Abstract
Objective:
To investigate the dimensions of the tensor veli palatini (TVP) muscle in adults with and without cleft palate.
Design:
Prospective study
Participants:
There were a total of 14 adult participants, eight non-cleft and six with cleft palate.
Methods:
Analysis and comparison of the TVP muscle and surrounding structures was completed using 3D MRI data and Amira 5.5 Visualization Modeling software. TVP muscle volume, hamular process distance, mucosal thickness, TVP muscle length, and TVP muscle diameter were used for comparison between participant groups based upon previous research methods.
Results:
Mann-Whitney U tests revealed a significantly smaller (U < .001, p = .002) TVP muscle volume in the cleft palate group (median = 536.22 mm3) compared to individuals in the non-cleft palate group (median = 895.19 mm3). The TVP muscle was also significantly shorter (U = 1.00, p = .003) in the cleft palate group (median = 18.04 mm) versus the non-cleft palate (median = 21.18 mm). No significant differences were noted for the other measured parameters.
Conclusion:
Significant differences in the TVP muscle volume and length among the non-cleft participants found in this study may insights into the reported increased incidence of otitis media with effusion (OME) seen in the cleft population. Results from this study contribute to our understanding of the underlying anatomic differences among individuals with cleft palate.
Keywords: Anatomy, magnetic resonance imaging, muscle function
INTRODUCTION
The Eustachian tube (ET) has three primary functions pertaining to the middle ear, including ventilation, middle ear clearance, and protection of the middle ear against nasopharyngeal pathogens (Gyanwali et al., 2016). Otitis media with effusion (OME), acute otitis media, cholesteatoma, and atelectasis are some of the pathological manifestations of deficiency in ET function (Bluestone and Bluestone, 2005). The development of middle ear pathology, as caused by ET dysfunction, begins with inflammation of the nasopharynx and ET mucosa and leads to congestion and subsequent blockage of the ET. This blockage creates a negative pressure inside the middle ear, often leading to effusion (OME) of the middle ear. The negative ear pressure may also lead to aspiration of pathogens present within the nasopharynx into the middle ear, resulting in further middle ear pathology (Bluestone and Bluestone, 2005). The susceptibility one has to this sequence of events depends largely on a person’s ET anatomy and the functionality of the primary muscle responsible for ET function, the tensor veli palatini (TVP) muscle. Contraction of the TVP muscle during a swallow or yawn results in dilation of the ET, allowing for drainage, ventilation, and the equalization of pressures in and outside the ear.
In normal anatomy, the TVP originates from the angular spine of the sphenoid bone, scaphoid fossa, and pterygoid fossa. Fibers also arise from the lateral membranous wall of the ET. Collectively, the fibers converge on the medial tendon, which passes around the hamulus and courses medially to form a horizontal palatal aponeurosis situated posterior to the hard palate and anterior to the soft palate. Using gross dissection, Barsoumain et al. (1998) described the dual nature of the TVP muscle explained by contributions of the TVP proper and dilator tubae. The dilator tubae is described as attaching to the hook of the ET and thereby contributing significantly to the opening of the ET. Although the primary function of the TVP is largely to promote normal ET function, it is likely that the TVP muscle plays a role in providing support to the bridging between the hard and soft palate via the extension of the tendon forming the palatal aponeurosis (Barsoumian et al., 1998). Specifically, the dilator tubae segment of the TVP muscle winds around the middle one third of the hamulus resulting in a possible tensile role to the anterior soft palate (Barsoumian et al., 1998). The contribution may therefore be more of adding durability to the stiffness of the anterior portion of the velum (Barsoumian et al., 1998).
Children with cleft palate can develop issues related to feeding, speech, hearing, language, facial growth, psychosocial and developmental factors, and dentition. Approximately 90% of patients with cleft palate have been reported to develop OME owing to dysfunction of the ET or TVP muscle abnormalities (Narayanan, 2013; Schonmeyr and Sadhu, 2014). The universally reported high incidence of middle ear effusion in children with a history of cleft palate has been shown to have a negative impact on the child’s overall development (Doyle et al., 1980; Grant et al., 1988; Schonweiler et al., 1994; Narayanan et al., 2013). Early appearance of middle ear effusion and recurrent OME may have a significant impact on auditory function, and consequently, speech and language acquisition. Delays in children’s development of speech and language, in turn, has a negative impact on academic performance and psychosocial development.
Dysfunction of the ET is commonly associated with middle ear infections among children with cleft palate (Fara and Dvorak, 1970; Cole et al., 1974; Paradise, 1975; Seif and Dellon, 1978; Doyle et al., 1980; Rood and Doyle, 1982; Aniansson et al., 2002.). Studies have observed a shorter ET (Sadler-Kimes et al., 1989), deformed cartilaginous portion of the ET (Shibahara and Sando, 1988; Sando and Takahashi, 1990; Matsune et al., 1991), and a decrease in the elastin at the hinge portion of the ET among individuals with a history of cleft palate (Matsune et al., 1992). Rajion et al. (2012) described a possible compression of the nasopharyngeal structures due to abnormalities of the nasopharyngeal and maxillary structures among those with cleft palate. This compression is suggested to have a negative impact on the patency of the ET at the nasopharynx (Maue-Dickson, 1980; Rajion et al., 2012). Rajion et al. (2012) also observed abnormal angulation of the hamulus among individuals with cleft palate and hypothesized that such variations lead to alterations in the orientation of the TVP muscle, and therefore, abnormal biomechanics of the tubal dilator. Shibahara and Sando (1988) also attribute ET dysfunction among individuals with a history of cleft palate to be a result of numerous ET cartilage abnormalities and the relationship of these structures relative to the TVP.
Terzi et al. (2016) used magnetic resonance imaging (MRI) to investigate the differences among individuals with chronic suppurative otitis media (CSOM) compared to those with normal ears. Results indicated no significant difference in paratubal structures including: TVP length and volume, diameter of the pharyngeal ET orifice, volume of the Ostamann fat pad, bimucosal ET lumen thickness, and mucosal thickness. This study, however, did not include individuals with cleft palate. Sapci et al. (2008) also found no difference in TVP electromyographic activity between individuals presenting with normal ears and individuals with chronic middle ear disease, all without cleft palate. However, one participant presented with a significant decrease in EMG activity and the individual was observed to have palatal pathologies. The authors emphasized the predisposing factor of palate pathologies, such as cleft palate, likely relates to TVP muscle abnormalities effecting overall middle ear health.
Investigations of the TVP among individuals with cleft palate have demonstrated variations from that of normal non-cleft anatomy. Matsune et al. (1991) observed a decreased or absent insertion of the TVP into the lateral lamina of the ET among unborn fetuses with cleft palate. The authors attribute this abnormality as a cause of the functional obstruction of the ET resulting in OME. However, data were collected via histology from unborn fetuses in which cause of death was not reported (Matsune et al., 1991; Matsune et al., 1992). Thus specimens from these studies may be more reflective of complex anatomies that extend beyond cleft palate. Additionally, disagreement persists within the literature regarding the role of the TVP muscle in the development of OME within the cleft palate population (Huang et al., 1997; Schonmeyr and Sadhu, 2014). Sehhati-Chafai-Leuwer et al. (2006) used MRI to examine the TVP muscle among 15 adult participants with cleft palate (14 with repaired cleft palate and one without a repaired palate). Although quantitative measures were not reported, the authors observed a visible discontinuity of the TVP muscle, particularly at the region alongside the levator veli palatini muscle and near the hamulus for individuals with a history of chronic middle ear disease. The present study was designed to build upon the findings reported by Sehhati-Chafai-Leuwer et al. (2006) and perform a quantitative linear and volumetric comparison between individuals with cleft palate and individuals without cleft palate.
The purpose of this study is to investigate the dimensions of the TVP muscle using high image resolution three-dimensional (3D) MRI of the soft palate among adults with and without cleft palate. Specifically, we examined if the length, diameter, and volume of the TVP muscle differ significantly between adults with and without cleft palate. It was hypothesized that individuals with repaired cleft palate would display differences in the TVP muscle, such as hypoplasia. We expected hypoplasia to be evidenced as a lower muscle volume. Additionally, we compared differences between groups regarding the hamular process width and mucosal thickness of the ET lumen at the nasopharyngeal orifice. We hypothesized the presence of recurrent OME would result in permanent changes to the mucosal thickness at the ET opening in the nasopharynx (Sando and Takahashi, 1990).
METHODS
Participants
In accordance with the local Institutional Review Boards, 14 English-speaking adults between 19 and 36 years of age were recruited to participate in this study. Participants included six adults (three males and three females) with repaired cleft palate (M = 24 years of age, SD = 6.8 years) and eight adults (two males and six females) without cleft palate (M = 22 years of age, SD = 4.8 years). Individuals without cleft palate were selected from a larger dataset (Perry et al., 2016) and were chosen because they represented individuals who were similar in age to those in the cleft group, indicated by self-identification no history of chronic ear infections on a participant history intake form, and had MRI data that was free of head movement as noted by motion artifacts. Ensuring minimal to no motion artifacts was important for all participants given the relatively small structures used as variables in the study. Inclusion criteria for the participants without cleft palate included a participant-reported absence of a history of hearing, neurological, swallowing, craniofacial, or musculoskeletal disorders. Patients with cleft palate were included if they had a repaired cleft palate and had an absence of syndrome diagnosis. Of the six adults with cleft palate, three had bilateral cleft lip and palate and three had cleft palate only. Cleft surgery had been performed in different hospitals, and specific details regarding the surgical procedures were not provided. However, all participants with cleft palate reported a history of middle ear infections on their patient questionnaire. Participants indicated, through self-report, no hearing loss or issue with middle ear infections/disease at the time of the study. Body mass index (BMI) was collected on all prospective participants to ensure that he/she was below the threshold BMI value for notable effects on the velopharyngeal anatomy (Finkelstein et al., 2014). Classifications of BMI was consistent with guidelines from the National Heart, Lung, and Blood Institute (National Institutes of Health, 2017).
Magnetic Resonance Imaging
All participants were imaged in the supine position using a Siemens 3 Tesla Trio (Erlangen, Germany) MRI scanner and a 12-channel Siemens Trio head coil. A high resolution T2-weighted 3D turbo spin echo (TSE) anatomical scan called Sampling Perfection with Application optimized Contrasts using different flip angle Evolution (SPACE) sequence was acquired with .8 mm isotropic spatial resolution in 4:52 minutes covering the anatomy of interest with a sagittal acquisition (25.6 X 19.2 X 15.4 cm). Parameters include a repetition time of 2,500 ms, echo time of 268 ms, and an echo-train length of 171. Parallel imaging acceleration with a factor of two in both phase encode and slice directions was used across the images.
Image analyses
The MRI data were imported into Amira 5.5 Visualization Modeling software (Visage Imaging GmbH, Berlin, Germany) complete with native Digital Imaging and Communication in Medicine (DICOM) support program which allowed for the preservation of geometric structures present in the data sets. The TVP muscle was visualized by slicing the 3D data along an oblique coronal plane at approximately 64 degrees from the sagittal plane. This angle allowed for visualization of the entire TVP muscle as it coursed along the ET and down to wrap around the pterygoid hamulus. Consecutive slices taken from the oblique coronal plane in which the TVP muscle was present were used to create a 3D volumetric reconstruction of the muscle, as described by Kotlarek et al. (2017) and Perry et al. (2013). A free-handed paintbrush tool in Amira segmentation editor was used to identify TVP muscle fibers, thereby creating a voxel set. Threshold analyses were used to ensure the selected voxels represented the muscle fibers, as determined by the voxel threshold obtained at the midline of the TVP muscle. The voxel set was subsequently used to calculate volumetric values of the TVP muscle.
Figure 1 shows the measures that were carried out for each participant. Similar to Terzi et al. (2016), variables for the present study included the length of the TVP, diameter of the TVP muscle, TVP muscle volume, bihamular distance, and mucosal thickness of the ET lateral lamina at the pharyngeal orifice. The TVP muscle length was obtained by measuring the muscle from the origin along the lateral lamina of the Eustachian tube to the point at which the muscle descends to wrap around the hamulus. This was obtained within the resliced oblique-coronal volume by choosing the image containing the longest portion of the muscle bilaterally. The right and left bundles were grouped to create a mean TVP muscle value for each participant. The diameter of the TVP muscle was obtained at the region of the greatest diameter of the muscle belly. As previously mentioned, the volume of the TVP muscle was calculated following manual segmentation of the muscle through sequential images and compared between participant groups Bihamular distance was measured as a straight line between the two hamular processes. The mucosal thickness of the ET lateral lamina was measured by drawing a reference line along the long axis of the ET and a corresponding perpendicular line that was used to determine the thickness of the ET lateral lamina.
FIGURE 1.
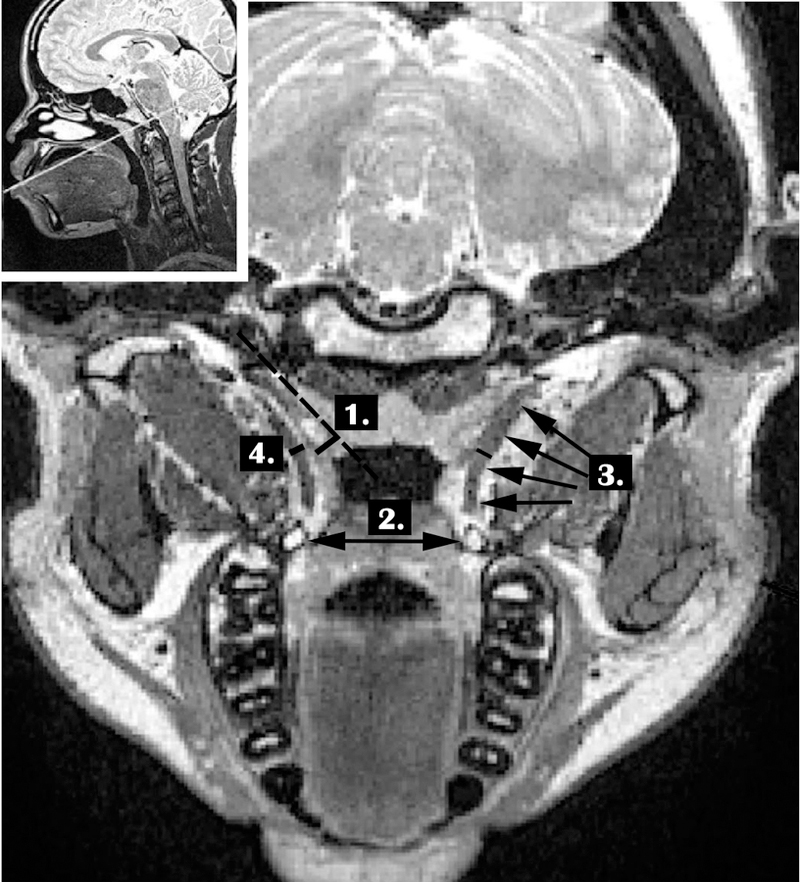
Demonstration of the measures obtained from the oblique coronal plane including: (1) mucosal thickness of the ET lateral lamina at the pharyngeal orifice (relative to the dotted reference line running through and middle of the ET), (2) bihamular distance (noted by double arrow), (3) TVP muscle length (noted by multiple arrows along the muscle contour), (4) TVP muscle diameter. The smaller figure in the upper left shows the plane (white line) from which these measures were derived placed upon the midsagittal image.
Statistical Analyses
Due to the unequal sample sizes between groups and the presence of outliers, non-parametric Mann-Whitney U tests (α<0.05) for independent samples were used to compare between the two groups (cleft and non-cleft) across the variables of interest. All assumptions associated with the use of a Mann-Whitney U test were met, including 1) variables in two categorical independent groups, 2) continuous dependent variables, 3) independent observations, and 4) the lack of normality, as determined using a Shapiro-Wilks test of normality (p < 0.05) and Q-Q Plot examination (demonstrating nonlinearity of data distributions). Qualitative analyses of the structures from visual inspection of the MRI data were also performed to compare between groups.
Inter- and intra-rater reliability measurements were obtained using an intra-class correlation coefficient (ICC) performed across 30% of participants. ICC estimates and their 95% confident intervals were calculated using SPSS statistical package version 23 (SPSS Inc, Chicago, IL) based on a 2-way mixed-effects model. The level of agreement for intra-rater agreement was between 0.877 and 0.964, indicating good to excellent reliability for measures of TVP volume, bihamular width, TVP length, and TVP diameter. Mucosal thickness showed moderate agreement at .639. Similarly, inter-rater agreement was between 0.874 and 0.901 indicating good reliability, with the lowest reliability for mucosal thickness.
RESULTS
Group medians, means, and standard deviations are presented in Table 1. A Mann-Whitney U test (Table 2) indicated the length of the TVP muscle was significantly (U = 1.00, p = .003) shorter for adults with cleft palate (median = 18.04 mm) compared to individuals without cleft palate (median = 21.18 mm). There was no significant difference (p > .05) between groups for measures of TVP muscle diameter. The TVP muscle volume was significantly (U < .001, p = .002) reduced for adults with cleft palate (median = 536.22 mm3) compared to adults without cleft palate (median = 895.19 mm3). There was no significant difference (p > .05) between groups for measures of bihamular width or mucosal thickness of the ET lumen at the nasopharyngeal orifice. The mean distance between hamular processes among groups differed by only 0.42 mm, and the differences between group means for mucosal thickness of the ET lumen and TVP muscle diameter were less than 0.2 mm. This demonstrates minimal difference between groups across these variables.
Table 1.
Median, mean, and standard deviation (SD) in millimeters (mm) and mm3 (for TVP volume) between groups across variables.
| Variable of Interest | Group | N | Median | Mean | SD | Std. Error Mean |
|---|---|---|---|---|---|---|
| TVP Muscle Length | Noncleft | 8 | 21.18 | 21.55 | 1.3 | 0.46 |
| Cleft | 6 | 18.04 | 17.59 | 1.93 | 0.79 | |
| TVP Muscle Diameter | Noncleft | 8 | 2.57 | 2.55 | 0.38 | 0.14 |
| Cleft | 6 | 2.56 | 2.68 | 0.63 | 0.26 | |
| TVP Muscle Volume | Noncleft | 8 | 895.19 | 987.63 | 301.55 | 106.61 |
| Cleft | 6 | 536.22 | 522.49 | 80.69 | 32.94 | |
| Bihamular Distance | Noncleft | 8 | 27.31 | 27.73 | 2.22 | 0.79 |
| Cleft | 6 | 27.54 | 28.15 | 3.25 | 1.33 | |
| Mucosal Thickness | Noncleft | 8 | 2.99 | 3.18 | 0.62 | 0.22 |
| Cleft | 6 | 3.03 | 3.09 | 0.43 | 0.18 |
Table 2.
Results of the Mann-Whitney U Tests are shown.
| TVP Length | TVP Diameter | TVP Volume | Bihamular Distance | Mucosal Thickness | |
|---|---|---|---|---|---|
| Mann-Whitney U | 1.000 | 22.000 | < .001 | 23.000 | 21.000 |
| Wilcoxon W | 22.000 | 58.000 | 21.000 | 59.000 | 42.000 |
| Z | −2.969 | −.258 | −3.098 | −.129 | −.387 |
| Asymp. Sig. (2-tailed) |
.003 | .796 | .002 | .897 | .699 |
| Exact Sig. [2*(1-tailed Sig.)] |
.001 | .852 | .001 | .950 | .755 |
Qualitative differences were also are apparent when comparing the TVP muscle in the cleft palate and non-cleft palate groups. Differences were primarily characterized as variations in the cohesiveness of the muscle from origin to insertion, overall size of the muscle, and consistency of the muscle location between participants. The TVP muscle was easily located in the non-cleft palate participants and presented as a consistent band of tissue from the origin to the insertion at the level of the hamulus. The shape of the muscle was well defined and marked by continuous attachment to the ET before it descends to wrap around the pterygoid hamulus. In contrast, the TVP muscle in the participants with cleft palate (Figure 2), was visibly shorter and less robust than that of individuals in the non-cleft palate group. Abnormality of the cohesiveness of the muscle was most notable near the hamular process. Occasionally, the right and left muscle bundles among the cleft palate group were not visible in a single image plane. Rather the right bundle and left were obtained on separate image planes. Additionally, the TVP muscle in individuals with cleft palate appeared to be displaced more medially, whereas individuals without cleft palate displayed a greater distance between the lateral pharyngeal walls of the pharynx and the TVP muscle belly. Figure 2 displays an oblique coronal image showing the TVP for all the participants between the two groups. The muscle is consistently visible in its entirety among individuals without cleft palate compared to individuals with cleft palate.
FIGURE 2.
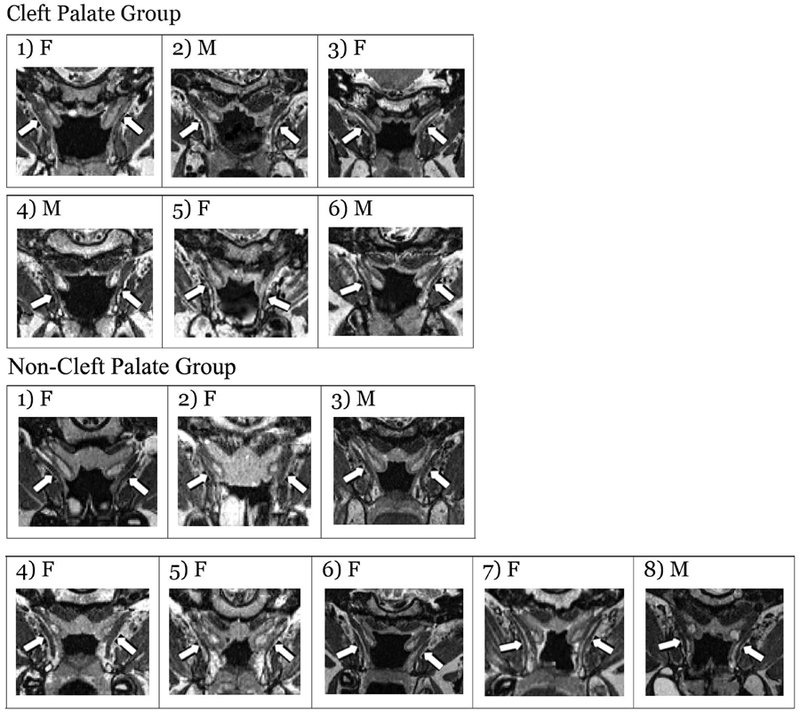
Oblique coronal images across each participant demonstrating the TVP muscle. The TVP muscle bundles are indicated with white arrows and the sex of the participant is noted as male (M) or female (F).
DISCUSSION
Qualitative differences in the present study demonstrated the TVP muscle to be positioned more medially compared to those without cleft palate. Although this study did not examine differences in the levator veli palatini muscle, the more medially displaced TVP muscle may also be attributed to hypoplasia of the levator muscle bellies, as reported previously (Ha et al., 2007; Perry et al., 2013; Kotlarek et al., 2017) and visibly evident in Figure 2 among those in the present study.
Observations in the present study as to the lack of continuity and integrity of the TVP muscle, particularly near the hamular process, is in agreement with findings by Sehhati-Chafai-Leuwer et al. (2006). Although surgical details were not available, it is possible differences in the TVP muscle, particularly at the hamular region, may be related to surgical attempt to separate the tensor tendon from the hamulus. As such, the separation may actually result in a greater separation or distance of the TVP belly from the insertion site at the hamular process. The TVP muscle was also not consistently visible in a single image plane among those with cleft palate. This may suggest inconsistencies in the positions of the right and left TVP muscle bundles among individuals with cleft palate. Because no participants had a unilateral cleft lip and palate, this noted asymmetry in the TVP position likely cannot be explained by the type of cleft palate. There were no differences in the hamular width between groups, and thus, the differences in the muscle positioning seen in the present study are likely not effected by bihamular distance.
The MRI methods in the present study, however, were aimed to capture the entire velopharyngeal system. In such, delineation between the TVP proper and dilator tubae described by Barsoumian et al. (1998) could not be identified. Using a high resolution volume reconstruction method and/or a diffuse tensor imaging approach could provide greater delineation of these muscle segments and variations between those with and without cleft palate. In cleft palate, controversy remains as to the role of surgery in restoring this normal TVP muscle position and function to promote improved ET function (Schonmeyr and Sadhu, 2014).
In agreement with other published studies showing a greater maxilla and hard palate width among those with cleft palate (Rajion et al., 2012), we expected to find a significant difference in the bihamular width between cleft palate and non-cleft palate study groups, with a greater width among those with cleft palate. We expected such difference would account for variations in the direction and course of the muscle from the origin to the hamulus. However, there was no significant difference in the bihamular width measure.
Results of this study suggest differences in the TVP muscle volume and length are features that may be common to those with repaired cleft palate. Differences in TVP muscle volume are likely related to the significant difference in TVP muscle length. The sites of origin of the TVP muscle could not be visualized using the MRI methods in the present study. Therefore, differences in the attachment of the TVP muscle to the ET cartilage and variations in the ET cartilage itself, as previously reported (Matsune et al., 1991; Matsune et al., 1992), could not be determined in the present study. These variations likely also contribute to alternations in the leverage action of the TVP and subsequent ET function. More research is needed to understand the variations in the functions of the TVP muscle using muscle imaging (MRI).
Efforts to repair the anatomical anomalies seen within the cleft palate population, and thereby reduce the risk of OME, have come in the form of new surgical techniques implemented during the repair of cleft palate (Flores et al., 2010). A more recent surgical technique called tensor tenopexy has sought to decrease the risk of OME in children with cleft palate by sparing the TVP muscle without compromising palatoplasty or significantly increasing the need for pharyngoplasty. Flores et al. (2010) and Tiwari et al. (2013) compared outcomes between children receiving tensor tenopexy and those receiving levator sling reconstruction with tensor transection. Significant improvements in otologic outcomes appear to only become apparent after 4 years of age with no significant differences observed in the infantile years for those receiving tensor tenopexy compared to those receiving palatoplasty with tensor transection (Flores et al., 2010).
Although Flores et al. (2010) and Tiwari et al. (2013) demonstrate possible improvements in middle ear health using these surgical techniques, neither study reported outcomes related to velopharyngeal function. It is not clear how TVP preservation using such surgical techniques effects levator veli palatini function and the subsequent effect on speech. For these reasons, Flores et al. (2010) emphasized the need for future studies to carefully balance the benefits of speech and ET function when evaluating surgical outcomes. This is particularly important given the essential role of normal hearing in establishing normal speech development.
These findings do not negate the importance of the surgery, as studies have shown that one-third of patients with standard cleft palate surgery continue to suffer from chronic otitis media or tubal dysfunction into adulthood (Gudiziol and Mann, 2006). These data suggest that there may be abnormalities of the TVP muscle (e.g., reduced muscle length and volume) that cannot be corrected with the tensor tenopexy which aims to correct the attachment, orientation, and location of the TVP muscle. Results from the present study suggest muscle deficiencies that go well beyond the muscle positioning. Specifically, deficiencies observed in the present study are related to factors that cannot be altered from surgery itself. Although the cause-and-effect of a decreased TVP muscle volume on OME has not been demonstrated within this study, it is logical to assume a hypoplastic muscle would have deleterious effects on normal function. Surgical procedures that alter the position of the muscle, therefore, may not sufficiently restore the normal function, particularly when dysfunction is due to muscle volume and overall length.
It is generally agreed that the levator veli palatini muscle alone cannot open the ET (Alper et al., 2012; Handzel et al., 2012). However, several studies have demonstrated the coordinated role of the levator veli palatini muscle with the TVP, particularly in assisting in opening the most anterior potion of the ET at the pharyngeal opening (Proctor, 1973; Shprintzen and Croft, 1981; Spauwen et al., 1991; Huang et al., 1997). Smith et al (2008) observed that children who underwent double-opposing Z-plasty required fewer ear tubes placed post-operatively compared to children who had a 2-flap palatoplasty. These findings demonstrate the likely contribution of the levator veli palatini muscle in overall middle ear health. Although the focus of this study was related to the TVP, future studies should examine dysmorphology found among both muscles and the relationship to middle ear health following varied surgical approaches.
It is not known if this reduced TVP length and volume found among the adult participants with cleft palate is a result of anatomic differences related solely to the presence of the cleft (evident at birth) or a result of hypoplasticity of the muscle developed over time. If it were determined that variations are not apparent at birth, this may lend greater support for surgical techniques that optimize the positioning of the muscle to ensure proper function and avoidance of muscle atrophy. Future studies should examine different age groups and determine if surgical techniques have a direct impact on the overall muscle morphology.
To our knowledge, this study is the first to provide quantitative comparisons between the TVP muscle between cleft palate and non-cleft palate anatomy using MRI in vivo. Due to the age-related morphological differences in the components of the ET and middle ear system (Sadler-Kimes et al., 1989), we aimed to examine the more stable auditory system found among adults. Additionally, the high-resolution MRI data may be more difficult to acquire among young children due to the length of time the individual must remain still. Future studies, however, should examine these differences among the cleft palate infant and child populations with careful consideration given to the likely impact of the age of the auditory system and the high variability in auditory function and disease among younger populations.
A limitation of this study is the unequal sample of male to female participants per group. It is likely that sex differences are apparent for the TVP muscle, similar to the sexual dimorphism found among the levator veli palatini muscle (Perry et al., 2014). Perry et al. (2014) demonstrated males display a significantly longer levator veli palatini muscle compared to females. It could be expected that the TVP may also follow the same observation with males showing a greater muscle mass and length compared to females. However, the non-cleft palate control group had a greater number of female participants (likely causing a lower mean TVP volume and length) compared to the cleft palate group, and we still rejected the null hypothesis for the study. This demonstrates that even with a possible sex bias, the participants with cleft palate still maintained a significant difference in TVP muscle volume and length. It would be expected that if the sample size were adequate for future comparisons based on sex, an even more pronounced difference between groups would be apparent. Because data were collected as part of a larger study, which aimed to examine muscles related to speech, participant self-report was used to determine status of hearing at the time of the MRI studies. This is a limitation of the present study. Specifically, a hearing screening at the time of the MRI study would have offered a more accurate determination of hearing status. Additional limitations related to this study include the relatively small sample size, lack of control for cleft type, and lack of surgical details for participants with cleft palate.
CONCLUSION
Data from the present study demonstrate a reduced TVP muscle volume and shorter muscle length in the participants with cleft palate when compared with the non-cleft palate participants, thereby providing a potential explanation for the increased incidence of OME seen in the cleft palate population. Results from this study contribute to our understanding of the underlying anatomic differences among individuals with cleft palate and may provide insights into the high incidence of recurrent OME among this population.
Acknowledgements:
This study was made possible by grant number 1R03DC009676–01A1 from the National Institute on Deafness and Other Communicative Disorders. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- Alper CM, Swarts JD, Singla A, Banks J, Doyle WJ. Relationship between the electromyographic activity of the paratubal muscles and Eustachian tube opening assessed by sonotubometry and videoendoscopy. Arch Otolaryngol Head Neck Surg 2012;138:741–746. [DOI] [PubMed] [Google Scholar]
- Aniansson G, Svensson H, Becker M, Ingvarsson L. Otitis media and feeding with breast milk of children with cleft palate. Scand J Plast Reconstr Surg Hand Surg 2002;36:9–15. [DOI] [PubMed] [Google Scholar]
- Barsoumian R, Kuehn DP, Moon JB, Canady JW. An anatomic study of the tensor veli palatini and dilatator tubae muscles in relation to Eustachian tube and velar function. Cleft Palate Craniofac J 1998;35(2):101–10. [DOI] [PubMed] [Google Scholar]
- Bluestone CD, Bluestone MB. Eustachian tube: structure, function, role in otitis media Hamilton: BC: Decker; 2005. [Google Scholar]
- Cole RM, Cole JE, Intaraprasong S. Eustachian tube function in cleft lip and palate patients. Arch Otolaryngol 1974;99:337–341. [DOI] [PubMed] [Google Scholar]
- Doyle WJ, Cantekin EI, Bluestone CD. Eustachian tube function in cleft palate children. Ann Otol Rhinol Laryngol Suppl 1980;89:34–40. [DOI] [PubMed] [Google Scholar]
- Fara M, Dvorak J. Abnormal anatomy of the muscles of palatopharyngeal closure in cleft palates: anatomical and surgical considerations based on the autopsies of 18 unoperated cleft palates. Plast Reconstr Surg 1970; 46:488–497. [PubMed] [Google Scholar]
- Finkelstein Y, Wolf L, Nachmani A, Lipowezky U, Rub M, Shemer S, Berger G. Velopharyngeal anatomy in patients with obstructive sleep apnea versus normal subjects. J Oral Maxillofac Surg 2014;72(7):1350–72. [DOI] [PubMed] [Google Scholar]
- Flores RL, Jones BL, Bernstein J, Karnell M, Canady J, Cutting CB. Tensor veli palatini preservation, transection, and transection with tensor tenopexy during cleft palate repair and its effects on Eustachian tube function. Plast Reconstr Surg 2010;125(1):282–289. [DOI] [PubMed] [Google Scholar]
- Grant HR, Quiney RE, Mercer DM, Lodge S. Cleft palate and glue ear. Archives of Disease in Childhood 1988;63:176–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudziol V, Mann WJ. Chronic Eustachian tube dysfunction and its sequelae in adult patients with cleft lip and palate. HNO 2006;54:684–688. [DOI] [PubMed] [Google Scholar]
- Gyanwali B, Li H, Xie L, Zhu M, Wu Z, He G, Tang A. The role of tensor veli palatini muscle (TVP) and levetor veli palatini [corrected] muscle (LVP) in the opening and closing of pharyngeal orifice of Eustachian tube. Acta Otolaryngol 2016;136(3):249–255. [DOI] [PubMed] [Google Scholar]
- Ha S, Kuehn DP, Cohen M, Alperlin M. Magnetic resonance imaging of the levator veli palatini muscle in speakers with repaired cleft palate. Cleft Palate Craniofac J 2007;44:494–501. [DOI] [PubMed] [Google Scholar]
- Handzel O, Poe D, Marchbanks RJ. Sychronous endoscopy and sonotubometry of the Eustachian tube: a pilot study. Otol Neurotol 2012;33:184–191. [DOI] [PubMed] [Google Scholar]
- Huang MH, Lee ST, Rajendran K. A fresh cadaveric study of the paratubal muscles: implications for Eustachian tube function in cleft palate. Plast Reconstr Surg 1997;100:833–842. [DOI] [PubMed] [Google Scholar]
- Kotlarek KJ, Perry JL, Fang X. Morphology of the levator veli palatini muscle in adults with repaired cleft palate. J Craniofac Surg 2017;28(3):833–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsune S, Sando I, Takahashi H. Abnormalities of lateral cartilaginous lamina and lumen of Eustachian tube in cases of cleft palate. Ann Otol Rhinol Laryngol 1991;100(11):909–913. [DOI] [PubMed] [Google Scholar]
- Matsune S, Sando I, Takahashi H. Elastin at the hinge portion of the Eustachian tube cartilage in specimens from normal participants and those with cleft palate. Ann Otol Rhinol Laryngol 1992;101:163–167. [DOI] [PubMed] [Google Scholar]
- Maue-Dickson W, Dickson DR. Anatomy and physiology related to cleft palate: current research and clinical implications. Plast Reconstr Surg 1980;65:83–90. [DOI] [PubMed] [Google Scholar]
- Narayanan DS, Pandian SS, Murugesan S, Kumar R. The incidence of secretory otitis media in cases of cleft palate. J Clin Diagn Res 2013;7(7):1383–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health. BMI Table Available at: https://www.nhlbi.nih.gov/health/educational/lose_wt/bmitools.htm. Accessed August 14, 2017.
- Paradise JL. Middle ear problems associated with cleft palate. An internationally oriented review. Cleft Palate J 1975;12:17–22. [PubMed] [Google Scholar]
- Perry JL, Kuehn DP, Sutton BP. Morphology of the levator veli palatini muscle using magnetic resonance imaging. Cleft Palate Craniofac J 2013;50:64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Kuehn DP, Sutton BP, Gamage JK. Sexual dimorphism of the levator veli palatini muscle: an imaging study. Cleft Palate Craniofac J 2014;51:544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Kuehn DP, Sutton BP, Gamage JK, Fang X. Anthropometric Analysis of the Velopharynx and Related Craniometric Dimensions in Three Adult Populations Using MRI. Cleft Palate Craniofac J 2016;53:e1–e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor B Anatomy of the Eustachian tube. Arch Otolaryngol 1973;97:2–8. [DOI] [PubMed] [Google Scholar]
- Rajion ZA, Al-Khatib AR, Netherway DJ, Townsend GC, Anderson PJ, McLean NR, Samsudin AR. The nasopharynx in infants with cleft lip and palate. Int J Pediatr Otorhinolaryngol 2012;76(2):227–34. [DOI] [PubMed] [Google Scholar]
- Rood SR, Doyle WH. The nasopharyngeal orifice of the auditory tube: implications for tubal dynamics anatomy. Cleft Palate J 1982;19:119–128. [PubMed] [Google Scholar]
- Sadler-Kimes D, Siegel MI, Todhunter JS. Age-related morphologic differences in the components of the Eustachian tube/middle ear system. Ann Otol Rhinol Laryngol 1989;98(11):854–858. [DOI] [PubMed] [Google Scholar]
- Sando I, Takahashi H. Otitis media in association with various congenital diseases: preliminary study. Ann Otol Rhinol Laryngol Suppl 1990;148:13–16. [DOI] [PubMed] [Google Scholar]
- Sapci T, Mercangoz E, Evcimik MF, Karavus A, Gozke E. The evaluation of the tensor veli palatini muscle function with electromyography in chronic middle ear diseases. Eur Arch Otorhinolaryngol 2008;265:271–278. [DOI] [PubMed] [Google Scholar]
- Schonmeyr B, Sadhu P. A review of the tensor veli palatine function and its relevance to palatoplasty. J Plast Surg Hand Surg 2014;48(1):5–9. [DOI] [PubMed] [Google Scholar]
- Schonweiler R, Schonweiler B, Schmelzeisen R. Hearing function and language skills of 417 children with cleft palates. HNO 1994;42:691–96. [PubMed] [Google Scholar]
- Sehhati-Chafai-Leuwer S, Wenzel S, Bschorer R, Seedorf H, Kucinski T, Maier H, Leuwer R. Pathophysiology of the Eustachian tube: relevant new aspects for the head and neck surgeon. J Craniomaxillofac Surg 2006;34(6):351–4. [DOI] [PubMed] [Google Scholar]
- Seif S, Dellon AL. Anatomic relationships between the human levator and tensor veli palatini and the Eustachian tube. Cleft Palate J 1978;15:329–336. [PubMed] [Google Scholar]
- Shibahara Y, Sando I. Histopathologic study of Eustachian tube in cleft palate patients. Ann Otol Rhinol Laryngol 1988;97:403–408. [DOI] [PubMed] [Google Scholar]
- Smith LK, Gubbels SP, MacArthur CJ, Milczuk HA. The effect of the palatoplasty method on the frequency of ear tube placement. Arch Otolaryngol Head Neck Surg 2008;134:1085–1089. [DOI] [PubMed] [Google Scholar]
- Spauwen PH, Hillen B, Lommen E, Otten E. Three-diemnsional computer reconstruction of the Eustachian tube and paratubal muscles. Cleft Palate Craniofac J 1991;28:217–219. [DOI] [PubMed] [Google Scholar]
- Terzi S, Beyazal Celiker F, Ozgur A, et al. The evaluation of Eustachian tube paratubal structures using magnetic resonance imaging in patients with chronic suppurative otitis media. Acta Otolaryngol 2016;136(7):673–676. [DOI] [PubMed] [Google Scholar]
- Tiwari R, Sharma RK, Panda NK, Munjal S, Makkar S. Tensor tenopexy: a clinical study to assess its effectiveness in improving Eustachian tube function and preventing hearing loss in patients with cleft palate. J Plast Reconstr Aethet Surg 2013;66:e239–e245. [DOI] [PubMed] [Google Scholar]


