Abstract
Objective
The aim of this meta-analysis was to clarify the role of Matrix metalloproteinase 1 (MMP-1) -1607 1G/2G (rs1799750) polymorphism on the osteoarthritis (OA) risk.
Methods
Articles were selected by retrieving the Web of Science, Embase and Pubmed. The strength of the association between -1607 1G/2G polymorphism and OA risk was assessed by odds ratios (ORs) with the corresponding 95% confidence interval (CI) for each study.
Results
No significant association between -1607 1G/2G polymorphism and OA risk was found in all the models overall (2G2G vs 1G1G, OR (95%CI) = 0.69 (0.36–1.32), P = 0.54; 2G2G + 2G1G vs 1G1G, OR (95%CI) = 0.88 (0.47–1.63), P = 0.69; 2G2G vs 2G1G + 1G1G, OR (95%CI) = 1.30 (0.68–2.47), P = 0.41; 2 G vs 1G, OR (95%CI) = 0.90 (0.86–1.54), P = 0.66). By subgroup analysis, significant association was found in the “< 60 years” group (2G2G vs 1G1G, OR (95%CI) = 3.46 (2.13–5.62), P = 0.00; 2G2G + 2G1G vs 1G1G, OR (95%CI) = 0.49 (0.31–0.79), P = 0.00; 2G2G vs 2G1G + 1G1G, OR (95%CI) = 2.74 (1.80–4.16, P = 0.00; 2 G vs 1G, OR (95%CI) = 0.56 (0.35–0.89), P = 0.01).
Conclusions
This meta-analysis showed that -1607 1G/2G polymorphism may increase the susceptibility to OA among the younger populations (<60 years). More studies with detailed information are needed to validate our conclusion.
Level of Evidence
Level I Diagnostic Study.
Keywords: MMP-1, rs1799750, Polymorphism, Osteoarthritis, Meta-analysis
Introduction
Osteoarthritis (OA) is a multifactorial disease and often occurs among middle-aged and elderly people.1 The irreversible cartilage damage is the main characteristic of OA. Matrix metalloproteinase 1 (MMP-1), a member of the family of Matrix metalloproteinases (MMPs), synthesized by chondrocytes, osteoblasts, and synovial cells, can affect the regulation of cartilage damage by degrading extracellular matrix (ECM) collagen types I, II, and III.2, 3 Low expression of MMP-1 in normal cells contributes to the remodeling of healthy cartilage.4 The expression of MMP-1 in the OA chondrocytes is higher than in normal chondrocytes, indicating that MMP-1 is involved in the pathogenesis of OA.5, 6 Many kinds of cells can express the MMP-1 gene, which is located on the long arm of chromosome 11.7 Various single nucleotide polymorphisms (SNPs) in the promoter region can alter the expression level of MMP-1. It has been confirmed that an insertion/deletion of guanine at position -1607 in human MMP-1 promoter can lead to two different alleles: 1G (containing one guanine) and 2G (containing two guanines); additionally, there is a direct link between the 2G allele and the high expression of MMP-18,9. Although many recent studies have sought to clarify the relationship between 1G/2G polymorphism and the incidence of OA, the conclusions are inconsistent.
Therefore, in this study, we conducted a meta-analysis to examine whether there is a correlation between the 1G/2G polymorphism and OA risk.
Methods
Search strategy
Previous studies with relevant information for conducting the meta-analysis were retrieved from the Web of science, Embase, and Pubmed (up to April 16, 2018) using a combination of the following keywords: matrix metalloproteinase 1 or MMP-1; osteoarthritis or OA; polymorphisms or polymorphism. Additionally, the references within the included articles and reviews were checked to avoid missing other qualifying studies. Xu and Xing independently selected the articles to minimize the deviation.
Inclusion and exclusion criteria
In this meta-analysis, the articles that provided information on MMP-1 were included. Simultaneously, the articles needed to meet the following criteria: (1) the number of cases and controls were provided; (2) genotype frequency and (or) allele frequency of the cases and controls were provided; (3) the research sample was independent of other research reports; and (4) other important information for the analysis was provided.
Data extraction
Two independent researchers collected the following information from all eligible articles: (1) the first author; (2) journal name; (3) publication year; (4) population information; (5) sample size; (6) phenotype information; (7) number of genotypes in cases and controls; (8) conclusions of studies.
Statistical analysis
The Hardy–Weinberg equilibrium (HWE) was used to assess the distribution of genotypes in the control populations. A meta-analysis was used to analyze the general data. First, a heterogeneity test was conducted by a chi-squared (χ2) test. If P < 0.05, the random effect model was adopted. If P < 0.05, the fixed effect model was adopted. Meta-regression analysis was used to look for possible sources of any heterogeneity. Funnel plots were used to evaluate the publication bias, and the results were further assessed using the Begg's and Egger's tests. The strength of the association between the 1G/2G polymorphism and OA risk was assessed by the odds ratios (ORs) and confidence interval (CI). STATA software was used for the meta-analysis (version 14; Stata Corporation, College Station, TX, USA). A P value < 0.05 was considered as significant difference.
Results
Characteristics of the studies
Based on the search terms, a total of 54 studies were selected. Among these, only 5 studies were eligible after applying the criteria, and 49 studies were excluded; the detailed process of study selection is shown in Fig. 1. The first author's name, genotyping method, diagnostic criteria, publication year, ethnicity, distributions of genotypes and alleles in OA cases and controls and HWE of controls for each study are listed in Table 1 and Table 2. The genotype distributions of the control groups were all consistent with the HWE.
Fig. 1.
Flowchart of the study selection.
Table 1.
Characteristics of the included studies.
| Study | Mean age (years) |
Ethnicity | OA type | Design | Surgery | Genotyping | Cases | Controls | |
|---|---|---|---|---|---|---|---|---|---|
| Case | Control | ||||||||
| Allah 2012 | 54.2 | 51.4 | Caucasian | Knee | PCC | NO | PCR-RFLP | 100 | 100 |
| Barlas 2009 | 61.7 | 62.3 | Caucasian | Knee | HCC | NO | PCR-RFLP | 156 | 81 |
| Lepetsos 2014 | 73.1 | 73.8 | Caucasian | Knee | HCC | YES | PCR-RFLP | 155 | 139 |
| Luo 2015 | 37.2 | 33.5 | Asian | Temporomandibular | PCC | YES | PCR | 206 | 185 |
| Yang 2015 | 70.1 | 71.0 | Asian | Knee | PCC | YES | PCR-RFLP | 207 | 207 |
PCR-RFLP, polymerase chain reaction-restriction fragment length polymorphism; HCC, Hospital based case-control study; PCC, Population based case-control study.
Table 2.
Distributions of genotypes and alleles among cases and controls.
| Study | Case |
Control |
PHWE | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1G1G | 1G2G | 2G2G | 1G | 2G | 1G1G | 1G2G | 2G2G | 1G | 2G | ||
| Allah 2012 | 27 | 46 | 27 | 100 | 100 | 50 | 40 | 10 | 140 | 60 | 0.63 |
| Barlas 2009 | 31 | 57 | 68 | 119 | 193 | 5 | 24 | 52 | 34 | 128 | 0.33 |
| Lepetsos 2014 | 28 | 64 | 63 | 120 | 190 | 34 | 58 | 47 | 126 | 152 | 0.06 |
| Luo 2015 | 49 | 91 | 66 | 140 | 157 | 63 | 93 | 29 | 156 | 122 | 0.10 |
| Yang 2015 | 27 | 88 | 92 | 142 | 272 | 20 | 89 | 98 | 129 | 285 | 0.97 |
HWE, Hardy–Weinberg equilibrium.
Quantitative synthesis
The results of the meta-analysis for 1G/2G polymorphism and OA risk are listed in Table 3.
Table 3.
Meta-analysis for 1G/2G polymorphism with OA risk.
| Category | na | 2G2G vs. 1G1G |
2G2G + 2G1G vs. 1G1G |
2G2G vs. 2G1G + 1G1G |
2G vs. 1G |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I2 (%) | Pb,c | OR (95% CI) | Pd | I2 (%) | Pb,c | OR (95% CI) | Pd | I2 (%) | Pb,c | OR (95% CI) | Pd | I2 (%) | Pb,c | OR (95% CI) | Pd | |||
| Total | 5 | 87.8 | 0.00 | 0.69 (0.36–1.32) | 0.54 | 80.9 | 0.00 | 0.88 (0.47–1.63) | 0.69 | 87.1 | 0.00 | 1.30 (0.68–2.47) | 0.41 | 89.0 | 0.00 | 0.90 (0.86–1.54) | 0.66 | |
| OA type | ||||||||||||||||||
| Knee | 4 | 88.3 | 0.00 | 1.06 (0.35–3.25) | 0.90 | 84.7 | 0.00 | 1.00 (0.43–2.34) | 0.98 | 84.7 | 0.00 | 1.09 (0.55–2.14) | 0.79 | 91.1 | 0.00 | 0.96 (0.52–1.76) | 0.90 | |
| Other | 1 | / | / | 2.92 (1.64–5.19) | 0.00 | / | / | 0.60 (0.38–0.9) | 0.02 | / | / | 2.53 (1.54–4.15) | 0.00 | / | / | 0.69 (0.50–0.96) | 0.03 | |
| Ethnicity | ||||||||||||||||||
| Asian | 2 | 90.6 | 0.00 | 1.22 (0.25–5.99) | 0.61 | 79.0 | 0.02 | 0.89 (0.39–2.03) | 0.79 | 90.7 | 0.00 | 1.48 (0.53–4.14) | 0.45 | 80.2 | 0.02 | 0.90 (0.55–1.47) | 0.68 | |
| Caucasian | 3 | 91.0 | 0.00 | 1.22 (0.25–5.99) | 0.80 | 87.4 | 0.00 | 0.91 (0.30–2.79) | 0.87 | 89.6 | 0.00 | 1.20 (0.42–3.48) | 0.72 | 93.6 | 0.00 | 0.90 (0.37–2.20) | 0.83 | |
| Age | ||||||||||||||||||
| <60 | 2 | 2.5 | 0.31 | 3.46 (2.13–5.62) | 0.00 | 41.3 | 0.19 | 0.49 (0.31–0.79) | 0.00 | 0.0 | 0.56 | 2.74 (1.80–4.16) | 0.00 | 69.7 | 0.06 | 0.56 (0.35–0.89) | 0.01 | |
| ≥60 | 3 | 83.2 | 0.00 | 0.66 (0.23–1.88) | 0.43 | 78.7 | 0.00 | 1.42 (0.59–3.39) | 0.43 | 78.6 | 0.00 | 0.82 (0.45–1.46) | 0.49 | 87.3 | 0.00 | 1.25 (0.71–2.19) | 0.44 | |
I2, 0–25: no heterogeneity; 25–50: modest heterogeneity; 50: high heterogeneity.
Number of studies.
P value for heterogeneity test.
Random effect model was used when P value < 0.05 for heterogeneity test; otherwise, fixed effect model was used.
P value for each test.
Overall population
After screening, 5 studies were finally selected for conducting the meta-analysis. Upon completion of whole analysis, no significant association was observed in all the models (2G2G vs. 1G1G, OR (95%CI) = 0.69 (0.36–1.32), P = 0.54; 2G2G + 2G1G vs. 1G1G, OR (95%CI) = 0.88 (0.47–1.63), P = 0.69; 2G2G vs. 2G1G + 1G1G, OR (95%CI) = 1.30 (0.68–2.47), P = 0.41; 2 G vs. 1G, OR (95%CI) = 0.90 (0.86–1.54), P = 0.66) (Table 3, Fig. 2).
Fig. 2.
Forest plot of the association between 1G/2G polymorphism and OA risk (2G vs. 1G).
Subgroup analysis
In our study, we found that a relationship between the 1G/2G polymorphism and OA risk only existed among the “< 60 years” group (2G2G vs. 1G1G, OR (95% CI) = 3.46 (2.13–5.62), P = 0.00; 2G2G + 2G1G vs. 1G1G, OR (95% CI) = 0.49 (0.31–0.79), P = 0.00; 2G2G vs. 2G1G + 1G1G, OR (95% CI) = 2.74 (1.80–4.16, P = 0.00; 2G vs. 1G, OR (95% CI) = 0.56 (0.35–0.89), P = 0.01). No significant association was found in other groups (Table 3, Fig. 3).
Fig. 3.
Forest plot of the association between 1G/2G polymorphism and OA risk in the “< 60 years” group (2G vs. 1G).
Test of heterogeneity
Heterogeneity was observed in all the subjects. Thus, a random effects model was adopted except for the “< 60 years” group. In order to find the possible sources of heterogeneity, we carried out a meta-regression analysis. However, no source of heterogeneity was found except for age. Next, based on the types of OA, ethnicity, and age, we carried out subgroup analyses.
Publication bias
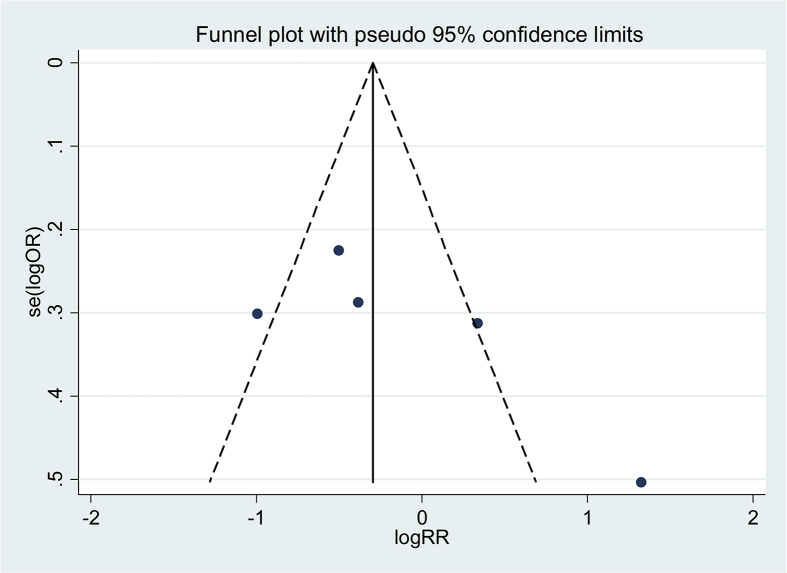
The potential publication bias was assessed qualitatively using funnel plots. Taking the allele contrast model (2G vs. 1G) as an example, we analyzed the results of the funnel plots and found no apparent asymmetry (Fig. 4). Moreover, the potential publication bias was tested by the Begg's and Egger's tests, for which the P values were all greater than 0.05 (Egger's: P = 0.89; Begg's: P = 0.80), indicating no publication bias.
Fig. 4.
Funnel plot for publication bias test (2G vs. 1G).
Discussion
Lately, there have been an increasing number of studies examining the association between genetic polymorphisms and the occurrence of OA. Genetic factors have been reported to play a key role in the occurrence of OA.10 Notably, family and twin studies have shown that genetic factors have a significant influence on more than half of the patients with OA.11, 12 Many genes have been reported to promote the occurrence and development of OA, although the effects were relatively minor.13 MMP-1 is one such important gene that has been most closely associated with OA.5, 18, 19 Recently, multiple studies were conducted to find the association between 1G/2G polymorphism and OA risk2, 14, 15, 16, 17; however, the results were inconsistent.
The current study aimed to conduct a meta-analysis to find an association between 1G/2G polymorphism and OA risk among different studies. In this meta-analysis, no significant association was demonstrated in any of the models, which is inconsistent with the conclusions of other studies on MMP-1 polymorphism. Many factors can lead to the occurrence of OA, such as different genetic backgrounds and lifestyles. Type II error could also lead to inaccuracy of the result of 1G/2G polymorphism. Recently, some genes, such as GDF5, FILIP1, and COG5, have been confirmed to have a close relationship with occurrence of OA by genome-wide association studies (GWAS); however, MMP-1 1G/2G polymorphism was not confirmed.20 Moreover, other factors, including age, sex, and environmental factors, are considered to be related to the occurrence of OA. Thus, we carried out subgroup-analysis and found that the relationship between 1G/2G polymorphism and OA risk only existed among the “< 60 years” group, but not among other groups. This result is consistent with some studies, where a significant association was found between 1G/2G SNP polymorphism and knee OA, when the average age of the population was about 50 years old.15, 16 It is not yet completely clear why this link exists only in young populations. However, we propose that at a young age, the pathogenic factors and pathogenesis may be relatively simple, and genes may play a leading role in the development of the disease. With aging, the internal and external environment of the body changes, likely allowing multiple other pathogenic factors to influence the pathogenesis, which becomes complex. Thus, the role of genes may become relatively weak at an older age. In addition, the differences in lifestyle and environmental factors among different groups of people are related to occurrence of OA and may also interact with genes.
This meta-analysis study has some inevitable limitations. First, there was considerable heterogeneity between studies on 1G/2G polymorphism, which may lead to misinterpretation of the meta-analysis results. Second, the total sample size from all eligible studies may not be enough to draw a robust conclusion. In addition, information on factors proven to be closely related to the occurrence of OA, such as smoking, trauma, overweight and drug therapy, were not available or considered in this study. Future studies including such detailed information may lead to more accurate conclusions.
Conclusions
In conclusion, the meta-analysis shows that 1G/2G polymorphism may increase the susceptibility to OA among the younger population. However, because of the existence of inevitable limitations, the conclusion should be carefully interpreted and more studies with detailed information are needed to validate our conclusion.
Funding statement
This work was supported by the National Natural Science Foundation of China (No. 81774334), Jiangsu Provincial Bureau of traditional Chinese Medicine program (No. YB2017023, No. 2015NL-068-02), Jiangsu provincial health and Family Planning Commission program (No. BJ15019), Jiangsu Key R & D project (No. BE2017774).
Conflicts of interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Acknowledgments
No acknowledgments.
Footnotes
Peer review under responsibility of Turkish Association of Orthopaedics and Traumatology.
Contributor Information
Bo Xu, Email: xubo12080@163.com.
Run-lin Xing, Email: xingrunlin@126.com.
Li Zhang, Email: zhang4462053@126.com.
Zheng-quan Huang, Email: 5964990@qq.com.
Nong-Shan Zhang, Email: 357076591@qq.com.
Jun Mao, Email: junmao1978@hotmail.com.
References
- 1.Zhang R., Yao J., Xu P. A comprehensive meta-analysis of association between genetic variants of GDF5 and osteoarthritis of the knee, hip and hand. Inflamm Res. 2015;64(6):405–414. doi: 10.1007/s00011-015-0818-9. [DOI] [PubMed] [Google Scholar]
- 2.Lepetsos P., Pampanos A., Kanavakis E. Association of MMP-1 -1607 1G/2G (rs1799750) polymorphism with primary knee osteoarthritis in the Greek population. J Orthop Res. 2014;32(9):1155–1160. doi: 10.1002/jor.22647. [DOI] [PubMed] [Google Scholar]
- 3.Kaspiris A., Khaldi L., Grivas T.B. Subchondral cyst development and MMP-1 expression during progression of osteoarthritis: an immunohistochemical study. Orthop Traumatol Surg Res. 2013;99(5):523–529. doi: 10.1016/j.otsr.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Vincenti M.P., Brinckerhoff C.E. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002;4(3):157–164. doi: 10.1186/ar401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandes J.C., Martel-Pelletier J., Lascau-Coman V. Collagenase-1 and collagenase-3 synthesis in normal and early experimental osteoarthritic canine cartilage: an immunohistochemical study. J Rheumatol. 1998;25(8):1585–1594. [PubMed] [Google Scholar]
- 6.Tetlow L.C., Adlam D.J., Woolley D.E. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum. 2001;44(3):585–594. doi: 10.1002/1529-0131(200103)44:3<585::AID-ANR107>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 7.Brinckerhoff C.E., Rutter J.L., Benbow U. Interstitial collagenases as markers of tumor progression. Clin Cancer Res. 2000;6(12):4823–4830. [PubMed] [Google Scholar]
- 8.Rutter J.L., Mitchell T.I., Buttice G. A single nucleotide polymorphism in the matrix metalloproteinase-1 promoter creates an Ets binding site and augments transcription. Cancer Res. 1998;58(23):5321–5325. [PubMed] [Google Scholar]
- 9.Arakaki P.A., Marques M.R., Santos M.C. MMP-1 polymorphism and its relationship to pathological processes. J Biosci. 2009;34(2):313–320. doi: 10.1007/s12038-009-0035-1. [DOI] [PubMed] [Google Scholar]
- 10.Lee Y.H., Rho Y.H., Choi S.J., Ji J.D., Song G.G. Osteoarthritis susceptibility loci defined by genome scan meta-analysis. Rheumatol Int. 2006;26(11):959–963. doi: 10.1007/s00296-006-0181-9. [DOI] [PubMed] [Google Scholar]
- 11.MacGregor A.J., Spector T.D. Twins and the genetic architecture of osteoarthritis. Rheumatology (Oxford) 1999;38(7):583–588. doi: 10.1093/rheumatology/38.7.583. [DOI] [PubMed] [Google Scholar]
- 12.Bijkerk C., Houwing-Duistermaat J.J., Valkenburg H.A. Heritabilities of radiologic osteoarthritis in peripheral joints and of disc degeneration of the spine. Arthritis Rheum. 1999;42(8):1729–1735. doi: 10.1002/1529-0131(199908)42:8<1729::AID-ANR23>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 13.Valdes A.M., Doherty M., Spector T.D. The additive effect of individual genes in predicting risk of knee osteoarthritis. Ann Rheum Dis. 2008;67(1):124–127. doi: 10.1136/ard.2007.075838. [DOI] [PubMed] [Google Scholar]
- 14.Barlas I.O., Sezgin M., Erdal M.E. Association of (-1,607) 1G/2G polymorphism of matrix metalloproteinase-1 gene with knee osteoarthritis in the Turkish population (knee osteoarthritis and MMPs gene polymorphisms) Rheumatol Int. 2009;29(4):383–388. doi: 10.1007/s00296-008-0705-6. [DOI] [PubMed] [Google Scholar]
- 15.Yang H.Y., Chuang S.Y., Fang W.H. Effect of RAGE polymorphisms on susceptibility to and severity of osteoarthritis in a Han Chinese population: a case-control study. Genet Mol Res. 2015;14(3):11362–11370. doi: 10.4238/2015.September.25.3. [DOI] [PubMed] [Google Scholar]
- 16.Abd-Allah S.H., Shalaby S.M., Pasha H.F., El-Shal A.S., Abou E.A. Variation of matrix metalloproteinase 1 and 3 haplotypes and their serum levels in patients with rheumatoid arthritis and osteoarthritis. Genet Test Mol Biomarkers. 2012;16(1):15–20. doi: 10.1089/gtmb.2011.0003. [DOI] [PubMed] [Google Scholar]
- 17.Luo S., Deng M., Long X., Li J., Xu L., Fang W. Association between polymorphism of MMP-1 promoter and the susceptibility to anterior disc displacement and temporomandibular joint osteoarthritis. Arch Oral Biol. 2015;60(11):1675–1680. doi: 10.1016/j.archoralbio.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Shlopov B.V., Lie W.R., Mainardi C.L., Cole A.A., Chubinskaya S., Hasty K.A. Osteoarthritic lesions: involvement of three different collagenases. Arthritis Rheum. 1997;40(11):2065–2074. doi: 10.1002/art.1780401120. [DOI] [PubMed] [Google Scholar]
- 19.Aigner T., Zien A., Gehrsitz A., Gebhard P.M., McKenna L. Anabolic and catabolic gene expression pattern analysis in normal versus osteoarthritic cartilage using complementary DNA-array technology. Arthritis Rheum. 2001;44(12):2777–2789. doi: 10.1002/1529-0131(200112)44:12<2777::aid-art465>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 20.Zeggini E., Panoutsopoulou K., Southam L. Identification of new susceptibility loci for osteoarthritis (arcOGEN): a genome-wide association study. Lancet. 2012;380(9844):815–823. doi: 10.1016/S0140-6736(12)60681-3. [DOI] [PMC free article] [PubMed] [Google Scholar]