Abstract
Introduction:
An increased risk of diagnosed chorioamnionitis in women vaccinated with Tdap during pregnancy was previously detected at two Vaccine Safety Datalink (VSD) sites. The clinical significance of this finding related to infant outcomes remains uncertain.
Methods:
Retrospective cohort study of singleton live births born to women who were continuously insured from 6 months prior to their last menstrual period through 6 weeks postpartum, with ≥1 outpatient visit during pregnancy from January 1, 2010 to November 15, 2013 at seven integrated United States health care systems part of the VSD. We re-evaluated the association between maternal Tdap and chorioamnionitis and evaluated whether specific infant morbidities differ among infants born to mothers who did and did not receive Tdap during pregnancy. We focused on 2 Tdap exposure windows: the recommended 27–36 weeks gestation or anytime during pregnancy. We identified inpatient diagnostic codes for transient tachypnea of the newborn (TTN), neonatal sepsis, neonatal pneumonia, respiratory distress syndrome (RDS), and newborn convulsions associated with an infant’s first hospitalization. A generalized linear model with Poisson distribution and log-link was used to estimate propensity score adjusted rate ratios (ARR) with 95% confidence intervals (CI).
Results:
The analyses included 197,564 pregnancies. Chorioamnionitis was recorded in 6.4% of women who received Tdap vaccination any time during pregnancy and 5.2% of women who did not (ARR [95% CI]: 1.23 [1.17, 1.28]). Compared with unvaccinated women, there were no significant increased risks (ARR [95% CI]) for TTN (1.04 [0.98, 1.11]), neonatal sepsis (1.06 [0.91, 1.23]), neonatal pneumonia (0.94 [0.72, 1.22]), RDS (0.91 [0.66, 1.26]), or newborn convulsions (1.16 [0.87, 1.53]) in infants born to Tdap-vaccinated women.
Conclusions and Relevance:
Despite an observed association between maternal Tdap vaccination and maternal chorioamnionitis, we did not find increased risk for clinically significant infant outcomes associated with maternal chorioamnionitis.
Keywords: Vaccination, Infant, Chorioamnionitis
1. Introduction
Due to ongoing pertussis outbreaks and newborn susceptibility to severe pertussis disease, the tetanus, diphtheria, and acellular pertussis (Tdap) vaccine has been recommended for routine use in pregnant women not previously vaccinated since 2010 in California, and since 2011 across the United States [1,2]. In 2012, the U.S. Advisory Committee on Immunization Practices (ACIP) revised these recommendations, advising that Tdap should be administered during every pregnancy, ideally between 27 – 36 weeks gestation [3].
To date, studies on maternal Tdap vaccination have demonstrated both the effectiveness [4] and general safety of this practice [5–10]. However, previous work by our group detected a small but statistically significant increased risk of clinical chorioamnionitis, (referred to as chorioamnionitis in this document and identified using ICD-9 code 658.41), following maternal Tdap at two Vaccine Safety Datalink (VSD) sites during 2010–2012 (adjusted risk ratio [ARR] [95% confidence interval (CI)]: 1.19 [1.13, 1.26]) [5]. This risk was slightly lower in those vaccinated during the recommended time period (27–36 weeks gestation) compared to unvaccinated women (ARR [95% CI]: 1.11 [1.03, 1.21]).
Chorioamnionitis, is an intrauterine infection affecting the tissues of either mixed maternal-fetal origin or fetal origin including an infection of the amniotic fluid, fetus, umbilical cord, placenta, or fetal membranes [11,12]. The key clinical signs or symptoms of chorioamnionitis include maternal fever, maternal or fetal tachycardia, uterine tenderness, and malodorous amniotic fluid [12]. The diagnosis of chorioamnionitis typically requires maternal fever >38.0 °C, in addition to one or two other physical exam findings. This clinical definition lacks both sensitivity and specificity and there is no uniformly accepted definition. Neonates exposed to chorioamnionitis are at higher risk for preterm birth [13] and neonatal infections such as neonatal sepsis [14–17] and pneumonia [16]. The association between maternal chorioamnionitis and respiratory distress syndrome (RDS) is less clear, with some studies showing decreased risk of RDS in infants exposed to chorioamnionitis [18,19] while others show an increased risk [20]. There have also been associations between chorioamnionitis or intrapartum maternal fever and neonatal seizures [16,21].
Although chorioamnionitis is often associated with preterm birth, in our previous study, we found no association between preterm birth and maternal Tdap vaccination [5]. Additionally, in the subset of infants born preterm, we did not detect increased risk of chorioamnionitis following maternal Tdap administration. Other studies have also shown no association between incidence of preterm birth and maternal Tdap administration [6,7,22,23]. Given our prior findings regarding a small but statistically significant association between maternal Tdap vaccination and chorioamnionitis, we felt it was important to determine whether neonatal outcomes differed among infants born to mothers who did and did not receive Tdap during pregnancy. In the current VSD study, we re-evaluated the association between maternal Tdap and chorioamnionitis and examined risks for specific infant morbidities following maternal Tdap vaccination.
2. Material and methods
The VSD is a collaboration between the Centers for Disease Control and Prevention (CDC) and several integrated health care systems in the United States [24]. We conducted an observational cohort study of pregnancies ending in a live birth at seven VSD sites (Northern California, Southern California, Colorado, Minnesota, Oregon, Washington and Wisconsin), to examine whether the previously detected increased risk for chorioamnionitis persisted and to determine clinical significance of this finding related to infant outcomes by evaluating risks for neonatal morbidities. Incidence rates for specific neonatal outcomes, chosen based on their relationship to chorioamnionitis, were compared between infants of mothers who received Tdap during pregnancy and infants born to unvaccinated women. This study was approved by the IRBs of all participating sites with a waiver of informed consent.
Pregnancies were identified using a validated algorithm based on administrative, electronic health record (EHR), and claims data [25]. The available data were limited to singleton live births, born January 1, 2010–November 15, 2013, to women continuously insured from 6 months prior to their last menstrual period (LMP) through 6 weeks postpartum, with at least 1 outpatient visit during pregnancy. Infants were required to have birth weight and gestational age available in the EHR or from linked birth certificate data. Diagnoses from each infant’s first inpatient hospital admission, limited to the first 7 days of life (i.e., birth hospitalization or first inpatient hospitalization for infants not born in a traditional hospital setting), were included. If an infant was discharged and readmitted within 3 days of discharge, all diagnoses were included.
Tdap administrations were identified from standardized VSD files, based on claims and EHR data, and assigned by gestational week of pregnancy, as previously described [26]. Consistent with our previous studies, women with Tdap administration recorded <8 days after their LMP or 8 days prior to delivery were excluded to account for uncertainty regarding the date of LMP and to avoid misclassification of postpartum vaccinations as occurring during pregnancy [5,27,28]. Women receiving live virus vaccines during pregnancy were also excluded. We focused on 2 Tdap exposure windows: 27–36 weeks gestation (consistent with current ACIP recommendations) and from ≥8 days after LMP through 8 days prior to birth, referred to as “any week during pregnancy.”
2.1. Outcomes
Chorioamnionitis was identified using ICD-9 code 658.41 from the mother’s medical record and was included as an outcome when 1 or more inpatient diagnoses were recorded during the delivery hospitalization. We examined 5 neonatal outcomes individually and as a composite outcome including any of the following: (1) transient tachypnea of the newborn (TTN); (2) neonatal sepsis; (3) neonatal pneumonia; (4) RDS; and (5) newborn convulsions. They were identified using ICD-9-CM codes associated with an infant’s first hospitalization and subsequent hospitalizations if admission occurred within 3 days following discharge as described above. If an infant was diagnosed with both TTN and RDS during the same admission, a hierarchy in classification was used in which only one diagnosis was included in analysis with RDS > TTN. We performed subgroup analyses for infants born at gestational age < 34 weeks, as some of the outcomes evaluated are increased in prematurity, particularly in early preterm births.
2.2. Statistical analyses
Descriptive statistics were used to compare baseline characteristics between vaccinated and unvaccinated. We included all births from all sites 2010–2013 for analyses of chorioamnionitis and neonatal outcomes following maternal Tdap vaccination at any time during pregnancy. Given that vaccine recommendations changed over time (by year and by state), analyses of neonatal outcomes associated with maternal Tdap vaccination from 27 to 36 weeks gestation included births in 2010–2013 for the California VSD sites and were limited to births in 2012–2013 for the other participating VSD sites. We constructed propensity scores for each comparison group: (1) maternal Tdap vaccination in any week during pregnancy versus unvaccinated at all sites, 2010–2013; (2) maternal Tdap vaccination between 27 – 36 weeks gestation versus unvaccinated at California sites, 2010–2013 and other VSD sites, 2012–2013. We used logistic regression to estimate propensity scores including the following variables: VSD site, pregnancy delivery year, site-year interaction, maternal age at delivery, gestational age at delivery, Kotelchuck Adequacy of Prenatal Care Utilization Index as derived from VSD data [29], hospitalization during the first 20 weeks gestation, smoking during pregnancy, mean census tract poverty level, race/ethnicity, pre-existing hypertension, and pre-existing heart, renal, or pulmonary conditions. A generalized linear model with Poisson distribution and log link was used to estimate rate ratios (RR) with 95% CIs. Poisson distribution with identity link with robust variance estimation was used to estimate rate differences (RD) with 95% CIs. In addition to unadjusted RR and RD, we obtained adjusted estimates by adding propensity scores to the models as quintiles. We estimated mean and 95% CI for length of hospitalization in log scale and retransformed into the original scale (geometric mean). We evaluated the difference using the median 2-sample test. All analyses were conducted using SAS version 9.3 (SAS Institute Inc., USA).
3. Results
There were 243,981 pregnancies ending in a live birth identified among women 14–44 years of age with pregnancy end dates during January 1, 2007–September 30, 2013 meeting minimum enrollment criteria. Women who received Tdap during the first or last week of pregnancy (n = 4915; 2.0%) or who received a live virus vaccine during pregnancy (n = 534; 1.0%) were excluded from the analyses. After applying all exclusion criteria, 197,564 (81.0%) pregnancies were included in the analyses (Fig. 1); 152,556 (77.2%) unexposed and 45,008 (22.8%) Tdap-exposed with 22,772 vaccinations between 27 – 36 weeks gestation during 2010–2013 in California and 2012–2013 for the other sites. Baseline comparisons of women who did or did not receive Tdap vaccine during pregnancy are shown in Table 1.
Fig. 1.
Flow chart of pregnancies ending in a live birth identified and exclusion criteria used to obtain study population at 7 Vaccine Safety Datalink sites – January 1, 2010–September 2013, 2013. GA = gestational age.
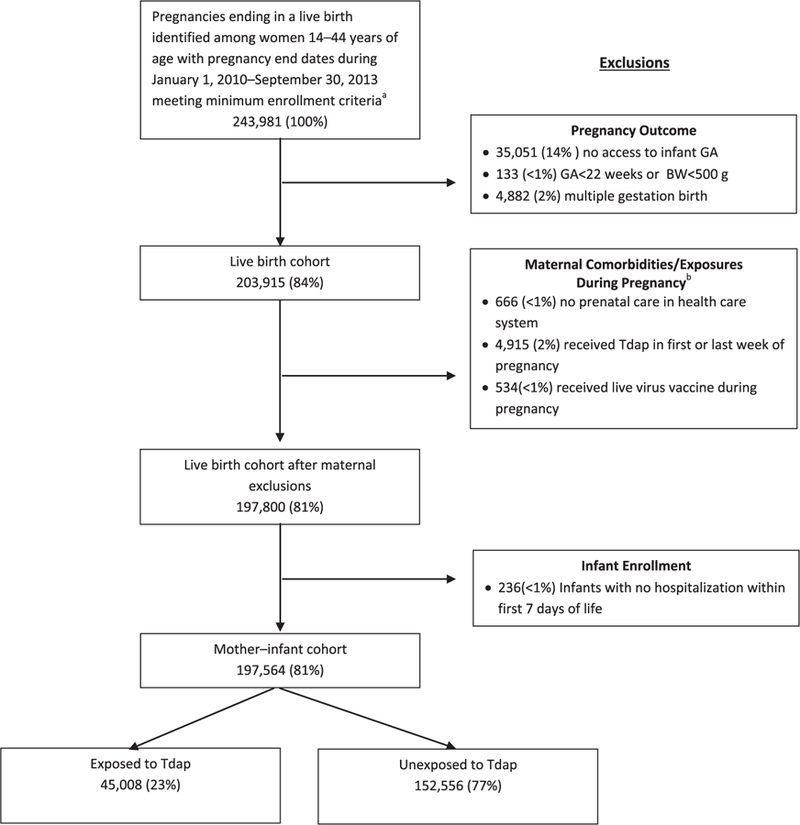
a Continuous insurance enrollment from 6 months prior to their last menstrual period through 6 weeks postpartum. b More than one condition may apply to a single pregnant female.
Table 1.
Baseline characteristics of Tdap vaccine exposed and unexposed pregnant women with a live birth at 7 Vaccine Safety Datalink sites, 2010–2013.
| Mother–infant cohort, N = 197,564 |
||
|---|---|---|
| Tdap unexposeda (n = 152,556; 77.2%) | Tdap exposed (n = 45,008; 22.8%) | |
| *Age at delivery, yᶲ | ||
| <18 | 1915 (1.3) | 314 (0.7) |
| 18–24 | 20053 (13.1) | 5658 (12.6) |
| 25–34 | 93,521 (61.3) | 28,155 (62.6) |
| ≥35 | 37,067 (24.3) | 10,881 (24.1) |
| *Race/ethnicityᶲ,b | ||
| Asian | 25,002 (16.4) | 7743 (17.2) |
| Black | 11,096 (7.3) | 2873 (6.4) |
| Hispanic | 43,921 (28.8) | 14,755 (32.8) |
| Other | 12,159 (8.0) | 4001 (8.9) |
| White | 60,378 (39.6) | 15,636 (34.7) |
| *Year of deliveryᶲ | ||
| 2010 | 45,404 (29.8) | 7184 (16.0) |
| 2011 | 39,400 (25.8) | 13,632 (30.3) |
| 2012 | 44,069 (28.9) | 10,453(23.2) |
| 2013 | 23683 (15.5) | 13,739 (30.5) |
| Medical care in first trimester | 144,626 (94.8) | 43,329 (96.3) |
| *Prenatal care indexᶲ | ||
| Adequate/plus | 112,167 (73.5) | 36,178 (80.4) |
| Intermediate | 34,249 (22.5) | 7799 (17.3) |
| Inadequate | 6140 (4.0) | 1031 (2.3) |
| *Hospitalized < 20 weeks gestationᶲ | 5242 (3.4) | 871 (2.2) |
| Received other vaccine during pregnancyᶲ,c | 76,231 (50.0) | 28,827 (64.0) |
| Received Tdap before pregnancyd | 47,481 (35.5) | 2826 (9.1) |
| Delivery via cesarean section | 41357 (27.1) | 12391 (27.5) |
| *Maternal co-morbiditiese | ||
| Smoking | 14,529 (9.5) | 4235 (9.4) |
| Diabetes mellitus | 2372 (1.6) | 686 (1.5) |
| Hypertensionψ | 3616 (2.4) | 933 (2.1) |
| Pulmonary diseaseψ | 10,400 (6.8) | 2863 (6.4) |
| Heart disease | 1640 (1.1) | 445 (1.0) |
| Renal disease | 1755 (1.2) | 486 (1.1) |
| *Vaccine safety datalink siteᶲ | ||
| A | 68,783 (45.1) | 16,461 (36.3) |
| B | 7592 (5.0) | 1476 (3.3) |
| C | 3760 (2.5) | 219 (0.5) |
| D | 1916 (1.3) | 120 (0.3) |
| E | 8556 (5.6) | 1524 (3.4) |
| F | 55932 (36.7) | 23,947 (53.2) |
| G | 6017 (3.9) | 1261 (2.8) |
| Poverty %f | 15.70% | 16.50% |
Indicates variable included in propensity score.
Indicates p-value <0.0001.
Indicates p-value <0.05.
Tdap unexposed may have received Tdap prior to pregnancy or after delivery.
Race and ethnicity were classified based on information available in each participating site’s medical record and included in the study due to differences in immunization rates by race and ethnicity.
Other vaccines administered during pregnancy included inactivated influenza (96%), hepatitis A or B, human papillomavirus [as previously described5].
Tdap before pregnancy includes Tdap vaccines administered during the two years before date of last menstrual period.
Diagnoses during inpatient admissions from 6 months before pregnancy through the end of the pregnancy.
Percentage of families in census tract whose income was below 150% of the federal poverty level.
Chorioamnionitis was recorded in 6.4% of women who received Tdap vaccination any time during pregnancy and 5.2% of women who did not (adjusted rate ratio [ARR] [95% CI]: 1.23 [1.17, 1.28]). This association was not found in women delivering at <34 weeks gestational age (ARR [95% CI]: 0.87 [0.59, 1.30]). Compared with unvaccinated women, there were no increased risks (ARR [95% CI]) for TTN (1.03 [0.96, 1.11]), neonatal sepsis (1.06 [0.91, 1.23]), neonatal pneumonia (0.94 [0.72, 1.22]), RDS (0.91 [0.66, 1.26)]), newborn convulsions (1.16 [0.87, 1.53]), or the composite outcome including any of these outcomes (1.04 [0.98, 1.11]) in infants born to women who had been vaccinated at any time during pregnancy. These results were similar when evaluating vaccinations given during the recommended time period and when stratifying the results by gestational age at birth (Table 2). For the full cohort, there was no clinical difference in infant mean length of hospital stay after birth for those whose mother received Tdap vaccine (2.1 days [95% CI 2.13, 2.15]) compared to those who did not receive Tdap (2.2 days [95% CI 2.22, 2.23]).
Table 2.
Incidence rates, rate differences (RD), and rate ratios (RR) of infant outcomes associated with receipt of Tdap during pregnancy at 7 Vaccine Safety Datalink sites, 2010–2013, (N = 197,564), by time period during pregnancy in which Tdap was administered and limiting results to infants born < 34 weeks gestation.
| Maternal Tdap administration during pregnancy at any time during pregnancy, 2010–2013 |
Adjusted measures of association 95% CI | |||||
|---|---|---|---|---|---|---|
| No (n = 152,556) |
Yes (n = 45,008) |
|||||
| n | Incidence rate % | n | Incidence rate % | RD in cases per 100 live births | RR | |
| Chorioamnionitis | 7970 | 5.22 | 2883 | 6.41 | 1.16 (0.9, 1.42) | 1.23 (1.17, 1.28) |
| Composite outcome | 4824 | 3.16 | 1288 | 2.86 | 0.11 (−0.07, 0.29) | 1.04 (0.98, 1.11) |
| TTNa | 3524 | 2.31 | 973 | 2.16 | 0.07 (−0.09, 0.22) | 1.03 (0.96, 1.11) |
| Neonatal sepsisb | 939 | 0.62 | 231 | 0.51 | 0.03 (−0.05, 0.10) | 1.06 (0.91, 1.23) |
| Pneumoniac | 371 | 0.24 | 73 | 0.16 | 0.00 (−0.04, 0.04) | 0.94 (0.72, 1.22) |
| RDSd | 215 | 0.14 | 49 | 0.11 | −0.01 (−0.05, 0.03) | 0.91 (0.66, 1.26) |
| Convulsions in newborne | 261 | 0.17 | 68 | 0.15 | 0.02 (−0.02, 0.06) | 1.16 (0.87, 1.53) |
| Maternal Tdap administration during recommended time period of pregnancy, 27–36 weeks, 2010–2013 |
||||||
| No (n = 133,882) |
Yes (n = 22,772) |
|||||
| n | Incidence rate % | n | Incidence rate % | RD in cases per 100 live births | RR | |
| Chorioamnionitis | 7109 | 5.31 | 1430 | 6.28 | 0.98 (0.65,1.32) | 1.20 (1.14, 1.28) |
| Composite outcome | 4157 | 3.10 | 653 | 2.87 | 0.13 (−0.11, 0.37) | 1.02 (0.94, 1.12) |
| TTN | 3061 | 2.29 | 520 | 2.28 | 0.20 (−0.02, 0.41) | 1.08 (0.98, 1.19) |
| Neonatal sepsis | 785 | 0.59 | 101 | 0.44 | −0.01 (−0.11, 0.08) | 0.90 (0.73, 1.11) |
| Pneumonia | 329 | 0.25 | 34 | 0.15 | −0.01 (−0.06, 0.05) | 0.82 (0.57, 1.17) |
| RDS | 168 | 0.13 | 21 | 0.09 | −0.02 (−0.06, 0.03) | 0.79 (0.50, 1.26) |
| Convulsions in newborn | 229 | 0.17 | 28 | 0.12 | −0.01 (−0.06, 0.04) | 0.88 (0.58, 1.31) |
| Maternal Tdap administration at any time during pregnancy, infants born < 34 weeks GA |
||||||
| No (n = 2711) |
Yes (n = 426) |
|||||
| n | Incidence rate % | n | Incidence rate % | RD in cases per 100 live births | RR | |
| Chorioamnionitis | 221 | 8.15 | 28 | 6.57 | −1.02 (−3.51, 1.47) | 0.87 (0.59, 1.30) |
| Composite outcome | 695 | 25.64 | 104 | 24.41 | 0.18 (−4.20, 4.57) | 1.02 (0.83, 1.26) |
| TTN | 291 | 10.73 | 51 | 11.97 | 0.84 (−2.48, 4.15) | 1.07 (0.79, 1.45) |
| Neonatal sepsis | 318 | 11.73 | 48 | 11.27 | 0.85 (−2.22, 3.93) | 1.11 (0.81, 1.51) |
| Pneumonia | 121 | 4.46 | 9 | 2.11 | −0.95 (−2.76, 0.86) | 0.60 (0.30, 1.19) |
| RDS | 46 | 1.70 | 5 | 1.17 | −0.26 (−1.59, 1.07) | 0.84 (0.33, 2.14) |
| Convulsions in newborn | 40 | 1.48 | 5 | 1.17 | −0.12 (−1.49, 1.24) | 0.98 (0.38, 2.50) |
TTN = transient tachypnea of the newborn; RDS = respiratory distress syndrome.
ICD-9 code 770.6.
ICD-9 codes 771.8, 771.81, 995.92, & 771.89.
ICD-9 codes 770.0 & 480.0–486.
ICD-9 codes 769, 770.84, & 770.87.
ICD-9 code 779.
4. Discussion
In this large, multi-site observational study, we found no increased risk for TTN, neonatal sepsis, neonatal pneumonia, RDS, newborn convulsions, or the composite outcome of any of these outcomes in infants following maternal Tdap vaccination. We are not aware of any direct biologic mechanism for an association between maternal Tdap and these infant outcomes due to the vaccine containing inactivated bacterial particles. However, our prior findings on maternal Tdap and chorioamnionitis provided rationale for the current investigations. This study extends our previous work evaluating risks for chorioamnionitis, expanding our study population by 36%, by including data from 5 additional VSD sites and adding one additional year of data, while still including the prior cohort.
Our initial and repeated detection of a small but positive association between chorioamnionitis and maternal Tdap, in the absence of increased risks for related infant outcomes, raises concern about the relevance of this finding. In our initial study, despite the observed statistical association between maternal Tdap and chorioamnionitis, we found no association with preterm birth, a major sequela of chorioamnionitis. Similarly, in our current analyses, although we again noted a positive association between maternal Tdap and chorioamnionitis, there was no increased risk for associated indicators of infant morbidity following maternal Tdap. In a study of pregnancy outcomes following maternal Tdap, Morgan, et al., found no difference in incidence of chorioamnionitis in the group who received Tdap compared to those that did not [23]. In our previous study, the positive predictive value of the ICD-9 code 658.41 for “possible chorioamnionitis,” defined as maternal temperature ≥38.0 °C and one additional clinical finding, was 0.78 (95% CI 0.72, 0.83), highlighting the difficulties of reliably diagnosing chorioamnionitis [5]. Future studies should consider using strict case definitions for chorioamnionitis rather than relying on diagnostic codes which may not be accurate.
One limitation of this study was the small number of infants born <34 weeks gestation, as much of the literature related to chorioamnionitis and neonatal outcomes has focused on early premature infants. Nevertheless, it has been shown that regardless of gestational age, prenatal inflammation, defined as clinical chorioamnionitis, maternal intrapartum temperature >38 °C, or histologic chorioamnionitis, is associated with adverse neonatal outcomes [30]. Although the majority of infants in our study were born >34 weeks gestational age, the lack of an association between maternal Tdap and infant infections, respiratory problems, and convulsions is important. The results for infants born at gestational age <34 weeks were consistent with results for the entire cohort, but the confidence intervals were larger.
A second limitation of this study was our reliance on electronic health record data, specifically diagnostic codes; we did not perform chart reviews to validate the infant outcomes. However, we did compare incidence rates of the outcomes of interest with published background rates. Incidence rates for all outcomes were similar to the expected background rates, except for TTN, which has been noted to have an incidence <1% [31,32] among infants born ≥37 weeks gestational age but in our study was 2.31% among all births. Another study, evaluating the respiratory morbidity in late preterm infants (born between 34 0/7 – 36 6/7 weeks gestation), showed an increased odds of diagnosis of TTN with decreasing gestational age; infants born at 34 weeks gestation had higher odds of TTN compared to those born at 39 or 40 weeks gestation (adjusted odds ratio [95% CI]:14.7 [11.7, 18.4]) [33]. The reason for the higher prevalence of TTN in our cohort compared to background rates is unclear but may be related to diagnostic coding practices using ICD-9 codes for which we were unable to confirm outcomes via chart review. It should be noted that two studies providing background rates for TTN were conducted in European countries, England [31] and Italy [32], and the majority of data from these studies is more than 10 years older than our data. A U.S. study conducted during 2012–2014 by Berenson, et al., reported an incidence of TTN of 4.8% and 3.6% in infants born to women who did not receive and who did receive Tdap during pregnancy [22]. This is higher than the incidence found in our study. Differences between countries and over time make it difficult to compare the background rates in our study and the Berenson study to the two European studies. The United States based study assessing short-term respiratory morbidity in late preterm births compared with term births during 2002–2008, showed higher incidence of TTN at earlier gestational ages, but incidence was still lower than in our study [33]. It is worthwhile noting that in our study, the prevalence of TTN was similar between both Tdap exposed and Tdap unexposed groups and, along with all outcomes evaluated, was higher in the group of infants born at <34 weeks gestation.
Finally, selection bias may have occurred due to our inclusion criteria requiring continuous insurance enrollment and a prenatal clinic visit, thus excluding many high risk pregnancies where adverse neonatal outcomes may be more common. However, the inclusion criteria also allowed for the exposure of interest to occur; maternal Tdap vaccination would be unlikely to occur if a patient does not present for prenatal care.
5. Conclusion
Continued examination of the safety of maternal Tdap administration is important for ongoing public health efforts aimed at decreasing infant morbidity and mortality associated with pertussis disease in the United States and abroad. Maternal pertussis vaccination has been demonstrated to be up to 93% effective in preventing pertussis infection among infants less than 8 weeks old [4]. Our study, along with prior work by our group and others [5–9,34,35], supports the safety of maternal Tdap vaccination for infant outcomes.
Acknowledgments
Funding/Support: The Centers for Disease Control and Prevention.
Role of the Funder/Sponsor: CDC participated in the design and conduct of the study and interpretation of the data, and approval of the manuscript.
Dr. Nicola Klein receives research support from GlaxoSmithKline, Sanofi Pasteur, Pfizer, Merck, and Protein Science, and MedImmune. Dr. Allison Naleway receives research support from Merck, Pfizer, and MedImmune. Dr. Cheetham has received research support from Bristol-Myers Squibb.
Abbreviations:
- Tdap
tetanus, diphtheria, and acellular pertussis
- ACIP
Advisory Committee on Immunization Practices
- VSD
Vaccine Safety Datalink
- RDS
respiratory distress syndrome
- TTN
transient tachypnea of the newborn
Footnotes
This work was completed while Dr. DeSilva was an employee of HealthPartners Institute. Dr. DeSilva now works for the Infectious Disease Epidemiology, Prevention, and Control Division at the Minnesota Department of Health.
Conflict of Interest Disclosures: The remaining co-authors have no financial conflicts of interest to report.
Publisher's Disclaimer: Disclaimer: Findings of this study represent those of the authors and do not necessarily represent the official position of the CDC.
References
- [1].Hewagama S, Walker SP, Stuart RL, et al. 2009 H1N1 influenza A and pregnancy outcomes in Victoria, Australia. Clin Infect Dis 2010;50(5):686–90. [DOI] [PubMed] [Google Scholar]
- [2].Centers for Disease Control and Prevention. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Tdap) in pregnant women and persons who have or anticipate having close contact with an infant aged <12 months –- Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep 2011;60(41):1424–6. [PubMed] [Google Scholar]
- [3].Centers for Disease Control and Prevention. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women–Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep 2013;62(7):131–5. [PMC free article] [PubMed] [Google Scholar]
- [4].Dabrera G, Amirthalingam G, Andrews N, et al. A case-control study to estimate the effectiveness of maternal pertussis vaccination in protecting newborn infants in England and Wales, 2012–2013. Clin Infect Dis 2015;60 (3):333–7. [DOI] [PubMed] [Google Scholar]
- [5].Kharbanda EO, Vazquez-Benitez G, Lipkind HS, et al. Evaluation of the association of maternal pertussis vaccination with obstetric events and birth outcomes. JAMA 2014;312(18):1897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Shakib JH, Korgenski K, Sheng X, Varner MW, Pavia AT, Byington CL. Tetanus, diphtheria, acellular pertussis vaccine during pregnancy: pregnancy and infant health outcomes. J Pediatr 2013; 163(5): 1422–1426 e1421–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Munoz FM, Bond NH, Maccato M, et al. Safety and immunogenicity of tetanus diphtheria and acellular pertussis (Tdap) immunization during pregnancy in mothers and infants: a randomized clinical trial. JAMA 2014;311(17):1760–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zheteyeva YA, Moro PL, Tepper NK, et al. Adverse event reports after tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccines in pregnant women. Am J Obstet Gynecol 2012; 207(1): 59 e51–57. [DOI] [PubMed] [Google Scholar]
- [9].Sukumaran L, McCarthy NL, Kharbanda EO, et al. Safety of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis and influenza vaccinations in pregnancy. Obstet Gynecol 2015;126(5):1069–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Moro PL, McNeil MM, Sukumaran L, Broder KR. The centers for disease control and prevention’s public health response to monitoring Tdap safety in pregnant women in the United States. Hum Vaccin Immunother 2015;11(12):2872–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hagberg H, Wennerholm UB, Savman K. Sequelae of chorioamnionitis. Curr Opin Infect Dis 2002;15(3):301–6. [DOI] [PubMed] [Google Scholar]
- [12].Tita AT, Andrews WW. Diagnosis and management of clinical chorioamnionitis. Clin Perinatol 2010;37(2):339–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Guzick DS, Winn K. The association of chorioamnionitis with preterm delivery. Obstet Gynecol 1985;65(1):11–6. [PubMed] [Google Scholar]
- [14].Yancey MK, Duff P, Kubilis P, Clark P, Frentzen BH. Risk factors for neonatal sepsis. Obstet Gynecol 1996;87(2):188–94. [DOI] [PubMed] [Google Scholar]
- [15].Wolfs TG, Jellema RK, Turrisi G, Becucci E, Buonocore G, Kramer BW. Inflammation-induced immune suppression of the fetus: a potential link between chorioamnionitis and postnatal early onset sepsis. J Matern Fetal Neonatal Med 2012;25(Suppl 1):8–11. [DOI] [PubMed] [Google Scholar]
- [16].Alexander JM, McIntire DM, Leveno KJ. Chorioamnionitis and the prognosis for term infants. Obstet Gynecol 1999;94(2):274–8. [DOI] [PubMed] [Google Scholar]
- [17].Soraisham AS, Singhal N, McMillan DD, Sauve RS, Lee SK, Canadian Neonatal N. A multicenter study on the clinical outcome of chorioamnionitis in preterm infants. Am J Obstet Gynecol 2009; 200(4): 372 e371–376. [DOI] [PubMed] [Google Scholar]
- [18].Watterberg KL, Demers LM, Scott SM, Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics 1996;97(2):210–5. [PubMed] [Google Scholar]
- [19].Been JV, Rours IG, Kornelisse RF, et al. Histologic chorioamnionitis, fetal involvement, and antenatal steroids: effects on neonatal outcome in preterm infants. Am J Obstet Gynecol 2009; 201(6): 587 e581–588. [DOI] [PubMed] [Google Scholar]
- [20].Jones MH, Corso AL, Tepper RS, et al. Chorioamnionitis and subsequent lung function in preterm infants. PLoS One 2013;8(12):e81193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lieberman E, Lang J, Richardson DK, Frigoletto FD, Heffner LJ, Cohen A. Intrapartum maternal fever and neonatal outcome. Pediatrics 2000;105(1 Pt 1):8–13. [DOI] [PubMed] [Google Scholar]
- [22].Berenson AB, Hirth JM, Rahman M, Laz TH, Rupp RE, Sarpong KO. Maternal and infant outcomes among women vaccinated against pertussis during pregnancy. Hum Vaccin Immunother 2016;12(8):1965–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Morgan JL, Baggari SR, McIntire DD, Sheffield JS. Pregnancy outcomes after antepartum tetanus, diphtheria, and acellular pertussis vaccination. Obstet Gynecol 2015;125(6):1433–8. [DOI] [PubMed] [Google Scholar]
- [24].Baggs J, Gee J, Lewis E, et al. The Vaccine Safety Datalink: a model for monitoring immunization safety. Pediatrics 2011;127(Suppl 1):S45–53. [DOI] [PubMed] [Google Scholar]
- [25].Naleway AL, Gold R, Kurosky S, et al. Identifying pregnancy episodes, outcomes, and mother-infant pairs in the Vaccine Safety Datalink. Vaccine 2013;31(27):2898–903. [DOI] [PubMed] [Google Scholar]
- [26].Kharbanda EO, Vazquez-Benitez G, Lipkind H, et al. Receipt of pertussis vaccine during pregnancy across 7 Vaccine Safety Datalink sites. Prev Med 2014;67:316–9. [DOI] [PubMed] [Google Scholar]
- [27].Kharbanda EO, Vazquez-Benitez G, Lipkind H, et al. Inactivated influenza vaccine during pregnancy and risks for adverse obstetric events. Obstet Gynecol 2013;122(3):659–67. [DOI] [PubMed] [Google Scholar]
- [28].Kharbanda EO, Vazquez-Benitez G, Shi WX, et al. Assessing the safety of influenza immunization during pregnancy: the Vaccine Safety Datalink. Am J Obstet Gynecol 2012;207(3 Suppl):S47–51. [DOI] [PubMed] [Google Scholar]
- [29].Nordin JD, Kharbanda EO, Vazquez Benitez G, et al. Maternal influenza vaccine and risks for preterm or small for gestational age birth. J Pediatr 2014; 164(5): 1051–1057 e1052. [DOI] [PubMed] [Google Scholar]
- [30].Bastek JA, Weber AL, McShea MA, Ryan ME, Elovitz MA. Prenatal inflammation is associated with adverse neonatal outcomes. Am J Obstet Gynecol 2014; 210 (5): 450 e451–410. [DOI] [PubMed] [Google Scholar]
- [31].Morrison JJ, Rennie JM, Milton PJ. Neonatal respiratory morbidity and mode of delivery at term: influence of timing of elective caesarean section. Br J Obstet Gynaecol 1995;102(2):101–6. [DOI] [PubMed] [Google Scholar]
- [32].Zanardo V, Simbi AK, Franzoi M, Solda G, Salvadori A, Trevisanuto D. Neonatal respiratory morbidity risk and mode of delivery at term: influence of timing of elective caesarean delivery. Acta Paediatr 2004;93(5):643–7. [DOI] [PubMed] [Google Scholar]
- [33].Consortium on Safe Labor Hibbard JU Wilkins I et al. Respiratory morbidity in late preterm births. JAMA 2010; 304(4): 419–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Walls T, Graham P, Petousis-Harris H, Hill L, Austin N. Infant outcomes after exposure to Tdap vaccine in pregnancy: an observational study. BMJ Open 2016;6(1):e009536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].DeSilva M, Vazquez-Benitez G, Nordin JD, et al. Tdap Vaccination During Pregnancy and Microcephaly and Other Structural Birth Defects in Offspring. JAMA 2016;316(17):1823–5. [DOI] [PubMed] [Google Scholar]


