Abstract
Objective
To examine risks for major structural birth defects in infants after first trimester inactivated influenza vaccine (IIV) exposures.
Study design
In this observational study, we used electronic health data from 7 Vaccine Safety Datalink sites to examine risks for selected major structural defects in infants after maternal IIV exposure. Vaccine exposures for women with continuous insurance enrollment through pregnancy who delivered singleton live births between 2004 and 2013 were identified from standardized files. Infants with continuous insurance enrollment were followed to 1 year of age. We excluded mother–infant pairs with other exposures that potentially increased their background risk for birth defects. Selected cardiac, orofacial or respiratory, neurologic, ophthalmologic or otologic, gastrointestinal, genitourinary and muscular or limb defects were identified from diagnostic codes in infant medical records using validated algorithms. Propensity score adjusted generalized estimating equations were used to estimate prevalence ratios (PRs).
Results
We identified 52 856 infants with maternal first trimester IIV exposure and 373 088 infants whose mothers were unexposed to IIV during first trimester. Prevalence (per 100 live births) for selected major structural birth defects was 1.6 among first trimester IIV exposed versus 1.5 among unexposed mothers. The adjusted PR was 1.02 (95% CI 0.94-1.10). Organ system-specific PRs were similar to the overall PR.
Conclusion
First trimester maternal IIV exposure was not associated with an increased risk for selected major structural birth defects in this large cohort of singleton live births.
Pregnant women and newborns have long been recognized as being at risk for increased morbidity and mortality from influenza infections.1 As such, pregnant women are a priority group for prevention through vaccination. Since 2004, the Advisory Committee on Immunization Practices has recommended that women who will be pregnant during the influenza season receive the inactivated influenza vaccine (IIV) in any trimester of pregnancy.2 Although initial adherence with these guidelines was low,3 a national survey of women pregnant during the 2014-2015 influenza season noted that about 50% reported receiving IIV before or during pregnancy.4
Data on the effectiveness and safety of maternal influenza vaccination have been reassuring.5-10 Nevertheless, general concerns about potential adverse events in infants after maternal receipt of influenza vaccine during pregnancy persist.11-13 Given that IIV is explicitly recommended during the first trimester, a period of fetal organogenesis, continued monitoring of birth outcomes after maternal vaccination is important. Existing studies on maternal IIV and birth defects in offspring have been limited by varied definitions of birth defect outcomes and have been underpowered for assessing risks associated with first trimester vaccine exposures.14,15 We used the Vaccine Safety Datalink (VSD) to identify a large, multisite cohort of pregnant women and their infants to examine risks for major structural birth defects after maternal receipt of IIV in the first trimester.
Methods
The VSD is a collaboration between the Centers for Disease Control and Prevention Immunization Safety Office and eight integrated healthcare systems. The aim of the VSD is to monitor the safety of vaccines routinely administered within the United States.16 We analyzed data from 7 participating VSD sites in 6 states (California, Colorado, Minnesota, Oregon, Washington, and Wisconsin) for the present study. The study was approved by the institutional review board at each participating site and the Centers for Disease Control and Prevention.
Our study population included pregnant women who delivered a live-born infant from January 1, 2004, through September 1, 2013, at a participating VSD site, identified using a validated algorithm17 and with linked birth records available. Women were required to have continuous insurance enrollment from 6 months before pregnancy through 6 weeks post-partum and have at least 1 medical encounter during pregnancy. Eligible women were also required to have, at minimum, a 1-week period during their first trimester overlap with a time period when influenza vaccine was available. For each influenza season, vaccine availability was defined as the time period between the first and last IIV administration within the cohort.
Infants surviving to 1 year of age were required to have at least 4 months of continuous insurance enrollment and at least 1 outpatient encounter. To help ensure identification of infants diagnosed with a severe major structural birth defect, those who died before 1 year of age or were hospitalized after birth for 30 days or longer were retained in the cohort even if they did not meet insurance enrollment or healthcare use criteria. We excluded women with multiple gestation pregnancies, those with prepregnancy or gestational diabetes, those with neoplasms, and those diagnosed with potentially teratogenic infections (eg, syphilis or toxoplasmosis) or exposures to teratogenic medications (eg, warfarin or phenytoin). In addition, women with exposures to live virus vaccine during their first trimester were excluded. Infants with birth weight of less than 350 g, gestational age of less than 20 weeks, or postnatally diagnosed chromosomal anomalies or congenital infections were also excluded. A full list of exclusions is available (Table I; available at www.jpeds.com).
Table I.
Exclusions applied, based on maternal or infant diagnoses or pharmacy fills
| Exclusion types | Name | ICD-9 code or other codes | Risk window for exclusion |
|---|---|---|---|
| Teratogenic medications | Acitretin | Not applicable, medications identified by Generic Product Identifier codes | One more pharmacy fill 6 months before pregnancy start through end of pregnancy |
| Amiodarone | |||
| Azathioprine | |||
| Bexarotene | |||
| Carbamazepine | |||
| Dronedarone | |||
| Isotretinoin | |||
| Lithium | |||
| Misoprostol | |||
| Methotrexate | |||
| Mycophenolate mofetil | |||
| Phenobarbital | |||
| Phenytoin | |||
| Primidone | |||
| Topiramate | |||
| Thalidomid | |||
| Valproic acid | |||
| Warfarin | |||
| Teratogenic infections | Cytomegalovirus | 078.5x | One or more diagnoses in mother’s records during pregnancy or in infant's records during first year of life |
| Syphilis | 091.x−097.x, 647.0x | ||
| Rubella | 056.xx, 647.5x, 655.3,771.0 | ||
| Toxoplasmosis | 130.x, 647.8x, 771.2 | ||
| Varicella | 052.x | ||
| Chromosomal anomalies | Trisomy 21 | 758.0x | One or more diagnoses in infant's records during the first year of life |
| Trisomy 13 | 758.1x | ||
| Trisomy 18 | 758.2x | ||
| Cri-du-chat | 758.31 | ||
| 22q11.2 deletion syndrome | 758.32, 279.11 | ||
| Miller-Dieker syndrome | 758.33 | ||
| Smith-Magenis syndrome | 758.33 | ||
| Gonadal dysgenesis | 758.6 | ||
| Klinefelter syndrome | 758.7 | ||
| Maternal comorbidities | Diabetes | 250.xx | One or more diagnoses from 6 months before pregnancy through first trimester |
| Malignant neoplasms | 140-208 | ||
| Live virus vaccines | Bacillus of Calmette and Guerin | Identified through Health Level-7 codes | One or more live virus vaccines administered in first trimester of pregnancy |
| Live attenuated influenza | |||
| Live oral typhoid | |||
| Measles-mumps-rubella | |||
| Rabies | |||
| Rotavirus | |||
| Varicela | |||
| Yellow fever |
Vaccinations were identified from standardized VSD files, which include data from electronic health records, medical and pharmacy claims, and linkage with state immunization information systems. Historical vaccines, administered at health fairs, schools, or the work place, were available if manually recorded at a healthcare visit. Using data on gestational age (in weeks) at birth and date of birth obtained from electronic health records or birth certificates, and based on clinician assessment at birth, we assigned a pregnancy start date to each pregnancy. Influenza vaccinations identified during pregnancy were categorized by gestational week of pregnancy. First trimester vaccination was defined as receipt of IIV before 14 weeks of gestation. Women receiving IIV in their second or third trimester or who did not receive IIV during pregnancy were classified as IIV unexposed during first trimester. The study period did include a period of monovalent H1N1 vaccine availability. Exposures to the monovalent H1N1 vaccine were captured in “other vaccines” received during pregnancy.
Major structural birth defects were identified from International Classification of Diseases, Ninth Revision (ICD-9) diagnostic codes occurring at outpatient, emergency, or inpatient visits in infant medical records. Our primary outcome was the presence of 1 or more prespecified major structural birth defects. As previously described, to improve specificity for our ICD-9–based outcomes, we applied outcome-specific algorithms.18 For example, congenital diaphragmatic hernia is defined as having 1 inpatient diagnosis by 3 months of age. Algorithms were developed and validated within a sample of the VSD population. These outcomes have been applied in a prior study on the safety of maternal pertussis vaccination.19 In secondary analyses, we examined structural birth defect outcomes by organ system (central nervous system, ophthalmologic or otologic, gastrointestinal, respiratory or orofacial, cardiac, genitourinary or renal, muscular or limb defects). We also examined severe cardiac defects, neural tube defects, microcephaly, and cleft lip or cleft palate as individual safety outcomes, applying outcome-specific algorithms for identification.
Descriptive statistics were used to compare baseline demographic variables, comorbidities, and healthcare use variables between first trimester exposed and unexposed women. Propensity scores, predicting the probability of influenza vaccination during first trimester, were developed using a regression generalized additive model with a smooth variable for maternal age and study week of last menstrual period. Other covariates, identified through diagnostic coding, pharmacy claims, or electronic health data and included in the propensity score were maternal race/ethnicity, neighborhood poverty level, Kotelchuck adequacy of prenatal care index,20,21 smoking during pregnancy, hospitalization in the first 20 weeks of pregnancy, comorbidities (ie, hypertension and other cardiovascular disease, lung disease, nutritional deficiencies, neurologic conditions, renal disease, rheumatic disorders and thyroid disorders), exposure to a possibly teratogenic medication (ie, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, aliskiren, paroxetine, trimethoprim, and trimetrexate), and VSD site. Model fit for the propensity score was evaluated by the C-statistic and Hosmer-Lemeshow goodness-of-fit test.
A generalized linear model with Poisson distribution and log link was used to estimate prevalence ratios (PRs) with 95% CIs. Poisson distribution with identity link with robust variance estimation was used to estimate prevalence differences (PDs) with 95% CIs. Our primary contrasts were between women with first trimester IIV exposures and those unexposed during their first trimester. In addition to unadjusted PRs and PDs, we obtained adjusted estimates by adding propensity scores to the models as quintiles. In secondary analyses, we report PRs and PDs for infants, comparing those with maternal first trimester IIV exposures with those with no IIV exposure during pregnancy. Based on our available sample size, in primary analyses, we had 80% power to detect a 0.23 per 100 live birth PD for selected major structural defects with a background rate of 1.6 per 100 live births. For severe cardiac defects, we had 80% power to detect an 8 per 10 000 live birth PD with a background rate of approximately 17 per 10 000 live births.18 All analyses were conducted using SAS version 9.3 (SAS Institute, Cary, North Carolina).
Results
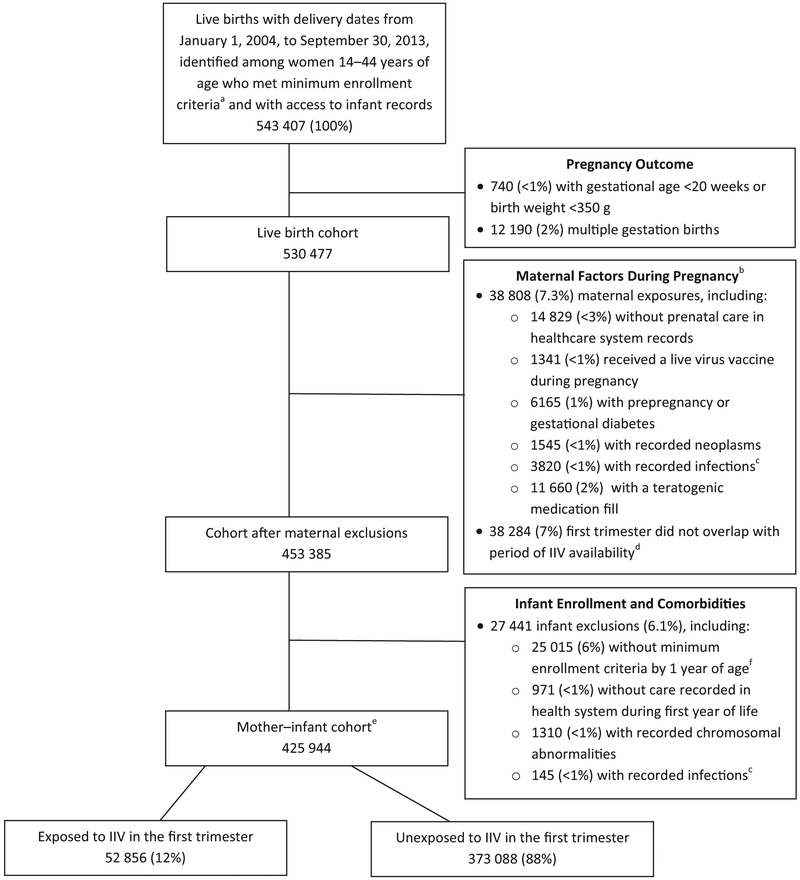
We initially identified 530 477 pregnant women with singleton live births meeting insurance enrollment criteria. Of these, 38 808 (7.3%) were excluded based on maternal factors (comorbidities, use of teratogenic medications, receipt of a live virus vaccine, or no recorded prenatal care in a VSD health system). An additional 38 284 (7.2%) were excluded because their first trimester of pregnancy did not overlap with influenza vaccine availability, as defined in the Methods. Of the 453 385 pregnant women remaining after maternal exclusions, 27 441 (6.1%) were excluded based on infant insurance enrollment, healthcare use, or clinical criteria (Figure 1; available at www.jpeds.com). Our final cohort consisted of 425 944 mother-infant pairs, of whom 52 856 mothers (12%) received IIV in their first trimester of pregnancy. Of the 373 088 women who did not receive IIV during their first trimester, 90 991 (24%) received IIV in their second or third trimester of pregnancy. Vaccinations occurred in 10 influenza seasons, with increases starting in the 2009-2010 season (Table II; available at www.jpeds.com).
Figure 1.
Flow chart of identified pregnancies ending in a live birth and exclusion criteria used to obtain mother–infant cohort at 7 Vaccines Safety Datalink sites, January 1, 2004, to September 30, 2013. aContinuous insurance enrollment from 6 months before the last menstrual period (LMP) through 6 weeks postpartum. bMore than 1 medical condition may apply to a single pregnant female. cToxoplasmosis, syphilis, varicella, rubella, or cytomegalovirus. dAfter excluding no medical care within the healthcare system during pregnancy, medical condition, teratogenic medication, or live virus vaccine. eincludes infants with mortality during the first year of life and infants hospitalized for more than 30 days after birth who were not required to meet insurance enrollment criteria. fFor infants surviving to 1 year, enrollment in a health insurance plan for 4 months during the first year and at least 1 month of insurance in the first 3 months of life.
Table II.
First trimester influenza vaccination, by season
| Influenza seasons | Vaccine composition | Unexposed (n = 373 088), n (%) | IIV Exposed in first trimester (n = 52 856), n (%) |
|---|---|---|---|
| 2003-2004 | A/New Caledonia/20/99-like (H1N1) A/Moscow/10/99-like (H3N2) B/Shanghai/361/2002-likeB |
32 854 (8.8) | 836 (1.6) |
| 2004-2005 | A/New Caledonia/20/99-like (H1N1) A/California/7/2004-like (H3N2) B/Shanghai/361/2002-likeB |
40 875 (11.0) | 3051 (5.8) |
| 2005-2006 | A/New Caledonia/20/99-like (H1N1) A/Fujian/411/2002-like (H3N2) B/Shanghai/361/2002-like |
41 892 (11.2) | 2804 (5.3) |
| 2006-2007 | A/New Caledonia/20/99-like (H1N1) A/Wisconsin/67/2005-like (H3N2) B/Malaysia/2506/2004-like |
41 643 (11.2) | 3777 (7.2) |
| 2007-2008 | A/Solomon Islands/3/2006-like (H1N1) A/Wisconsin/67/2005-like (H3N2) B/Malaysia/2506/2004-like |
41 217 (11.1) | 4175 (7.9) |
| 2008-2009 | A/Brisbane/59/2007-like (H1N1) A/Brisbane/10/2007-like (H3N2) B/Florida/4/2006-like |
40 466 (10.9) | 4757 (9.0) |
| 2009-2010 | A/Brisbane/59/2007-like (H1N1) A/Brisbane/10/2007-like (H3N2) B/Brisbane/60/2008-like |
36 315 (9.7) | 8648 (16.4) |
| 2010-2011 | A/California/7/2009-like (H1N1) A/Perth/16/2009-like (H3N2) B/Brisbane/60/2008 |
37 230 (10.0) | 7836 (14.8) |
| 2011-2012 | A/California/7/2009-like (H1N1) A/Perth/16/2009-like (H3N2) B/Brisbane/60/2008 |
37 851 (10.2) | 8423 (15.9) |
| 2012-2013 | A/California/7/2009-like (H1N1) A/Victoria/361/2011-like (H3N2) B/Wisconsin/1/2010-like |
22 745 (6.1) | 8549 (16.2) |
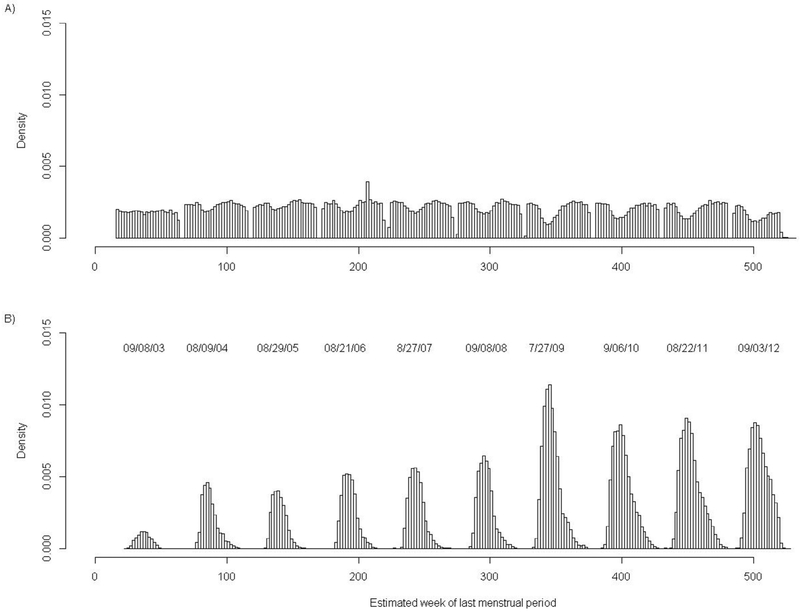
In our final cohort, the majority of pregnant women were aged 25-34 years (62%), 39% were white non-Hispanic, 29% were Hispanic, and 16% were Asian (Table III). Most women received medical care in their first trimester and had an adequate/plus Kotelchuck prenatal care index.21 As compared with women unexposed to IIV in the first trimester, women exposed to IIV were significantly more likely to have been aged 25 years or older, white, non-Hispanic, have received medical care in their first trimester, or have had preexisting lung disease. Women exposed to IIV in the first trimester were also more likely to have received another inactivated vaccine during the first trimester (41% vs 23%). Other vaccines identified included the tetanus, diphtheria, acellular pertussis vaccine (77%), monovalent H1N1 vaccine (22%), and meningococcal vaccine (<1%) (Table I). Even after excluding women whose first trimester did not overlap with periods of influenza vaccine availability, the strongest predictor of receiving IIV in first trimester was the timing of the start of pregnancy or last menstrual period being in September (Figure 2; available at www.jpeds.com). Model fit for the propensity was adequate. The C statistic was 0.685 and the P value was less than .0001 (8 df) for the Hosmer-Lemeshow test.
Table III.
Baseline characteristics of inactivated first trimester IlV-exposed and IIV-unexposed women with a live birth at 7 VSD sites, 2004-2013
| Unexposed (n = 373 088), n (%) |
IIV Exposed in first trimester (n = 52 856), n (%) |
|
|---|---|---|
| Age at delivery, years | ||
| <18 | 5270 (1.4) | 399 (0.8) |
| 18-24 | 50 972 (13.7) | 5212 (9.9) |
| 25-34 | 229 684 (61.6) | 33 967 (64.3) |
| ≥35 | 87 162 (23.4) | 13 278 (25.1) |
| Race/ethnicity | ||
| Asian | 57 535 (15.4) | 8922 (16.9) |
| Black, non-Hispanic | 28 280 (7.6) | 2467 (4.7) |
| Hispanic | 110 879 (29.7) | 13 821 (26.2) |
| Other | 32 738 (8.8) | 4376 (8.3) |
| White, non-Hispanic | 143 656 (38.5) | 23 270 (44.0) |
| Poverty, %* | 16% (11%) | 15% (10%) |
| Medical care in first trimester | 333 547 (89.4) | 50 716 (96.0) |
| Prenatal Care Index | ||
| Adequate/plus | 259 874 (69.7) | 39 428 (74.6) |
| Intermediate | 78 002 (20.9) | 11 273 (21.3) |
| Inadequate | 35 212 (9.4) | 2155 (4.1) |
| Hospitalized before 20 weeks of gestation | ||
| No hospitalization | 363 884 (97.5) | 51 465 (97.4) |
| 1 hospitalization | 7653 (2.1) | 1156 (2.2) |
| ≥2 hospitalizations | 1551 (0.4) | 235 (0.4) |
| Received other vaccine during pregnancy† | 84 267 (22.6) | 21 734 (41.1) |
| Received possibly teratogenic medication before or during pregnancy‡ | 21 756 (5.8) | 2985 (5.6) |
| Comorbidities§ | ||
| Heart disease (excluding hypertension) | 3435 (0.9) | 607 (1.1) |
| Hypertension | 7021 (1.9) | 1113 (2.1) |
| Renal disease | 3532 (0.9) | 517 (1.0) |
| Lung disease | 21 103 (5.7) | 3593 (6.8) |
| Anemia | 12 353 (3.3) | 1955 (7.1) |
| Neurologic | 1468 (0.4) | 266 (0.5) |
| Rheumatologic | 1251 (0.3) | 215 (0.4) |
| Thyroid | 2415 (0.6) | 496 (0.9) |
| Ever smoked cigarettes | 28 260 (7.6) | 3762 (7.1) |
Percentage of families in census tract whose income was below 150% of the federal poverty level.
Other vaccines administered in first trimester included monovalent H1N1 inactivated vaccine (21.8%), meningococcal (0.5%), and acellular pertussis vaccines (77.7%).
Possibly teratogenic medications: angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, aliskiren, paroxetine, trimethoprim and trimetrexate (women with exposures to confirmed teratogens were excluded).
Based on diagnoses at inpatient, outpatient, or emergency visits from 6 months before pregnancy through the end of the pregnancy.
Figure 2.
Histogram of estimated week at last menstrual period (LMP) by vaccination status, for the final study cohort, across 10 influenza seasons. A, Women who did not receive IIV during first trimester. B, Women vaccinated with IIV during first trimester. Week 0 corresponds to the week starting January 1, 2003. Peaks in first trimester IIV administration, by week of LMP, by influenza season, were as follows: 2003-2004 (LMP = week 37 or September 8, 2003); 2004-2005 (LMP = week 85 or August 9, 2004); 2005-2006 (LMP = week 139 or August 29, 2005); 2006-2007 (LMP = week 190 or August 21, 2006); 2007-2008 (LMP = week 243 or August 27, 2007); 2008-2009 (LMP = week 297 or September 8, 2008); 2009-2010 (LMP = week 343 or July 27, 2009); 2010-2011 (LMP = week 400 or September 6, 2010); 2011-2012 (LMP = week 450 or August 22, 2011); and 2012-2013 (LMP = week 504 or September 3, 2012).
Among 52 856 women who were exposed to IIV in the first trimester, 865 (1.6 per 100 live births) had an infant with a selected major structural defect versus 5730 (1.5 per 100 live births) in the first trimester unexposed group. The adjusted PR for having a selected major structural birth defect after first trimester IIV was 1.02 (95% CI 0.94-1.10). The most common subgroup of major structural birth defects observed was cardiac defects, occurring in 58 per 10 000 live births among first trimester IIV-exposed women versus 56 per 10 000 live births in unexposed women. The adjusted PR for cardiac defects was 1.0 (95% CI 0.89-1.10). A subset of 14 severe cardiac defects, likely to require urgent or emergent intervention at birth, occurred in 13 per 10 000 live births among first trimester IIV-exposed women and 12 per 10 000 live births among unexposed women, with an adjusted PR of 0.99 (95% CI 0.76-1.28) (Table IV).
Table IV.
Selected birth defect outcomes and first trimester maternal IIV exposure as compared with unexposed in first trimester
| Birth defect types | Unexposed in first trimester (n = 373 088) |
IIV Exposed in first trimester (n = 52 856) |
Adjusted PD 95% CI, P value |
Adjusted PR, P value |
|---|---|---|---|---|
| Selected major structural birth defects* | 5730 (1.5 per 100 live births) | 865 (1.6 per 100 live births) | 0.03% (−0.10 to .15), .63 | 1.02 (0.94-1.10), .63 |
| Selected major structural birth defects by organ system | n (rate per 10 000 live births) | n (rate per 10 000 live births) | Per 10 000 live births | |
| Any cardiac | 2076 (56) | 308 (58) | 0 (−7 to 7), .97 | 1.00 (0.89-1.10), .97 |
| Severe cardiac | 453 (12) | 68 (13) | 0 (−4 to 3), .83 | 0.99 (0.76, 1.30), .92 |
| Central nervous system | 596 (16) | 87 (16) | 0 (−4 to 4), .91 | 1.0 (0.8, 1.3), .98 |
| Neural tube defects | 95 (3) | 9 (2) | −1 (−2 to −1), .04 | 0.59 (0.30, 1.20), .14 |
| Microcephaly | 419 (11) | 62 (12) | 0 (−3 to 4), .69 | 1.05 (0.80, 1.39), .70 |
| Ophthalmologic or otologic | 156 (4) | 23 (4) | 0 (−2 to 2), .90 | 1.05 (0.67-1.70), .82 |
| Gastrointestinal | 856 (23) | 114 (22) | 0 (−4 to 4), .98 | 0.99 (0.81-1.20), .92 |
| Genitourinary or renal | 1443 (39) | 247 (47) | 3 (−3 to 9), .36 | 1.06 (0.93-1.20), .39 |
| Muscular or limb defects | 309 (8) | 44 (8) | −0 (−3 to 2), .73 | 0.92 (0.67, 1.30), .63 |
| Respiratory or orofacial | 500 (13) | 73 (14) | 0 (−3 to 4), .90 | 1.00 (0.77, 1.30), .99 |
| Cleft lip and/or cleft palate | 484 (13) | 68 (13) | 0 (−4 to 3), .92 | 0.97 (0.74, 1.30), .81 |
Selected major structural birth defects include the following diagnoses, with outcome-specific algorithms applied: neural tube defects (spina bifida [741.0x and 741.9x], encephalocele, cranial meningocele, or encephalomyelocele [742.0]), other central nervous system (microcephaly [742.1], holoprosencephaly [742.2]), ophthalmologic (anophthalmia or microphthalmia [743.00 and 743.10-743.12], cataracts and other lens defects [743.2x and 743.30-743.36]), otologic (anotia or microtia [744.01 and 744.23]), severe cardiac (single ventricle, tricuspid atresia, Ebstein anomaly, hypoplastic left heart, hypoplastic right heart, common truncus, transposition, atrioventricular septal defects, tetralogy of Fallot, aortic valve atresia/stenosis, coarctation, total anomalous pulmonary venous return, and anomalous coronary artery [745.0, 745.1x, 745.2-745.3, 745.6x, 745.7, 746.00, 746.01, 746.1-746.3, 746.7, 746.85, 747.1x, 747.22, and 747.41]), other cardiac (septal defects, heterotaxy, partial anomalous pulmonary venous return [745.4, 745.8, 745.9, 759.3, and 747.42]), cleft lip or cleft palate 749.0, 749.00-749.04, 749.1, 749.10-749.14, 749.2, and 749.20-749.25), respiratory (choanal atresia [748.0]), gastrointestinal (esophageal atresia with or without tracheoesophageal fistula [750.3], pyloric stenosis [750.5], intestinal atresia or stenosis [751.1 and 751.2], biliary atresia [751.61]), genitourinary or renal (second- or third-degree hypospadias [752.61], renal agenesis or hypoplasia [753.0], renal dysplasia [753.15], congenital hydronephrosis [753.2x], bladder exstrophy [753.5], posterior urethral valve and/or prune belly [753.60 and 756.71]), and muscular or limb defects (limb deficiency [755.2-755.9], sacral agenesis [756.13], diaphragmatic hernia [756.6], gastroschisis or omphalocele [756.72, 756.73, and 756.79]).
Findings for all additional subgroups of major structural birth defects, stratified by organ system, were consistent, with no increased risks after first trimester maternal IIV exposure compared with those without first trimester maternal IIV exposure. There was also no increased risk observed for neural tube defects, microcephaly, or cleft lip and/or cleft palate after first trimester maternal IIV exposure (Table IV). Results were consistent in secondary analyses, where women with first trimester IIV exposures were compared with women who were unexposed to IIV throughout pregnancy (Table V).
Table V.
Secondary analyses: selected birth defects with first trimester IIV exposure as compared to IIV unexposed in pregnancy
| Birth defect types | Unexposed to IIV during pregnancy (n = 282 091) |
IIV Exposed in first trimester (n = 52 552)* |
Adjusted Prevalence difference 95% CI, P value |
Adjusted Prevalence ratio, P value |
|---|---|---|---|---|
| Selected major structural birth defects† | 4284 (1.5 per 100 live births) | 861 (1.6 per 100 live births) | 0.04% (−0.09 to 0.16), .55 | 1.02 (0.94-1.10), .55 |
| Selected major structural birth defects by organ system | n (rate per 10 000 live births) | n (rate per 10 000 live births) | Per 10 000 live births | |
| Any cardiac | 1566 (56) | 306 (58) | −1 (−9 to 6), .70 | 0.98 (0.87-1.10), .79 |
| Severe cardiac | 353 (13) | 68 (13) | −1 (−5 to 2), .52 | 0.93 (0.71,1.20), .57 |
| Central nervous system | 438 (16) | 86 (16) | 1 (−3 to 5), .57 | 1.05 (0.83, 1.30), .68 |
| Neural tube defects | 67 (2) | 9 (2) | –1 (−2 to 0), .16 | 0.67 (0.33, 1.40), .27 |
| Microcephaly | 309 (11) | 62 (12) | 1 (−2 to 5), .40 | 1.11 (0.84, 1.50), .44 |
| Ophthalmologic or otologic | 112 (4) | 23 (4) | 0 (−2 to 2), .71 | 1.09 (0.68-1.80), .72 |
| Gastrointestinal | 659 (23) | 113 (22) | 0 (−5 to 4), .84 | 0.97 (0.79-1.20), .77 |
| Genitourinary or renal | 1057 (37) | 247 (47) | 4 (−4 to 10), .26 | 1.09 (0.94-1.30), .26 |
| Muscular or limb defects | 230 (8) | 44 (8) | −0 (−3 to 2), .73 | 0.93 (0.67, 1.30), .67 |
| Respiratory or orofacial | 385 (14) | 73 (14) | 0 (−4 to 4), .97 | 1.0 (0.77, 1.30), .98 |
| Cleft lip and/or cleft palate | 372 (13) | 68 (13) | 0 (−4 to 3), .86 | 0.97 (0.74, 1.30), .84 |
In these secondary analyses, n = 301 women with a first trimester IIV exposure excluded as they also had a second or third trimester IIV exposure.
Selected major structural birth defects include the following diagnoses, with outcome-specific algorithms applied: neural tube defects (spina bifida [741.0x and 741.9x], encephalocele, cranial meningocele, or encephalomyelocele [742.0]), other central nervous system (microcephaly [742.1], holoprosencephaly [742.2]), Ophthalmologic (anophthalmia or microphthalmia [743.00 and 743.10-743.12], cataracts and other lens defects [743.2x and 743.30-743.36]), otologic (anotia or microtia [744.01 and 744.23]), severe cardiac (single ventricle, tricuspid atresia, Ebstein anomaly, hypoplastic left heart, hypoplastic right heart, common truncus, transposition, atrioventricular septal defects, tetralogy of Fallot, aortic valve atresia/stenosis, coarctation, total anomalous pulmonary venous return, and anomalous coronary artery [745.0, 745.1x, 745.2-745.3, 745.6x, 745.7, 746.00, 746.01, 746.1-746.3, 746.7, 746.85, 747.1x, 747.22, and 747.41]), other cardiac (septal defects, heterotaxy, partial anomalous pulmonary venous return [745.4, 745.8, 745.9, and 759.3, and 747.42]), cleft lip or cleft palate (749.0, 749.00-749.04, 749.1, 749.10-749.14, 749.2, and 749.20-749.25), respiratory (choanal atresia [748.0]), gastrointestinal (esophageal atresia with or without tracheoesophageal fistula [750.3], pyloric stenosis [750.5], intestinal atresia or stenosis [751.1 and 751.2], biliary atresia [751.61]), genitourinary or renal (second- or third-degree hypospadias [752.61], renal agenesis or hypoplasia [753.0], renal dysplasia [753.15], congenital hydronephrosis [753.2x], bladder exstrophy [753.5], posterior urethral valve and/or prune belly [753.60 and 756.71]), muscular or limb defects (limb deficiency [755.2-755.9], sacral agenesis [756.13], diaphragmatic hernia [756.6], gastroschisis or omphalocele [756.72, 756.73, and 756.79]).
Discussion
We examined risks for more than 50 prespecified major structural birth defects among singleton, live births to 425 944 women, including 52 856 (12%) with IIV exposure in their first trimester. In this large observational study, we did not observe an increased risk for selected major structural birth defects after first trimester vaccination. Because IIV is currently recommended for all women who will be pregnant during periods of influenza circulation,22 these data should provide reassurance for women considering first trimester vaccination.
Our findings add to the current literature supporting the absence of an association between early maternal vaccination and major structural birth defects in offspring. In 2014, McMillan et al14 systematically reviewed 12 studies of maternal influenza vaccination, including approximately 4000 women with first trimester exposures. Across these studies, odds ratios for first trimester IIV and birth defects (where specific birth defect outcomes varied across studies) ranged from 0.67 to 2.18; no study reviewed reported an association that was statistically significant.14 In 2015, Polyzos et al23 conducted a meta-analysis of largely overlapping studies of 4733 women exposed to IIV in the first trimester and reported an odds ratio of 0.98 (95% CI 0.95-1.20) for major birth defects in offspring. More recently, studies from US and Swedish cohorts, including nearly 15 000 first trimester IIV exposures, along with 1 US-based case-control study, have demonstrated safety of first trimester influenza vaccination with respect to birth defects in offspring.24-26
One strength of our study was our large population of women with first trimester IIV exposure. As such, we were able to examine subgroups of major structural birth defects, along with a few individual birth defects. Our findings were consistent across all subgroups when stratified by organ system or specific birth defect. Our adjusted PRs generally ranged from 90 to 1.05, with tight CIs around these point estimates. In addition, our birth defect outcomes, ICD-9–based algorithms, were developed and validated for use in the VSD population, consistent with recommendations for maternal vaccine safety surveillance.15 As part of this process, we determined that ICD-9 codes were valid for identifying defects by system but were prone to error for distinguishing similar defects, such as differentiating cleft palate with cleft lip from isolated cleft palate. Thus, a majority of outcomes analyzed were for groups of defects and not isolated defects. As previously described, for most groups of defects, background rates in the VSD were similar to those from population-based surveillance systems.18
Additional strengths of our study were that we were able to systematically identify and exclude pregnant women and infants who were a priori at increased risk for major structural birth defects owing to comorbidities (eg, diabetes), or drug exposures (eg, oral retinoids or valproate), diagnosed chromosomal anomalies, or congenital infections. Also, we were able to identify and adjust for other important potential confounders through the use of a propensity score. Of note, even after excluding mothers whose first trimester did not overlap with influenza vaccine availability (eg, last menstrual period is May 1,and thus first trimester ends August 13) the strongest predictor of maternal first trimester vaccination was timing of pregnancy start. Thus, as in our approach, calendar month of conception or pregnancy start would be important to account for in observational studies of maternal vaccine or drug exposures, especially if there is also seasonality for the outcomes. For some birth defects, seasonality has been described, although results have varied by study.27-29
Several limitations should be noted. First, our selected major structural birth defect outcomes were based on ICD-9 codes and were not confirmed by clinical review or adjudication of cases. To improve specificity, we applied outcome-specific algorithms, developed and validated for use in the VSD. These algorithm outcomes would be classified as Brighton level 3, or meeting case definitions with the lowest level of diagnostic certainty.15 Achieving a higher level of diagnostic certainty would require additional review of clinical, laboratory, or imaging data, which is not routinely available in our automated VSD files. Manual review of charts for more than 5000 infants with major structural birth defects identified in this study was cost prohibitive. Also, because our birth defect definitions were consistent across IIV-exposed and -unexposed groups, potential misclassification of outcomes would likely be nondifferential. Additionally, because our list of outcomes was prespecified to only include those with valid and specific ICD-9 codes available, our outcomes may differ from those applied in other studies of maternal vaccine safety. Furthermore, our approach was not designed to detect syndromes or associations of defects. Other limitations were that we were only able to identify and account for risks for birth defects if they were documented in electronic healthcare data. For example, we were able to identify and exclude women with potential exposures to teratogenic prescription medications, but we could not determine whether women were taking folic acid or whether there was maternal alcohol or illicit drug use during the first trimester. Similarly, the potential that influenza vaccines administered through health fairs or the workplace would not be captured in our standardized files could lead to some misclassification of exposure status.30 Our cohort was limited to women with continuous insurance coverage; nearly all had a medical visit during first trimester. As such, infants in our study were likely at lower risk than the general US population for having a major structural birth defect. Finally, owing to limitations in our data sources, we were unable to include birth defects identified in stillbirths, spontaneous abortions, or resulting in therapeutic abortions; therefore, our results are specific to pregnancies ending in a singleton live birth.
In this large observational study, we did not observe increased risks for first trimester maternal IIV exposure and major structural birth defects in offspring. This study supports the safety of current recommendations for IIV to be administered to women who may be pregnant, in any trimester, during influenza season. ∎
Acknowledgments
Funded by the Centers for Disease Control and Prevention (200-2012-53526 [to E.K]). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. N.K. receives research support from GlaxoSmithKline, Sanofi Pasteur, Pfizer, Merck, and Protein Science, and MedImmune. A.N. receives research support from GlaxoSmithKline, Pfizer, Merck, and MedImmune. T.C. receives research support from Bristol-Myers Squibb. M.J. receives research support from Sanofi Pasteur. The other authors declare no conflicts of interest.
Glossary
- ICD-9
International Classification of Diseases, Ninth Revision
- IIV
Inactivated influenza vaccine
- PD
Prevalence difference
- PR
Prevalence ratio
- VSD
Vaccine Safety Datalink
Footnotes
We thank Leslie Kuckler, MPH, Beth Molitor, MBA, and Avalow Olsen, BS (HealthPartners Institute), for their assistance with data collection and James G. Donahue, DVM, PhD (Marshfield Clinic), for his thoughtful review of the manuscript.
References
- 1.Neuzil KM, Reed GW, Mitchel EF, Simonsen L, Griffin MR. Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. Am J Epidemiol 1998;148:1094–102. [DOI] [PubMed] [Google Scholar]
- 2.Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB, Centers for Disease C, et al. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2004;53(RR-6):1–40. [PubMed] [Google Scholar]
- 3.Groom HC, Henninger ML, Smith N, Koppolu P, Cheetham TC, Glanz JM, et al. Influenza vaccination during pregnancy: influenza seasons 2002-2012, Vaccine Safety Datalink. Am J Prev Med 2016;50:480–8. [DOI] [PubMed] [Google Scholar]
- 4.Ding H, Black CL, Ball S, Donahue S, Fink RV, Williams WW, et al. Influenza vaccination coverage among pregnant women—United States, 2014-15 influenza season. MMWR Morb Mortal Wkly Rep 2015;64:1000–5 [DOI] [PubMed] [Google Scholar]
- 5.Kharbanda EO, Vazquez-Benitez G, Lipkind H, Naleway A, Lee G, Nordin JD, et al. Inactivated influenza vaccine during pregnancy and risks for adverse obstetric events. Obstet Gynecol 2013;122:659–67. [DOI] [PubMed] [Google Scholar]
- 6.Nordin JD, Kharbanda EO, Vazquez Benitez G, Lipkind H, Vellozzi C, Destefano F. Maternal influenza vaccine and risks for preterm or small for gestational age birth. J Pediatr 2014;164:1051–7, e2. [DOI] [PubMed] [Google Scholar]
- 7.Pasternak B, Svanstrom H, Molgaard-Nielsen D, Krause TG, Emborg HD, Melbye M, et al. Risk of adverse fetal outcomes following administration of a pandemic influenza A(H1N1) vaccine during pregnancy. JAMA 2012;308:165–74. [DOI] [PubMed] [Google Scholar]
- 8.Zaman K, Roy E, Arifeen SE, Rahman M, Raqib R, Wilson E, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med 2008;359:1555–64. [DOI] [PubMed] [Google Scholar]
- 9.Steinhoff MC, Omer SB, Roy E, El Arifeen S, Raqib R, Dodd C, et al. Neonatal outcomes after influenza immunization during pregnancy: a randomized controlled trial. CMAJ 2012;184:645–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nordin JD, Kharbanda EO, Benitez GV, Nichol K, Lipkind H, Naleway A, et al. Maternal safety of trivalent inactivated influenza vaccine in pregnant women. Obstet Gynecol 2013;121:519–25. [DOI] [PubMed] [Google Scholar]
- 11.Yuen CY, Tarrant M. Determinants of uptake of influenza vaccination among pregnant women—a systematic review. Vaccine 2014;32:4602–13. [DOI] [PubMed] [Google Scholar]
- 12.Chamberlain AT, Seib K, Ault KA, Orenstein WA, Frew PM, Malik F, et al. Factors associated with intention to receive influenza and tetanus, diphtheria, and acellular pertussis (Tdap) vaccines during pregnancy: a focus on vaccine hesitancy and perceptions of disease severity and vaccine safety. PLoS Curr 2015;7:pii: ecurrents.outbreaks.d37b61bceebae5a7a06d40a301cfa819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henninger ML, Irving SA, Thompson M, Avalos LA, Ball SW, Shifflett P, et al. Factors associated with seasonal influenza vaccination in pregnant women. J Womens Health (Larchmt) 2015;24:394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McMillan M, Porritt K, Kralik D, Costi L, Marshall H. Influenza vaccination during pregnancy: a systematic review of fetal death, spontaneous abortion, and congenital malformation safety outcomes. Vaccine 2015;33:2108–17. [DOI] [PubMed] [Google Scholar]
- 15.DeSilva M, Munoz FM, McMillan M, Kawai AT, Marshall H, Macartney KK, et al. Congenital anomalies: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 2016;34:6015–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baggs J, Gee J, Lewis E, Fowler G, Benson P, Lieu T, et al. The Vaccine Safety Datalink: a model for monitoring immunization safety. Pediatrics 2011;127(Suppl 1):S45–53. [DOI] [PubMed] [Google Scholar]
- 17.Naleway AL, Gold R, Kurosky S, Riedlinger K, Henninger ML, Nordin JD, et al. Identifying pregnancy episodes, outcomes, and mother-infant pairs in the Vaccine Safety Datalink. Vaccine 2013;31:2898–903. [DOI] [PubMed] [Google Scholar]
- 18.Kharbanda EO, Vazquez-Benitez G, Romitti PA, Naleway AL, Cheetham TC, Lipkind HS, et al. Identifying birth defects in automated data sources in the Vaccine Safety Datalink. Pharmacoepidemiol Drug Saf 2017;26:412–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeSilva M, Vazquez-Benitez G, Nordin JD, Lipkind HS, Romitti PA, DeStefano F, et al. Tdap vaccination during pregnancy and microcephaly and other structural birth defects in offspring. JAMA 2016;316:1823–5. [DOI] [PubMed] [Google Scholar]
- 20.Kotelchuck M An evaluation of the Kessner Adequacy of Prenatal Care Index and a proposed Adequacy of Prenatal Care Utilization Index. Am J Public Health 1994;84:1414–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotelchuck M The Adequacy of Prenatal Care Utilization Index: its US distribution and association with low birthweight. Am J Public Health 1994;84:1486–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grohskopf LA, Sokolow LZ, Broder KR, Olsen SJ, Karron RA, Jernigan DB, et al. Prevention and control of seasonal influenza with vaccines. MMWR Recomm Rep 2016;65:1–54. [DOI] [PubMed] [Google Scholar]
- 23.Polyzos KA, Konstantelias AA, Pitsa CE, Falagas ME. Maternal influenza vaccination and risk for congenital malformations: a systematic review and meta-analysis. Obstet Gynecol 2015;126:1075–84. [DOI] [PubMed] [Google Scholar]
- 24.Louik C, Kerr S, Van Bennekom CM, Chambers C, Jones KL, Schatz M, et al. Safety of the 2011-12, 2012-13, and 2013-14 seasonal influenzavaccines in pregnancy: preterm delivery and specific malformations, a study from the case-control arm of VAMPSS. Vaccine 2016;34:4450–9. [DOI] [PubMed] [Google Scholar]
- 25.Chambers CD, Johnson DL, Xu R, Luo YJ, Louik C, Mitchell AA, et al. Safety of the 2010-11, 2011-12, 2012-13, and 2013-14 seasonal influenza vaccines in pregnancy: birth defects, spontaneous abortion, preterm delivery, and small for gestational age infants, a study from the cohort arm of VAMPSS. Vaccine 2016;34:4443–9. [DOI] [PubMed] [Google Scholar]
- 26.Ludvigsson JF, Strom P, Lundholm C, Cnattingius S, Ekbom A, Ortqvist A, et al. Risk for congenital malformation with H1N1 influenza vaccine: a cohort study with sibling analysis. Ann Intern Med 2016;165:848–55. [DOI] [PubMed] [Google Scholar]
- 27.Luteijn JM, Dolk H, Addor MC, Arriola L, Barisic I, Bianchi F, et al. Seasonality of congenital anomalies in Europe. Birth Defects Res A Clin Mol Teratol 2014;100:260–9. [DOI] [PubMed] [Google Scholar]
- 28.Caton AR. Exploring the seasonality of birth defects in the New York State Congenital Malformations Registry. Birth Defects Res A Clin Mol Teratol 2012;94:424–37. [DOI] [PubMed] [Google Scholar]
- 29.Chung MK, Lao TT, Ting YH, Hung Suen SS, Leung TY, Kin Lau T. Is there seasonality in the incidence of oral-facial clefts? J Matern Fetal Neonatal Med 2011;doi: 10.3109/14767058.2011.629251. [DOI] [PubMed] [Google Scholar]
- 30.Sy LS, Liu IL, Solano Z, Cheetham TC, Lugg MM, Greene SK, et al. Accuracy of influenza vaccination status in a computer-based immunization tracking system of a managed care organization. Vaccine 2010;28:5254–9. [DOI] [PubMed] [Google Scholar]