The disruption of photorespiration in GDC knockdown rice plants alters leaf photorespiratory 13CO2 fractionation and carbon isotope exchange.
Keywords: 13C discrimination, C4 photosynthesis, CO2 exchange, GDC knockdown, leaf dark respiration, photorespiration, rice
Abstract
The influence of reduced glycine decarboxylase complex (GDC) activity on leaf atmosphere CO2 and 13CO2 exchange was tested in transgenic Oryza sativa with the GDC H-subunit knocked down in leaf mesophyll cells. Leaf measurements on transgenic gdch knockdown and wild-type plants were carried out in the light under photorespiratory and low photorespiratory conditions (i.e. 18.4 kPa and 1.84 kPa atmospheric O2 partial pressure, respectively), and in the dark. Under approximately current ambient O2 partial pressure (18.4 kPa pO2), the gdch knockdown plants showed an expected photorespiratory-deficient phenotype, with lower leaf net CO2 assimilation rates (A) than the wild-type. Additionally, under these conditions, the gdch knockdown plants had greater leaf net discrimination against 13CO2 (Δo) than the wild-type. This difference in Δo was in part due to lower 13C photorespiratory fractionation (f) ascribed to alternative decarboxylation of photorespiratory intermediates. Furthermore, the leaf dark respiration rate (Rd) was enhanced and the 13CO2 composition of respired CO2 (δ13CRd) showed a tendency to be more depleted in the gdch knockdown plants. These changes in Rd and δ13CRd were due to the amount and carbon isotopic composition of substrates available for dark respiration. These results demonstrate that impairment of the photorespiratory pathway affects leaf 13CO2 exchange, particularly the 13C decarboxylation fractionation associated with photorespiration.
Introduction
In C3 plants, Rubisco operates in the leaf mesophyll cells, where CO2 and O2 compete to react with ribulose-1,5-bisphosphate (RuBP). The carboxylation of RuBP results in the formation of two molecules of 3-phosphoglycerate (3-PGA) that are integrated into the Calvin–Benson cycle. Alternatively, the oxygenation of RuBP produces one molecule of 3-PGA and one 2-phosphoglycolate (2-PG). The 2-PG is primarily recycled via photorespiration through a complex and energy-consuming set of reactions, which spans the chloroplasts, cytosol, peroxisomes, and mitochondria (Bauwe et al., 2010; Betti et al., 2016). By scavenging 2-PG, photorespiration removes a strong inhibitor of enzymes in photosynthetic carbon metabolism (Anderson, 1971; Kelly and Latzko, 1976; Peterhansel et al., 2013b; Walker et al., 2016) and recovers up to one molecule of 3-PGA for every two molecules of 2-PG. Nevertheless, a minimum of one out of four 2-PG carbon atoms is released as CO2 by the glycine decarboxylase complex (GDC) and can be lost by the plant (Bauwe, 2018).
The GDC is an atypical mitochondrial four-protein system, comprised of three enzymes (P-, T-, and L-protein) and the H-protein, which is a small lipoylated protein (Somerville and Ogren, 1982; Douce et al., 2001; Bauwe, 2018). GDC plays a critical role in the photorespiratory cycle by catalyzing the conversion of two molecules of glycine into serine and one molecule of CO2 and NH3 (Somerville, 2001; Maurino and Peterhansel, 2010). However, in the absence of the H component, the GDC cannot oxidize glycine (Douce et al., 2001; Parys and Jastrzębski, 2008), which can accumulate. In C3 plants, the impaired activity of the H-subunit leads to a knockdown (KD) of GDC activity and a photorespiratory phenotype (Ewald et al., 2007). Plants with reduced GDC activity typically have lower rates of leaf photosynthesis, a depletion of Calvin cycle metabolites, an impairment of photorespiratory nitrogen re-assimilation, and the accumulation of photorespiratory metabolites (e.g. glycine) under current ambient CO2 and O2 partial pressures (Wingler et al., 2000; Timm and Bauwe, 2013; Lin et al., 2016).
This buildup of leaf photorespiratory metabolites can have a negative feedback effect on Calvin cycle activity. For example, glyoxylate produced by glycolate oxidation negatively impacts on the activation state of Rubisco (Wingler et al., 1999; Peterhansel et al., 2010). Additionally, disruption of the photorespiratory pathway may lead to alternative decarboxylation reactions of accumulated pools of photorespiratory intermediates, such as glyoxylate and hydroxypyruvate in the peroxysomes (Wingler et al., 1999, 2000; Tcherkez, 2006; Peterhansel et al., 2010), and an increase in the ratio of moles of photorespiratory CO2 released per mole of O2 reacting with RuBP (α; Cousins et al., 2008, 2011; Walker and Cousins, 2013; Timm et al., 2018). Furthermore, the accumulation of photorespiratory intermediates could also affect the rates of leaf CO2 evolved in the dark (Rd, μmol CO2 m−2 s−1) and the 13C composition of Rd (δ13CRd, ‰) (Ghashghaie et al., 2003; Tcherkez et al., 2003).
The multiple leaf metabolic reactions simultaneously consuming and releasing CO2 in the light make it difficult to determine how changes in photorespiration affect rates of leaf net CO2 assimilation (A), mesophyll CO2 conductance (gm), refixation of (photo)respired CO2, and mitochondrial non-photorespiratory respiration rates (RL). However, photosynthesizing leaves discriminate against 13C during CO2 diffusion from the atmosphere to the chloroplast stroma (through both the air and liquid phases), and during carboxylation, photorespiration, and mitochondrial non-photorespiratory respiration processes, with a specific 13C fractionation for each diffusional or biochemical step (Evans et al., 1986). The observed leaf net discrimination against 13C in the light (Δo, ‰) can be modeled with four 13C fractionation terms (‰): Δi, which accounts for the 13C discrimination during CO2 diffusion from the atmosphere to the intercellular air space and for the Rubisco 13C fractionation (~29‰, Ubierna and Farquhar, 2014; von Caemmerer et al., 2014); Δgm, which accounts for the 13C discrimination during CO2 diffusion in the liquid phase to chloroplast stroma and depends on the magnitude of gm; and Δf and Δe which are associated with photorespiration and mitochondrial non-photorespiratory respiration activity, respectively (von Caemmerer and Evans, 1991; Flexas et al., 2008; Tazoe et al., 2011; Evans and von Caemmerer, 2013). Δf is primarily attributed to the glycine–serine reaction catalyzed by GDC, which releases CO2 depleted in 13C compared with substrate and tends to decrease Δo (Farquhar et al., 1982; Ghashghaie et al., 2003; Lanigan et al., 2008). In contrast, Δe may increase or decrease Δo in relation to the difference between 13C composition (‰) of CO2 entering the leaf chamber during measurements and in the plant growth chamber (Gillon and Griffiths, 1997; Ghashghaie et al., 2003).
The photorespiratory fractionation (f, ‰) estimated in vivo in multiple C3 species ranges between 8‰ and 16.2‰ relative to photosynthetic products (Ghashghaie et al., 2003; Evans and von Caemmerer, 2013), with 11‰ predicted from the theory (Tcherkez, 2006). However, under photorespiratory conditions, when Rubisco oxygenation exceeds the capacity of the photorespiratory recycling of 2-PG or in the presence of disruption of the photorespiratory pathway, Δf and f may vary due to changes in α associated with alternative decarboxylation of photorespiratory intermediates (Cousins et al., 2008, 2011; Walker and Cousins, 2013). Alternative photorespiratory bypasses may occur in the chloroplasts (e.g. glyoxylate may be enzymatically reduced back to glycolate or further oxidized to CO2, but with no RuBP regenerated; see Kebeish et al., 2007), peroxysomes (non-enzymatic decarboxylation of glyoxylate to formate using H2O2 as oxidizing agent may lead to formation of serine; catalase may be also involved as reported in Wingler et al., 1999), mitochondria (enzymatic oxidation of glycolate to glyoxylate with release of CO2 and synthesis of glycine; see Niessen et al., 2007), and cytosol (enzymatic reduction of hydroxypiruvate to glycerate; see Timm et al., 2008).
The aim of the present study was to test how changes in carbon flux through the photorespiratory pathway influenced leaf CO2 and 13CO2 isotope exchange, both in the light and in the dark, in transgenic plants of Oryza sativa with the GDC H-subunit KD in mesophyll cells. Both gdch-KD and wild-type (WT) plants were grown under low photorespiratory conditions (atmospheric CO2 partial pressure of 184.2 Pa) to minimize any pleiotropic effects. In the light, measurements of leaf–atmosphere CO2 and stable carbon isotope exchange were performed under low photorespiratory and photorespiratory conditions (atmospheric O2 partial pressure of 1.84 kPa or 18.4 kPa, respectively, and CO2 partial pressure of 27.6 Pa). The disruption of the photorespiratory pathway in the gdch-KD plants was characterized by leaf photosynthetic traits, Δo, Δf, f, α, Rd, and δ13CRd, compared with the WT.
Materials and methods
Plant material
Generation of GDC-H knockdown transgenic rice lines
The generation and the characterization of three Oryza sativa gdch-KD transgenic lines, including gdch-38, was previously described by Lin et al. (2016). Line gdch-38 was selected for analysis in the present study since in Lin et al. (2016) it had shown a more consistent photorespiratory-deficient phenotype under different O2:CO2 growing and measuring conditions compared with the other two gdch-KD lines. Untransformed O. sativa cv. IR64 line A009 (WT) was used as negative control for comparison with the gdch-KD line.
Plant growth conditions
Two batches of 10 transgenic gdch-38 line (T4 generation) and 10 WT plants of O. sativa cv. IR64 were grown consecutively in a controlled-environment growth chamber (Gch; Bigfoot series, BioChambers Inc., Winnipeg, MB, Canada) at the School of Biological Sciences at Washington State University, Pullman, WA (USA). All plants were individually grown in 4 liter free drainage pots; soil, irrigation, and fertilization were as in Giuliani et al. (2013).
The daily photoperiod was 14 h, from 8.00 h to 22.00 h standard time. Light was provided by F54T5/841HO Fluorescent 4100 K and 40 W halogen incandescent bulbs (Philips) and was supplied in a bell-shaped pattern; that is, with increasing photosynthetic photon flux density (PPFD) during the first 2 h, a maximum PPFD of 600 μmol photons m−2 s−1 incident on the plant canopy for 10 h, and decreasing PPFD in the last 2 h. Air temperature (tair) was set at 22 °C in the dark period; after switching on the light, tair tracked the PPFD pattern; that is, it ramped during the first 2 h from 22 °C to 26 °C, then 26 °C for 10 h, and decreased to 22 °C in the last 2 h photoperiod. Air relative humidity was maintained at ~70%, corresponding to a maximum air vapor pressure deficit (VPD) of ~1.6 kPa. During the light period, the CO2 partial pressure (pCO2) in the Gch atmosphere was elevated to 184.2 Pa (2000 μmol mol−1). The 13C composition of the atmospheric CO2 during the light period (δ13CGch) was −41.6‰ and −30.6‰ for the first and second batch of grown plants, respectively. The δ13CGch was determined as described in Supplementary Methods S1 at JXB online, and was a proxy of the 13C composition of the CO2 in the tank used (during the second plant growing cycle a new tank was needed and no tank with 13CO2 composition comparable with the previous one was available).
Leaf biochemical analysis
Protein content
Protein immunoblot analysis was performed to determine the leaf abundance of GDC H-, P-, and T-subunits in fully expanded leaves of 4- to 5-week-old transgenic gdch-KD and WT plants. For each genotype, two separate protein extractions were performed, each one using the leaf tissue collected from two plants, according to Koteyeva et al. (2015). Protein concentration was determined for each extract with an RC DC protein quantification kit (Bio-Rad, Hercules, CA, USA) and 20 µg of protein per extract were separated by 10% (w/v) SDS–PAGE for the GDC P-subunit or 15% (w/v) for GDC H- and T-subunits. Proteins were then transferred to a nitrocellulose membrane and immunoblots (n=2 for both gdch-KD and WT) were performed according to Koteyeva et al. (2015) with primary antibodies for anti-Pisum sativum L. GDC H-, P-, and T-subunits (1:10 000) raised in rabbit (courtesy of Dr D. Oliver, Iowa State University). The L-subunit was not detected because antibodies were unavailable. The band intensities were quantified with ImageJ 1.37 software (NIH, USA).
Malate content
The leaf portions used for photosynthesis analysis in gdch-KD and WT plants (n=5) were sampled immediately after the leaf–atmosphere gas exchange measurements and frozen in liquid N2. Malate content per unit leaf surface area (mmol malate m−2) was then determined with a spectrophotometry-based assay as described by Hatch (1979), with modifications by Edwards et al. (1982).
Leaf physiological analysis
Coupled measurements of leaf–atmosphere CO2, H2O, and 13CO2 exchange
Measurements were performed in Pullman, WA, USA with a mean atmospheric pressure of 92.1 kPa. Two LI-6400XT portable gas analyzers (LI-COR Biosciences, Lincoln, NE, USA; detecting 12CO2) operating as open systems were coupled to a tunable diode laser absorption spectroscope, which detects 12CO2 and 13CO2 isotopologs (TDLAS model TGA200A, Campbell Scientific, Inc., Logan, UT, USA; Bowling et al., 2003; Barbour et al., 2007; Ubierna et al., 2011; Stutz et al., 2014; Sun et al., 2014). Additional technical information on the system setup are available in Supplementary Methods S2.
For the leaf photosynthesis measurements, each LI-COR was equipped with a 2×3 cm leaf chamber (Lch) assembled with an LED light source (6400-02B; LI-COR Biosciences). Alternatively, leaf dark respiration measurements were performed using an 8×10 cm custom-built Lch having an adaxial glass window, and with a volume of ~100 cm3 (Barbour et al., 2007, based on Sharkey et al., 1985). The chamber had a hollowed stainless steel frame sealed with a closed-cell foam gasket and was connected to a circulating water bath for temperature control in the lumen. Before dark respiration measurements, the leaf portions included in the Lch were exposed to the light, which was supplied by a LI-COR 6400-18 light source placed adjacent to the glass window.
Protocol for coupled measurements of leaf–atmosphere CO2, H2O, and 13CO2 exchange
The mid to distal portions of two fully expanded leaves from the same stem on 4- to 5-week-old plants (n=4 for gdch-KD; n=5 for WT) grown under δ13CGch of −41.6‰ were used for leaf photosynthetic measurements. The leaves were positioned to cover the 6 cm2 Lch section area. Measurements were taken from 10.00 h until 16.00 h standard time under an O2 partial pressure (pO2) of 18.4 kPa (approximately the current atmospheric pO2) and 1.84 kPa, pCO2 (Ca) of 27.6 Pa, and 13C composition of CO2 (from a pressurized tank) entering the Lch (δin) of −48.0‰. PPFD was set at 1500 µmol photons m−2 s−1, leaf temperature (tleaf) at 25 °C, and leaf to air VPD was kept between 1.0 kPa and 1.5 kPa. The airflow rate through the LI-COR system was 300 µmol s−1 (~0.48 l min−1). In particular, a Ca below current ambient pCO2 (which was ~37 Pa) was chosen to amplify, under a pO2 of 18.4 kPa, the signals of the photorespiratory-deficient phenotype in the gdch-KD plants compared with the WT.
Under each experimental O2 condition, leaf portions were acclimated for ~30 min and data were recorded for ~30–40 min. The rate of net CO2 assimilation per unit (one side) leaf surface area (A, µmol CO2 m−2 s−1), stomatal conductance to CO2 diffusion (gsC, μmol CO2 m−2 s−1 Pa−1), intercellular pCO2 (Ci, Pa), and the ratio Ci/Ca were determined.
For leaf dark respiration measurements, gdch-KD and WT plants (n=4) grown at a δ13CGch of both −41.6‰ and −30.6‰ were used. Two plants per day (one gdch-KD and one WT) were taken out of the Gch at 9.30 h standard time and the mid to distal portions of 8–9 fully expanded leaves, similar to those used for the photosynthetic analysis, were enclosed in the custom-built Lch to cover the section area of ~76 cm2. Leaf portions were first exposed to a PPFD of 750 µmol photons m−2 s−1 for 20 min, 500 µmol photons m−2 s−1 for 15 min (at tleaf of 25 °C), and 100 µmol photons m−2 s−1 for 5 min (at tleaf of 30 °C). Measurements were taken under a pO2 of 1.84 kPa or 18.4 kPa for plants grown at a δ13CGch of −41.6‰ or −30.6‰, respectively. Ca was set at 35.0 Pa, and the airflow rate through the LI-COR was changed from 700 µmol s−1 to 500 µmol−1, and from 500 µmol s−1 to 350 µmol s−1 tracking the decreasing PPFD. A CO2 cartridge from a set of cartridges with δ13C from −6.2‰ to −4.8‰ was used, one per day, as CO2 source (the mean δin for all experimental conditions is shown in Supplementary Table S1). The different (higher) δ13CO2 composition entering the Lch with respect to the Gch (−41.6‰) was chosen to have the leaf carbon assimilates produced in the Lch with dissimilar (higher) δ13C signatures compared with those previously produced in the Gch. After 40 min of leaf light exposure, darkness was imposed in the Lch. Leaf CO2 evolution was measured at a pO2 of 18.4 kPa and tleaf of 30 °C for 195 min to determine the dynamics of the dark respiration rate per unit (one side) of leaf surface area (Rd, µmol CO2 m−2 s−1) and corresponding δ13C (δ13CRd, ‰). The tleaf was set at 30 °C to enhance the precision of the dark measurements. Additionally, three plants (n=3) of the gdch-KD line and of the WT were taken out of the growth chamber at 12.00 h standard time 3 d after their use for measurements, and darkened at 25 °C for 24 h. Subsequently, leaf dark CO2 evolution was measured at a tleaf of 30 °C and a pO2 of 18.4 kPa to determine Rd(24h) (µmol CO2 m−2 s−1) and δ13CRd(24h) (‰). The blade portions used for dark measurements on WT and gdch-KD plants were sampled and dried in a ventilated oven at 55 °C for 48 h to determine leaf dry mass per (one side) unit of leaf surface area (LMA, g m−2).
For each gdch-KD and WT plant used for leaf photosynthesis measurements, the 13C signature of leaf dry matter (δ13Cdm, ‰) and leaf total N content as a fraction (%) of dry matter were determined as described in Supplementary Methods S3, and the leaf total N content per unit leaf surface area (g m−2) was calculated. The descriptions, values, and units of abbreviations and symbols are listed in Table 1.
Table 1.
Description of the abbreviations, symbol, value (as in Evans and von Caemmerer, 2013), and unit of the environmental parameters and leaf variables used in this study
| Abbreviation | Description | |
|---|---|---|
| Gch | Growth chamber | |
| GDC | Glycine decarboxylase complex | |
| gdch-KD | Transgenic GDC H-subunit knockdown | |
| Lch | Leaf chamber | |
| LEDR | Light-enhanced dark respiration | |
| NH3 | Ammonia | |
| NH4+ | Ammonium cation | |
| PDH | Pyruvate dehydrogenase | |
| RuBP | Ribulose 1,5-bisphosphate | |
| TCA | Tricarboxylic acid | |
| 2-PG | 2-phosphoglycolate | |
| 3-PGA | 3-phosphoglycerate | |
| Symbol | Environmental parameters/leaf variables | Value and unit |
| A | Net CO2 assimilation rate per unit (one side) leaf surface area | µmol CO2 m−2 s−1 |
| a | 13C fractionation during CO2 diffusion (in air) through stomata | 4.4‰ |
| b 3 | Rubisco 13C fractionation | 29.0‰ |
| C a | CO2 mole fraction or CO2 partial pressure set in the leaf chamber | µmol mol−1; Pa |
| C c | CO2 mole fraction or CO2 partial pressure in the chloroplast | µmol mol−1; Pa |
| C i | CO2 mole fraction or CO2 partial pressure in the intercellular air space | µmol mol−1; Pa |
| C in | CO2 mole fraction entering the leaf chamber | µmol mol−1 |
| C out | CO2 mole fraction leaving the leaf chamber | µmol mol−1 |
| C s | CO2 mole fraction at the leaf surface | µmol mol−1 |
| f | Photorespiratory 13CO2 fractionation | ‰ |
| g m | Mesophyll conductance to CO2 diffusion from the substomatal cavity to the chloroplast stroma | µmol CO2 m−2 s−1 Pa−1 |
| g sC | Stomatal conductance to CO2 diffusion | µmol CO2 m−2 s−1 Pa−1 |
| LMA | Leaf dry mass per (one side) unit surface area | g m−2 |
| pCO2 | Partial pressure of CO2 | Pa |
| pO2 | Partial pressure of O2 | kPa |
| PPFD | Photosynthetic photon flux density | µmol photons m−2 s−1 |
| R d | Dark respiration rate per unit (one side) leaf surface area | µmol CO2 m−2 s-1 |
| R d(24h) | R d after 24 h dark | µmol CO2 m−2 s−1 |
| R d(30min) | R d after 30 min dark | µmol CO2 m−2 s−1 |
| R d(3h) | R d after 3 h dark | µmol CO2 m−2 s−1 |
| R d(6min) | R d after 6 min dark | µmol CO2 m−2 s−1 |
| R L | Light mitochondrial non-photorespiratoy respiration rate per unit (one side) leaf surface area | µmol CO2 m−2 s−1 |
| t | Correction factor for ternary effects | ‰ |
| t air | Air temperature | °C |
| t leaf | Leaf temperature | °C |
| VPD | Vapor pressure deficit | kPa |
| α | Moles of CO2 released in the photorespiratory pathway per mole of O2 reacting with RuBP | mol CO2 mol−1 O2 |
| Δe | 13C discrimination associated wtih mitochondrial non-photorespiratory respiration | ‰ |
| Δf | 13C discrimination associated with photorespiration | ‰ |
| Δgm | 13C discrimination associated with mesophyll conductance to CO2 diffusion | ‰ |
| Δi | 13C discrimination due to carboxylation, boundary layer and stomatal CO2 diffusion | ‰ |
| Δo | Observed (instantaneous) leaf net discrimination against 13CO2 in the light | ‰ |
| Γ | CO2 compensation point | µmol mol−1; Pa |
| Γ* | CO2 compensation point in absence of mitochondrial non-photorespiratory respiration | µmol mol−1; Pa |
| δin | δ13C of CO2 entering the leaf chamber | ‰ |
| δout | δ13C of CO2 leaving the leaf chamber | ‰ |
| δRdGch_substr | Fractional contribution of respiratory substrates from Gch carbon assimilates to δ13C of dark-evolved CO2 | ‰/‰ |
| δRdLch_substr | Fractional contribution of respiratory substrates from Lch carbon assimilates to δ13C of dark-evolved CO2 | ‰/‰ |
| δ13C | 13C composition of CO2 | ‰ |
| δ13Cdm | 13C signature of leaf dry matter | ‰ |
| δ13CGch | 13C composition of atmospheric CO2 in the growth chamber during the photoperiod | ‰ |
| δ13CLch_Ph | Representative δ13C of carbon assimilates produced in the Lch | ‰ |
| δ13CRd | δ13C of CO2 evolved by leaves in the dark | ‰ |
| δ13CRd(24h) | δ13C of CO2 evolved by leaves after 24 h dark | ‰ |
| δ13CRd(30min) | δ13C of CO2 evolved by leaves after 30 min dark | ‰ |
| δ13CRd(3h) | δ13C of CO2 evolved by leaves after 3 h dark | ‰ |
| δ13CRd(6min) | δ13C of CO2 evolved by leaves after 6 min dark | ‰ |
Leaf net 13CO2 discrimination in the light and mesophyll conductance to CO2 diffusion
The observed leaf net discrimination against 13CO2 in the light (Δo, ‰) was calculated by mass balance from the TDLAS measurements according to Evans et al. (1986). Under photorespiratory conditions (18.4 kPa pO2), the 13CO2 fractionation for photorespiration (f, ‰) in the gdch-KD plants was calculated based on Evans and von Caemmerer (2013). Briefly, the value of f was determined by modeling the leaf net discrimination against 13CO2 (Δo) as a function of the 13C discrimination fractions associated with CO2 diffusion from the atmosphere to the intercellular air space and with carboxylation (Δi), with CO2 diffusion in liquid phase to chloroplast stroma (Δgm), mitochondrial non-photorespiratory respiration (Δe), and photorespiration (Δf). The equation Δo=Δi−Δgm−Δf−Δe can be rearranged so that Δf=Δi−Δo−Δgm−Δe and f can be estimated by substituting Δf with to get . An f value of 16.2‰ was taken from Evans and von Caemmerer (2013) and assumed for WT plants. The input parameters needed to calculate f include the leaf mitochondrial respiration rate in the light (RL, µmol CO2 m−2 s−1), the CO2 compensation point in the absence of mitochondrial non-photorespiratory respiration (Γ*, μmol mol−1), and mesophyll CO2 conductance (gm, mol CO2 m−2 s−1). Values of RL at a tleaf of 25 °C were modeled for both genotypes from the corresponding Rd at 30 °C after 3 h in the dark [Rd(3h), μmol CO2 m−2 s−1] following leaf photosynthesis under atmospheric pO2 of 18.4 kPa using the temperature response function in Bernacchi et al. (2001). The Γ* was modeled based on von Caemmerer (2000), as described in Supplementary Methods S4, and was significantly different (P<0.05) between WT and gdch-KD plants, 45.0±1.7 SE (n=4) μmol mol−1 and 53.3±0.6 SE (n=3) μmol mol−1, respectively. Finally, gm was estimated based on leaf–atmosphere CO2 and 13CO2 exchange data, according to Evans and von Caemmerer (2013). Specifically, 13C-based gm was calculated in the gdch-KD and WT plants at 1.84 kPa pO2, but only in WT plants under 18.4 kPa pO2, using an f value of 16.2‰. The 13C-based gm cannot be calculated in gdch-KD plants at 18.4 kPa pO2 because gm and f are not independent variables in the applied procedure. Therefore, at 18.4 kPa, the gm values of gdch-KD plants were set the same as for the WT. This assumes that the 13C-based gm integrates the within-leaf resistances affecting CO2 movement across the cell wall, plasma membrane, and the chloroplast membranes, and that this cumulative resistance does not differ between gdch-KD and WT plants. This assumption is supported by the fact that the 18O-based gm, which was determined by analysis of leaf–atmosphere 18O exchange according to Ubierna et al. (2017), Kolbe and Cousins (2018), and Sonawane and Cousins (2019), was not significantly different between the gdch-KD and WT plants at 18.4 kPa pO2 (Supplementary Table S2). The 18O-based gm is not strictly associated with the biochemistry of photosynthesis as is the 13C-based gm and therefore cannot be used to estimate f. The values of 13C-based gm for gdch-KD and WT plants at each pO2 were used to calculate the corresponding pCO2 in the chloroplasts (Cc, Pa) by applying Fick’s first law.
The Γ* was defined in terms of Rubisco kinetic properties according to Jordan and Ogren (1984), and the estimate of CO2 released per O2 reacting with RuBP (α) was determined for the gdch-KD plants versus α set equal to 0.5 in the WT as described in Supplementary Methods S5. A sensitivity analysis for the dependency of f on Γ* and α is also described in Supplementary Methods S5.
13C composition of leaf dark-evolved CO2 and contributions of leaf chamber and growth chamber assimilates to substrates feeding leaf dark respiration
The 13C composition of the dark-evolved CO2 determining Rd (δ13CRd, ‰) was calculated according to Barbour et al. (2007) as described by Evans et al. (1986).
The substrates feeding leaf dark respiration were from carbon assimilates produced in the Lch and in the Gch. Given δ13CRd(i) as the mean values of δ13C for dark-evolved CO2 at time i from light–dark transition, the fractional contribution of Lch assimilates to δ13CRd(i) (δRdLch_substr(i), ‰/‰) was calculated for gdch-KD and WT plant types after leaf photosynthesis under both O2 levels as
| (1) |
where δ13CRd(i) was determined by steps of 3 min over 195 min in the dark; δ13CRd(24h) is the mean δ13CRd after 24 h in the dark as shown in Supplementary Table S1; and δ13CLch_Ph (‰) is the representative δ13C of gdch-KD or WT carbon assimilates produced in the Lch at a pO2 of 1.84 kPa or 18.4 kPa before the light–dark transition (values are shown in Supplementary Table S1). The assumptions underlying Equation 1 and the calculation of δ13CLch_Ph are reported in Supplementary Methods S6.
Based on the total fractional contributions of Lch and Gch carbon assimilates to δ13CRd equal to 1.0, the complementing fractional contribution of Gch assimilates to δ13CRd(i) [δRdGch_substr(i), ‰/‰] was calculated for both plant types after leaf photosynthesis under both O2 levels as
| (2) |
In addition, to make a combined analysis of the data collected in the two O2 experimental conditions possible, the δ13CRd generated from plants grown at the more depleted δ13CGch were edited to cancel out the bias in the δ13CGch effect on δ13CRd with respect to the other batch of plants. In particular, the δ13CRd following leaf photosynthesis at the lower O2 experimental level were edited through the procedure described in Supplementary Methods S7.
Leaf CO2 compensation points in the presence of RL
Leaf–atmosphere gas exchange measurements were taken with an LI-6400XT portable gas analyzer equipped with the 2×3 cm Lch on gdch-KD and WT plants (n=4) at a PPFD of 1500 µmol photons m−2 s−1, tleaf of 25 °C, leaf to air VPD between 1.0 kPa and 1.5 kPa, Ca decreasing from 35.0 Pa to 3.7 Pa, and at a pO2 of 1.84 kPa or 18.4 kPa. For each leaf, a least square regression analysis of the response of A (µmol CO2 m−2 s−1) to Ci (Pa) was applied to the initial slope (for Ci≤9.2 Pa) to determine the CO2 compensation point in the presence of RL (Γ, Pa).
Statistical analysis
Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). A linear mixed effects model (PROC MIXED) was used with plant type (gdch-KD and WT) and O2 level (pO2 of 1.84 kPa and 18.4 kPa) as fixed factors and leaves as random factor nested within plant type. The effects of plant type, O2 level, and plant type×O2 level interaction on A, gsC, Ci, Ci/Ca, Δo, Cc, Γ, Rd(6m), δ13CRd(6m), Rd(30m), δ13CRd(30m), Rd(3h), and δ13CRd(3h) were assessed. A PROC MIXED procedure was applied as a one-way ANOVA to determine the plant type (fixed factor) effect on the following traits: total N content, malate content, Γ*, Δo, Δi, Δi−Δo, Δgm, Δe, Δf, Rd(24h), δ13CRd(24h), LMA, and δ13Cdm. A one-sample t-test (P<0.05) was applied to test the difference of f or α modeled for the gdch-KD plants compared with a constant f or α value assumed in the WT. A two-sample t-test (P<0.05) was applied to test the difference between gdch-KD and WT gm at a pO2 of 1.84 kPa, and WT gm at the two O2 levels. For each plant type, a three-parameter non-linear model was fit to the Rd and δ13CRd responses determined over the 195 min in the dark after leaf photosynthesis at each O2 experimental level. In particular, the δ13CRd values associated with the lower O2 level had been first edited as described in Supplementary Methods S7, and then used for the analysis. The fitting model y=θ1e–θ2x+θ3 was employed where x are minutes from 0 to 195 by steps of three, and y are Rd or δ13CRd values; θ1, θ2, and θ3 are the range, slope, and lower asymptote (or floor) parameters, respectively, which were determined using non-linear least squares with the iterative Gauss–Newton algorithm. Specifically, for Rd or δ13CRd responses, the range parameter corresponds to the difference between initial and lower asymptote values, and the slope is the exponential rate of change. An extra sum of squares F-test was applied to define the significance (P<0.05) of the effects of plant type and O2 level (main effects), and plant type×O2 level interactions on the three parameters of Rd or δ13CRd fitting models.
Results
Leaf GDC protein and malate content
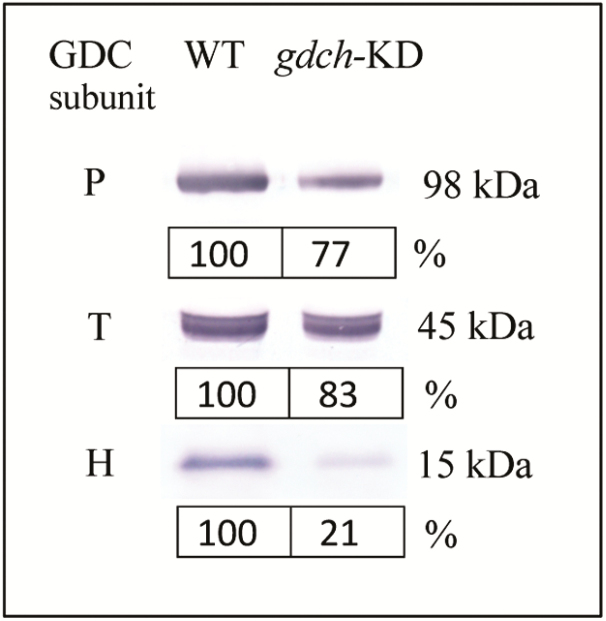
Leaves of gdch-KD plants had 21% (±2 SE) H-protein content compared with the WT, while the P- and T-protein content was 77% (±6 SE) and 83% (±2 SE) of that of the WT, respectively (Fig. 1; n=2). Malate content in leaf samples taken immediately after measurements of leaf photosynthesis were 0.49±0.08 mmol m−2 and 0.38±0.02 mmol m−2 (mean ±SE; n=5) in gdch-KD and WT plants, respectively (P=0.29).
Fig. 1.
Immunoblot analysis for GDC P-, T-, and H-subunits in mature leaves of gdch-KD compared with WT plants. The protein molecular weight of each subunit (kDa) is shown. Subunit protein abundances for gdch-KD plants are mean percentage values of the WT (n=2).
Leaf physiological analysis
Leaf photosynthetic responses
There was no observable difference in growth phenotypes between the gdch-KD and WT plants when they were grown under 184.2 Pa pCO2 (2000 μmol mol−1). However, at approximately current ambient CO2 and O2 partial pressures, the net rate of CO2 assimilation (A) was lower in the gdch-KD compared with the WT but there was no significant difference in A between plant types under low photorespiratory conditions, when pO2 was reduced to 1.84 kPa (Table 2). There was, however, a significant effect of O2 level on gsC (negative effect) and Ci/Ca (positive effect) but not a plant type effect (Table 2). There was a significant plant type×O2 level interaction on Δo, which showed higher values for the gdch-KD compared with the WT at a pO2 of 18.4 kPa, but no difference at a pO2 of 1.84 kPa (Table 2). There was no significant plant type effect on gm at a pO2 of 1.84 kPa (P=0.586), and no O2 effect on gm in the WT (P=0.701; Table 2). There was, however, a significant effect of plant type on Cc, which showed comparable values in the gdch-KD and WT plants at 1.84 kPa pO2 and higher values in the gdch-KD plants compared with the WT at 18.4 kPa pO2 (modeled based on equal gm in both transgenic and WT plants). In addition, O2 level had a positive effect on Cc (Table 2). The Γ in the gdch-KD compared with WT plants was significantly lower under 1.84 kPa pO2 but higher under 18.4 kPa pO2 (Table 3). There was no significant difference in leaf N content between plant types, with means of 2.30±0.08 SE g m−2 and 2.31±0.14 SE g m−2 in gdch-KD and WT leaves, respectively (n=4).
Table 2.
Leaf photosynthetic traits estimated on gdch-KD and WT plants under approximately current ambient and below current ambient O2 levels (pO2 of 18.4 kPa and 1.84 kPa, respectively) at Ca of 27.6 Pa.
| Plant-type | pO2 | A | g sC | C i | C i /C a | g m a | C c | Δo |
|---|---|---|---|---|---|---|---|---|
| (kPa) | (µmol CO2 m−2 s−1) | (µmol CO2 m−2 s−1 Pa−1) | (Pa) | (µmol CO2 m−2 s−1 Pa−1) | (Pa) | (‰) | ||
| gdch-KD | 1.84 | 24.7±1.4 | 3.47±0.53 | 18.1±0.8 | 0.66±0.03 | 4.56±0.43 | 12.6±1.4 | 14.9±0.7 |
| 18.4 | 6.3±0.3 | 1.37±0.11 | 22.1±0.2 | 0.80±0.01 | 20.6±0.2 | 23.7±0.5 | ||
| WT | 1.84 | 21.6±1.2 | 2.78±0.43 | 18.5±0.9 | 0.67±0.03 | 3.80±0.76 | 12.6±0.5 | 14.3±0.8 |
| 18.4 | 14.3±0.8 | 2.45±0.31 | 20.5±0.5 | 0.74±0.02 | 4.07±0.14 | 17.0±0.6 | 17.8±0.3 | |
| Significance | Plant type | P=0.050 | P=0.629 | P=0.411 | P=0.411 | – | P=0.031 | P=0.003 |
| pO2 | P<0.001 | P=0.018 | P=0.003 | P=0.003 | – | P=0.000 | P<0.001 | |
| Plant type×pO2 | P=0.002 | P=0.057 | P=0.171 | P=0.171 | – | P=0.033 | P=0.003 |
Values are the mean±SE (n=4). Significance (P<0.05) of the effects of plant type, pO2, and plant type×pO2 interaction were evaluated by SAS PROC MIXED.
a No significant differences were evaluated by a two sample t-test (significance for P<0.05) between the gdch-KD and WT gm values at a pO2 of 1.84 kPa (P=0.586), and the WT gm values at the two pO2 values (P=0.701).
Table 3.
CO2 compensation points (Γ) determined under low photorespiratory (1.84 kPa pO2) and photorespiratory (18.4 kPa pO2) conditions on gdch-KD and WT plants
| Plant-type | pO2 | Γ |
|---|---|---|
| (kPa) | (Pa) | |
| gdch-KD | 1.84 | 0.25±0.06 |
| 18.4 | 5.48±0.04 | |
| WT | 1.84 | 0.62±0.12 |
| 18.4 | 4.54±0.18 | |
| Significance | Plant type | P=0.055 |
| pO2 | P<0.001 | |
| Plant type×pO2 | P=0.001 |
Values are the mean ±SE (n=3 for gdch-KD at a pO2 of 18.4 kPa; n=4 otherwise). Significance (P<0.05) for the effects of plant type, pO2, and plant type×pO2 interaction was evaluated by SAS PROC MIXED.
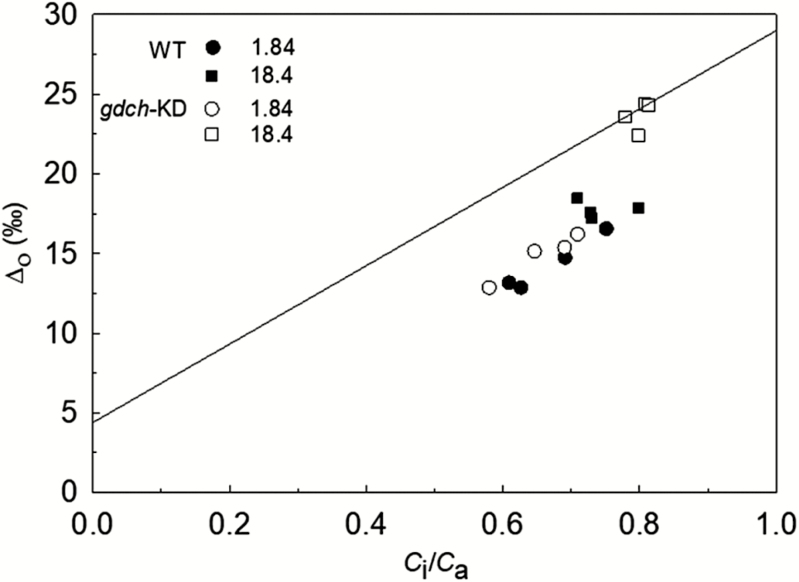
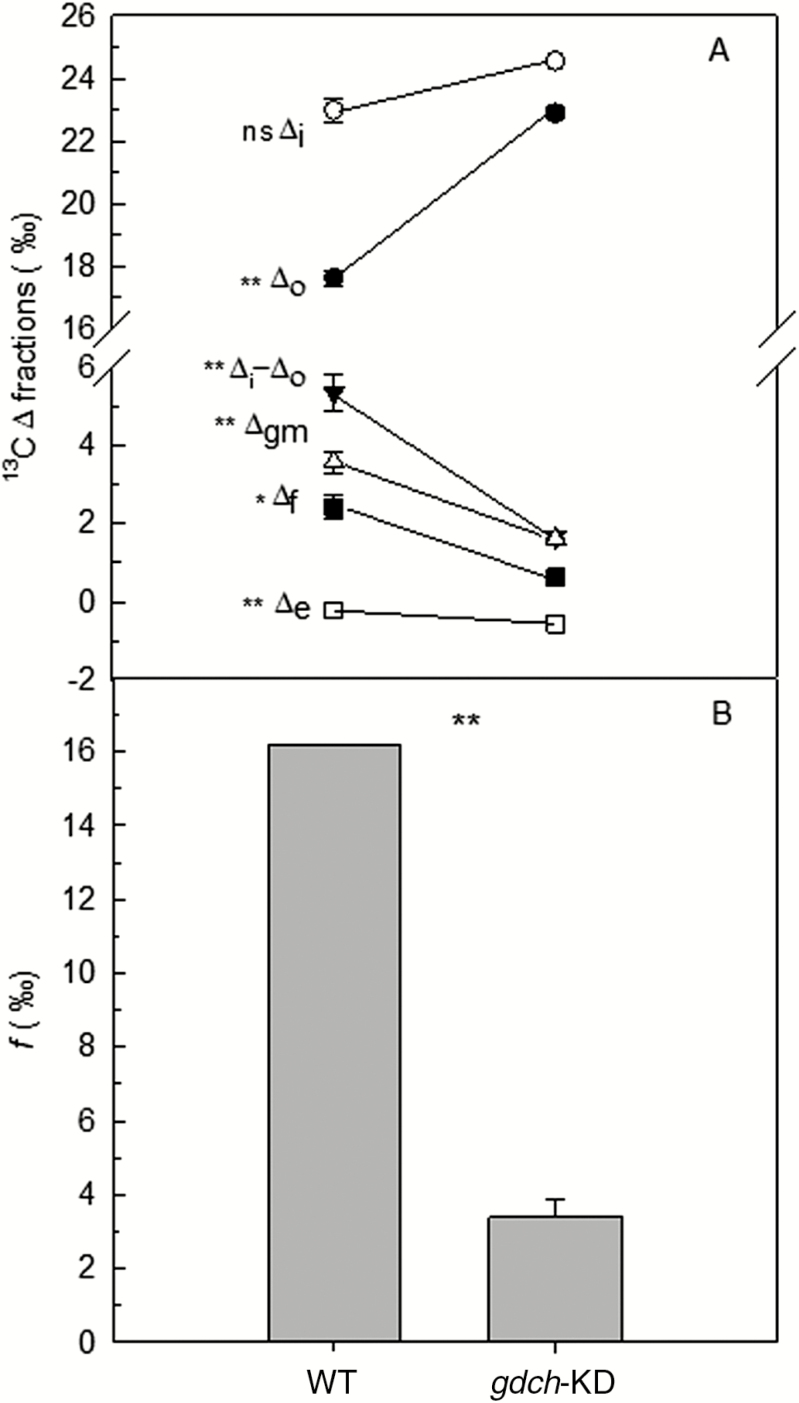
Δo plotted versus Ci/Ca showed a similar response in the gdch-KD and WT plants at 1.84 kPa pO2 but was significantly greater in the gdch-KD compared with WT plants at 18.4 kPa pO2 (Fig. 2; see Table 2 for statistical analysis). The gdch-KD plants had significantly lower Δgm, Δf, and Δe compared with the WT under a pO2 of 18.4 kPa (Fig. 3A). Additionally, the gdch-KD plants had a significantly lower f with mean values of 3.4±0.5‰ SE (n=4) compared with 16.2‰ in the WT under approximately current ambient pO2 (Fig. 3B; P<0.001). A significantly higher α was determined at 18.4 kPa pO2 in gdch-KD plants, with a mean value of 0.59±0.01 SE (n=3), versus 0.5 assumed for the WT (P<0.01). There was a negative linear dependency of f on Γ* and on α (Supplementary Methods S5; Fig. S1A and B, respectively); however, there was a positive linear dependency of f on gm and RL, with a greater sensitivity to gm (Supplementary Fig. S2A ands B, respectively).
Fig. 2.
Leaf 13CO2 net discrimination in the light (Δo) versus Ci/Ca under a pO2 of 1.84 kPa and 18.4 kPa for individual gdch-KD and WT plants. The line represents the leaf 13CO2 net discrimination modeled in relation to Ci/Ca as Δ13Cmod=a+(b3−a)×Ci/Ca (Farquhar et al., 1982) where a=4.4‰ and b3=29.0‰. Δ13Cmod is a proxy of Δi as described by Evans and von Caemmerer (2013). Open symbols are for gdch-KD and filled symbols for WT plants. Circles are for a pO2 of 1.84 kPa and squares for a pO2 of 18.4 kPa.
Fig. 3.
Leaf 13CO2 net discrimination and discrimination fractions in the light, and 13CO2 photorespiratory fractionation for gdch-KD versus WT plants determined based on Evans and von Caemmerer (2013). (A) Observed leaf net 13CO2 discrimination in the light (Δo), and modeled 13C discrimination fractions for gdch-KD (n=3) and the WT (n=4) at an atmospheric pO2 of 18.4 kPa. Δi is the additive 13CO2 discrimination during CO2 diffusion from atmosphere to intercellular air space and due to carboxylation; Δi−Δo is comprised of three terms: Δgm, which is the 13CO2 fractionation fraction during CO2 diffusion in the liquid phase to chloroplast stroma, and Δe and Δf, which are the 13C fractionation fractions associated with light mitochondrial non-photorespiratory respiration and photorespiration, respectively. Δf was calculated as Δf=Δi−Δo−Δgm−Δe. Values are mean ±SE. (B) 13CO2 fractionation for photorespiration (f) in gdch-KD plants calculated at an atmospheric pO2 of 18.4 kPa from versus f of 16.2‰ in the WT. Values for gdch-KD plants are the mean ±SE (n=3). Significance (P<0.05) of the effect of plant type on the variables in (A) was evaluated by SAS PROC MIXED as a one-way ANOVA; * for 0.01<P<0.05; ** for P<0.01. Significance in (B) was evaluated by one-sample t-test (P<0.05). ** for P<0.01.
Leaf dark respiration responses
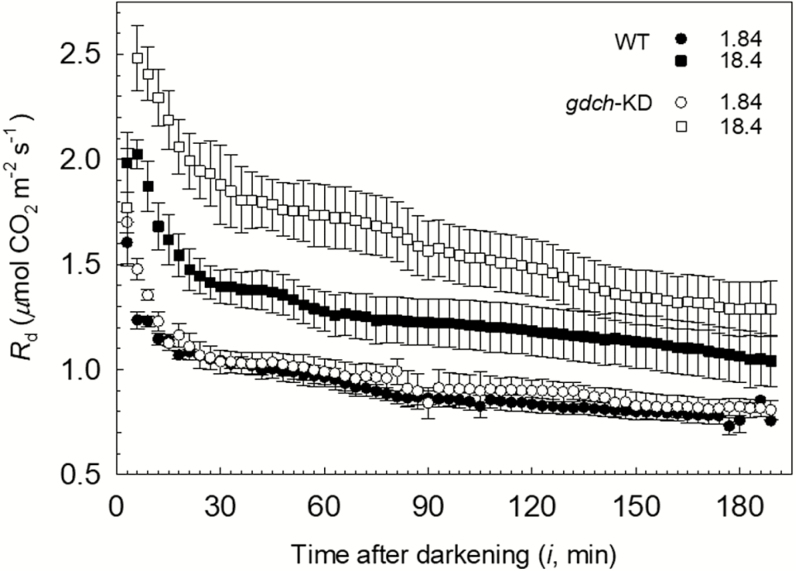
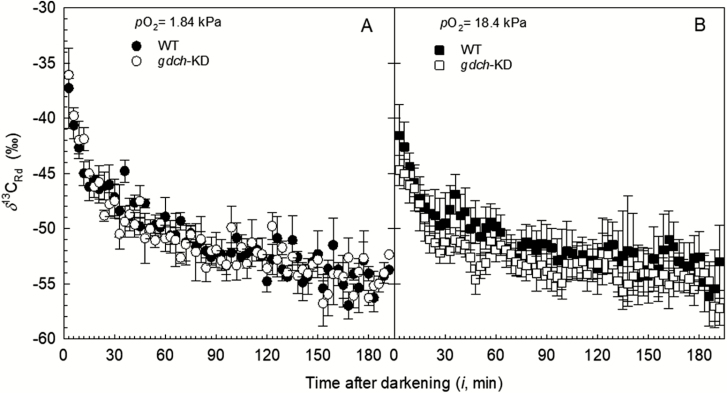
In the gdch-KD and WT plants, the Rd showed a hyperbolic decrease over the 3 h in the dark after leaf light exposure under different O2 levels, with a noticeable rapid decline in the first hour; however, Rd was higher following leaf photosynthesis at a pO2 of 18.4 kPa compared with 1.84 kPa. (Fig. 4). A significant positive O2 effect on Rd responses of gdch-KD and WT plants was inferred based on significantly higher floor (μmol CO2 m−2 s−1; P<0.0001) and range (μmol CO2 m−2 s−1; P<0.0001) parameters after leaf photosynthesis at pO2 of 18.4 kPa compared with 1.84 kPa in the non-linear model fit to the Rd responses (Supplementary Tables S3, S4). In particular, based on the spot measurements, a significant positive O2 effect was determined on Rd(6min) (together with a significant plant type effect), Rd(30min), and Rd(3h) (Table 4). A significant plant type effect on Rd responses was inferred based on a statistically larger range (P=0.023) and less steep rate of exponential change (slope, μmol CO2 m−2 s−1 min−1; P<0.0001) for the gdch-KD versus WT plants (Supplementary Tables S3, S4). After leaf photosynthesis at a pO2 of 18.4 kPa, a significant plant type effect on Rd (with higher Rd determined in the gdch-KD plants versus the WT; see Fig. 4) was driven by the significantly less steep Rd slope (P<0.001) in gdch-KD compared with WT plants. In addition, following leaf photosynthesis at a pO2 of 18.4 kPa, a change in Rd for ~75% of the Rd range occurred in WT plants within the first 30 min after light–dark transition; in contrast, this fractional variation took ~90 min in the gdch-KD plants (Fig. 4). The mean values of RL inferred from Rd(3h) were 0.59±0.03 SE μmol CO2 m−2 s−1 for gdch-KD and 0.56±0.03 SE μmol CO2 m−2 s−1 for WT plants after leaf photosynthesis under a pO2 of 1.84 kPa (n=4). In contrast, RL was 0.98±0.12 SE μmol CO2 m−2 s−1 for gdch-KD and 0.82±0.09 SE μmol CO2 m−2 s−1 for WT plants after leaf exposure to a pO2 of 18.4 kPa (n=4). For the RL values, a non-significant plant type effect and a significant effect of the O2 level can be inferred from the significance of Rd(3h) (see Table 4). In addition, no significant difference in leaf dry mass per unit surface area (LMA) was determined between gdch-KD and WT plants, with values of 43.6±2.7 SE g m−2 and 44.5±1.5 SE g m−2 (n=4), respectively.
Fig. 4.
Dynamics of leaf dark respiration rate (Rd) determined during ~3 h in the dark on gdch-KD (open symbols) and WT (filled symbols) plants after leaf photosynthesis under a pO2 of 1.84 kPa (circles) or 18.4 kPa (squares). Symbols correspond to the mean ±SE (n=4) determined every 3 min.
Table 4.
Leaf dark respiration rates (Rd) at 30 °C and 13CO2 composition of dark-evolved CO2 (δ13CRd) determined on gdch-KD versus the WT after 6 min [Rd(6min) and δ13CRd(6min); n=4], 30 min [Rd(30min) and δ13CRd(30min); n=4], 3 h [Rd(3h) and δ13CRd(3h); n=4], and 24 h [Rd(24h) and δ13CRd(24h); n=3] in the dark following leaf exposure to light under approximately current ambient and below current ambient O2 levels (pO2 of 18.4 kPa and 1.84 kPa, respectively)
| Plant-type | pO2 | R d(6min) | δ13CRd(6min) | R d(30min) | δ13CRd(30min) | R d(3h) | δ13CRd(3h) | R d(24h) | δ13CRd(24h) |
|---|---|---|---|---|---|---|---|---|---|
| (kPa) | (µmol CO2 m-2 s-1) | (‰) | (µmol CO2 m−2 s−1) | (‰) | (µmol CO2 m−2 s−1) | (‰) | (µmol CO2 m−2 s−1) | (‰) | |
| gdch-KD | 1.84 | 1.48±0.05 | −39.2±0.9* | 1.04±0.06 | −47.3±1.6* | 0.81±0.04 | −56.0±0.7* | 0.79±0.02 | −58.0±0.5* |
| WT | 1.84 | 1.23±0.04 | −40.6±1.2* | 1.04±0.01 | −47.2±1.7* | 0.77±0.04 | −54.1±0.7* | 0.69±0.02 | −58.6±1.0* |
| Significance | P=0.045 | P=0.705 | |||||||
| gdch-KD | 18.4 | 2.59±0.29 | −45.6±1.7 | 1.98±0.20 | −54.1±0.9 | 1.34±0.17 | −55.6±1.2 | 0.69±0.06 | −58.1±0.1 |
| WT | 18.4 | 2.13±0.07 | −43.2±0.9 | 1.47±0.08 | −50.4±2.8 | 1.12±0.13 | −52.5±1.8 | 0.74±0.08 | −58.6±0.6 |
| Significance | Plant type | P=0.042 | P=0.723 | P=0.110 | P=0.349 | P=0.215 | P=0.132 | P=0.596 | P=0.410 |
| pO2 | P=0.0001 | P=0.032 | P=0.0009 | P=0.035 | P=0.004 | P=0.429 | – | – | |
| Plant type×pO2 | P=0.407 | P=0.238 | P=0.069 | P=0.375 | P=0.326 | P=0.753 | – |
Values are the mean ±SE; the asterisks indicate means from δ13CRd values edited according to Supplementary Method S7. Significance (P<0.05) of the effects of plant type, pO2, and plant type×pO2 interaction was evaluated by SAS PROC MIXED. The effect of plant type on Rd(24h) and δ13CRd(24h) was evaluated at a pO2 of 1.84 kPa or 18.4 kPa by one-way ANOVA (significance for P<0.05).
In gdch-KD and WT plants, the δ13CRd estimated over the 3 h after light–dark transition showed a negative hyperbolic pattern, with most of the δ13CRd variation occurring in the first 30 min (Fig. 5A, B). A tight positive correlation between Rd and δ13CRd over the 3 h dark period was determined after leaf photosynthesis at a pO2 of 1.84 kPa for both plant types (r>0.90). After leaf photosynthesis at a pO2 of 18.4 kPa, a positive correlation between Rd and δ13CRd with r=0.75 and r=0.78 was determined for gdch-KD and the WT, respectively. Statistical analysis of a non-linear model fit to the δ13CRd responses showed a significantly lower δ13CRd range after leaf photosynthesis at a pO2 of 18.4 kPa (‰; P<0.0001) compared with 1.84 kPa pO2. In contrast, the floor parameter was non-significantly different between the O2 levels (Supplementary Tables S5, S6). These statistical results indicate a significant effect of the O2 level during previous leaf light exposure on the δ13CRd values of both plant types over 3 h in the dark (with lower δ13CRd at a pO2 of 18.4 kPa, and higher δ13CRd at a pO2 of 1.84 kPa). The spot δ13CRd measurements also showed significantly lower δ13CRd(6min) and δ13CRd(30min) after leaf photosynthesis at a pO2 of 18.4 kPa compared with 1.84 kPa (Table 4). There was no significant plant type effect on the δ13CRd over the 3 h in the dark (Table 4; Supplementary Table S6) and there was no difference for δ13Cdm between gdch-KD and the WT (Supplementary Table S1).
Fig. 5.
13CO2 composition associated with Rd (δ13CRd) determined during ~3 h in the dark in gdch-KD (open symbols) and WT (filled symbols) plants. (A) δ13CRd after leaf photosynthesis under a pO2 of 1.84 kPa edited (see Supplementary Methods S7) to remove the effect of a lower atmospheric δ13CO2 compared with (B) while growing the plants. (B) Distributions of δ13CRd after leaf photosynthesis under a pO2 of 18.4 kPa. Symbols correspond to the mean ±SE (n=4) determined every 3 min.
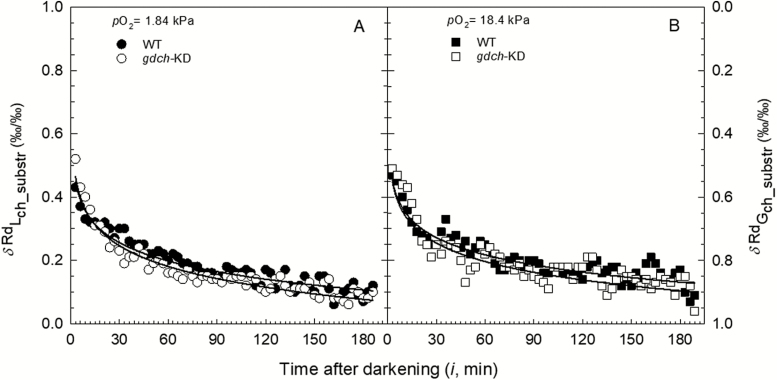
Over the 3 h after the light–dark transition, the fractional contribution of Lch assimilates to δ13CRd (δRdLch_substr, ‰/‰) showed a decreasing hyperbolic pattern for both gdch-KD and WT plants (Fig. 6), with no significant differences between plant types and O2 levels.
Fig. 6.
Distributions over ~3 h in the dark of the fractional contributions (total contribution equal to 1.0) to δ13CRd of recent Lch carbon assimilates (δRdLch_substr, ‰/‰) and Gch assimilates (δRdGch_substr, ‰/‰) for gdch-KD (open symbols) and WT (filled symbols) after leaf light exposure at a pO2 of (A) 1.84 kPa and (B) 18.4 kPa. The first values are at 3 min after light–dark transition. Symbols correspond to the mean determined every 3 min (n=4). Continuous lines represent logarithmic trend lines (R2 >0.90) for gdch-KD (lower) and the WT (higher), respectively.
Discussion
Altered photorespiratory metabolism and leaf photosynthetic traits
Based on leaf protein analysis, gdch-KD plants had ~21, 77, and 83% of GDC H-, P-, and T-protein abundance, respectively, compared with the WT. Previous studies reported how GDC activity is linearly correlated with H-protein accumulation (Wingler et al., 1997; Lin et al., 2016). Additionally, in agreement with Lin et al. (2016), the gdch-KD plants in the current study showed an expected photorespiratory-deficient phenotype. Under photorespiratory conditions, a disruption of the photorespiratory pathway negatively affects the rate of net CO2 assimilation (A) due to accumulation of metabolites that inhibit the Calvin–Benson cycle and restrict RuBP regeneration (Wingler et al., 2000). Specifically, leaf glycine level is a sensitive indicator of altered photorespiratory carbon flow (Blackwell et al., 1988; Timm et al., 2012). For example, gdch-KD mutants of Arabidopsis, barley, and rice had substantial increases in leaf contents of glycine under ambient pO2 (Bauwe and Kolukisaoglu, 2003; Lin et al., 2016). This accumulation of glycine and its precursors (P-glycolate, glycolate, and glyoxylate) in the gdch-KD plants has been suggested to alter photorespiratory carbon metabolism (Peterhansel et al., 2010, 2013a). These changes have important implications for understanding and modeling leaf carbon metabolism, because they may influence the stoichiometry of CO2 released per oxygenation reaction (α) and the CO2 compensation point (Γ) (see Cousins et al., 2008, 2011; Walker and Cousins, 2013).
In the present study, the gdch-KD plants had greater Γ compared with the WT under 18.4 kPa pO2, as previously reported by Lin et al. (2016). This may be partially due to enhanced RL, which Igamberdiev et al. (2004) and Bykova et al. (2005) reported was needed to compensate for the lack of photorespiratory regulation of redox and energy balance (Igamberdiev et al., 2001). The increase in Γ in the gdch-KD plants could also be associated with a higher α leading to an increasing Γ* compared with the WT. It has been previously suggested that α increased in Arabidopsis mutants lacking peroxysomal hydroxypuruvate reductase (Cousins et al., 2008) and the peroxysomal malate dehydrogenase (Cousins et al., 2011). However, these previous publications did not determine whether these disruptions to photorespiration influenced leaf CO2 isotope exchange.
Leaf net 13C discrimination in the light and photorespiratory 13C fractionation
Under leaf photorespiratory conditions, the change in leaf net discrimination against 13CO2 (Δo) in the gdch-KD plants compared with WT plants was caused by a higher Ci/Ca, lower Δgm, greater Δe, and lower Δf (Fig. 3A). However, the lower Δgm in the gdch-KD plants with respect to the WT was due to a reduction in A, since WT gm was applied to both plant types (see Δgm equation in Evans and von Caemmerer, 2013). In addition, a difference in Δe between gdch-KD and WT plants was related to proportional changes in RL/(A+RL) and (Ci−Γ*) (see Δe equation in Evans and von Caemmerer, 2013).
The term Δf is dependent on , but, despite the higher Γ* in the gdch-KD relative to WT plants, it was lower in the transgenic plants caused by the lower f. In WT plants, f is primarily attributed to the 13C discrimination associated with the decarboxylation of glycine catalyzed by GDC (Tcherkez et al., 2004; Tcherkez, 2006). While Rooney (1988) determined an in vitro f of 7–8‰ for Glycine max (soybean), Igamberdiev et al. (2001, 2004) reported f for several species between 9.8 and 13.7‰, and Ghashghaie et al. (2003) reported an f of >9–11‰ for Senecio species. Additionally, Evans and von Caemmerer (2013) determined an in vivo f of 16.2‰ in Nicotiana tabacum. Based on Farquhar et al. (1982), and according to O’Leary (1988) and Tcherkez (2006),
| (3) |
where glycine is assumed to have the same 13C signature of recently fixed carbon. Therefore, an increase in δ13C of the released CO2 during photorespiration corresponds to a linear decrease in f. There is also a negative linear dependency of f on Γ* and α, as shown in Supplementary Methods S5; Fig. S1A, B.
In C3 plants, most of the CO2 released by photorespiration tends to be through GDC (Badger, 1985; Bauwe et al., 2010). However, previous reports have suggested that alternative reactions can release CO2 when the flux of glycolate into the photorespiratory cycle exceeds its metabolic capacity, or when the traditional photorespiratory pathway has been genetically disrupted (Cousins et al., 2008, 2011; Timm et al., 2008; Peterhansel et al., 2013a). The GDC multienzyme system requires all subunits to function (Douce et al., 2001); in the present study, since a low level of the H-subunit was determined in gdch-KD plants, some residual activity for GDC is expected in the transgenic plants. A change in 13C fractionation associated with the knockdown of GDC activity is therefore unlikely because the products of the glycine decarboxylation reaction (NH4+, NADH, and methylene-tetrahydrofolate) can be readily processed by downstream reactions in the glycolate pathway (Bauwe et al., 2010; Maurino and Peterhansel, 2010). Thus, the higher α and lower f in the gdch-KD compared with the WT suggest an increased flow of photorespiratory carbon through alternative decarboxylation reactions, independent of the GDC, and a buildup of photorespiratory metabolites.
Leaf dark respiration and 13C isotopic composition of dark-evolved CO2
Leaves of C3 plants in the first 30 min after light–dark transition largely respire metabolites (carbohydrates and organic acids) recently produced in the light (Cornic, 1973; Rademacher et al., 2002; Barbour et al., 2007; Werner et al., 2009; Werner and Gessler, 2011; Lehmann et al., 2015, 2016) and show high rates of CO2 evolution (named as LEDR, light-enhanced dark respiration; Atkin et al., 1998). While the activity of pyruvate dehydrogenase (PDH) and metabolism in the tricarboxylic acid (TCA) cycle are the major mitochondrial decarboxylations in the dark, they are partially inhibited in the light (Ghashghaie et al., 2003; Tcherkez et al., 2005, 2008; Barbour et al., 2007). It has been suggested that LEDR mostly depends on a buildup of malate and fumarate in the light, which are then rapidly decarboxylated after the light–dark transition (Atkin et al., 1998; Barbour et al., 2007; Tcherkez et al., 2012). However, there is evidence for species-specific differences (Lehmann et al. 2016; Gessler et al., 2017). Overall, Rd in the gdch-KD and WT plants showed an expected negative hyperbolic pattern during 3 h in the dark following leaf photosynthesis. The results of the present study indicate that leaf photosynthesis under photorespiring conditions, before the light–dark transition, led to an additional buildup of TCA cycle substrates in the gdch-KD plants compared with the WT. In fact, the gdch-KD plants had a significantly higher Rd over 3 h dark following leaf photosynthesis under the approximately current ambient O2 level (with leaf blades having no significantly different LMA), compared with the WT; this suggests a greater accumulation of metabolites in the light, in particular photorespiratory intermediates, as respiratory substrates to feed Rd. More precisely, a restricted photorespiratory pathway in the light may lead to an accumulation of 2-carbon metabolites in the gdch-KD plants.
The increase in LEDR has been reported to come from the decarboxylation of 13C heavier metabolites, primarily malate, and the decline in LEDR rates and δ13CRd over time due to a decrease in malate availability (Barbour et al. 2007; Gessler et al., 2009). In the gdch-KD plants, the cumulative leaf respired CO2 over 30 min after leaf photosynthesis at a pO2 of 18.4 kPa was 4.1 mmol CO2 m−2 higher with respect to the WT; theoretically, if this enhancement of Rd in the gdch-KD plants was due to malate alone this would require ~1 mmol malate m−2. The non-significant differences in the leaf malate content determined during the light period between gdch-KD and the WT suggest that metabolites other than malate, such as photorespiratory intermediates, may have contributed to the greater LEDR rates in the gdch-KD plants compared with the WT. A substantial part of the malate in leaves is also stored in vacuoles, as observed in C4 plants (Hatch, 1979; Arrivault et al. 2017), and not readily available for LEDR.
In the gdch-KD and WT plants presented here, the δ13CRd decreased during the 3 h dark period, tracking the decline in Rd (Fig. 5A, B). Over the 3 h of darkness, there was an increase in the contribution to δ13CRd from respiratory substrates generated during plant growth (δRdGch_substr, ‰/‰) for both plant types and O2 experimental conditions. Regardless of plant type or O2 treatment, the δRdLch_substr went from ~50% after 6 min from the light–dark transition to ~30% after 30 min in the dark, while after 3 h in the dark it represented only ~10% [see Fig. 6; data of δ13CRd(3h) approaching δ13CRd(24h) are shown in Table 4]. Tcherkez et al. (2010) estimated on sunflower (Helianthus annuus) that recent assimilates provide 40–60% of the substrates for Rd (via a pool with a half-life of several hours) both in the light and in the dark. A similar contribution of recent assimilates to Rd was determined by Nogués et al. (2004) on French bean (Phaseolus vulgaris) leaves during ~2 h in the dark following illumination, which indicates that leaf respiration was fed by a mixture of recent and older substrates.
The tendency for a lower leaf δ13CRd in gdch-KD plants compared with the WT following leaf light exposure under photorespiratory conditions may partially depend on the higher Δo in the gdch-KD plants during leaf photosynthesis in the Lch at approximately current ambient pO2. A greater Δo would cause (recent) carbon assimilates synthetized in the Lch (Supplementary data Table S1) to produce more depleted respiratory substrates and a lower δ13CRd in the gdch-KD compared with the WT. It is also possible that higher Δo in the gdch-KD compared with WT plants during growth under enriched atmospheric pCO2 and current ambient pO2 may have produced Gch assimilates feeding Rd over 3 h after the light–dark transition with slightly lower δ13C compared with the WT.
The 13C fractionation during leaf dark respiration can change depending on species and environmental conditions (Ghashghaie et al., 2003; Priault et al., 2009; Werner et al., 2009; Lehmann et al., 2016). In addition, δ13CRd is influenced by the isotopic signatures of respiratory substrates, from diverse non-homogeneous isotope distributions in the substrates (positional effects) and the different relative activities of decarboxylation pathways. However, decreasing δ13CRd over time is mainly dependent on the origin of respiratory substrates, where CO2 released from pyruvate decarboxylation is 13C enriched (compared with total organic matter) but relatively 13C depleted from acetyl-CoA metabolism through the TCA cycle (Tcherkez et al., 2003). Under continuous darkness and constant tair, it has been shown that δ13CRd decreases due to a switch in respiratory substrates from carbohydrates to more 13C-depleted substrates such as lipids or proteins (Ghashghaie et al., 2003; Tcherkez et al., 2003).
The similar δ13CRd(24h) in gdch-KD versus WT plants implies that the long-term substrates for the TCA cycle produced in the Gch were 13C isotopically similar. This is further supported by similar leaf δ13Cdm between the gdch-KD and WT plants. Interestingly, δ13CRd(24h) was more depleted than δ13Cdm, in agreement with Tcherkez et al. (2003) who had found that CO2 evolved in the dark by French bean leaves in a condition of carbohydrate starvation had a lower δ13C than total leaf organic matter. This denotes potential changes in dark respiration substrates, such as carbohydrate oxidation producing 13C-enriched CO2 and β-oxidation of fatty acids producing 13C-depleted CO2 when compared with total organic matter.
Conclusions
Under photorespiratory conditions, the gdch-KD plants had altered 13C discrimination fractions in the light, with a lower Δf caused by a reduced f. This change in Δf and the lower Δgm lead to a higher Δo in the gdch-KD plants in comparison with the WT. The lower f in the gdch-KD plants was attributed to a greater α compared with the WT, suggesting the occurrence of alternative photorespiratory reactions in the GDC-impaired plants. In addition, the enhanced Rd in the gdch-KD compared with WT plants after photorespiratory leaf photosynthesis indicated that the photorespiratory disruption led to an additional buildup of metabolites in the light that were decarboxylated by the TCA cycle in the dark. The tendency for a more depleted δ13CRd in the gdch-KD plants compared with the WT after photorespiratory leaf photosynthesis was mainly ascribed to a higher Δo before the light–dark transition and differences in the δ13C of the substrates feeding Rd. These results indicate that an alteration in photorespiratory carbon metabolism can have a significant effect on leaf CO2 exchange and 13CO2 discrimination, both in the light and in the dark.
Supplementary data
Supplementary data are available at JXB online.
Methods S1. Estimate of the 13CO2 composition of the growth chamber atmosphere during the light period.
Methods S2. Additional technical information on the system setup to measure online leaf atmosphere CO2, H2O, and 13CO2 exchange.
Methods S3. Estimate of the 13C signature and total N content in the leaf biomass.
Methods S4. Estimate of the CO2 compensation point in the absence of mitochondrial non-photorespiratory respiration.
Methods S5. Estimate of α, and evaluation of the sensitivity of f to Γ* and α (shown in Fig. S1).
Methods S6. Estimate of the fractional contribution of respiratory substrates from leaf chamber and growth chamber carbon assimilates to the 13C composition of dark-evolved CO2.
Methods S7. Editing of the 13C composition of dark-evolved CO2 for plants grown at an atmospheric 13C composition of −41.6‰.
Fig. S1 Sensitivity of the f parameter to Γ* and α.
Fig. S2. Sensitivity of the f parameter to gm, RL, and e'.
Table S1. Data used to calculate the fractional contributions of leaf chamber and growth chamber carbon assimilates to 13C composition of leaf dark-evolved CO2.
Table S2. Values of 18O-based gm.
Table S3. Statistics for the model used to fit leaf dark respiration rates.
Table S4. Significance for the model used to fit leaf dark respiration rates.
Table S5. Statistics for the model used to fit the 13C composition of leaf dark respiration rates.
Table S6. Significance for the model used to fit 13C composition of leaf dark respiration rates.
Author contributions
SK, SC, H-CL, RAC, WPQ, and JMH generated the transgenic plant material; SvC, RG, RTF, GEE, and ABC planned and designed the experiments; RG performed leaf gas and isotope exchange measurements and analysis; NK performed the biochemical analysis; RG, SvC, RTF, GEE, and ABC interpreted the data; and RG, ABC, and GEE developed the manuscript.
Acknowledgements
Research was funded by a C4 Rice Project grant from The Bill and Melinda Gates Foundation to IRRI (2012–2015) and to the University of Oxford (2015–2019); by the National Science Foundation, grant MCB-1146928; by the National Science Foundation, grant MRI-0923562; and by the Russian Science Foundation, grant 16-16-00089. We thank Charles A. Cody for plant growth management, Dr Raymond W. Lee for 13C analysis on leaf biomass, and Dr Todd Coffey and Dr Marc A. Evans for statistical advice.
References
- Anderson LE. 1971. Chloroplast and cytoplasmic enzymes. II. Pea leaf triose phosphate isomerases. Biochimica et Biophysica Acta 235, 237–244. [DOI] [PubMed] [Google Scholar]
- Arrivault S, Obata T, Szecówka M, Mengin V, Guenther M, Hoehne M, Fernie AR, Stitt M. 2017. Metabolite pools and carbon flow during C4 photosynthesis in maize: 13CO2 labeling kinetics and cell type fractionation. Journal of Experimental Botany 68, 283–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkin OK, Evans JR, Siebke K. 1998. Relationship between the inhibition of leaf respiration by light and enhancement of leaf dark respiration following light treatment. Functional Plant Biology 25, 437–443. [Google Scholar]
- Badger MR. 1985. Photosynthetic oxygen exchange. Annual Review of Plant Physiology 36, 27–53. [Google Scholar]
- Barbour MM, McDowell NG, Tcherkez G, Bickford CP, Hanson DT. 2007. A new measurement technique reveals rapid post-illumination changes in the carbon isotope composition of leaf-respired CO2. Plant, Cell & Environment 30, 469–482. [DOI] [PubMed] [Google Scholar]
- Bauwe H. 2018. Photorespiration—damage repair pathway of the Calvin–Benson cycle. In: Logan DC, ed, Annual Plant Reviews 50. Hoboken, NJ: Wiley-Blackwell, 293–342. [Google Scholar]
- Bauwe H, Hagemann M, Fernie AR. 2010. Photorespiration: players, partners and origin. Trends in Plant Science 15, 330–336. [DOI] [PubMed] [Google Scholar]
- Bauwe H, Kolukisaoglu U. 2003. Genetic manipulation of glycine decarboxylation. Journal of Experimental Botany 54, 1523–1535. [DOI] [PubMed] [Google Scholar]
- Bernacchi CJ, Singsaas EL, Pimentel C, Portis Jr AR, Long SP. 2001. Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant, Cell & Environment 24, 253–259. [Google Scholar]
- Betti M, Bauwe H, Busch FA, et al. 2016. Manipulating photorespiration to increase plant productivity: recent advances and perspectives for crop improvement. Journal of Experimental Botany 67, 2977–2988. [DOI] [PubMed] [Google Scholar]
- Blackwell RD, Murray AJ, Lea PJ, Kendall AC, Hall NP, Turner JC, Wallsgrove RM. 1988. The value of mutants unable to carry out photorespiration. Photosynthesis Research 16, 155–176. [DOI] [PubMed] [Google Scholar]
- Bowling DR, Sargent SD, Tanner BD, Ehleringer JR. 2003. Tunable diode laser absorption spectroscopy for stable isotope studies of ecosystem–atmosphere CO2 exchange. Agricultural and Forest Meteorology 118, 1–19. [Google Scholar]
- Bykova NV, Keerberg O, Pärnik T, Bauwe H, Gardeström P. 2005. Interaction between photorespiration and respiration in transgenic potato plants with antisense reduction in glycine decarboxylase. Planta 222, 130–140. [DOI] [PubMed] [Google Scholar]
- Cornic G. 1973. Etude de l’inhibition de la respiration par la lumière chez la moutarde blanche Sinapis alba L. Physiologie Végétale 11, 663–679. [Google Scholar]
- Cousins AB, Pracharoenwattana I, Zhou W, Smith SM, Badger MR. 2008. Peroxisomal malate dehydrogenase is not essential for photorespiration in Arabidopsis but its absence causes an increase in the stoichiometry of photorespiratory CO2 release. Plant Physiology 148, 786–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins AB, Walker BJ, Pracharoenwattana I, Smith SM, Badger MR. 2011. Peroxisomal hydroxypyruvate reductase is not essential for photorespiration in Arabidopsis but its absence causes an increase in the stoichiometry of photorespiratory CO2 release. Photosynthesis Research 108, 91–100. [DOI] [PubMed] [Google Scholar]
- Douce R, Bourguignon J, Neuburger M, Rébeillé F. 2001. The glycine decarboxylase system: a fascinating complex. Trends in Plant Science 6, 167–176. [DOI] [PubMed] [Google Scholar]
- Edwards GE, Ku MSB, Hatch MD. 1982. Photosynthesis in Panicum milioides, a species with reduced photorespiration. Plant & Cell Physiology 23, 1185–1195. [Google Scholar]
- Evans JR, Sharkey TD, Berry JA, Farquhar GD. 1986. Carbon isotope discrimination measured concurrently with gas exchange to investigate CO2 diffusion in leaves of higher plants. Functional Plant Biology 13, 281–292. [Google Scholar]
- Evans JR, von Caemmerer S. 2013. Temperature response of carbon isotope discrimination and mesophyll conductance in tobacco. Plant, Cell & Environment 36, 745–756. [DOI] [PubMed] [Google Scholar]
- Ewald R, Kolukisaoglu U, Bauwe U, Mikkat S, Bauwe H. 2007. Mitochondrial protein lipoylation does not exclusively depend on the mtKAS pathway of de novo fatty acid synthesis in Arabidopsis. Plant Physiology 145, 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar GD, O’Leary M, Berry JA. 1982. On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Functional Plant Biology 9, 121– 137. [Google Scholar]
- Flexas J, Ribas-Carbó M, Diaz-Espejo A, Galmés J, Medrano H. 2008. Mesophyll conductance to CO2: current knowledge and future prospects. Plant, Cell & Environment 31, 602–621. [DOI] [PubMed] [Google Scholar]
- Gessler A, Roy J, Kayler Z, et al. 2017. Night and day—circadian regulation of night-time dark respiration and light-enhanced dark respiration in plant leaves and canopies. Environonmental Experimental Botany 137, 14–25. [Google Scholar]
- Gessler A, Tcherkez G, Karyanto O, Keitel C, Ferrio JP, Ghashghaie J, Kreuzwieser J, Farquhar GD. 2009. On the metabolic origin of the carbon isotope composition of CO2 evolved from darkened light-acclimated leaves in Ricinus communis. New Phytologist 181, 374–386. [DOI] [PubMed] [Google Scholar]
- Ghashghaie J, Badeck F-W, Lanigan G, Nogués S, Tcherkez G, Deléens E, Cornic G, Griffiths H. 2003. Carbon isotope fractionation during dark respiration and photorespiration in C3 plants. Phytochemistry Reviews 2, 145–161. [Google Scholar]
- Gillon JS, Griffiths H. 1997. The influence of (photo) respiration on carbon isotope discrimination in plants. Plant, Cell & Environment 20, 1217–1230. [Google Scholar]
- Giuliani R, Koteyeva N, Voznesenskaya E, Evans MA, Cousins AB, Edwards GE. 2013. Coordination of leaf photosynthesis, transpiration, and structural traits in rice and wild relatives (genus Oryza). Plant Physiology 162, 1632–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch MD. 1979. Mechanism of C4 photosynthesis in Chloris gayana: pool sizes and kinetics of 14CO2 incorporation into 4-carbon and 3-carbon intermediates. Archives of Biochemistry and Biophysics 194, 117–127. [DOI] [PubMed] [Google Scholar]
- Igamberdiev AU, Bykova NV, Lea PJ, Gardeström P. 2001. The role of photorespiration in redox and energy balance of photosynthetic plant cells: a study with a barley mutant deficient in glycine decarboxylase. Physiologia Plantarum 111, 427–438. [DOI] [PubMed] [Google Scholar]
- Igamberdiev AU, Mikkelsen T, Ambus P, Bauwe H, Lea PJ, Gardeström P. 2004. Photorespiration contributes to stomatal regulation and carbon isotope fractionation: a study with barley, potato and Arabidopsis plants deficient in glycine decarboxylase. Photosynthesis Research 81, 139–152. [Google Scholar]
- Jordan DB, Ogren WL. 1984. The CO2/O2 specificity of ribulose 1,5-bisphosphate carboxylase/oxygenase: dependence on ribulosebisphosphate concentration, pH and temperature. Planta 161, 308–313. [DOI] [PubMed] [Google Scholar]
- Kebeish R, Niessen M, Thiruveedhi K, Bari R, Hirsch HJ, Rosenkranz R, Stäbler N, Schönfeld B, Kreuzaler F, Peterhänsel C. 2007. Chloroplastic photorespiratory bypass increases photosynthesis and biomass production in Arabidopsis thaliana. Nature Biotechnology 25, 593–599. [DOI] [PubMed] [Google Scholar]
- Kelly GJ, Latzko E. 1976. Inhibition of spinach-leaf phosphofructokinase by 2-phosphoglycollate. FEBS Letters 68, 55–58. [DOI] [PubMed] [Google Scholar]
- Kolbe AR, Cousins AB. 2018. Mesophyll conductance in Zea mays responds transiently to CO2 availability: implications for transpiration efficiency in C4 crops. New Phytologist 217, 1463–1474. [DOI] [PubMed] [Google Scholar]
- Koteyeva NK, Voznesenskaya EV, Edwards GE. 2015. An assessment of the capacity for phosphoenolpyruvate carboxykinase to contribute to C4 photosynthesis. Plant Science 235, 70–80. [DOI] [PubMed] [Google Scholar]
- Lanigan GJ, Betson N, Griffiths H, Seibt U. 2008. Carbon isotope fractionation during photorespiration and carboxylation in Senecio. Plant Physiology 148, 2013–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann MM, Rinne KT, Blessing C, Siegwolf RT, Buchmann N, Werner RA. 2015. Malate as a key carbon source of leaf dark-respired CO2 across different environmental conditions in potato plants. Journal of Experimental Botany 66, 5769–5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann MM, Wegener F, Barthel M, Maurino VG, Siegwolf RT, Buchmann N, Werner C, Werner RA. 2016. Metabolic fate of the carboxyl groups of malate and pyruvate and their influence on δ13C of leaf-respired CO2 during light enhanced dark respiration. Frontiers in Plant Science 7, 739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Karki S, Coe RA, et al. 2016. Targeted knockdown of GDCH in rice leads to a photorespiratory-deficient phenotype useful as a building block for C4 rice. Plant & Cell Physiology 57, 919–932. [DOI] [PubMed] [Google Scholar]
- Maurino VG, Peterhansel C. 2010. Photorespiration: current status and approaches for metabolic engineering. Current Opinion in Plant Biology 13, 249–256. [DOI] [PubMed] [Google Scholar]
- Niessen M, Thiruveedhi K, Rosenkranz R, Kebeish R, Hirsch HJ, Kreuzaler F, Peterhänsel C. 2007. Mitochondrial glycolate oxidation contributes to photorespiration in higher plants. Journal of Experimental Botany 58, 2709–2715. [DOI] [PubMed] [Google Scholar]
- Nogués S, Tcherkez G, Cornic G, Ghashghaie J. 2004. Respiratory carbon metabolism following illumination in intact French bean leaves using 13C/12C isotope labeling. Plant Physiology 136, 3245–3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary MH. 1988. Carbon isotopes in photosynthesis. Bioscience 38, 328–336. [Google Scholar]
- Parys E, Jastrzębski H. 2008. Mitochondria from leaf mesophyll cells of C4 plants are deficient in the H protein of glycine decarboxylase complex. Journal of Plant Physiology 165, 1061–1069. [DOI] [PubMed] [Google Scholar]
- Peterhansel C, Blume C, Offermann S. 2013a Photorespiratory bypasses: how can they work? Journal of Experimental Botany 64, 709–715. [DOI] [PubMed] [Google Scholar]
- Peterhansel C, Horst I, Niessen M, Blume C, Kebeish R, Kürkcüoglu S, Kreuzaler F. 2010. Photorespiration. The Arabidopsis Book 8, e0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterhansel C, Krause K, Braun HP, Espie GS, Fernie AR, Hanson DT, Keech O, Maurino VG, Mielewczik M, Sage RF. 2013b Engineering photorespiration: current state and future possibilities. Plant Biology 15, 754–758. [DOI] [PubMed] [Google Scholar]
- Priault P, Wegener F, Werner C. 2009. Pronounced differences in diurnal variation of carbon isotope composition of leaf respired CO2 among functional groups. New Phytologist 181, 400–412. [DOI] [PubMed] [Google Scholar]
- Rademacher T, Häusler RE, Hirsch HJ, Zhang L, Lipka V, Weier D, Kreuzaler F, Peterhänsel C. 2002. An engineered phosphoenolpyruvate carboxylase redirects carbon and nitrogen flow in transgenic potato plants. The Plant Journal 32, 25–39. [DOI] [PubMed] [Google Scholar]
- Rooney MA. 1988. Short-term carbon isotopic fractionation in plants. PhD thesis, University of Wisconsin, Madison, WI, USA. [Google Scholar]
- Sharkey TD, Berry JA, Raschke K. 1985. Starch and sucrose synthesis in Phaseolus vulgaris as affected by light, CO2, and abscisic acid. Plant Physiology 77, 617–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville CR. 2001. An early Arabidopsis demonstration. Resolving a few issues concerning photorespiration. Plant Physiology 125, 20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville CR, Ogren WL. 1982. Genetic modification of photorespiration. Trends in Biochemical Sciences 7, 171–174. [Google Scholar]
- Sonawane BV, Cousins AB. 2019. Uncertainties and limitations of using carbon‐13 and oxygen‐18 leaf isotope exchange to estimate the temperature response of mesophyll CO2 conductance in C3 plants. New Phytologist 222, 122–131. [DOI] [PubMed] [Google Scholar]
- Stutz SS, Edwards GE, Cousins AB. 2014. Single-cell C4 photosynthesis: efficiency and acclimation of Bienertia sinuspersici to growth under low light. New Phytologist 202, 220–232. [DOI] [PubMed] [Google Scholar]
- Sun W, Ubierna N, Ma JY, Walker BJ, Kramer DM, Cousins AB. 2014. The coordination of C4 photosynthesis and the CO2-concentrating mechanism in maize and Miscanthus × giganteus in response to transient changes in light quality. Plant Physiology 164, 1283–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazoe Y, von Caemmerer S, Estavillo GM, Evans JR. 2011. Using tunable diode laser spectroscopy to measure carbon isotope discrimination and mesophyll conductance to CO₂ diffusion dynamically at different CO2 concentrations. Plant, Cell & Environment 34, 580–591. [DOI] [PubMed] [Google Scholar]
- Tcherkez G. 2006. How large is the carbon isotope fractionation of the photorespiratory enzyme glycine decarboxylase? Functional Plant Biology 33, 911–920. [DOI] [PubMed] [Google Scholar]
- Tcherkez G, Bligny R, Gout E, Mahe´ A, Hodges M, Cornic G. 2008. Respiratory metabolism of illuminated leaves depends on CO2 and O2 conditions. Proceedings of the National Academy of Sciences, USA 105, 797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tcherkez G, Boex-Fontvieille E, Mahé A, Hodges M. 2012. Respiratory carbon fluxes in leaves. Current Opinion in Plant Biology 15, 308–314. [DOI] [PubMed] [Google Scholar]
- Tcherkez G, Cornic G, Bligny R, Gout E, Ghashghaie J. 2005. In vivo respiratory metabolism of illuminated leaves. Plant Physiology 138, 1596–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tcherkez G, Farquhar GD, Badeck F, Ghashghaie J. 2004. Theoretical considerations about carbon isotope distribution in glucose of C3 plants. Functional Plant Biology 31, 857–877. [DOI] [PubMed] [Google Scholar]
- Tcherkez G, Nogués S, Bleton J, Cornic G, Badeck F, Ghashghaie J. 2003. Metabolic origin of carbon isotope composition of leaf dark-respired CO2 in French bean. Plant Physiology 131, 237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tcherkez G, Schäufele R, Nogués S, et al. 2010. On the 13C/12C isotopic signal of day and night respiration at the mesocosm level. Plant, Cell & Environment 33, 900–913. [DOI] [PubMed] [Google Scholar]
- Timm S, Bauwe H. 2013. The variety of photorespiratory phenotypes—employing the current status for future research directions on photorespiration. Plant Biology 15, 737–747. [DOI] [PubMed] [Google Scholar]
- Timm S, Florian A, Arrivault S, Stitt M, Fernie AR, Bauwe H. 2012. Glycine decarboxylase controls photosynthesis and plant growth. FEBS Letters 586, 3692–3697. [DOI] [PubMed] [Google Scholar]
- Timm S, Giese J, Engel N, Wittmiß M, Florian A, Fernie AR, Bauwe H. 2018. T-protein is present in large excess over the other proteins of the glycine cleavage system in leaves of Arabidopsis. Planta 247, 41–51. [DOI] [PubMed] [Google Scholar]
- Timm S, Nunes-Nesi A, Pärnik T, Morgenthal K, Wienkoop S, Keerberg O, Weckwerth W, Kleczkowski LA, Fernie AR, Bauwe H. 2008. A cytosolic pathway for the conversion of hydroxypyruvate to glycerate during photorespiration in Arabidopsis. The Plant Cell 20, 2848–2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubierna N, Farquhar GD. 2014. Advances in measurements and models of photosynthetic carbon isotope discrimination in C3 plants. Plant, Cell & Environment 37, 1494–1498. [DOI] [PubMed] [Google Scholar]
- Ubierna N, Gandin A, Boyd RA, Cousins AB. 2017. Temperature response of mesophyll conductance in three C4 species calculated with two methods: 18O discrimination and in vitro Vpmax. New Phytologist 214, 66–80. [DOI] [PubMed] [Google Scholar]
- Ubierna N, Sun W, Cousins AB. 2011. The efficiency of C4 photosynthesis under low light conditions: assumptions and calculations with CO2 isotope discrimination. Journal of Experimental Botany 62, 3119–3134. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S. 2000. Biochemical models of leaf photosynthesis. No. 2. Collingwood, Australia: CSIRO Publishing. [Google Scholar]
- von Caemmerer S, Evans JR. 1991. Determination of the average partial pressure of CO2 in chloroplasts from leaves of severa1 C3 plants. Australian Journal of Plant Physiology 18, 287–305. [Google Scholar]
- von Caemmerer S, Ghannoum O, Pengelly JJ, Cousins AB. 2014. Carbon isotope discrimination as a tool to explore C4 photosynthesis. Journal of Experimental Botany 65, 3459–3470. [DOI] [PubMed] [Google Scholar]
- Walker BJ, Cousins AB. 2013. Influence of temperature on measurements of the CO2 compensation point: differences between the Laisk and O2-exchange methods. Journal of Experimental Botany 64, 1893–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BJ, VanLoocke A, Bernacchi CJ, Ort DR. 2016. The costs of photorespiration to food production now and in the future. Annual Review of Plant Biology 67, 107–129. [DOI] [PubMed] [Google Scholar]
- Werner C, Gessler A. 2011. Diel variations in the carbon isotope composition of respired CO2 and associated carbon sources: a review of dynamics and mechanisms. Biogeosciences 8, 2437–2459. [Google Scholar]
- Werner C, Wegener F, Unger S, Nogués S, Priault P. 2009. Short-term dynamics of isotopic composition of leaf-respired CO2 upon darkening: measurements and implications. Rapid Communications in Mass Spectrometry 23, 2428–2438. [DOI] [PubMed] [Google Scholar]
- Wingler A, Lea PJ, Leegood RC. 1997. Control of photosynthesis in barley plants with reduced activities of glycine decarboxylase. Planta 202, 171–178. [Google Scholar]
- Wingler A, Lea PJ, Leegood RC. 1999. Photorespiratory metabolism of glyoxylate and formate in glycine-accumulating mutants of barley and Amaranthus edulis. Planta 207, 518–526. [Google Scholar]
- Wingler A, Lea PJ, Quick WP, Leegood RC. 2000. Photorespiration: metabolic pathways and their role in stress protection. Philosophical Transactions of the Royal Society B: Biological Sciences 355, 1517–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.