Abstract
Maize is a cold-sensitive species, but selective breeding programs have recently succeeded in producing plants strikingly well adapted to the cold springs of a temperate climate, showing the potential for improved cold tolerance. The aim of the present study was to determine whether the adaptation of some inbred lines to spring chills is due to their increased true cold tolerance or whether it only represents an avoidance mechanism, which was the sole mode of adaptation during early stages of agricultural dispersal of maize towards higher latitudes. By characterizing numerous physiological features of several lines of different cold sensitivity, we show that a combination of both avoidance and tolerance is involved. A novel avoidance mechanism was found that favored unhindered development of the photosynthetic apparatus through protection of the shoot apex below soil level due to a shortened mesocotyl. It seems to be mediated by increased seedling photosensitivity at early growth stages. True tolerance involved improved protection of the cell membrane against cold injury at temperatures close to 0 °C and stimulation of light-induced processes (accumulation of anthocyanins, carotenoids, and chlorophyll, proper development of chloroplasts) at temperatures in the range of 10–14 °C, likely also related to the increased photosensitivity and mediated by gibberellin signaling.
Keywords: Avoidance, chloroplasts, early growth, gibberellins, light-induced processes, mesocotyl, photosynthetic apparatus, shoot apex, tolerance, Zea mays
High photosensitivity of seedlings of some modern maize inbred lines keeps the growing shoot below soil level and protects it against cold injury allowing for unhindered development at low temperatures.
Introduction
Among the many thermophilic plants introduced into temperate climates, maize appears to be particularly successful. It is now a high yield staple crop even in high latitude regions. The initial maize adaptation to the temperate climate was achieved by shortening of the growth cycle allowing avoidance of the harmful effects of cold at the start of vegetation, which is a major concern in spring in those regions. The consequence was, however, limited yield potential. Thus, any further progress in the adaptation of maize to a temperate climate requires an improved early growth under cold conditions that would allow early sowing to prolong the vegetation season (Frascaroli and Revilla, 2018).
The early stages of maize growth comprise diverse processes potentially susceptible to cold: emergence, juvenile leaf development, and the formation of the photosynthetic apparatus allowing a shift from heterotrophic to autotrophic growth, i.e. the VE–V2 growth stage (http://www.channel.com/agronomics/Documents/AgronomicContentPDF/GrowthStages%20GuidesChannel.pdf). Until the present, in most studies on this subject, cold sensitivity was evaluated either at emergence, i.e. the VE growth stage (Di Frenza et al., 2017), or for the juvenile, but already autotrophic, leaves, i.e. the V3–V5 growth stages (Szalai et al., 2018), while the intermediate stages were largely ignored, even though they are considered highly sensitive to cold stress (Leipner and Stamp, 2009; Frascaroli and Revilla, 2018). That neglect was not without justification, since the first and second leaves are usually small, short-lived, and depend exclusively on kernel resources, and therefore appear not to be particularly important for the further fate of the plant. On the other hand, subjecting maize at this stage to ca 13 °C can have dramatic consequences, since, in extremis, seedlings have been observed to die having fully exhausted the seed reserves (Hardacre and Eagles, 1980; Sowiński et al., 2005). Additionally, since the mature embryo contains five or six leaf primordia (Scheridan and Clark, 1994) and successive phytomers are produced soon after germination, a cold spell at this stage could compromise the just-initiated developmental processes thereby affecting the duration of further vegetative growth as well as the date of flowering (Sowiński et al., 2005).
Emergence is possible even below 13 °C in maize, but the photosynthetic apparatus cannot form below 15 °C in some maize plants (Hardacre and Eagles, 1980), which underscores the role of photosynthesis in maize cold sensitivity at early growth stages. Indeed, in seedlings developed at low temperatures, disturbances of chloroplast ultrastructure appear that cannot be repaired after the onset of favorable conditions (Nie et al., 1995). Apart from the formation of the photosynthetic apparatus, also cell divisions, elongation, and differentiation are crucial for early growth, but how these processes are related to the cold-inhibition of this stage is unknown. However, numerous isolated observations addressing this question at somewhat later stages have been published. Shoot meristem temperature was found to determine the leaf elongation rate (Barlow et al., 1977; Giauffret et al., 1995; Ben-Haj-Salah and Tardieu, 1995; Tardieu, 2003) and the number of leaves also depended on temperature (Tollenar and Hunter, 1983; Warrington and Kanemasu, 1983; Giauffret et al., 1995). At the V3–V5 growth stage, low temperature affected cell divisions (Ben-Haj-Salah and Tardieu, 1995), and cold nights enhanced expression of cell cycle inhibitors and caused down-regulation of positive regulators of cell division (Rymen et al., 2007). Whether the effect is similar at the earlier V1 stage is unknown.
Despite the well-documented general cold sensitivity of maize, recent data have demonstrated substantial progress in the adaptation of modern inbred lines to a temperate climate (Li et al., 2016; Sobkowiak et al., 2016). Some such lines demonstrate perfect early vigor even when grown for a long period at low temperatures or exposed to severe cold spells (Sobkowiak et al., 2016). Other studies found freezing to inflict almost no damage on leaves of some maize inbred lines (Li et al., 2016), while earlier reports evidenced serious leaf lesions at temperatures below 0 °C (Greaves, 1996). This diversity suggests that decades of artificial selection have improved maize cold tolerance at early growth stages. Studying the response of the new plants to low temperature should provide crucial information on the mechanism(s) of maize cold tolerance and its potential to eventually attain cold resistance.
In view of the apparent contradiction between the tenor of the rich literature on maize cold sensitivity on the one hand and the recent progress in maize breeding for temperate climates on the other, we decided to look again at the problem at the physiological level by studying modern maize inbred lines that demonstrate perfect early vigor under cold. A priori, two main mechanisms of this adaptation should be considered, tolerance and avoidance (terms after Levitt, 1980). Tolerance refers to processes that allow an organism to withstand a stress, while avoidance is the use of a barrier of any kind (physical, chemical, etc.) against the stressor. As regards early growth, cold tolerance could involve several processes reported earlier to be affected by cold in maize. They include cell membrane functioning, reported to be susceptible to injury by cold (Janowiak and Markowski, 1994); the rate of early growth, taking separately the emergence and further shoot growth since the cold sensitivity of these two phases seems to be determined by different mechanisms (Hodges et al., 1997); and the development of the photosynthetic apparatus, including chloroplast ultrastructure, electron transport efficiency, and biosynthesis of chlorophyll, anthocyanins and carotenoids (for review see Greaves, 1996; Foyer et al., 2002; Farooq et al., 2009; Leipner and Stamp, 2009; Frascaroli and Revilla, 2018).
With respect to the avoidance mechanism, the most important trait is earliness of the material, i.e. shortening of the period between sowing and flowering and further kernel production. This mechanism allows late sowing that limits the risk of cold affecting early growth stages. Protection of plants against cold could also be achieved by increased production of epicuticular waxes (Vigh et al., 1981) and by assuming a xeromorphic body shape (Verheul et al., 1996), both likely preventing water loss and secondary water stress under cold conditions (Strigens et al., 2013). Recently, a weaker photomorphogenic response has been suggested as another factor possibly involved in the adaptation of modern maize to a temperate climate (Markelz et al., 2003). Such a feature would improve shading avoidance at the lower insolation of higher latitudes. At face value, it has little to do with the seedling behavior at low temperatures. However, the photomorphogenic response also involves the development of the photosynthetic apparatus, which is one of the key targets of cold in maize. One may thus hypothesize that the photomorphogenic response and the response to cold are inter-related.
The aim of the present study was to determine whether the advantage of the inbred lines well adapted to cold springs in a temperate climate over those not fully adapted reflects their increased true cold tolerance or whether it still is due exclusively to avoidance.
We investigated the early development (up to the V2 stage) of several maize inbred lines of strongly diversified cold sensitivity at low temperature. A diversified set of physiological approaches was used with the development of the photosynthetic apparatus as the main focus. A combination of both avoidance and tolerance mechanisms related to increased photosensitivity was found to be involved in the improved cold tolerance of modern maize inbred lines.
Material and methods
Plant material
Five inbred lines of dent-type maize (Zea mays ssp. indentata) were used in the project: B73 (Stiff Stalk Synthetic), S160 (Canadian Gene Pool), S50676 (Stiff Stalk Synthetic/Iodent), S68911 (Stiff Stalk Synthetic/Iodent), and S84854 (Iodent). The S68911, S84854, S50676, and S160 inbred lines are early material produced by the Plant Breeding Smolice Co., Poland. These lines (except S84854) have been used in earlier projects of our group (Sobkowiak et al., 2016), including re-sequencing of their genomes (deposited at iPlant Collaborative). Field data on the early vigor of these inbred lines determined at the Smolice location (western Poland) in 2013–2017 are shown in Supplementary Fig. S1 at JXB online together with relevant field temperature data. The B73 inbred line, widely used in breeding programs in the USA, is a late inbred line (Giauffret et al., 1995) showing an arrest of growth in the cold (Riva-Roveda et al., 2016) that poorly adapted to the temperate climate (our unpublished field observations). B73 is considered intermediately cold tolerant (Rodríguez et al., 2013) or even cold sensitive (Joets et al., 2018).
Growth conditions
Kernels were sown in the soil at a depth of 4 cm, unless indicated otherwise, and germinated in a custom-made growth chamber under a 14 h/10 h photoperiod, light irradiance of 600 µmol quanta m−2 s−1 (MASTER GreenPower 600 W 400 V E40, Philips), air humidity 70% RH and temperature conditions depending on the experimental variant (see further). Further growth of seedlings was carried out in the same conditions. For control and cold-subjected plants, the temperature regimes were 24 °C/20 °C and 14 °C/10 °C, respectively. Plants were grown in PVC tubes of 50 cm height and 11 cm diameter, nine plants per tube, five tubes per inbred line. To prevent the soil warming up under the strong light source, the tubes were nearly fully immersed in an externally cooled water bath (2.0×0.6 m) at 20 or 10 °C for control and cold-grown plants, respectively. Soil temperature was monitored permanently with the use of six sensors connected to a data recorder. The tubes were located throughout the bath in a completely randomized design, and to ensure uniform conditions throughout the experiment, their positions were rotated randomly every 2 d.
Membrane injury
Membrane injury was estimated by determining electrolyte leakage from plant tissue before and after cold treatment. Plants were germinated in the dark at 24 °C for a week and then transferred to a dark cold chamber at −2, +2, or +8 °C for 24, 48, or 72 h. At time points indicated, 2-cm-long fragments of mesocotyl were collected (three seedlings per sample), rinsed in distilled water, and transferred to glass tubes with 10 ml of deionized water. The tubes were shaken for 2.5 h at room temperature. Electro-conductivity of water in each tube was measured before sample transfer, after the 2.5 h bath, and after boiling of the material. Electrolyte leakage was calculated as the percentage of total electrolyte released by boiling. Three independent, sequential biological experiments were conducted with up to 10 samples per variant (line, temperature, time).
Plant early growth and biometrics
The rate of early development was determined by daily estimation of the percentage of plants entering growth stages VE (germination) and at V1 (full development of the first leaf). For finer timing of the early growth, the beginning of the development of the first, second, third, and fourth leaves marked by the appearance of leaf tips was determined. For biometric analysis, leaf area, shoot length, and mesocotyl length were measured at V1 stage. Five independent, sequential experiments were conducted, with 60 seedlings per inbred line per experiment.
Photosynthetic apparatus characteristics
Photosynthetic apparatus efficiency
Chlorophyll fluorescence was determined in the middle region of the first leaf blade. Measurements were performed with a Chlorophyll Fluorometer (PAM 200, H. Waltz, Germany) at 24 °C between 3 and 6 h after the light had been switched on in the growth chamber. Maximal quantum yield of PS II electron transport, Fv/Fm (ratio of variable to maximum efficiency), was estimated with plants kept in darkness for 30 minutes. Effective quantum yield of PS II electron transport, ΦPSII (where ΦPSII=(Fm′−F)/Fm′, where Fm′ is maximal fluorescence yield of the illuminated sample with all PS II centers closed and F is fluorescence yield measured briefly before application of a saturation pulse; Klughammer and Schreiber, 2008), was estimated using induction curves with quenching analysis at a 10 ms/pulse sampling rate and actinic light similar to that used in the growth chamber. Measurements were repeated in three independent, sequential experiments, each with six to nine plants per inbred line per temperature regime.
Pigment content
Chlorophyll content analysis was performed according to Lichtenthaler and Buschmann, (2001). In brief, 50 mg of fresh tissue from the middle part of the first leaf was extracted in 80% pre-cooled acetone and absorbance at 646.6 and 663.6 nm was measured on a Shimadzu UV-160A spectrophotometer. Carotenoid content (ibid.) was determined in the same extract as absorbance at 470 nm. Anthocyanin was measured according to Mori and Sakurai (1995) in methanol–1% HCl extract as absorbance at 525 nm. Independent, sequential experiments were repeated three times, with five samples per experiment.
Chloroplast ultrastructure
The middle part of the first leaf was collected for transition electron microscopy. Two 2-mm2 pieces were cut from each leaf and placed in a fixative (50 mM cacodylate buffer, pH 7.0, 2% (v/v) glutaraldehyde) for 2 h with a fixative change after 1 h. Next, the tissue was postfixed in 1% osmium tetroxide for 2 h. After washing in fresh cacodylate buffer and water, the material was dehydrated in ethanol graded series, embedded in Spurr’s resin according to the manufacturer, cut into ultrathin sections of 80 nm with a Leica ultramicrotome, immobilized on copper slot grids and contrasted in 2% aqueous uranyl acetate. The samples were analysed with a JEM-1400 (JEOL, Japan) electron microscope at 80 kV at the Laboratory of Electron Microscopy, Nencki Institute of Experimental Biology, Warsaw, and photographed with a high resolution CCD MORADA (SiS-Olympus, Germany) digital camera. Samples were taken from three different plants per inbred line per each of two independent, sequential experiments.
Effect of sowing depth and soil temperature on photosynthetic apparatus efficiency
Kernels were sown at a depth of 0.5 or 4 cm and were germinated and then grown until V1 stage at three different soil temperatures measured (±0.5 °C) in the top soil layer (4 cm): 23, 24, or 25 °C for control plants and 13, 14, or 15 °C for cold-treated plants. Air temperature was 24 °C/22 °C or 14 °C/10 °C, respectively. The same tank was used to control temperature of soil-containing tubes as before (see ‘Growth conditions’) but the cooling water was provided from one side only and the tank water was not mixed. As a result, a stable temperature gradient formed along the tank. Tubes were grouped in three sections of different temperature, two tubes per line in each section, in a completely random design. Within each section tube positions were rotated daily. Basic chlorophyll fluorescence parameters (Fv/Fm and ΦPSII) were then determined as above. Independent, sequential experiments were repeated twice, with nine plants per experimental variant (line, sowing depth, soil temperature).
Seedling photosensitivity
Effect of light on mesocotyl elongation
Photosensitivity of seedlings was assessed by measuring mesocotyl length of seedlings grown under a 14 h/10 h photoperiod with weak white light (LED, NS1, Valoya, Finland) or under LED lamps (Valoya) of different colors: blue (450 nm), red (660 nm), or far red (730 nm), at 24 °C/22 °C or 14 °C/10 °C. Light fluence for all light sources was in the range of 2–5 µmol quanta m−2 s−1. As a control, seedlings grown in darkness were used. Kernels were sown on wet paper, 20 per inbred line. Independent, sequential experiments were repeated three times.
Expression of photoreceptor genes
Expression of maize orthologs of Arabidopsis genes PHYA1, PHYA2, PHYB1, PHYB2, PHYC1, PHYC2, PHOT1, PHOT2, and CRY2 was determined in the coleoptile of etiolated 7-day-old seedlings by RT-qPCR with primers designed as described earlier (Sobkowiak et al., 2016; see Supplementary Table S1). Seedlings were grown under constant darkness and thermoperiod of 24 °C/22 °C, and were collected around noon. Expression was calculated against ubiquitin and actin transcript levels. Independent, sequential experiments were repeated three times, with three samples (five coleoptiles each) per experiment and three technical repeats per sample.
Response to gibberellins
Kernels were germinated and seedlings grown as described in ‘Growth conditions’ but with addition of gibberellin. To avoid gibberellin dilution in the soil, seedlings were grown individually in 16 mm-diameter, 4.5 cm-long open tubes filled with soil. GA3 solution (10−5 M) was applied below the soil surface directly above the kernel (2 ml per seedling twice a day starting 48 h after sowing). As a control, water was used instead of the gibberellin solution. Mesocotyl length was determined at VE stage. Independent, sequential experiments were repeated six times, with nine seedlings per line per experiment.
Additionally, the gibberellin sensitivity was estimated for seedlings grown on filter paper under weak white light (as above at optimal temperature) and sprayed with gibberellin solution (GA3, 10−5 M), or water as a control, once a day, starting 48 h after sowing. Plants grown in darkness and sprayed with gibberellin or water were used as a reference. Fifteen seedlings were used per experiment per line.
Cold-response of an extended set of maize inbred lines
Eighteen inbred lines representing different heterotic groups (Table 1; 15 of them were kindly supplied by Mr Mark J. Millard, USDA, ARS, Iowa State University, Regional Plant Introduction Station) were examined for a correlation between their cold sensitivity, mesocotyl length and photosensitivity. For comparison, three lines studied in the main study (B73, S160, and S68911) were also included. Plants were grown as described in ‘Growth conditions’. The basic chlorophyll fluorescence parameters, Fv/Fm and ΦPSII, of the plants developed under cold treatment were used as a measure of the cold sensitivity. The measurements were performed as before (‘Photosynthetic apparatus characteristics’). In the same experiment, mesocotyl length was determined for cold-treated plants as before (‘Plant early growth and biometrics’). Photosensitivity (ratio of mesocotyl length of VE plants germinated under weak white light to that of dark-germinated ones) was determined for plants grown under optimal temperature as before (‘Seedling photosensitivity’). Three independent, sequential experiments for control plants and four for cold-treated ones, each with four to six plants per line per experiment, were run.
Table 1.
Extended set of 21 maze inbred lines used for correlation study
| Inbred line | Subpopulation | Origin | |
|---|---|---|---|
| Dent | |||
| 1. | B73 | SS | CCB |
| 2. | LH74 | SS | Holden’s Foundation Seeds |
| 3. | S03198 | SS/Iodent | Plant Breeding Smolice Co. Ltd |
| 4. | S68911 | Iodent/SS | Plant Breeding Smolice Co. Ltd |
| 5. | PH207 | Iodent | Pioneer Hi-Bred International Inc. |
| 6. | KY21 | NSS | KY |
| 7. | M162W | NSS | NATAL |
| 8. | MBPM | NSS | PVP |
| 9. | MBUB | NSS | DeKalb Plant Genetics |
| 10. | Oh43 | NSS | CCB |
| 11. | W22 | NSS | CCB |
| 12. | CML333 | TS | CIMMYT |
| 13. | Mo18W | TS | CBB |
| 14. | M37W | MIX | SoAfr |
| 15. | Oh7 | MIX | CCB |
| 16. | A188 | MINN | NCB |
| 17. | Mo17 | Lancaster | CCB |
| 18. | S160 | Canadian Gene Pool | Plant Breeding Smolice Co. Ltd |
| Flint | |||
| 19. | F2 | F2 | OP Lacaune |
| 20. | S018693 | Flint/BSS | Plant Breeding Smolice Co. Ltd |
| 21. | S61328 | F2/EP1 | Plant Breeding Smolice Co. Ltd |
Subpopulations after Flint-Garcia et al. (2005). MIX, mixed; NSS, Non Stiff Stalk Synthetic; SS, Stiff Stalk Synthetic; TS, tropical/subtropical. The three lines used in other experiments are shown in bold.
Statistical analysis
All analyses were performed in R 3.3.2 (R Core Team, 2016). Data were compared by non-orthogonal analysis of variance (ANOVA results are shown in Table S2) and differences were considered significant for the F-ratio probability <0.05. Statistical analysis for electrolyte leakage proportion data was performed with the use of Beta Regression with the betareg function (Cribari-Neto and Zeileis, 2010).
To find significant factor(s) determining expression of a trait, a regression tree with P set at 0.05 was used. The trees were calculated with the ctree function from the partykit package (Hothorn and Zeileis, 2015). For the electrolyte leakage and gibberellin experiments the post hoc analysis was performed with the use of LSD.test in the agricolae package with false discovery rate (FDR) correction at P<0.05 (de Mendiburu, 2016).
A heatmap representing relative transcript levels was created with the heatmap.2 function in the gplots package (Warnes et al., 2016).
Correlation coefficients were calculated in Microsoft Excel. Significance level of correlation coefficients was taken from the public-access Correlation Coefficient Table (https://www.statisticssolutions.com/table-of-critical-values-pearson-correlation/).
Results
The inbred lines studied in this report were chosen so as to represent different levels of fitness under cold spring conditions as estimated by early vigor in the field (see Supplementary Fig. S1). Early vigor was determined at the stage of the fifth leaf in selective years, when the minimal temperatures near the soil surface dropped to or even below 0 °C in mid-spring (third decade of April/first decade of May, Supplementary Fig. S1). Among the four locally produced inbred lines studied, three (S50676, S68911, and S84854) showed almost perfect and one (S160) poor early vigor under cold spring conditions. The fifth line, B73, has not been evaluated for early vigor, but is considered to be intermediately cold tolerant (Rodríguez et al., 2013) or cold sensitive (Joets et al., 2018). For an additional measure of the cold sensitivity of the lines, their susceptibility to cold injury was determined by quantifying electrolyte leakage from the mesocotyl at around 0 °C (Table 2). Little membrane injury was found for S84854 and very strong injury for S160, while B73, S50676, and S68911 showed intermediate levels of electrolyte leakage. No tissue injury was found at 8 °C (Table 2) or higher temperatures (data not shown) in any of these inbred lines.
Table 2.
Cell membrane injury in maize seedlings at low temperatures
| Electrolyte released (%) | |||||
|---|---|---|---|---|---|
| Inbred line | Temperature (°C) | 0 h | 24 h | 48 h | 72 h |
| B73 | −2 | 4.80±0.4 a |
17.88±1.27 de |
26.00±1.78 e |
49.25±2.44 e |
| +2 | 5.86±0.31 ab |
8.21±0.93 ab |
17.95±1.35 d |
27.13±1.72 c |
|
| +8 | 4.97±0.67 ab |
5.91±1.25 ab |
6.31±1.51 a |
5.04±1.34 a |
|
| S160 | −2 | 7.4±0.49 bcd |
29.18±1.81 f |
58.86±2.26 h |
61.35±2.49 f |
| +2 | 8.11±0.36 cd |
16.59±1.42 cde |
37.17±1.93 fg |
70.63±1.86 f |
|
| +8 | 8.31±0.85 bcd |
10.54±1.80 abcd |
11.96±2.32 abcd |
9.41±2.09 ab |
|
| S50676 | −2 | 5.98±0.44 ab |
11.38±1.06 bc |
28.64±19.42 ef |
43.09±2.42 de |
| +2 | 7.43±0.35 bcd |
7.97±0.9 ab |
9.30±1.00 ab |
13.09±1.21 b |
|
| +8 | 5.27±0.61 ab |
8.39±1.52 ab |
8.54±1.94 ab |
8.34±1.96 ab |
|
| S68911 | −2 | 5.79±0.41 ab |
21.00±1.48 e |
42.53±2.20 g |
48.99±2.44 e |
| +2 | 8.11±0.37 d |
8.22±0.85 ab |
16.67±1.34 cd |
28.41±1.58 c |
|
| +8 | 5.32±0.69 ab |
10.10±0.91 abcd |
8.41±1.93 ab |
7.33±1.82 ab |
|
| S84854 | −2 | 6.30±0.43 abcd |
11.37±1.1 bc |
32.25±1.82 ef |
33.59±2.21 cd |
| +2 | 6.46±0.32 abc |
5.83±0.75 a |
12.77±1.15 bcd |
13.64±12.23 b |
|
| +8 | 5.35±0.68 ab |
8.89±1.98 abc |
9.14±2.22 abc |
7.73±1.88 ab |
|
Mesocotyl fragments (2 cm long, three per sample) were cut from seedlings subjected to low temperatures for 1–3 d and electrolyte leakage following 150 min of shaking in water at room temperature was quantified. Data are normalized to amount of electrolyte released by boiling. Three independent experiments were conducted with up to 10 samples per experimental variant. Mean ±SE is shown. Within columns, different letters mark significant differences (P<0.05) estimated with the use of Beta Regression (betareg) in the R program.
Early growth and biometrics
Plant development under controlled conditions was characterized by the time of completion of the initial stages of growth, VE–V2. For a more detailed analysis, we determined the timing of the appearance of the first, second, third, and fourth leaf tips (Fig. 1). Only data for plants grown at low temperature are presented since under optimal conditions all inbred lines showed similar kinetics of development, with the B73 line leading over the other lines by several hours.
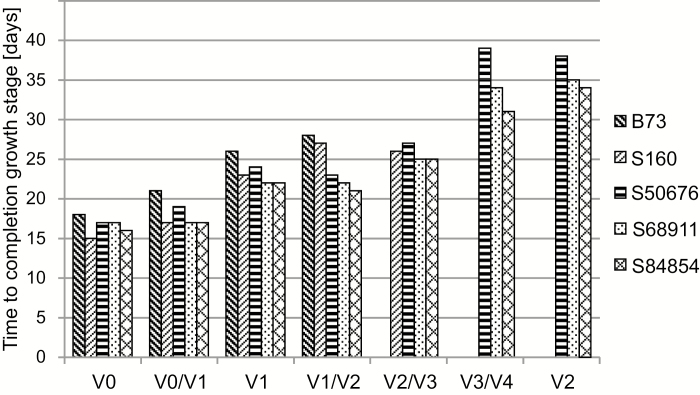
Fig. 1.
Development kinetics of maize seedlings at low temperature. Kernels were germinated and seedlings grown at 14 °C/10 °C, as described in ‘Material and methods’. Time to completion of successive growth stages is shown. VE, emergence; V1, V2, full development of first, second leaf in at least 50% of seedlings; VE/V1, V1/V2, V2/V3, V3/V4, appearance of tips of first–fourth leaf, respectively, in at least 50% of seedlings. Missing bar signifies given leaf failing to develop before termination of experiment (45 d). Three independent experiments were run, each with 60 kernels per inbred line.
Germination was completed at the low temperature in around 2 weeks, with 90–100% of kernels emerging for all the inbred lines except B73, where the emergence was below 70%. Further early development was similar in all lines but soon a delay of development was observed in the B73 line and, to a lesser extent, in S160. Most notably, their development stopped at the third leaf tip appearance stage and neither the fourth leaf tip appearance stage nor the V2 stage was reached during the course of the experiment (45 d). The other inbred lines continued the development with the appearance of successive leaf tips and completing the V2 stage, upon which the experiment was terminated. However, their growth was markedly slower than under optimal conditions, where all the lines completed the V2 stage in 7 d.
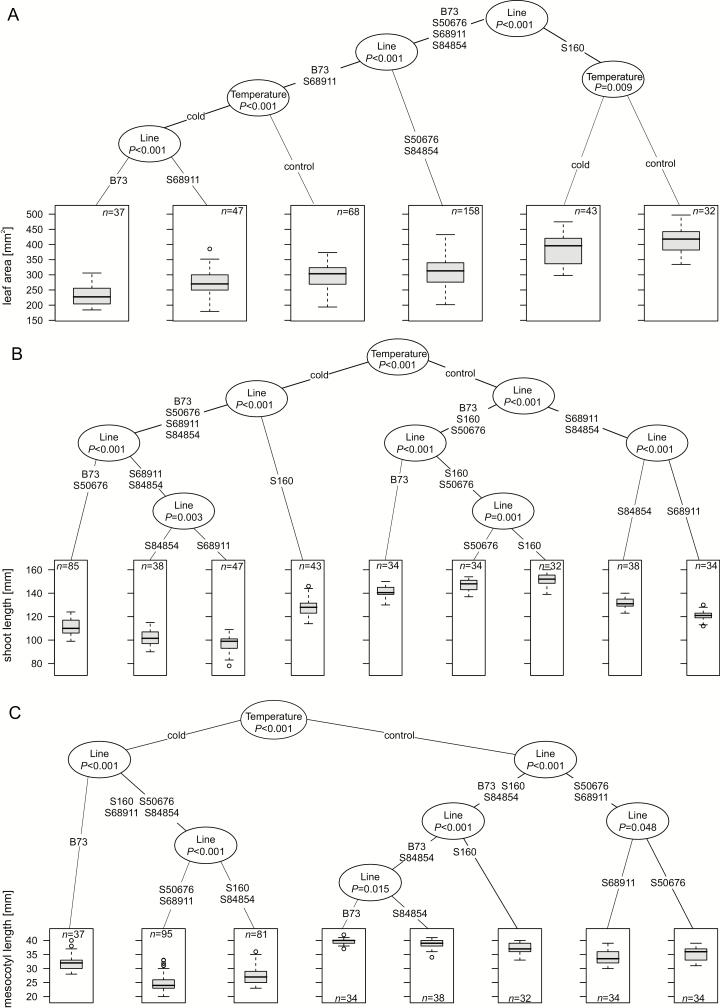
To quantify the early growth at the optimal and low temperatures, leaf area, shoot length, and mesocotyl length were determined at the V1 stage. These biometric parameters were then analysed by clustering and regression trees were constructed (Fig. 2). Profound differences in the growth patterns and of the effects of the cold were observed among the lines. The S160 and S68911 lines showed opposite shoot shapes, with the former being the tallest with the largest leaves and the latter the shortest with the smallest leaves. That was so at both growth conditions, albeit the shoot length decreased substantially in both those lines grown at the low temperatures, while leaf area changed little. Also in the other inbred lines, intermediate between those two extremes regarding their morphology, the cold response of the shoot length was more pronounced than that of the leaf area.
Fig. 2.
Biometric characteristics of maize seedlings at V1 stage. Seedlings were grown at 24 °C/22 °C (control) or 14 °C/10 °C (cold) until full development of first leaf and basic biometric parameters were determined. Five independent experiments, each with 60 kernels per experimental variant, were run. Number of plants actually measured for each parameter (n) is shown. The values were used to construct regression tree using ctree from Partykit (Hothorn and Zeileis, 2015). In boxplots, boxes represent second and third quartile with median indicated by thick line; whiskers represent half of first and half of fourth quartile. Branch lengths are arbitrary; numbers at nodes show statistical differences between two branches. Regression trees shown are for leaf area (in mm2) (A), shoot length (in mm) (B), and mesocotyl length (in mm) (C).
To sum up the results of the growth and biometric analyses, there was no clear association between the timing of the early stages of development in the cold and shoot morphology at the V1 stage. One should notice, however, that two of the three inbred lines capable of efficiently continuing the development beyond the V1 stage, S50676 and S68911, had a short mesocotyl both at the optimal and especially at the low temperature.
Photosynthetic apparatus
Early development of the photosynthetic apparatus was estimated by determining chlorophyll fluorescence, ultrastructure of chloroplasts, and the content of chlorophyll, anthocyanins, and carotenoids, all in the fully developed first leaf.
Photosynthetic apparatus efficiency
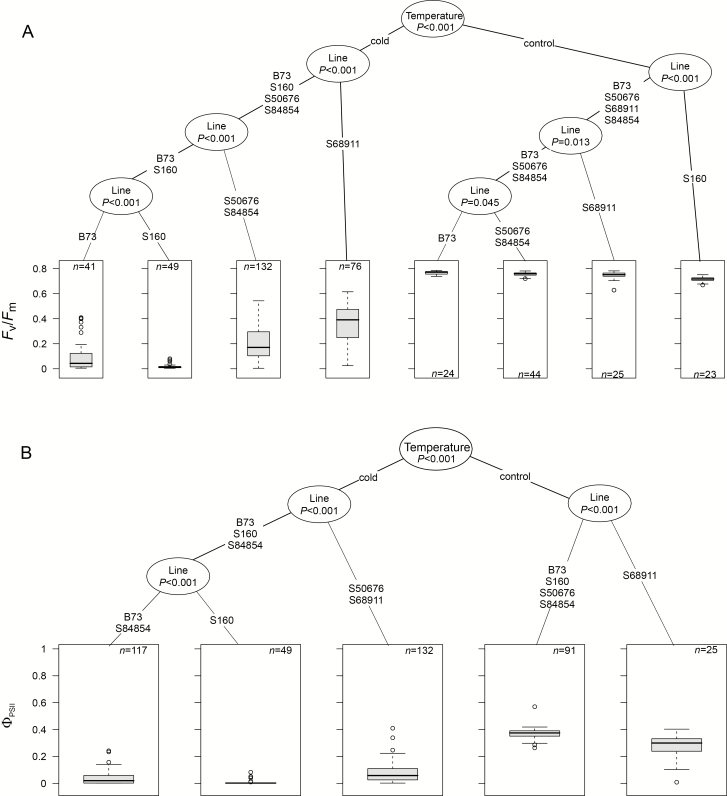
Chlorophyll fluorescence parameters are shown in Fig. 3. Control plants showed nearly identical similar efficiency of the photosynthetic apparatus in all the inbred lines, with the Fv/Fm values between 0.72 and 0.78. In the cold-grown plants, Fv/Fm was much lower in all the lines and also became highly diversified. Clustering divided the lines into three groups comprising S160 with an extremely low value (ca 0.05), B73, S50676, and S84854 with slightly higher values (0.1–0.2), and S68911 with a fairly high Fv/Fm (ca 0.4), but still markedly lower than in the control (Fig. 3A). ΦPSII data reflected the Fv/Fm determinations, with minor differences (Fig. 3B).
Fig. 3.
Photosynthetic characteristics of maize seedlings at V1 stage. Seedlings were grown as described for Fig. 2 and basic photosynthetic parameters were determined for first leaf. Three independent experiments, each with six to nine plants per experimental variant, were run. Data were processed and are presented as described for Fig. 2. Regression trees shown are for Fv/Fm (A) and ΦPSII (B).
Pigment content
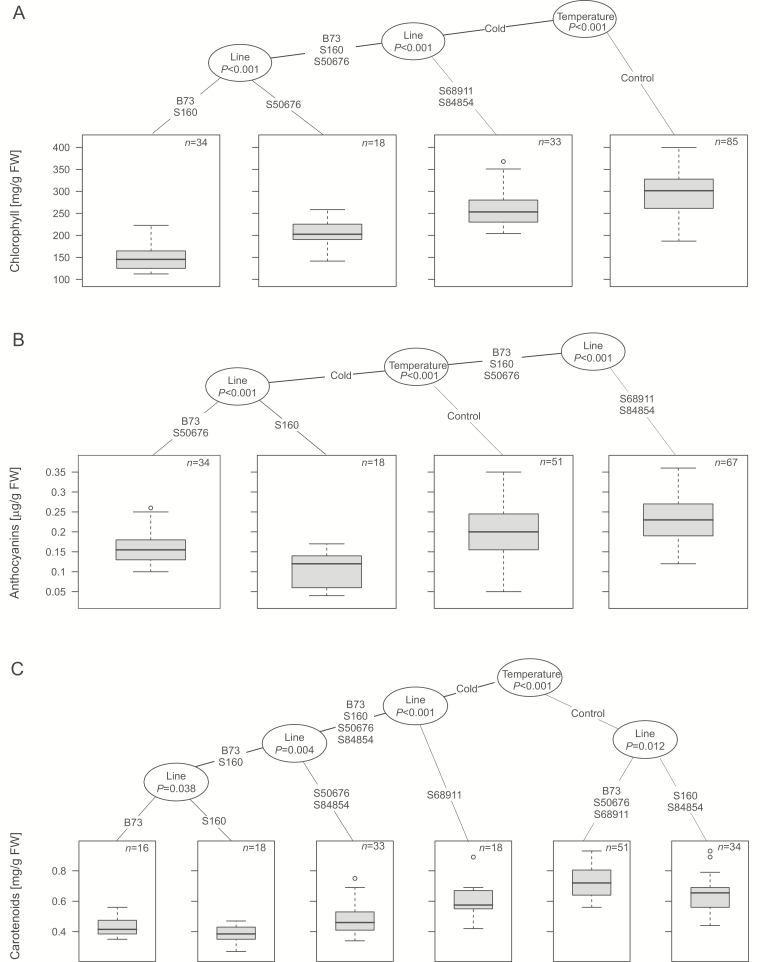
To complement the functional characteristics of the photosynthetic apparatus, we determined the content of chlorophyll, carotenoids, and anthocyanins in the first leaf and again sorted the data by clustering (Fig. 4). Here the low temperature had a highly diversified effect in different lines. In general, the B73 and S160 lines showed the most pronounced drop of pigment content, while S68911 and S84854 were the least affected by cold, with their carotenoids showing no drop at all.
Fig. 4.
Pigment content in the first leaf of maize seedlings at V1 stage. Seedlings were grown as described for Fig. 2 and pigment content was determined spectrophotometrically in middle part of first leaf, as described in ‘Material and methods’. Three independent experiments were run, each with five seedlings per experimental variant. Data were processed and are presented as described for Fig. 2. Regression trees shown are for chlorophyll content (in mg/g FW) (A), anthocyanin content (in µg/g FW) (B), and carotenoid content (in mg/g FW) (C).
Chloroplast ultrastructure
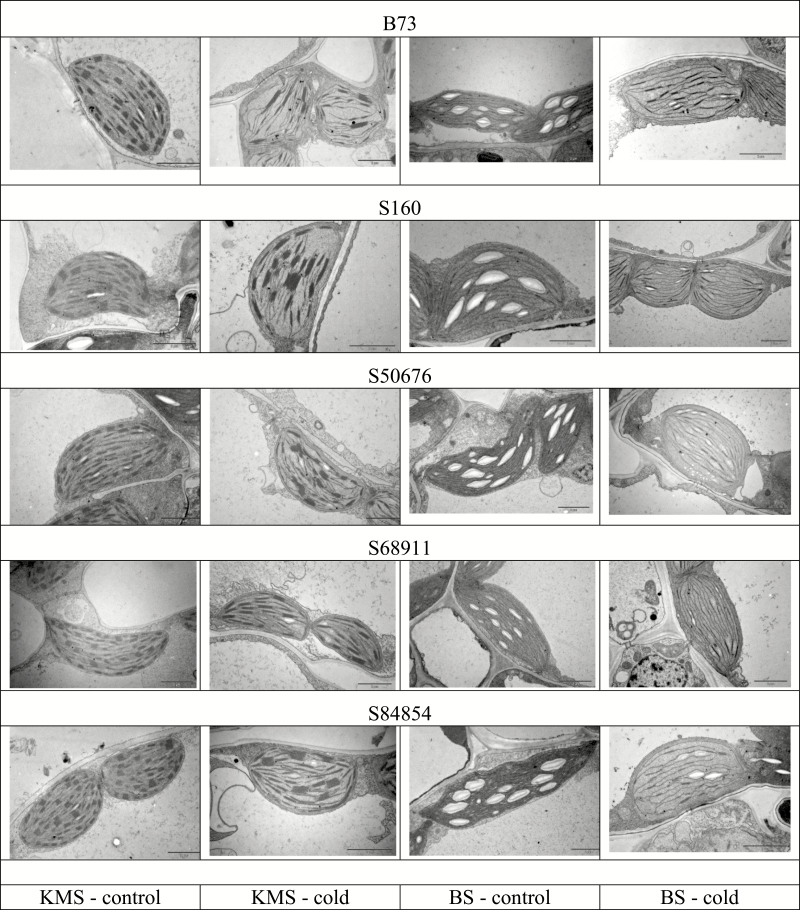
In terms of chloroplast ultrastructure, the response to low temperature of different inbred lines (Fig. 5) largely reflected the functional data. In control conditions the chloroplasts had normal ultrastructure in all the lines, with a developed system of thylakoids and grana in Kranz mesophyll (KMS) chloroplasts and a system of thylakoids without grana in bundle sheath (BS) chloroplasts. Plastoglobules were scarce and peripheral reticulum poorly developed. Starch grains were visible in BS chloroplasts, indicating effective carbon metabolism. Chloroplasts developed under cold conditions showed many anomalies, in particular in the B73 and S160 inbred lines and, to a lesser extent, in S50676 and S84854. The KMS chloroplasts had regions without thylakoids and abnormal grana, and contained numerous plastoglobules. The BS chloroplasts had irregular thylakoids and grana, and lacked starch grains. In contrast, in the S68911 line, only the BS chloroplasts demonstrated some membrane appression, while the KMS chloroplasts had a normal ultrastructure, indistinguishable from that from control conditions.
Fig. 5.
Ultrastructure of chloroplasts in the first leaf of maize seedlings at V1 stage. Seedlings were grown as described for Fig. 2 and middle part of first leaf was sampled for electron microscopy viewing as described in ‘Material and methods’. BS, bundle sheath; KMS, Kranz mesophyll. Scale bars represent 2 µm.
Effect of sowing depth and soil temperature on photosynthetic apparatus efficiency
To obtain more information about the determinants of early development of the photosynthetic apparatus, the effect of sowing depth (shallow sowing vs standard 4-cm-deep sowing) on the response of seedlings to low temperatures was studied. We assumed that deep sowing protected the developing photosynthetic apparatus in seedlings with a short mesocotyl and therefore these lines sown just under the soil surface should exhibit a substantially stronger cold inhibition of the photosynthetic apparatus. To allow even subtle effects of suboptimal early growth conditions to be noticed, less severe cold treatment was used than before. We also applied two ranges of soil temperatures instead of single values for control (23, 24, and 25 °C) and low-temperature conditions (13, 14, and 15 °C). Air temperatures were as in the earlier experiments. For the control conditions neither the single-degree differences in temperature nor sowing depth affected the Fv/Fm, which for all lines was nearly identical as in the earlier experiments, in the range of 0.72–0.78 (see Supplementary Fig. S2). Also control ΦPSII was insensitive to the small temperature differences and sowing depth, and was similar for all the lines (Supplementary Fig. S3), albeit higher than that obtained earlier for the soil temperature of 20 °C.
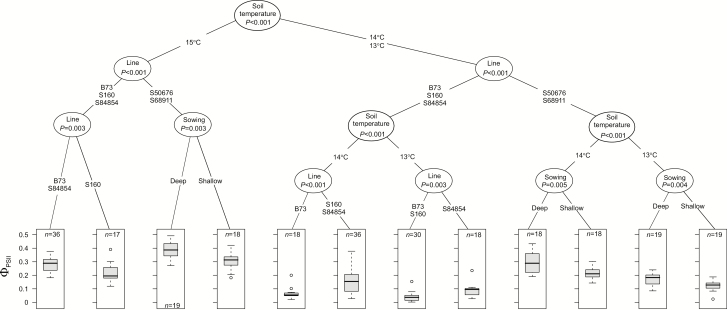
The sub-optimal soil temperatures decreased the Fv/Fm values to different extents in the five inbred lines (see Supplementary Fig. S4). Also the single-degree differences in the low-temperature range had a significant effect on the diminution of Fv/Fm, lower temperatures causing larger decreases. In all three temperatures S68911 showed the highest Fv/Fm and S160 the lowest. Sowing depth did not affect these values. Also ΦPSII was significantly lower for seedlings grown at low soil temperatures than that in the controls, and again this effect was sensitive to the single-degree differences (Fig. 6; compare with Supplementary Fig. S4). The drop in ΦPSII compared with control values was the least in the S68911 and S50676 lines. Notably, shallow sowing aggravated the negative effect of low temperature on ΦPSII only in those lines.
Fig. 6.
Effect of sowing depth on effective quantum yield of electron transport in photosystem II of maize seedlings at V1 stage. Kernels were sown at different soil depths (0.5 or 4 cm) and grown at soil temperatures of 23, 24, or 25 °C (controls) or 13, 14, or 15 °C. Air thermoperiod was 24 °C/22 °C or 14 °C/10 °C, respectively. ΦPSII was determined in the first leaf. Two independent experiments, each with 6–9 plants per experimental variant, were run. Data were processed and are presented as described for Fig. 2. Regression tree shown is for the cold-treated plants only. Data for control plants are shown in Supplementary Fig. S3.
Seedling photosensitivity
Effect of light on mesocotyl elongation
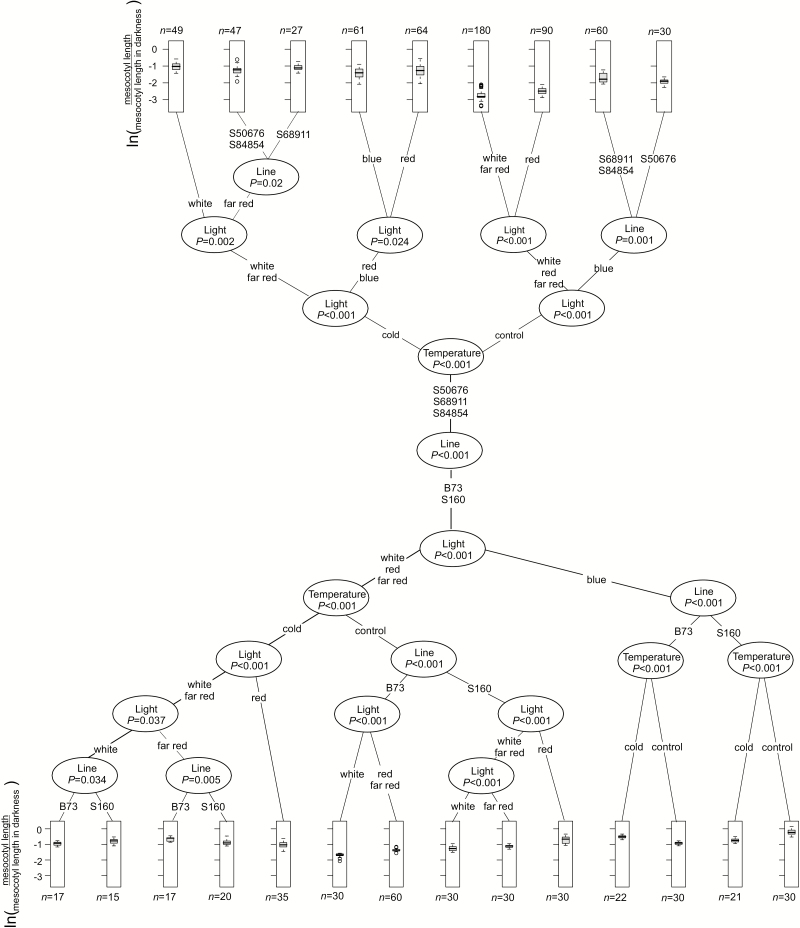
The distinct differences in shoot length and especially in the length of the mesocotyl observed among the inbred lines (Fig. 2) suggested possible differences in their photosensitivity. To analyse the photomorphogenic response of the seedlings mesocotyl elongation was studied under weak light of different colors: blue, far red, red, and white against darkness as reference, in control and low-temperature conditions (Fig. 7).
Fig. 7.
Photomorphogenic response of maize seedlings to light of different colors. Kernels were germinated and seedlings grown at 24 °C/22 °C or 14 °C/10 °C under light indicated and mesocotyl length was determined at VE stage. Three independent experiments were run, each with 10 seedlings per experimental variant. Data were normalized to mesocotyl length of seedlings grown in parallel in darkness (scotomorphogenesis), expressed as natural logarithm and are presented as described for Fig. 2.
In control conditions the photosensitivity (inhibition of mesocotyl elongation) of S50676, S68911, and S84854 seedlings was much stronger than that of B73 and S160 ones at all the spectral conditions. The most pronounced difference was found for blue light, which had almost no influence on the B73 and S160 lines. The low temperature by itself strongly inhibited mesocotyl elongation, and illumination decreased it further in a pattern identical to that observed at the optimal temperature—that is, B73 and S160 were the least responsive and blue light was the least effective.
Expression of photoreceptor genes
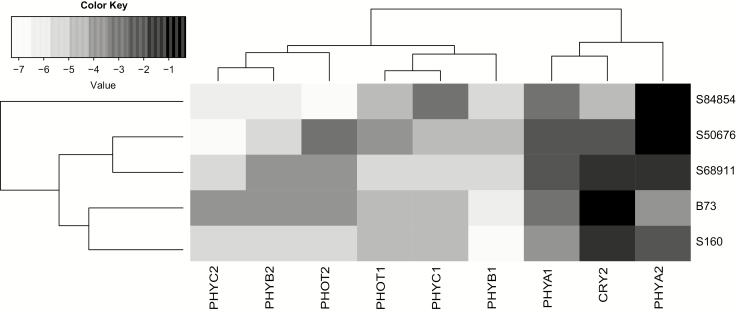
This markedly diversified photosensitivity of the lines could be due to different levels of relevant photoreceptors. We checked this assumption by determining the level of expression (transcript level) of several photoreceptor-encoding genes in the coleoptile of etiolated seedlings (Fig. 8). The differences between the lines were complex, but clustering showed that the two cold-tolerant lines, S50676 and S68911, were highly similar to each other, as were the two cold-sensitive ones (B73 and S160). The PHYA2 and PHYB1 genes showed the most consistent differences in expression between the highly photosensitive lines S50676, S68911, and S84854 relative to B73 and S160, albeit the differences were rather small.
Fig. 8.
Expression level of genes encoding photoreceptors in coleoptile of etiolated maize seedlings grown at optimal temperature. Indicated transcripts were quantitated by RT-qPCR relative to ubiquitin and actin transcript levels as described in ‘Material and methods’. Three independent experiments were run, each with three samples of five coleoptiles per inbred line, determined in triplicate. Shading intensity on heatmap is according to ΔCt values.
Response to gibberellins
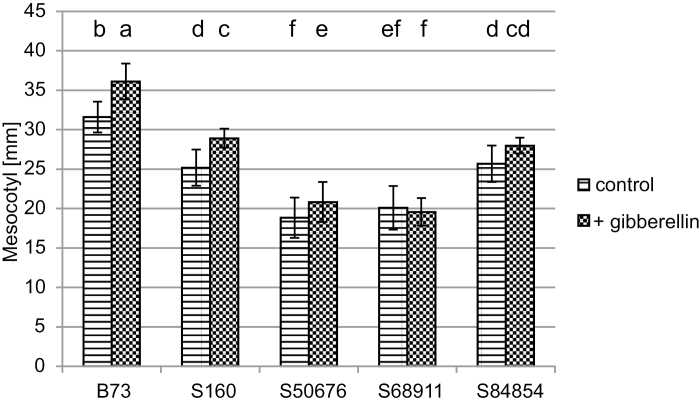
To probe the basis of the varied photosensitivity of the inbred lines further, we determined the effect of external gibberellins on mesocotyl elongation in those lines grown in soil at the optimal temperature (Fig. 9). Gibberellin stimulated mesocotyl elongation in the B73 and S160 lines by ca 15%, in S50676 by ca 11%, and in S84854 by ca 9%; in the latter case the effect was not statistically significant. No effect was observed for the S68911 line. Similar effects of external gibberellin were found for seedlings grown on filter paper under weak white light at optimal temperature and sprayed with GA3 (results not shown).
Fig. 9.
Effect of external gibberellin on mesocotyl length. Plants were grown at 24 °C/22 °C individually in bottomless tubes and GA3 solution (2 ml, 10−5 M) was applied directly on soil twice a day starting 48 h after sowing. Mesocotyl length was determined at VE stage. Six independent experiments were run, each with nine seedlings per line. Statistical analysis for mesocotyl length was performed with the use of agricolae package (R). LSD was estimated with FDR correction (P<0.05). Different letters mark significantly different values.
Cold response of an extended set of maize inbred lines
To verify that the observed relation between cold sensitivity and mesocotyl length and photosensitivity is not random among the five inbred lines assayed, we determined these parameters in a larger set of 18 lines representing diverse heterotic groups (Table 1). Three of them had been developed specifically for the local climate and showed good (S018693) or very good (S03195 and S61328) early vigor in the field under cold-spring conditions (see Supplementary Fig. S1); their photosensitivity and mesocotyl length were not known. The remaining 15 lines were selected from ca 350 inbred lines from across the world used in an independent project aiming to define the genetic basis of mesocotyl length); they represented a wide spectrum of this parameter and their cold sensitivities were not known, except for PH207 and F2, which are assumed to be cold tolerant (Revilla et al., 2014; Joets et al., 2018). For comparison, we assayed again three lines showing different cold sensitivity from the earlier part of the present study, B73, S160 and S68911.
To gauge the lines’ cold sensitivity, Fv/Fm and ΦPSII were compared for plants grown under optimal conditions vs plants subjected to low temperature, exactly as earlier. In control conditions, Fv/Fm was nearly identical for all the lines (0.70–0.78; Supplementary Fig. S5), while ΦPSII showed some variation (0.38–0.55; Supplementary Fig. S6). The plants subjected to cold treatment showed a very wide range of the response of these two parameters (see Supplementary Figs S5, S6), indicating a wide range of the cold sensitivity of the set. Fv/Fm dropped to between 0.05 and 0.40, and ΦPSII to between 0.01 and 0.15. Taking these values together, lines S03195, S61328, and S68911 turned out to be the least affected, and LH74, M37W, Mo17, S160, and W22 the most affected by cold.
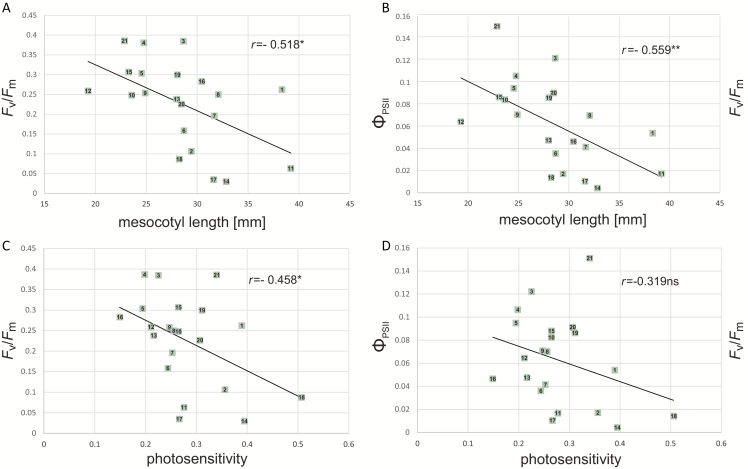
Then, mesocotyl length was determined for V1 seedlings grown under cold, and photosensitivity for seedlings grown in optimal conditions, and these parameters were correlated with the photosynthetic parameters described above. Mesocotyl length was highly and significantly correlated with the photosynthetic parameters of cold-grown plants, i.e. their cold sensitivity (Fig. 10A, B). Notably, these correlations were even stronger (r=−0.64 for Fv/Fm and −0.61 for ΦPSII) and more significant when the three lines investigated in the earlier parts of the study were excluded from calculations (results not shown). In turn, seedling photosensitivity was correlated significantly only with Fv/Fm (Fig. 10D). For ΦPSII the correlation coefficient was slightly below significance level (Fig. 10C).
Fig. 10.
Correlations between basic chlorophyll fluorescence parameters, mesocotyl length and photosensitivity in 21 maize inbred lines. Mesocotyl length and Fv/Fm and ΦPSII of the first leaf were determined for plants grown at 14 °C/10 °C, photosensitivity (ratio of mesocotyl length of seedlings grown under weak white light to that of dark-grown seedlings) for plants grown at 24 °C/22 °C. (A) Mesocotyl length vs Fv/Fm; (B) mesocotyl length vs ΦPSII; (C) photosensitivity vs Fv/Fm; (D) photosensitivity vs ΦPSII. *P<0.05; **P<0.01. Number/inbred line: 1, B73; 2, LH74; 3, S03198; 4, S68911; 5, PH207; 6, KY21; 7, M162W; 8, MBPM; 9, MBUB; 10, Oh43; 11, W22; 12, CML333; 13, Mo18W; 14, M37W; 15, Oh7; 16, A188; 17, Mo17; 18, S160; 19, F2; 20, S018693; 21, S61328.
Discussion
To assess what features allow modern maize plants to adapt better to a temperate climate, different characteristics of plant growth and functioning were studied, relevant to the three physiological mechanisms most frequently postulated to cause the maize cold sensitivity: membrane injury, disturbance of photosynthetic apparatus development, and inhibition of growth. In terms of classical plant stress physiology (Levitt, 1980), that is avoidance versus tolerance, we found that both these strategies appear to be involved in the successful adaptation of maize to a temperate climate.
Detailed analysis of mechanisms involved in cold tolerance of cold-spring-adapted materials
Among the five inbred lines studied, three—S84854, S68911, and S50676—show perfect or nearly perfect early vigor following cold spells under field conditions (Supplementary Fig. S1). Two of these lines were studied earlier at the V3 growth stage and S68911 was found to be cold tolerant while S50676 was medium cold tolerant (Sobkowiak et al. 2016). These three tolerant lines were compared with the B73 and S160 inbred lines. B73 is a late line (Giauffret et al., 1995) and has not been evaluated under field conditions for early vigor but has been shown to arrest growth at a low temperature at the V5 growth stage (Riva-Roveda et al., 2016) and is considered intermediately cold tolerant (Rodríguez et al., 2013) or cold sensitive (Joets et al., 2018). The S160 line has been well characterized regarding both its physiological and its molecular responses to low temperature, showing poor early vigor under field conditions and strong cold sensitivity (Sowiński et al., 2005; Sobkowiak et al., 2016).
The field characteristics of the inbred lines tested were only partially reflected by the extent of membrane injury at low temperatures (Table 1). While the most cold-sensitive line, S160, also showed the most extensive membrane injury, similar to the injury caused by sub-zero temperatures (Janowiak and Markowski, 1994; Janda et al., 1999; Li et al., 2016), and S84854 and S50676, showing good early vigor, were the least injured near 0 °C, the third line of similar early vigor, S68911, showed much stronger membrane injury. Moreover, at ≥8 °C the extent of membrane injury was virtually identical for all the lines, indicating that diversified membrane injury cannot be the basis of the spectacular differences in the cold sensitivity of these lines found in this study at moderately low temperatures (>10 °C).
With respect to their development at low temperatures, the studied lines could be clearly divided into two groups: three that were able to continue shoot development after the V1 stage (S50676, S68911, and S84854) and two that were not (B73 and S160) (Fig. 1). In the former, the photosynthetic apparatus of the first leaf of cold-grown seedlings performed well, as estimated by chlorophyll fluorescence (Fig. 3) and chloroplast ultrastructure (Fig. 5), while in the latter it did not, showing both structural damage and negligible photosynthetic efficiency. Notably, the Fv/Fm and ΦPSII values determined in the first leaf of plants grown at the optimal temperature were close to the values reported for the third leaf (Sobkowiak et al., 2016), assumed to be the first fully autotrophic one (Stamp, 1984). Even more strikingly, the V1-stage chloroplasts contained starch granules in control plants, which suggests proper operation of photosynthetic carbon assimilation and reduction. By stressing these features we do not intend to imply a substantial role of the first leaf as a source of assimilates for the seedling, but rather to show that the chloroplast fluorescence parameters are a good indicator of the effects of stress on the photosynthetic apparatus also at this very early growth stage. Thus, in line with earlier data (Hardacre and Eagles, 1980; Sowiński et al., 2005), one may conclude that formation of the photosynthetic apparatus, i.e. the transition from heterotrophic to autotrophic stage of growth, is a major factor determining further development following consumption of kernel reserves.
Earlier studies on chloroplast development in maize grown at low temperatures reported pronounced ultrastructural injuries not only in the cold-sensitive (Nie et al., 1995; Kratsch and Wise, 2000), but also in cold-tolerant plants (Kutik et al., 1999; Sowiński et al. 2005). In stark contrast, the cold-tolerant inbred line S68911 showed virtually normal chloroplast development at low temperatures although their photosynthetic efficiency showed some cold inhibition. Thus, it seems that in some recently developed maize inbred lines well adapted to cold spring conditions, the forming chloroplasts have virtually lost susceptibility to low temperature. This feature could be related to the ability of the cold-tolerant seedlings to maintain the synthesis of chlorophyll, anthocyanins, and carotenoids in leaves during growth at low temperature (Fig. 4). Elevated levels of anthocyanins (Pietrini et al., 2002; Rodríguez et al., 2014) and carotenoids (Haldimann, 1998; Aroca et al., 2001), potentially protecting the photosynthetic apparatus developing in the cold (Van Breusegem et al., 1998), have been found earlier in flint plants representing good cold tolerance.
Two of the three cold-tolerant lines studied here, S68911 and S84854, presented a xeromorphic growth habit (short, chunky plants) as seedlings (Fig. 2). This trait could limit the loss of water at low temperatures and secondary water deficit. Xeromorphic morphology has been reported as specific to cold-tolerant flint maize plants grown at low temperatures both under field and controlled (Verheul et al., 1996; Sowiński et al., 2003; Strigens et al., 2013) conditions. These two xeromorphic lines exhibited the fastest development of consecutive leaves during early growth at low temperatures. Notably, the smallest seedlings (S68911) also had the best-performing photosynthetic apparatus among the inbred lines tested.
Integrated analysis of the mechanisms of cold tolerance of cold-spring-adapted materials
The analysis presented above followed the traditional approach to the question of maize cold sensitivity, amply represented in the literature since the 1970s, viewing the poor cold performance as due to a single major cause, i.e. defective early development, or deficient antioxidant accumulation, or defective formation of the photosynthetic apparatus, etc. However, we found here that in fact several of these processes are affected by low temperature in a similar manner and, crucially, this response is different for the cold-sensitive and the cold-tolerant maize lines. We propose that this is due to a common, previously neglected reason, i.e. differential photomorphogenic regulation. Indeed, inhibition of mesocotyl elongation (Markelz et al., 2003), accumulation of carotenoids and chlorophylls (Rajasekhar et al., 1981; Toledo-Ortiz et al., 2010; Pogson et al., 2015), and of anthocyanins (Duke et al., 1976; Singh et al., 1999) are all induced by light at a very early growth stage likely through phytochrome signaling. One should bear in mind that although chloroplast biogenesis is induced by light absorbed by protochlorophyllide oxidoreductase (Kowalewska and Mostowska, 2016), some steps of this process likely involve phytochromes as well (Pogson et al., 2015).
We noticed that seedlings of the cold-tolerant inbred lines showed pronounced inhibition of mesocotyl elongation during the growth at low temperatures under standard illumination (Fig. 2). Elongation of the mesocotyl is a convenient measure of photosensitivity, as it (as well as leaf expansion) is inhibited by light during early development. Notably, this response has been found to vary strongly between maize plants (Markelz et al., 2003). When the photosensitivity was studied here in more detail, the S50676, S68911, and S84854 lines turned out to be much more sensitive to light than S160 and B73, at both the optimal and the low temperature (Fig. 7). Blue light had the most diversified effect on these two groups of lines, suggesting an involvement of cryptochromes, blue light photoreceptors. Expression of genes encoding diverse photoreceptors did not differ in a clear-cut pattern between the cold-tolerant and cold-sensitive inbred lines, offering no strong clue as to the molecular mechanism of their different photosensitivity, albeit the slightly higher expression of a PHYB1 ortholog in the former seems interesting (Fig. 8). Notably, in Arabidopsis PHYB1 is involved in temperature sensing (Jung et al., 2016).
We reasoned that the observed differences in photosensitivity gauged by measuring mesocotyl length might stem from diverse sensitivity to gibberellin, a plant growth regulator known to stimulate mesocotyl elongation (Dubois et al., 2010). Indeed, the effect of externally applied gibberellin on mesocotyl elongation was markedly less pronounced in the cold-tolerant inbred lines, especially S68911, than in the cold-sensitive ones (Fig. 9). One may envisage diversification of the gibberellin signaling pathway among maize inbred lines as a foundation of their diversified cold tolerance. Whether this is indeed the case can only be verified in a dedicated study, but we found provisional confirmation of this hypothesis when resequencing the genomes of three inbred lines studied in this work: S160, S50676, and S68911 (see Supplementary Table S3). Among the many nucleotide sequence differences deemed highly functionally relevant, several concern genes encoding transcription factors related to gibberellin response, e.g. bzr7 and zim23, and GIBBERELLIN INSENSITIVE DWARF1 (GID1) encoding a GA receptor. Notably, also genes directly related to photomorphogenic response were found to differ markedly among these three lines, e.g. bhlh47 and bhlh60 encoding orthologs of Arabidopsis phytochrome interacting factors. Numerous other genes related to gibberellin signaling contain polymorphisms of predicted minor significance. Thus, there are genetic differences between some of the inbred lines studied here experimentally that could constitute a molecular basis of the observed physiological differences. A direct confirmation of the causal relationships between the genetic and physiological characteristics will require detailed studies.
Apart from the inhibition of mesocotyl elongation, the effects of light also comprise stimulation of the development of the photosynthetic apparatus and accumulation of chlorophyll, anthocyanins, and carotenoids. All these features set apart the cold-tolerant inbred lines from the sensitive ones and thus could be related to their higher photosensitivity. This poses the question whether the short mesocotyl is only a consequence of the elevated photosensitivity or whether it also has an adaptive character under cold.
A short mesocotyl causes the shoot apex to remain below the soil level for a prolonged time, while in the cold-sensitive (and less photosensitive) seedlings the apex is elevated above the soil already at the V1 stage. One may hypothesize that under field conditions the prolonged fairly deep underground localization of the shoot apical meristem could allow the plant to avoid low temperatures at night and in the morning during cold spells. At emergence, the shoot apex in maize remains below the soil surface and starts to elevate during juvenile growth. When the air temperature drops below 0 °C, the soil temperature can be higher by as much as 5–10 °C at the depth of 10 cm (Głowacka et al., 2015) and 2–6 °C higher at 4–8 cm (agriculture data for farmers or https://www.globe.gov/web/ggic/overview/previous-iops/march-2012), the routine sowing depth for maize in most soils. Thus, a delayed elevation of the shoot apex above the soil could protect the crucial meristem and new leaf primordia from cold injury and allow further undisturbed leaf development after the onset of favorable conditions. This trait of postulated key importance under field conditions in a temperate climate could have a less marked effect under controlled conditions of a growth chamber, where the temperatures of soil and air are similar. Alternatively, even in a growth chamber, a prolonged burial in the soil could minimize the stressful effect of fairly strong (close to natural) illumination (600 µmol quanta m−2 s−1) in the cold at very early stages of photosynthetic apparatus formation, and also protect the meristem by helping to maintain normal water potential despite the low temperature. In accordance, we found that the two inbred lines with the shortest mesocotyls, S50676 and S68911, demonstrated substantially higher ΦPSII at low temperatures when sown deeply than when sown shallowly, while there was no such effect of sowing depth in the other lines (Fig. 6). Since ΦPSII is a good indicator of the quantum efficiency of CO2 assimilation in maize (Genty et al., 1989), one may conclude that owing to the longer lasting protective action of the soil layer against low temperature stress, the two lines in question can develop a higher photosynthetic capacity than the other lines studied. Our hypothesis that a short mesocotyl should protect the forming seedling against cold was strongly corroborated by the analysis of 21 inbred lines from diverse heterotic groups (Fig. 10). As predicted, mesocotyl length correlated well with the cold sensitivity of those lines.
The observed high photosensitivity of the cold-tolerant inbred lines, helping the seedlings to avoid the adverse effects of cold on early growth, stands in apparent contrast with the idea that the selection pressure accompanying maize adaptation in northern, temperate regions has led to a decrease of its photosensitivity (Salamini, 1985) possibly due to diminution of the shade avoidance response (Markelz et al., 2003). However, the latter idea was based on the distribution of the short mesocotyl trait only among subpopulations from the Americas, South Africa and South Asia, but not those from Europe and Central Asia, where the most intensive breeding for a temperate climate has been taking place in recent decades.
Apart from the avoidance mechanism postulated above, protecting the shoot apex against low temperatures at early growth stages, there could exist also a true tolerance mechanism based on a direct link between the increased photosensitivity and improved cold tolerance, involving an enhanced stimulation of development of the photosynthetic apparatus (chloroplast ultrastructure and pigment accumulation) at low temperatures. This is clearly observed in the S68911 inbred line. It showed the strongest photosensitivity, especially in the cold, was the least sensitive to gibberellins, and was the only inbred line demonstrating perfect development of KMS chloroplasts and almost perfect development of BS chloroplasts at low temperatures, accompanied by the highest photosynthetic activity. We believe that the astonishing cold tolerance of the photosynthetic apparatus of the S68911 line could be directly related to the modification of the gibberellin signaling pathway reflected by the plant’s insensitivity to externally applied gibberellin and xeromorphic growth habit. This would be the first indication of a mechanism directly connecting two key aspects of plant physiology, photosensitivity and cold tolerance. That photosensitivity and cold sensitivity at the VE/V1 growth stage are indeed interrelated is strongly supported by the outcome of the analysis of the larger set of inbred lines documenting a negative correlation between these two parameters. Although a correlation does not prove a causal relationship, we believe that the phenomenon deserves further exploration.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Early vigor of seven locally developed maize inbred lines, maximum and minimum air temperatures, and minimum temperatures near ground (°C) at Smolice (western Poland) station during April–September of 2013–2017.
Fig. S2. Effect of sowing depth on maximal quantum yield of electron transport in photosystem II of maize seedlings at V1 stage grown at optimal temperatures.
Fig. S3. Effect of sowing depth on maximal quantum yield of electron transport in photosystem II of maize seedlings at V1 stage grown at low temperatures.
Fig. S4. Effect of sowing depth on effective quantum yield of electron transport in photosystem II of maize seedlings at V1 stage grown at optimal temperatures.
Fig. S5. Maximal quantum yield of electron transport in photosystem II of maize seedlings from extended set of inbred lines at V1 stage.
Fig. S6. Effective quantum yield of electron transport in photosystem II of maize seedlings at V1 stage.
Table S1. Primers for RT-qPCR
Table S2. Raw results of ANOVA
Table S3. Sequence variants of genes potentially involved in gibberellin signaling in three maize inbred lines.
Acknowledgements
We thank Mr Mark J. Millard, USDA, ARS, Iowa State University, Regional Plant Introduction Station for supplying most of the inbred lines used in this study. This work was supported by grants 2012/05/B/NZ9/03407 and 2017/27/B/NZ9/00995 from the National Science Centre (NCN), Poland.
References
- Aroca R, Irigoyen JJ, Sanchez-Diáz M. 2001. Photosynthetic characteristics and protective mechanisms against oxidative stress during chilling and subsequent recovery in two maize varieties differing in chilling sensitivity. Plant Science 161, 719–726. [Google Scholar]
- Barlow EWR, Boersma L, Young JL. 1977. Photosynthesis, transpiration, and leaf elongation in corn seedlings at suboptimal soil temperatures. Agronomy Journal 69, 95–100 [Google Scholar]
- Ben-Haj-Salah H, Tardieu F. 1995. Temperature affects expansion rate of maize leaves without change in spatial distribution of cell length (analysis of the coordination between cell division and cell expansion). Plant Physiology 109, 861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribari-Neto F, Zeileis A. 2010. Beta regression in R. Journal of Statistical Software 34, 1–24. [Google Scholar]
- de Mendiburu F. 2016. agricolae: Statistical procedures for agricultural research. R package version 1.2-4. http://CRAN.R-project.org/package=agricolae [Google Scholar]
- Di Frenza M, Hog B, Grant J, Barth S. 2017. Transcriptomic response of maize primary roots to low temperatures at seedling emergence. PeerJ 5, e2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois PG, Olsefski GT, Flint-Garcia S, Setter TL, Hoekenga OA, Brutnell TP. 2010. Physiological and genetic characterization of end-of-day far-red light response in maize seedlings. Plant Physiology 154, 173–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke SO, Fox SB, Naylor AW. 1976. Photosynthetic independence of light-induced anthocyanin formation in Zea seedlings. Plant Physiology 57, 192–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq M, Aziz T, Wahid A, Lee D-J, Siddique KHM. 2009. Chilling tolerance in maize: agronomic and physiological approaches. Crop and Pasture Science 60, 501–516. [Google Scholar]
- Flint-Garcia SA, Thuillet AC, Yu J, Pressoir G, Romero SM, Mitchell SE, Doebley J, Kresovich S, Goodman MM, Buckler ES. 2005. Maize association population: a high-resolution platform for quantitative trait locus dissection. The Plant Journal 44, 1054–1064. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Vanacker H, Gomez LD, Harbinson J. 2002. Regulation of photosynthesis and antioxidant metabolism in maize leaves at optimal and chilling temperatures: review. Plant Physiology and Biochemistry 40, 659–668. [Google Scholar]
- Frascaroli E, Revilla P. 2018. Genomics of cold tolerance in maize. In: Bennetzen J, Flint-Garcia S, Hirsch C, Tuberosa R, eds. The maize genome. Cham: Springer Nature Switzerland, 287–303. [Google Scholar]
- Giauffret C, Bonhomme R, Derieux M. 1995. Genotypic differences for temperature response of leaf appearance rate and leaf elongation rate in field-grown maize. Agronomie 15, 123–137 [Google Scholar]
- Genty B, Briantas J-M, Baker NR. 1989. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimical and Biophysical Acta 990, 87–92. [Google Scholar]
- Głowacka K, Jørgensen U, Kjeldsen JB, Kørup K, Spitz I, Sacks EJ, Long SP. 2015. Can the exceptional chilling tolerance of C4 photosynthesis found in Miscanthus × giganteus be exceeded? Screening of a novel Miscanthus Japanese germplasm collection. Annals of Botany 115, 981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves JA. 1996. Improving suboptimal temperature tolerance in maize—the search for variance. Journal of Experimental Botany 296, 307–323. [Google Scholar]
- Haldimann P. 1998. Low growth temperature-induced changes to pigment composition and photosynthesis in Zea mays genotypes differing in chilling sensitivity. Plant, Cell & Environment 21, 200–208. [Google Scholar]
- Hardacre AK, Eagles HA. 1980. Comparisons among populations of maize for growth at 13°C. Crop Sciences 20, 780–784. [Google Scholar]
- Hodges DM, Andrews CJ, Johnson DA, Hamilton RI. 1997. Sensitivity of maize hybrids to chilling and their combining abilities at two developmental stages. Crop Sciences 37, 850–856. [Google Scholar]
- Hothorn T, Zeileis AA. 2015. Modular toolkit for recursive partitioning in R. Journal of Machine Learning Research 16, 3905–3909. [Google Scholar]
- Janda T, Szalai G, Tari I, Páldi E. 1999. Hydroponic treatment with salicylic acid decreases the effects of chilling injury in maize (Zea mays L.) plants. Planta. 208, 175–180. [Google Scholar]
- Janowiak F, Markowski A. 1994. Changes in leaf water relations and injuries in maize seedlings induced by different chilling conditions. Journal of Agronomy and Crop Science 172, 19–28. [Google Scholar]
- Joets J, Vitte C, Charcosset A. 2018. Draft assembly of the F2 European maize genome sequence and its comparison to the B73 genome sequence: a characterization of genotype-specific regions. In: Bennetzen J, Flint-Garcia S, Hirsch C, Tuberosa R, eds. The maize genome. Cham: Springer Nature Switzerland, 3–12. [Google Scholar]
- Jung JH, Domijan M, Klose C, et al. 2016. Phytochromes function as thermosensors in Arabidopsis. Science 354, 886–889. [DOI] [PubMed] [Google Scholar]
- Klughammer C, Schreiber U. 2008. Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the Saturation Pulse method. PAM Application Notes. 1, 27–35. [Google Scholar]
- Kowalewska Ł, Mostowska A. 2016. Biogenesis of thylakoid membranes correlation of structure and function. In: Pessarakli M, ed. Handbook of photosynthesis, 3rd edn.Boca Raton: CRC Press, 3–15. [Google Scholar]
- Kratsch HA, Wise RR. 2000. The ultrastructure of chilling stress. Plant, Cell & Environment. 23, 337–350. [Google Scholar]
- Kutik J, Kočová, Holá D, Körnerová M. 1999. The development of chloroplasts ultrastructure and Hill reaction activity during leaf ontogeny in different maize genotypes. Photosynthetica 36, 497–507. [Google Scholar]
- Leipner J, Stamp P. 2009. Chilling stress in maize seedlings. In: Bennetzen JL, Hake S, eds. Handbook of maize: its biology. New York: Springer-Verlag, 291–310. [Google Scholar]
- Levitt J. 1980. Response of plants to environmental stresses. New York: Academic Press. [Google Scholar]
- Li Z, Hu G, Liu X, et al. 2016. Transcriptome sequencing identified genes and gene ontologies associated with early freezing tolerance in maize. Frontiers in Plant Science 7, 1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler HK, Buschmann C. 2001. Chlorophylls and carotenoids: measurement and characterization by UV-VIS spectroscopy. In: Wrolstad RE, Acree TE, An H, Decker EA, Penner MH, Reid DS, Schwartz SJ, Shoemaker CF, Sporns P, eds. Current protocols in food analytical chemistry. New York: John Wiley and Sons, F4.3.1–F4.3.8. [Google Scholar]
- Markelz NH, Costich DE, Brutnell TP. 2003. Photomorphogenic responses in maize seedling development. Plant Physiology 133, 1578–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Sakurai M. 1995. Effects of riboflavin and increased sucrose on anthocyanin production in suspended strawberry cell cultures. Plant Science 110, 147–153. [Google Scholar]
- Nie G-Y, Robertson EJ, Fryer MJ, Leech RM, Baker NR. 1995. Response of the photosynthetic apparatus in maize leaves grown at low temperature on transfer to normal growth temperature. Plant, Cell & Environment 18, 1–12. [Google Scholar]
- Pietrini F, Ianelli MA, Massacci A. 2002. Anthocyanin accumulation in the illuminated surface of maize leaves enhances protection from phot-inhibitory risks at low temperature, without further limitation to photosynthesis. Plant, Cell & Environment 25, 1251–1259. [Google Scholar]
- Pogson BJ, Ganguly D, Albrecht-Borth V. 2015. Insights into chloroplast biogenesis and development. Biochimica et Biophysica Acta 1847, 1017–1024. [DOI] [PubMed] [Google Scholar]
- Rajasekhar VK, Rao LVM, Guha-Mukherjee S, Sopory SK. 1981. Phytochrome control of chlorophyll and carotenoid accumulation in Sorghum bicolor. Plant and Cell Physiology 22, 773–780. [Google Scholar]
- R Core Team , 2016.The R project for statistical computing. Vienna: R Foundation for Statistical Computing.https://www.r-project.org/ [Google Scholar]
- Revilla P, Rodríguez VM, Ordas A, et al. 2014. Cold tolerance in two large maize inbred panels adapted to European climates. Crop Sciences 54, 1981–1991. [Google Scholar]
- Riva-Roveda L, Escale B, Giauffret C, Périlleux C. 2016. Maize plants can enter a standby mode to cope with chilling stress. BMC Plant Biology 16, 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez VM, Butrón A, Rady MOA, Soenas P, Revilla P. 2014. Identification of quantitative trait loci involved in the response to cold stress in maize (Zea mays L.). Molecular Breeding 33, 363–371. [Google Scholar]
- Rodríguez VM, Velasco P, Garrido JL, Revilla P, Ordás A, Butrón A. 2013. Genetic regulation of cold-induced albinism in the maize inbred line A661. Journal of Experimental Botany 64, 3657–3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rymen B, Fiorani F, Kartal F, Vandepoele K, Inzé D, Beemster GT. 2007. Cold nights impair leaf growth and cell cycle progression in maize through transcriptional changes of cell cycle genes. Plant Physiology 143, 1429–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamini F. 1985. Photosensitivity in maize: evaluation, genetics, and breeding for insensitivity. In: Bandolini A, Salamini F, eds. Breeding strategies for maize production improvement in the tropics. Florence: Food and Agriculture Organization of the United Nations and Istituto Agronomico per L’Oltremare, 143–157 [Google Scholar]
- Scheridan WF, Clark JK. 1994. Fertilization and embryogeny in maize. In: Freeling M, Walbot V, eds. The maize handbook. New York: Springer-Verlag, 3–10. [Google Scholar]
- Singh A, Tamil Selvi M, Sharma R. 1999. Sunlight-induced anthocyanin pigmentation in maize vegetative tissues. Journal of Experimental Botany 50, 1619–1625. [Google Scholar]
- Sobkowiak A, Jończyk M, Adamczyk J, et al. 2016. Molecular foundations of chilling-tolerance of modern maize. BMC Genomics 17, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowiński P, Rudzińska-Langwald A, Adamczyk J, Kubica I, Fronk J. 2005. Recovery of maize seedling growth, development and photosynthetic efficiency after initial growth at low temperature. Journal of Plant Physiology 162, 67–80. [DOI] [PubMed] [Google Scholar]
- Sowiński P, Rudzińska-Langwald A, Kobus P. 2003. Changes in plasmodesmata frequency in vascular bundles of maize seedling leaf induced by growth at suboptimal temperatures in relation to photosynthesis and assimilate export. Environmental and Experimental Botany 50, 183–196. [Google Scholar]
- Stamp P. 1984. Chilling tolerance of young plants demonstrated on the example of maize (Zea mays L.). Berlin: Paul Parey, 7. [Google Scholar]
- Strigens A, Freitag NM, Gilbert X, Grieder C, Riedelsheimer C, Schrag TA, Messmer R, Melchinger AE. 2013. Association mapping for chilling tolerance in elite flint and dent maize inbred lines evaluated in growth chamber and field experiments. Plant, Cell & Environment 36, 1871–1887. [DOI] [PubMed] [Google Scholar]
- Szalai G, Majláth I, Pál M, Gondor OK, Rudnóy S, Oláh C, Vanková R, Kalapos B, Janda T. 2018. Janus-faced nature of light in the cold acclimation processes of maize. Frontiers in Plant Science 9, 850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardieu F. 2003. Virtual plants: modelling as a tool for the genomics of tolerance to water deficit. Trends in Plant Science 8, 9–14. [DOI] [PubMed] [Google Scholar]
- Toledo-Ortiz G, Huqb E, Rodríguez-Concepción M. 2010. Direct regulation of phytoene synthase gene expression and carotenoid biosynthesis by phytochrome-interacting factors. Proceedings of the National Academy of Sciences, USA 107, 11626–11631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollenar M, Hunter RB. 1983. A photoperiod and temperature sensitive period for leaf number of maize. Crop Sciences 23, 457–460 [Google Scholar]
- Van Breusegem F, Van Montagu M, Inzé D. 1998. Engineering stress tolerance in maize. Outlook on Agriculture. 27, 115–124. [Google Scholar]
- Verheul MJ, Picatto C, Stamp P. 1996. Growth and development of maize (Zea mays L.) seedlings under chilling conditions in the field. European Journal of Agronomy 5, 31–43. [Google Scholar]
- Vigh L, Horvȃth I, Farkas T, Mustȃrdy LA, Faludi-Daniel A. 1981. Stomatal behavior and cuticular properties of maize leaves of different chilling-resistance during cold treatment. Physiologia Plantarum 51, 287–290. [Google Scholar]
- Warnes GR, Bolker B, Bonebakker L, et al. 2016. gplots: Various R programming tools for plotting data. R package version 3.0.1. ttps://CRAN.Rproject.org/package=gplots [Google Scholar]
- Warrington IJ, Kanemasu ET. 1983. Corn growth response to temperature and photoperiod I. Seedling emergence, tassel initiation, and anthesis. Agronomy Journal 75, 749–754. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.