and semi-arid regions—Australian cultivar Keel and Syrian landrace Arta—with respect to their response to drought, heat, and combined drought and heat treatments. Proteomic analysis revealed an increase in proteins involved in both protein biosynthesis and degradation (proteases), protein folding mediated by chaperones and redox homeostasis; as well as proteins involved in energy metabolism, including photosynthesis-related proteins (e.g. the thermotolerant Rubisco activase B isoform) and proteins related to anaerobic processes (glycolysis), indicating an increased need to reduce the risks of oxidative damage.
pQTL mapping for modern breeding programmes
In order to utilize the results of proteomic studies in modern breeding programmes, dissection of G×E effects on the relative abundance of different proteins is essential, and this can be achieved through pQTL mapping. Genetic mapping studies aim to determine QTLs underlying differences in quantitative traits within mapping populations derived from parental genotypes with contrasting values of the given phenotypic traits. Several QTLs underlying traits related to growth, development and yield have been mapped in drought-treated wheat and barley. The mapping populations were mostly derived from crosses between genotypes of different geographical origin revealing contrasting values of phenotypic traits under water-deficit conditions (reviewed in Kosová et al., 2014). By analogy with QTLs, protein quantitative trait loci (pQTLs) can be mapped based on differences in relative abundance of proteins in a population derived from parental genotypes with contrasting phenotypes (Box 2).
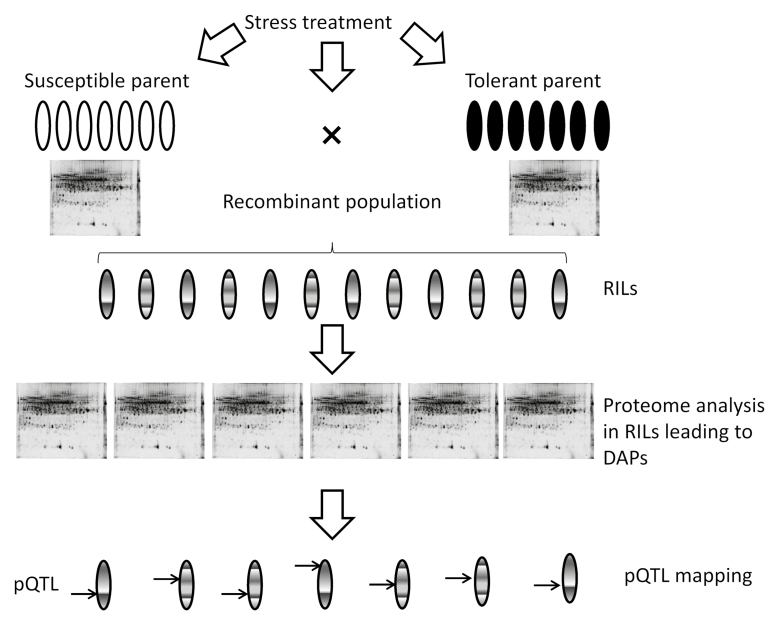
Box 2. pQTL mapping
A mapping approach using protein quantitative trait loci (pQTLs) is based on principles analogous to those of QTL mapping of other quantitative phenotypic traits. Parents revealing contrasting traits such as tolerance to given environmental conditions (e.g. water deficit) are crossed, giving rise to a recombinant population (recombinant inbred lines, RILs) with mixed characteristics. Next, quantitative proteomic analysis of both parents and recombinants leads to the identification of proteins with differentially abundant proteins (DAPs) in the mapping population in response to the given environmental stress. Finally, the data on different protein abundance is matched to a given QTL in the mapping population leading to pQTL mapping. The pQTL mapping can then be used in modern breeding programmes aimed at improvement of quantitative traits including environmental stress tolerance.
Unlike genetic mapping studies aimed at determining QTLs underlying phenotypic traits, studies aimed at determining pQTLs are scarce because of the need to evaluate quantitative changes in proteins within very large proteome sets. Nevertheless, the 2-DE technique is a good choice for pQTL mapping studies since fine but important differences in protein spot densities enable a dissection of the relative abundance of different protein isoforms. Distinct and often opposite patterns of quantitative change may be apparent in isoforms with potentially distinct biological functions.
In the study by Rodziewicz et al. (2019) the authors developed an innovative approach enabling them to assess, in total, 408 sets of 2-DE gels from leaf and root samples covering 100 recombinant inbred lines (RILs) derived from German semi-dwarf cultivar Maresi and Syrian breeding line Cam/B1/CI, i.e. genotypes revealing different drought-response strategies. Their analysis led to mapping of pQTLs underlying quantitative differences in the relative abundance of drought-responsive proteins with previously identified QTLs for yield-related traits (Mikołajczak et al., 2016, 2017). The drought-responsive proteins included betaine aldehyde dehydrogenase, thioredoxin O, ATP synthase β subunit, glutamine synthetase, HSP70, phenylalanine ammonia lyase, translocase subunit secA, δ-1-pyrroline-5-carboxylate synthetase and luminal binding protein; the yield-related traits included 1000-grain weight, length of main spike, number of spikelets per lateral spike, grain weight per main spike, and heading date. The authors’ effective management of laborious analyses of alterations in the relative abundance of protein spots in very large 2-DE gel sets is invaluable, and a necessary prerequisite for pQTL mapping. Such mapping associated with improved stress tolerance will enable breeders to apply proteomic data in modern breeding programmes.
Acknowledgments
The work was supported by an institutional project of the Ministry of Agriculture of the Czech Republic (MZe) MZe-RO0419 and by projects of the Czech Ministry of Agriculture QK1710302, QK1910197 and QK1910269.
References
- Blum A. 2009. Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crops Research 112, 119–123 . [Google Scholar]
- Condon AG, Richards RA, Rebetzke GJ, Farquhar GD.. 2004. Breeding for high water-use efficiency. Journal of Experimental Botany 55, 2447–2460 . [DOI] [PubMed] [Google Scholar]
- Ford KL, Cassin A, Bacic A.. 2011. Quantitative proteomic analysis of wheat cultivars with differing drought stress tolerance. Frontiers in Plant Science 2, 44 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosová K, Holková L, Prášil IT, Prášilová P, Bradáčová M, Vítámvás P, Čapková V.. 2008. Expression of dehydrin 5 during the development of frost tolerance in barley (Hordeum vulgare). Journal of Plant Physiology 165, 1142–1151 . [DOI] [PubMed] [Google Scholar]
- Kosová K, Vítámvás P, Prášilová P, Prášil IT.. 2013. Accumulation of WCS120 and DHN5 proteins in differently frost-tolerant wheat and barley cultivars grown under a broad temperature scale. Biologia Plantarum 57, 105–112 . [Google Scholar]
- Kosová K, Vítámvás P, Urban MO, Kholová J, Prášil IT.. 2014. Breeding for enhanced drought resistance in barley and wheat—drought-associated traits, genetic resources and their potential utilization in breeding programmes. Czech Journal of Genetics and Plant Breeding 50, 247–261 . [Google Scholar]
- Kosová K, Vítámvás P, Urban MO, Prášil IT, Renaut J.. 2018. Plant abiotic stress Proteomics: the major factors determining alterations in cellular proteome. Frontiers in Plant Science 9, 122 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikołajczak K, Kuczyńska A, Krajewski P, et al. 2017. Quantitative trait loci for plant height in Maresi × CamB barley population and their associations with yield-related traits under different water regimes. Journal of Applied Genetics 58, 23–35 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikołajczak K, Ogrodowicz P, Gudyś K, et al. 2016. Quantitative trait loci for yield and yield-related traits in spring barley populations derived from crosses between European and Syrian cultivars. PLOS ONE 11, e0155938 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passioura JB. 2012. Phenotyping for drought tolerance in grain crops: when is it useful to breeders? Functional Plant Biology 39, 851–859 . [DOI] [PubMed] [Google Scholar]
- Rebetzke GJ, Condon AG, Richards RA, Farquhar GD.. 2002. Selection for reduced carbon isotope discrimination increases aerial biomass and grain yield of rainfed bread wheat. Crop Science 42, 739–745 . [Google Scholar]
- Reynolds MP, Mujeeb-Kazi A, Sawkins M.. 2005. Prospects for utilizing plant-adaptive mechanisms to improve wheat and other crops in drought- and salinity-prone environments. Annals of Applied Biology 146, 239–259 . [Google Scholar]
- Rodziewicz P, Chmielewska K, Sawikowska A, et al. 2019. Identification of drought responsive proteins and related pQTLs in barley. Journal of Experimental Botany 70, 2823–2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins JA, Habte E, Templer SE, Colby T, Schmidt J, von Korff M.. 2013. Leaf proteome alterations in the context of physiological and morphological responses to drought and heat stress in barley (Hordeum vulgare L.). Journal of Experimental Botany 64, 3201–3212 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban MO, Vašek J, Klíma M, Krtková J, Kosová K, Prášil IT, Vítámvás P.. 2017. Proteomic and physiological approach reveals drought-induced changes in rapeseeds: Water-saver and water-spender strategy. Journal of Proteomics 152, 188–205 . [DOI] [PubMed] [Google Scholar]
- Vítámvás P, Kosová K, Musilová J, Holková L, Mařík P, Smutná P, Klíma M, Prášil IT.. 2019. Relationship between dehydrin accumulation and winter survival in winter wheat and barley grown in the field. Frontiers in Plant Science 10, 7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vítámvás P, Kosová K, Prášilová P, Prášil IT.. 2010. Accumulation of WCS120 protein in wheat cultivars grown at 9 degrees C or 17 degrees C in relation to their winter survival. Plant Breeding 129, 611–616 . [Google Scholar]
- Vítámvás P, Urban MO, Škodáček Z, Kosová K, Pitelková I, Vítámvás J, Renaut J, Prášil IT.. 2015. Quantitative analysis of proteome extracted from barley crowns grown under different drought conditions. Frontiers in Plant Science 6, 479 . [DOI] [PMC free article] [PubMed] [Google Scholar]