Graphical abstract
Abbreviations: ADMA, asymmetric dimethylarginine; AVMA, American Veterinary Medical Association; AKI, acute kidney injury; ANOVA, analysis of variance; ARRIVE, animal research: reporting of in vivo experiments; BNMN, Bi-nucleated cells with micronuclei; CBPI, cytokinesis block proliferation index; CIN, contrast-induced nephropathy; CKD, chronic kidney disease; CM, contrast medium; ESI, electrospray ionization; GFR, glomerular filtration rate; KIM-1, kidney injury molecule-1; LC–MS, liquid chromatography mass spectrometry; MN, micronuclei; NO, nitric oxide; NGAL, meutrophil gelatinase–associated lipocalin; OECD, Organisation for Economic Co-operation and Development; RBF, renal blood flow; ROS, reactive oxygen species; SCR, serum creatinine; SD, standard deviation; SDMA, symmetric dimethylarginine; TPT, touch preparation technique
Keywords: Asymmetric dimethylarginine, Contrast media, Kidney, Models, Animal, Iopromide, Nephropathy, Nephrotoxicity, Symmetric dimethylarginine
Highlights
-
•
Pathophysiology of contrast induced nephropathy is complex and obscure.
-
•
Iopromide increases sCr, SDMA and ADMA levels.
-
•
Iopromide increases significantly micronuclei frequency in lymphocytes.
-
•
Iopromide enhances cell degeneration and apoptosis in renal tissues.
Abstract
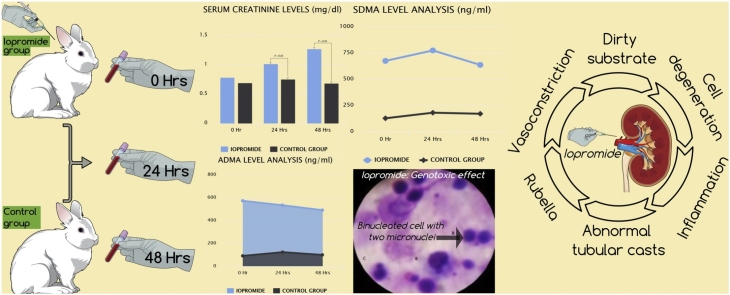
Identification of novel biomarkers of contrast-induced nephropathy (CIN) that may more accurately detect renal function changes; reflect kidney damage; assist monitoring; and elucidate pathophysiology attract considerable scientific attention nowadays. To evaluate novel biomarkers of nephrotoxicity in blood/tissue samples of a CIN model, 10 New Zealand white rabbits were divided into group 1 (n = 5; iopromide) and group 2 (n = 5; control). Blood was drawn at 0 h (immediately), 24 h and 48 h after contrast medium (CM) administration. Animals were euthanized at 48 h and kidneys were removed. Serum creatinine (sCr)/symmetric-asymmetric dimethylarginine (SDMA-ADMA) levels were measured. CM genotoxic/cytotoxic effect was investigated 48 h post-CM exposure using micronucleus assay in lymphocytes. Cytological examination was conducted using touch preparation technique (TPT). All animals in group 1 developed CIN: mean sCr levels increased by 68.2% within 48 h. Significant SDMA-ADMA level elevation was observed at 0 h and 24 h with insignificant drop at 48 h in group 1, remaining normal in group 2 at all time-points. Significant increase in bi-nucleated cells with micronuclei and micronuclei frequency was detected in group 1. Cytokinesis block proliferation index was reduced insignificantly in group 1. TPT revealed degenerative lesions/inflammation, cell degeneration, abnormal uterine tubular casts and rubella in kidneys of all animals in group 1. Group 2 presented normal cells.
1. Introduction
Iodinated contrast media (CM) are pharmaceutical substances administered for better medical imaging. CM are categorized into ionic/non-ionic and into high-, low- and iso-osmolar based on their water solubility and osmolarity, respectively [1]. Improvements in the safety profile of modern CM compared to older agents have largely been attributed to ionicity and osmolality reduction; low/iso-osmolar, non-ionic CM are preferred over high-osmolar, ionic CM [[2], [3], [4]]. Nevertheless, even after choosing the most suitable CM for each case, a variety of side effects may occur after exposure. CM administration exerts cytotoxic effects on renal tubular epithelial cells and promotes hemodynamic changes through renal vasoconstriction to severe renal damage and cellular apoptosis [5,6]. CM cause renal vasoconstriction by increasing adenosine and endothelin, thus changing blood flow from the marrow to the cortex. Consequently, the renal ability to infiltrate (glomerular filtration rate [GFR]) is decreased [7,8]. Reducing renal blood flow, in addition to reducing GFR, trigger the release of reactive oxygen species (ROS) through oxidative stress and osmotic necrosis induced directly by CM in tubular cells, although a direct renal oxidative stress action of CM has been challenged by in vitro studies [9]. As a result, acute necrosis of the renal tubules and renal hypoxia due to endothelial/tubular transport dysfunction are observed [[10], [11], [12]]. Apoptosis caused by activation of kinase stress/endogenous apoptotic pathway may be another consequence of CM administration [13].
The most common side effects after parenteral administration of CM apart from organ toxicity (with kidney being the most affected organ) [14] are hypersensitivity reactions [15]. Local side effects are attributed to extravasation but administration typically involves small volumes, and therefore it rarely results in serious injuries. Systemic side effects may occur early (<20 min) or late (>20 min) after administration, and may be attributed to anaphylactoid reaction or to effects due to the osmolarity/chemotoxicity of CM. Clinical reactions vary from minor, to intermediate and severe that can be life-threatening. In a recent systematic review, the incidence of immediate hypersensitivity skin reactions to CM was reported to be 0.12%–1.15% (most frequently urticaria, rashes, pruritus and limited facial edema), whereas the incidence of delayed reactions was reported to be 0.03%–10.1% (most frequently cutaneous manifestations) [16].
Contrast-induced nephropathy (CIN) is considered a reversible acute renal failure observed after the administration of CM that may exert their nephrotoxic effects through various mechanisms, including oxidative stress and apoptosis but the exact pathophysiology remains obscure and no standard diagnostic criteria apply [14]. Efforts towards the identification of novel biomarkers of CIN that more accurately detect changes in renal function, reflect kidney damage, assist monitoring, elucidate pathophysiology; and mainly towards the development of effective prevention strategies attract considerable scientific attention nowadays, representing an exciting topic of basic and clinical research given that the value of using compounds with antioxidant properties other than sodium bicarbonate remains controversial, warranting further investigation [14]. In this context, we have been extensively working during the last years by testing novel potential biomarkers of nephrotoxicity and compounds potentially preventing CIN in animal models, including various natural antioxidants and medications [[17], [18], [19], [20], [21], [22], [23]]. The aim of the present study was to evaluate novel biomarkers of nephrotoxicity in blood/tissue samples of a CIN animal model.
2. Experimental section
Ten New Zealand white male rabbits weighting approximately 3.5 kg were used in this protocol. The animal model was based on a previous study for evaluation of CIN in rabbits [24]. After receiving ethical approval for animal experimentation [23], the present study was conducted and conformed under National and European Union Directions/Directives/Guidelines (taken into account: the American Veterinary Medical Association (AVMA) Guidelines for the Euthanasia of Animals: 2013 Edition, DIRECTIVE 2010/63/EU, The Animal Research: Reporting of In Vivo Experiments (ARRIVE) Guidelines) regarding the care and treatment of laboratory animals so as to reach the required criteria and minimize possible suffering. All procedures were followed out by veterinarians. All euthanasia procedures were done so as to exclude any non-acceptable methods.
The animals were housed in individual metal cages under a 12 h dark/light cycle and steady ambient temperature between 20 and 23 °C. Commercial rabbit pellets and drinking water were provided ad libitum. After an acclimatization period of one week, the animals were divided into two groups: group 1 (n = 5; CM [iopromide]: Ultravist® 370, Bayer Healthcare, Berlin, Germany) and group 2 (n = 5; controls: NaCl 0.9%). Before the administration of CM, animals were weighed and anesthetized by intramuscular administration of xylazine hydrochloride (Xylapan®, Vetoquinol S.A., Lure Cedex, France) at a single dose of 4 mg/kg and ketamine hydrochloride (Narketan®, Vetoquinol S.A., Lure Cedex, France) at a single dose of 40 mg/kg. An intravenous catheter was placed in a marginal ear vein for the administration of CM over a period of 30 min at a single dose of 20.3 ml/kg (768.86 mg iopromide/ml, 370 mg iodine/ml, 7.5 g iodine/kg). Blood was drawn at three distinct time points: 0 h (immediately after the administration of CM/NaCl 0.9%), 24 h and 48 h after the administration of CM/NaCl 0.9%. The animals were euthanized at 48 h post CM/ NaCl 0.9% infusion, using pentobarbital sodium (Dolethal®, Vetoquinol S.A., Lure Cedex, France) intravenously at a single dose of 5 ml/animal and both their kidneys were removed.
Blood samples were stored in collection tubes without anticoagulant. Serum was isolated after centrifugation of the samples at 4000 rpm for 10 min at 21 °C. The supernatant was stored at −20 °C until biochemical analysis (serum creatinine [sCr] level measurement) using an automatic biochemical analyzer (Architect c4000, Abbott, Abbott Park, Illinois, U.S.A). Standard methanolic solutions of symmetric dimethylarginine (SDMA) and asymmetric dimethylarginine (ADMA) (Sigma Aldrich, St Louis, MO, USA) were prepared in concentrations of 0, 100, 250, 500, 1000 ng/ml, and spiked serum samples were prepared in concentrations of 0, 100, 200, 400 and 600 ng/ml. Plasma was isolated from peripheral blood by centrifugation at 4000 rpm for 10 min at 9 °C and stored at −20 °C. Extraction method has been previously described [25]. In brief, 50 μl of plasma were mixed with 60 μl buffer (1 ml formic acid, 1 g ammonium formate and 200 ml H2O) and 375 μl acetonitrile. After centrifugation at 10,000 x rpm for 5 min, 20 ul of the supernatant was used. SDMA/ADMA levels were measured using liquid chromatography mass spectrometry (LC–MS) (Shimadzu LC–MS 2010 EV) with electrospray ionization (ESI) in positive mode and single quadrupole mass filter. Separation of analytes was achieved using a LC-Si Supelco column 15 cm x 4.6 mm, 3 μm (Sigma‐Aldrich, St. Louis, MO, USA). A gradient of water with 0.1% formic acid and 10 mM ammonium acetate (solvent A) and methanol-acetonitrile with 10 mM ammonium acetate 50–50 (solvent B) were selected as the mobile phase with a flow rate 0.35 ml/min. The retention time of SDMA/ADMA was 9.96 and 10.39 min, respectively. The genotoxic and cytotoxic effect of CM was investigated at 48 h post-exposure using the micronucleus (MN) assay in lymphocytes. The MN test is an official regulatory “tool” in the European Legislation (B.12, Regulation 440/2008/EC) validated by the Organization for Economic Co-operation and Development (OECD) (OECD TG 474, 1997). Whole blood (0.5 ml) was added to 6.5 ml Ham’s F-10 medium, 1.5 ml fetal calf serum, and 0.3 ml phytohemagglutinin (to stimulate cell division). Cultures were incubated at 37 °C for a period of 72 h. Cytochalasin-B (6 μg/ ml) was added 44 h after culture initiation. Cells were collected by centrifugation 72 h post incubation. A mild hypotonic solution of Ham’s F-10 medium and milli-Q water (1:1, v/v) was added to the cell solution and left for 3 min at room temperature. Cells were fixed with a methanol:acetic acid solution (5:1, v/v) placed on microscope slides and stained with Giemsa [[26], [27], [28]]. These slides were then placed under a Nikon Eclipse E200 microscope where the bi-nucleated (BN) cells and MN can be viewed. One thousand BN cells with intact cytoplasm were scored per slide for each sample, in order to calculate MN frequency. Standard criteria were used for MN scoring [26,28]. The cytokinesis block proliferation index (CBPI) was also calculated. CBPI was calculated by the equation: CBPI = M1 + 2M2 + 3 x (M3 + M4) / N; M1, M2, M3, M4 correspond to the number of cells with one, two, three, and four nuclei, respectively and N is the total number of cells. These parameters were calculated by counting 2000 cells to determine possible cytotoxic effects [28,29]. Cytological examination of tissues was conducted using the touch preparation technique (TPT). Organs were cross sectioned/captured on a slide. Samples were fixed via alcohol, stained with Pap smear and observed with light microscope (20x) [30].
Outcome variables of interest (biomarkers of renal dysfunction) were treated as continuous and were tested for normality and equality of variances using the Shapiro-Wilk W test and Levene F test, respectively. They were subsequently compared between groups using one-way analysis of variance (ANOVA) or Mann-Whitney U, test as appropriate. Comparisons over time (time points: 0 h, 24 h and 48 h) was performed using repeated measures ANOVA or Friedman’s non-parametric test, as appropriate. Descriptive statistics for all measured biomarkers were presented as mean (standard deviation (SD)) regardless of variable distributions for uniformity purposes. Data were analyzed using IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp. Two-tailed p < 0.050 was considered statistically significant.
3. Results
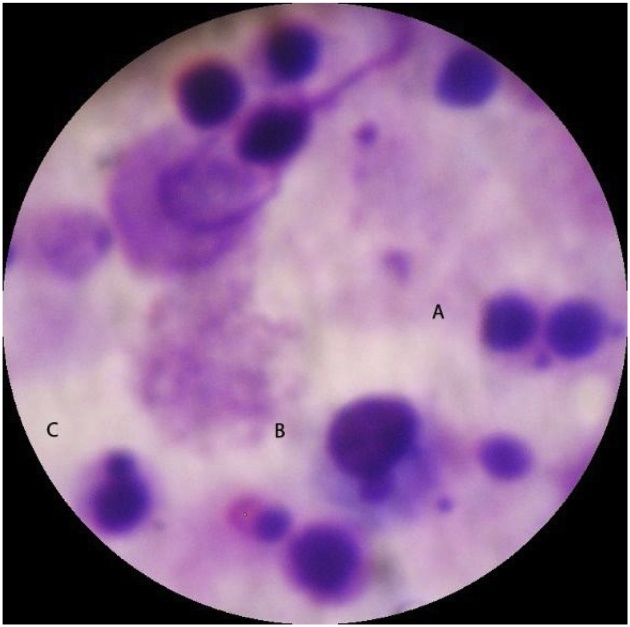
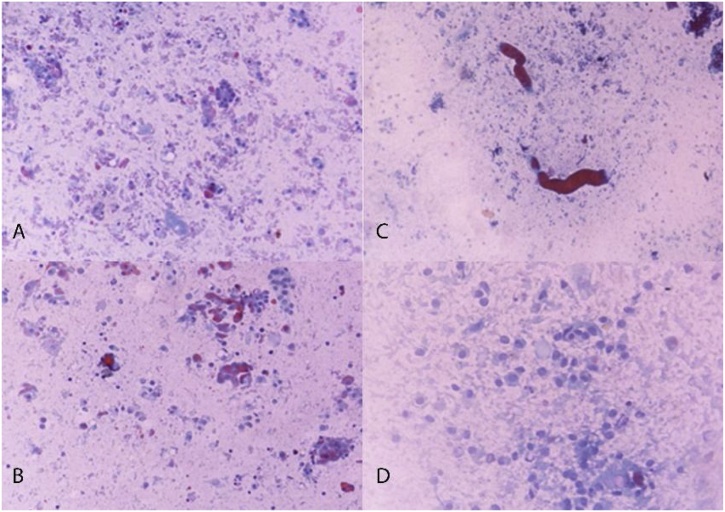
Each animal in group 1 developed acute kidney injury (AKI) after CM exposure, namely CIS. None of the animals in group 2 developed CIN. Mean sCr levels increased significantly in group 1 compared to group 2 at both 24 h and 48 h time points. Mean sCr levels increased by 68.2% within 48 h in group 1; no elevation in mean sCr levels was observed within 48 h in group 2. Biochemical analysis results are summarized in Table 1. A statistically significant elevation of both SDMA-ADMA levels was observed immediately (0 h) and 24 h after CM exposure, while the levels decreased slightly at 48 h without reaching normal values in group 1. Contrariwise, in group 2, SDMA/ADMA levels fluctuated over time within normal limits. SDMA-ADMA levels analysis results are summarized in Table 2. A statistically significant increase in mean number of BNMN cells and MN frequency was detected in group 1 compared to group 2 (15.33 ± 0.47 vs 6.33 ± 0.94 and 16.33 ± 0.94 vs 7.33 ± 0.47), respectively. CBPI was reduced in group 1 but the difference was not statistically significant between groups (1.42 ± 0.02 vs 1.55 ± 0.01). A microscopic picture taken during BNMN cells and MN scoring process is shown in Fig. 1. TPT revealed intense degenerative lesions, dirty substrate with intense grade inflammation, cell degeneration and abnormal uterine tubular casts as well as rubella, in both kidneys of all animals in group 1 in contrast to group 2 (Figs. 2A-D). Contrariwise, animals in group 2 presented normal cells of the urinary tubules, glomeruli, pelvis and normal red blood cells.
Table 1.
Biochemical analysis results.
| Serum Creatinine level (mg/dl) |
||||
|---|---|---|---|---|
| Time Interval | 0 h | 24h | 48h | |
| Groups | Mean (SD) | p value | ||
| 1 | 0.78 (0.18) | 1.01 (0.12) | 1.27 (0.64) | 0.022 |
| 2 | 0.69 (0.33) | 0.75 (0.20) | 0.68 (0.21) | 0.091 |
| p value | 0.690 | 0.032 | 0.008 | |
Table 2.
SDMA-ADMA levels analysis results.
| Group 1 |
Group 2 |
|||||
|---|---|---|---|---|---|---|
| Time | Mean | SD | Mean | SD | p value | |
| SDMA | 0 h | 673.7 | 521.9 | 126.5 | 70.9 | 0.049 |
| 24h | 773.4 | 585.4 | 179.8 | 116.5 | 0.050 | |
| 48h | 635.5 | 487.5 | 169.7 | 84.7 | 0.068 | |
| p value | 0.491 | 0.339 | ||||
| ADMA | 0 h | 572.0 | 451.8 | 90.4 | 93.5 | 0.048 |
| 24h | 533.0 | 352.2 | 122.9 | 95.5 | 0.036 | |
| 48h | 490.1 | 398.1 | 98.7 | 61.3 | 0.062 | |
| p value | 0.823 | 0.195 | ||||
Fig. 1.
A microscopic picture taken during the scoring process. (A) Binucleated cell with two micronuclei. (B and C) Mononucleated lymphocyte cells with present MN. According to the methods of this assay, such MN are not considered measurable.
Fig. 2.
Touch preparations (right kidney): A) Group 2: juxtaglomerular region, normal; B) Group 2: medulla, normal; C) Group 1: juxtaglomerular region, apoptosis; D) Group 1: medulla, cell degeneration.
4. Discussion
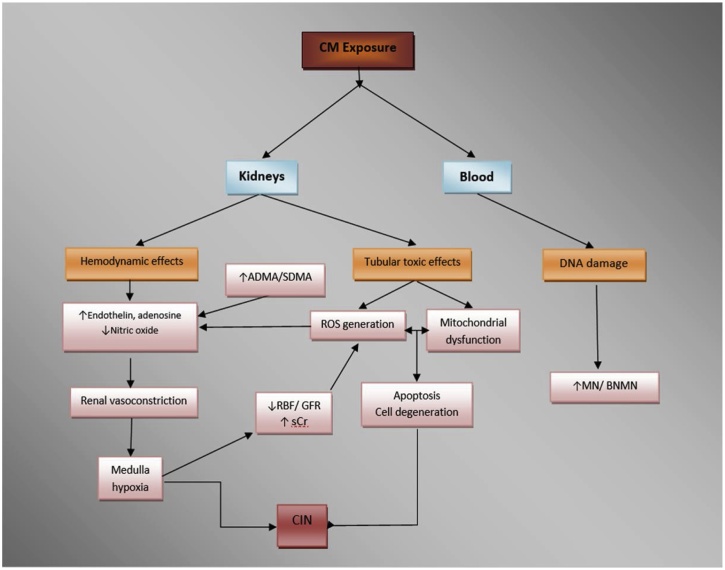
CIN is a reversible form of acute renal failure that begins after and is attributed to intravascular exposure to iodinated CM during angiographic or other imaging procedures such as urography [14]. Exact pathophysiology remains obscure with several factors appearing to be implicated, including among others renal ischemia, particularly medullar; ROS formation; reduction of nitric oxide (NO) production; and tubular epithelial/vascular endothelial injury (Fig. 3) [14]. CIN is associated with increased morbidity, hospital stay and mortality, especially in high risk-patients, who may be considered candidates for the application of prevention strategies. According to the American College of Radiology guidelines, risk factors that may warrant renal function assessment prior to intravascular iodinated CM exposure include [31]: 1) age >60 years; 2) history of renal disease; 3) history of hypertension; 4) history of diabetes mellitus; and 5) metformin uptake. Recognition of major risk factors facilitates detection of those at increased risk for CIN and helps research efforts to evaluate the effectiveness of potential prevention strategies.
Fig. 3.
Pathophysiology of contrast induced nephropathy (CIN) - Modified from [14].
CM: contrast media, CKD: chronic kidney disease, RBF: renal blood flow, GFR: glomerular filtration rate, sCr: serum creatinine, ROS: reactive oxygen species, MN: micronuclei, BNMN: binucleated cells with micronuclei, ADMA: asymmetric dimethylarginine, SDMA: symmetric dimethylarginine.
CIN has been previously recognized as relative elevation of baseline sCr level (25%–50%) and/or absolute baseline increase of 0.5–2.0 mg/dl [31] that generally appears within the first 48 h after CM administration, reaching a peak within the following five days [32] but no standard diagnostic criteria exist [14]. sCr, although widely used in the past, is considered nowadays neither a real-time biomarker of changing renal function since it rises slowly relative to the amount of filtration function lost in CIN, delaying diagnosis by an average of 48–72 h; nor a sensitive/specific one for slight alterations in GFR [33]. Due to low sensitivity/specificity, sCr increase cannot differentiate CIN from generic post-contrast AKI i.e., deterioration in kidney function that occurs within 48 h after administration of iodinated CM regardless of the cause [31]. More recently, however, according to the criteria developed by the Acute Kidney Network consensus group, the diagnosis of AKI is set if within 48 h after CM exposure (or after another nephrotoxic event) one of the following occurs [34]: i) absolute sCr increase ≥0.3 mg/dl (>26.4 μmol/l); ii) % increase in sCr ≥ 50% (≥1.5-fold above baseline); iii) urine output reduced to ≤0.5 ml/kg/h for at least 6 h.
CIN may be monitored by biomarkers reflecting kidney function changes (e.g. sCr or cystatin C and urine flowrate) or kidney damage (e.g., kidney injury molecule-1 (KIM-1), neutrophil gelatinase-associated lipocalin (NGAL), interleukin-18, etc.) extensively reviewed elsewhere [14]. The aim of the present study was to evaluate potentially novel biomarkers in blood/tissue samples of a CIN animal model that may more accurately detect renal function changes; reflect kidney damage; assist monitoring; and elucidate pathophysiology. For this purpose, New Zealand white rabbits were used due to their susceptibility to CIN as previously described [24]. To the best of our knowledge, apart from a routine biochemical analysis (evaluation of sCr levels), the present study is the first one to investigate SDMA-ADMA levels and evaluate the genotoxic/cytotoxic effect of CM as well as a TPT-based renal cytology in a CIN animal model.
The results of biochemical analysis confirmed the validity/adequacy of our model to investigate CIN based on modern diagnostic criteria. Concretely, all animals in group 1 developed AKI after CM exposure; Acute Kidney Network consensus group criteria for AKI diagnosis were fulfilled in each case of group 1 (absolute sCr increase ≥0.3 mg/dL; and % increase in sCr ≥ 50% (≥1.5-fold above baseline) within 48 h after CM exposure) [34]. Mean sCr levels increased significantly (by 68.2%) within 48 h in group 1. Mean sCr levels did not differ significantly at 0 h between groups but increased significantly in group 1 compared to group 2 at both 24 h and 48 h time points.
SDMA-ADMA levels analysis revealed a statistically significant elevation for both, immediately (0 h)/24 h after CM exposure and a slight decrease at 48 h without reaching normal values. Therefore, it appears that CM exposure results in an immediate significant elevation of SDMA-ADMA levels that remain constantly high over time. Contrariwise, in group 2, SDMA-ADMA levels fluctuated over time within normal limits. Based on the evidence provided by the present study it is concluded, that SDMA-ADMA are absolute indicators of renal dysfunction, their levels are directly affected by CM administration and the results are observed in real time. It has been reported that SDMA-ADMA are endogenous inhibitors of NO synthesis and elevated levels indicate renal damage, leading to renal hypoxia/ischemia [35]. It has also been reported that patients with chronic kidney disease (CKD) present high SDMA-ADMA levels [36], which are usually used as biomarkers of renal dysfunction in human [37]. However, to the best of our knowledge, SDMA-ADMA levels have never been evaluated as CIN biomarkers in animal models or human studies.
The results of genotoxicity/cytotoxicity assessment indicate that CM may affect cells at DNA level. In group 1, a statistically significant increase of BNMN/MN frequency was observed compared to group 2. Chemotoxic effects of CM occur due to direct molecular toxicity and their physiological properties [38,39]. Chromosomal damage and MN in lymphocytes of patients undergoing imaging based on CM has been demonstrated [[40], [41], [42], [43]]. These studies revealed that chromosomal damage and MN induction were due to two mechanisms. The first refers to induction of chromosomal damage through photoelectric interaction of radiation (X-rays) with the iodine atoms of CM, which emit photoelectrons that reach nearby cell structures. The second refers to chemical nature of CM, which may also induce chromosomal damage [44]. Finally, recent studies indicate the mutagenic effects of iodinated CM in animal models distant from the irradiated field [45,46].
TPT cytological observations showed that the kidneys of the animals in group 2 retained normal cells in shape and size as well as normal distribution of red blood cells. On the other hand, the kidneys of the animals in group 1 presented cell lesions as a result of CM-induced ROS production, apoptosis and cell degeneration [47]. In addition to the obvious degenerative lesions, a submerged substrate with severe inflammation was also observed. Furthermore, abundant urinary tract casts are protein structures (Tamm-Horsfall protein) that precipitate inside the renal tubules, resulting in tubule damage. Therefore, casts are nephropathy markers [48]. Finally, abundance of red blood cells occurs in cases of nephropathy, where erythrocytes have an abnormal distribution [49].
Author contributions
conception, C.M., A.T.; design, C.M., A.T.; data acquisition, I.F., P.S., A.K., and I.T.; data analysis, I.F., P.S., A.K., and I.T.; interpretation of data for the work, C.M., I.F., P.K., G.G., I-E.Z., P.S., A.K., I.T., T.B., G.L., K.T. D.K., and A.T.; drafting the work, C.M., I.F., and P.K.; revising the work critically for important intellectual content, C.M., G.G., I-E.Z., P.S., A.K., I.T., T.B., G.L., K.T., D.K., and A.T.
Funding
The authors would like to thank the Special Research Account of University of Crete for supporting this study (ELKE No 3550, No 3963, No 4920) and ToxPlus S.A.
Conflicts of interest
The authors declare no conflict of interest. AT is the Editor-in-Chief for the journal, but had no personal involvement in the reviewing process, or any influence in terms of adjudicating on the final decision, for this article.
Acknowledgments
The authors thank Mrs. D. Pantartzi, scientific secretary at the Department of Urology, University of Crete Medical School for the administrative and technical support.
Contributor Information
Charalampos Mamoulakis, Email: mamoulak@uoc.gr.
Irene Fragkiadoulaki, Email: eirinimbg@hotmail.gr.
Phaedra Karkala, Email: toxlab.uoc@gmail.com.
Georgios Georgiadis, Email: geokosgeo@yahoo.gr.
Ioannis-Erineos Zisis, Email: renoszisis@gmail.com.
Alexandra Kalogeraki, Email: kalogerakimed@yahoo.gr.
Ioannis Tsiaoussis, Email: tsiaoussis@uoc.gr.
Tatyana Burykina, Email: burykina58@mail.ru.
George Lazopoulos, Email: g.lazopoulos@med.uoc.gr.
Konstantinos Tsarouhas, Email: ktsarouhas14@yahoo.gr.
Aristides Tsatsakis, Email: tsatsaka@uoc.gr.
References
- 1.Buschur M., Aspelin P. Contrast media: history and chemical properties. Interv. Cardiol. Clin. 2014;3:333–339. doi: 10.1016/j.iccl.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Solomon R. Contrast media: are there differences in nephrotoxicity among contrast media? Biomed Res. Int. 2014;2014 doi: 10.1155/2014/934947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morcos S.K., Thomsen H.S., Webb J.A. Contrast-media-induced nephrotoxicity: a consensus report. Contrast media safety committee, european society of urogenital radiology (ESUR) Eur. Radiol. 1999;9:1602–1613. doi: 10.1007/s003300050894. [DOI] [PubMed] [Google Scholar]
- 4.Pannu N., Wiebe N., Tonelli M. Alberta kidney disease, N. Prophylaxis strategies for contrast-induced nephropathy. JAMA. 2006;295:2765–2779. doi: 10.1001/jama.295.23.2765. [DOI] [PubMed] [Google Scholar]
- 5.Golshahi J., Nasri H., Gharipour M. Contrast-induced nephropathy; A literature review. J. Nephropathol. 2014;3:51–56. doi: 10.12860/jnp.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiss N., Hamar P. Histopathological evaluation of contrast-induced acute kidney injury rodent models. Biomed Res. Int. 2016;2016 doi: 10.1155/2016/3763250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andreucci M., Faga T., Pisani A., Sabbatini M., Michael A. Acute kidney injury by radiographic contrast media: pathogenesis and prevention. Biomed Res. Int. 2014;2014 doi: 10.1155/2014/362725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caiazza A., Russo L., Sabbatini M., Russo D. Hemodynamic and tubular changes induced by contrast media. Biomed Res. Int. 2014;2014 doi: 10.1155/2014/578974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tungjai M., Sukantamala S., Malasaem P., Dechsupa N., Kothan S. An evaluation of the antioxidant properties of iodinated radiographic contrast media: an in vitro study. Toxicol. Rep. 2018;5:840–845. doi: 10.1016/j.toxrep.2018.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keaney J.J., Hannon C.M., Murray P.T. Contrast-induced acute kidney injury: how much contrast is safe? Nephrol. Dial. Transplant. 2013;28:1376–1383. doi: 10.1093/ndt/gfs602. [DOI] [PubMed] [Google Scholar]
- 11.Pisani A., Riccio E., Andreucci M., Faga T., Ashour M., Di Nuzzi A., Mancini A., Sabbatini M. Role of reactive oxygen species in pathogenesis of radiocontrast-induced nephropathy. Biomed Res. Int. 2013;2013 doi: 10.1155/2013/868321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tumlin J., Stacul F., Adam A., Becker C.R., Davidson C., Lameire N., McCullough P.A., Panel C.I.N.C.W. Pathophysiology of contrast-induced nephropathy. Am. J. Cardiol. 2006;98:14K–20K. doi: 10.1016/j.amjcard.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 13.Ramponi S., Grotti A., Morisetti A., Vultaggio S., Lorusso V. Effects of iodinated contrast media on endothelium: an in vitro study. Toxicol. In Vitro. 2007;21:191–196. doi: 10.1016/j.tiv.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Mamoulakis C., Tsarouhas K., Fragkiadoulaki I., Heretis I., Wilks M.F., Spandidos D.A., Tsitsimpikou C., Tsatsakis A. Contrast-induced nephropathy: basic concepts, pathophysiological implications and prevention strategies. Pharmacol. Ther. 2017;180:99–112. doi: 10.1016/j.pharmthera.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Sodagari F., Mozaffary A., Wood C.G., 3rd, Schmitz B., Miller F.H., Yaghmai V. Reactions to both nonionic iodinated and gadolinium-based contrast media: incidence and clinical characteristics. AJR Am. J. Roentgenol. 2018;210:715–719. doi: 10.2214/AJR.17.18655. [DOI] [PubMed] [Google Scholar]
- 16.Iordache A.M., Docea A.O., Buga A.M., Mitrut R., Albulescu D., Zlatian O., Ianosi S., Ianosi G., Neagoe D., Sifaki M. The incidence of skin lesions in contrast media-induced chemical hypersensitivity. Exp. Ther. Med. 2019;17:1113–1124. doi: 10.3892/etm.2018.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fragkiadoulaki I., Stivaktakis P., Kalogeraki A., Tsiaousis I., Kalliantasi A., Karzi V., Stratidakis A., Mamoulakis C., Tsatsakis A. Micronuclei frequency and blood cell number in rabbit contast-induced nephrotoxicity model with antioxidants as a preventive strategy. Toxicol. Lett. 2017;280S:s125. [Google Scholar]
- 18.Fragiadoulaki I., Tsitsimpikou C., Vakonaki E., Alegakis A., Kaloudis K., Kanaki K., Nathena D., Stivaktakis P., Tzatzarakis M., Mamoulakis C. Contast-induced nephropathy in animal model. Toxicol. Lett. 2017;280S:s140. [Google Scholar]
- 19.Tsatsakis A.M., Fragkiadoulaki I., Kalogeraki A., Tsiaoussis I., Karkala F., Kalliantasi K., Stivaktakis P., Fragkiadaki P., Psycharakis C., Mamoulakis C. Natural antioxidants prevent contrast-induced nephropathy in an animal model: results from a cytological study. Toxicol. Lett. 2018 [Google Scholar]
- 20.Fragkiadoulaki I., Mamoulakis C., Alegakis A., Tzatzarakis M.N., Karzi V., Stratidakis A., Renieri E., Vardavas A., Leon G., Tsitsimpikou C. Natural antioxidants prevent contrast-induced nephropathy by enhancing nitric oxide synthesis in an animal model. Toxicol. Lett. 2018;295S:s243. [Google Scholar]
- 21.Zisis I.E., Fragkiadoulaki I., Alegakis A., Karkala F., Vakonaki E.K., Apalaki E., Vaki G.A., Kovatsi L., Tsatsakis A.M., Mamoulakis C. 295S. 2018. p. s245. (Natural Antioxidants Prevent Contrast-Induced Nephropathy in an Animal Model: Results from a Biochemical Study). [Google Scholar]
- 22.Tsarouhas K., Tsitsimpikou C., Papantoni X., Lazaridou D., Koutouzis M., Mazzaris S., Rezaee R., Mamoulakis C., Georgoulias P., Nepka C. Oxidative stress and kidney injury in trans-radial catheterization. Biomed. Rep. 2018;8:417–425. doi: 10.3892/br.2018.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsamouri M.M., Rapti M., Kouka P., Nepka C., Tsarouhas K., Soumelidis A., Koukoulis G., Tsatsakis A., Kouretas D., Tsitsimpikou C. Histopathological evaluation and redox assessment in blood and kidney tissues in a rabbit contrast-induced nephrotoxicity model. Food Chem. Toxicol. 2017;108:186–193. doi: 10.1016/j.fct.2017.07.058. [DOI] [PubMed] [Google Scholar]
- 24.Lauver D.A., Carey E.G., Bergin I.L., Lucchesi B.R., Gurm H.S. Sildenafil citrate for prophylaxis of nephropathy in an animal model of contrast-induced acute kidney injury. PLoS One. 2014;9 doi: 10.1371/journal.pone.0113598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martens-Lobenhoffer J., Kielstein J.T., Oye C., Bode-Boger S.M. Validated high performance liquid chromatography-UV detection method for the determination of daptomycin in human plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2008;875:546–550. doi: 10.1016/j.jchromb.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 26.Stivaktakis P., Vlastos D., Giannakopoulos E., Matthopoulos D.P. Differential micronuclei induction in human lymphocyte cultures by imidacloprid in the presence of potassium nitrate. ScientificWorldJournal. 2010;10:80–89. doi: 10.1100/tsw.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vardavas A.I., Stivaktakis P.D., Tzatzarakis M.N., Fragkiadaki P., Vasilaki F., Tzardi M., Datseri G., Tsiaoussis J., Alegakis A.K., Tsitsimpikou C. Long-term exposure to cypermethrin and piperonyl butoxide cause liver and kidney inflammation and induce genotoxicity in New Zealand white male rabbits. Food Chem. Toxicol. 2016;94:250–259. doi: 10.1016/j.fct.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 28.Vardavas A.I., Ozcagli E., Fragkiadaki P., Stivaktakis P.D., Tzatzarakis M.N., Alegakis A.K., Vasilaki F., Kaloudis K., Tsiaoussis J., Kouretas D. The metabolism of imidacloprid by aldehyde oxidase contributes to its clastogenic effect in New Zealand rabbits. Mutat. Res. 2018;829-830:26–32. doi: 10.1016/j.mrgentox.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Stivaktakis P.D., Kavvalakis M.P., Tzatzarakis M.N., Alegakis A.K., Panagiotakis M.N., Fragkiadaki P., Vakonaki E., Ozcagli E., Hayes W.A., Rakitskii V.N. Long-term exposure of rabbits to imidaclorpid as quantified in blood induces genotoxic effect. Chemosphere. 2016;149:108–113. doi: 10.1016/j.chemosphere.2016.01.040. [DOI] [PubMed] [Google Scholar]
- 30.Diamantis A., Beloukas A.I., Kalogeraki A.M., Magiorkinis E. A brief chronicle of cytology: from Janssen to Papanicolaou and beyond. Diagn. Cytopathol. 2013;41:555–564. doi: 10.1002/dc.22887. [DOI] [PubMed] [Google Scholar]
- 31.ACR Committee on Drugs and Contrast Media . 10.2 ed. American College of Radiology; 2016. Contrast Media. [DOI] [PubMed] [Google Scholar]
- 32.Wi J., Ko Y.G., Kim J.S., Kim B.K., Choi D., Ha J.W., Hong M.K., Jang Y. Impact of contrast-induced acute kidney injury with transient or persistent renal dysfunction on long-term outcomes of patients with acute myocardial infarction undergoing percutaneous coronary intervention. Heart. 2011;97:1753–1757. doi: 10.1136/hrt.2010.218677. [DOI] [PubMed] [Google Scholar]
- 33.Sterling K.A., Tehrani T., Rudnick M.R. Clinical significance and preventive strategies for contrast-induced nephropathy. Curr. Opin. Nephrol. Hypertens. 2008;17:616–623. doi: 10.1097/MNH.0b013e32830f45a3. [DOI] [PubMed] [Google Scholar]
- 34.Mehta R.L., Kellum J.A., Shah S.V., Molitoris B.A., Ronco C., Warnock D.G., Levin A., Acute Kidney Injury, N Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alan C., Kurt H.A., Topaloglu N., Ersay A.R., Cakir D.U., Basturk G. Nitric oxide and asymmetric dimethyl arginine (ADMA) levels in an experimental hydronephrotic kidney caused by unilateral partial ureteral obstruction. Int. Braz J Urol. 2016;42:614–620. doi: 10.1590/S1677-5538.IBJU.2015.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boelaert J., Schepers E., Glorieux G., Eloot S., Vanholder R., Lynen F. Determination of asymmetric and symmetric dimethylarginine in serum from patients with chronic kidney disease: UPLC-MS/MS versus ELISA. Toxins (Basel) 2016:8. doi: 10.3390/toxins8050149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khan Z., Pandey M. Role of kidney biomarkers of chronic kidney disease: an update. Saudi J. Biol. Sci. 2014;21:294–299. doi: 10.1016/j.sjbs.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romano G., Briguori C., Quintavalle C., Zanca C., Rivera N.V., Colombo A., Condorelli G. Contrast agents and renal cell apoptosis. Eur. Heart J. 2008;29:2569–2576. doi: 10.1093/eurheartj/ehn197. [DOI] [PubMed] [Google Scholar]
- 39.Namasivayam S., Kalra M.K., Torres W.E., Small W.C. Adverse reactions to intravenous iodinated contrast media: a primer for radiologists. Emerg. Radiol. 2006;12:210–215. doi: 10.1007/s10140-006-0488-6. [DOI] [PubMed] [Google Scholar]
- 40.Norman A., Adams F.H., Riley R.F. Cytogenetic effects of contrast media and triiodobenzoic acid derivatives in human lymphocytes. Radiology. 1978;129:199–203. doi: 10.1148/129.1.199. [DOI] [PubMed] [Google Scholar]
- 41.Cochran S.T., Khodadoust A., Norman A. Cytogenetic effects of contrast material in patients undergoing excretory urography. Radiology. 1980;136:43–46. doi: 10.1148/radiology.136.1.7384520. [DOI] [PubMed] [Google Scholar]
- 42.Sinues B., Nunez E., Bernal M.L., Alcala A., Saenz M.A., Conde B. Micronucleus assay in biomonitoring of patients undergoing excretory urography with diatrizoate and ioxaglate. Mutat. Res. 1991;260:337–342. doi: 10.1016/0165-1218(91)90019-i. [DOI] [PubMed] [Google Scholar]
- 43.Cochran S.T., Norman A. Induction of micronuclei in lymphocytes of patients undergoing excretory urography with ioversol. Invest. Radiol. 1994;29:210–212. doi: 10.1097/00004424-199402000-00016. [DOI] [PubMed] [Google Scholar]
- 44.Adams F.H., Norman A., Mello R.S., Bass D. Effect of radiation and contrast media on chromosomes. Preliminary report. Radiology. 1977;124:823–826. doi: 10.1148/124.3.823. [DOI] [PubMed] [Google Scholar]
- 45.Deimling L.I., Machado F.L., Welker A.G., Peres L.M., Santos-Mello R. Micronucleus induction in mouse polychromatic erythrocytes by an X-ray contrast agent containing iodine. Mutat. Res. 2009;672:65–68. doi: 10.1016/j.mrgentox.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 46.Belle M.B., Leffa D.D., Mazzorana D., De Andrade V.M. Evaluation of the mutagenic effect of the iodinated contrast medium Urografina(R) 292 using the micronucleus test in mouse bone marrow cells. An. Acad. Bras. Cienc. 2013;85:737–744. doi: 10.1590/S0001-37652013000200018. [DOI] [PubMed] [Google Scholar]
- 47.Rezaee M.A., Mohammadpour A.H., Imenshahidi M., Mahmoudi M., Sankian M., Tsarouhas K., Tsakalof A., Tsatsakis A.M., Moallem S.A. Protective effect of erythropoietin on myocardial apoptosis in rats exposed to carbon monoxide. Life Sci. 2016;148:118–124. doi: 10.1016/j.lfs.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 48.Rampoldi L., Scolari F., Amoroso A., Ghiggeri G., Devuyst O. The rediscovery of uromodulin (Tamm-Horsfall protein): from tubulointerstitial nephropathy to chronic kidney disease. Kidney Int. 2011;80:338–347. doi: 10.1038/ki.2011.134. [DOI] [PubMed] [Google Scholar]
- 49.Salvagno G.L., Sanchis-Gomar F., Picanza A., Lippi G. Red blood cell distribution width: a simple parameter with multiple clinical applications. Crit. Rev. Clin. Lab. Sci. 2015;52:86–105. doi: 10.3109/10408363.2014.992064. [DOI] [PubMed] [Google Scholar]