Key Points
Question
In patients with chronic obstructive pulmonary disease (COPD) and also with increased cardiovascular risk, does aclidinium bromide, 400 μg twice daily, increase major adverse cardiovascular events (MACE) and decrease COPD exacerbations?
Findings
In this randomized study of 3589 patients with COPD and also with increased cardiovascular risk, the estimated MACE hazard ratio (aclidinium vs placebo) was 0.89 over 3 years; the 1-sided 97.5% CI of 1.23 did not exceed the noninferiority margin of 1.8. For moderate to severe COPD exacerbations, the estimated rate ratio (aclidinium vs placebo) was 0.78 during the first year and was statistically significant.
Meaning
Aclidinium, compared with placebo, reduced COPD exacerbations and did not result in an inferior risk of MACE.
Abstract
Importance
There is concern that long-acting muscarinic antagonists increase cardiovascular morbidity or mortality in patients with chronic obstructive pulmonary disease (COPD).
Objective
To determine the cardiovascular safety (noninferiority) and efficacy (superiority) of aclidinium bromide, 400 μg twice daily, in patients with COPD and cardiovascular disease or risk factors.
Design, Setting, and Participants
Multicenter, randomized, placebo-controlled, double-blind, parallel-design study conducted at 522 sites in North America. A total of 3630 patients with moderate to very severe COPD and either a history of cardiovascular disease or at least 2 atherothrombotic risk factors were randomized; follow-up occurred for up to 3 years until at least 122 major adverse cardiovascular events (MACE) occurred. The first patient was enrolled on October 16, 2013 and the last on August 22, 2016. The final patient completed follow-up on September 21, 2017.
Interventions
Patients were randomized to receive aclidinium (n = 1812) or placebo (n = 1818) by dry-powder inhaler, twice daily for up to 3 years.
Main Outcomes and Measures
The primary safety end point was time to first MACE over up to 3 years (hazard ratio [HR] 1-sided 97.5% CI noninferiority margin = 1.8). The primary efficacy end point was the annual COPD exacerbation rate during the first year of treatment. Secondary outcomes included an expanded MACE definition (time to first MACE or serious cardiovascular event of interest) and annual rate of exacerbations requiring hospitalization.
Results
Among 3589 patients analyzed (mean age, 67.2 years; 58.7% male), 2537 (70.7%) completed the study. Of these, 69 (3.9%) aclidinium and 76 (4.2%) placebo patients had a MACE (HR, 0.89; 1-sided 97.5% CI, 0-1.23); the expanded MACE definition included 168 (9.4%) aclidinium vs 160 (8.9%) placebo patients with events (HR, 1.03; 1-sided 97.5% CI, 0-1.28). Annual moderate to severe exacerbation rates (aclidinium, 0.44; placebo, 0.57; rate ratio, 0.78; 2-sided 95% CI, 0.68-0.89; P < .001) and rate of exacerbations requiring hospitalization (aclidinium, 0.07; placebo, 0.10; rate ratio, 0.65; 2-sided 95% CI, 0.48-0.89; P = .006) decreased significantly with aclidinium vs placebo. The most common adverse events were pneumonia (aclidinium, 109 events [6.1%]; placebo, 105 events [5.8%]), urinary tract infection (aclidinium, 93 events [5.2%]; placebo, 89 events [5.0%]), and upper respiratory tract infection (aclidinium, 86 events [4.8%]; placebo, 101 events [5.6%]).
Conclusions and Relevance
Among patients with COPD and increased cardiovascular risk, aclidinium was noninferior to placebo for risk of MACE over 3 years. The rate of moderate to severe COPD exacerbations was reduced over the first year.
Trial Registration
ClinicalTrials.gov Identifier: NCT01966107
This randomized clinical trial compares the effects of the long-acting muscarinic antagonist aclidinium bromide vs placebo on major adverse cardiovascular events and chronic obstructive pulmonary disease (COPD) exacerbations in patients with COPD and increased cardiovascular risk.
Introduction
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality worldwide.1 It is associated with increased risk of cardiovascular morbidity,2 with approximately one-third of COPD deaths being due to cardiovascular disease.3,4 Although long-acting muscarinic antagonists (LAMAs) are widely used for maintenance bronchodilation in patients with COPD, there is controversy surrounding their cardiovascular safety.5,6,7,8,9,10,11
Several meta-analyses and epidemiological studies have demonstrated an increased risk of cardiovascular events following treatment with either short-acting muscarinic antagonists (SAMAs) or LAMAs.5,8,9,10 In contrast, large-scale clinical trials of the LAMA tiotropium have not demonstrated an increase in major adverse cardiovascular events (MACE; a composite of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke),6,11,12 and the US Food and Drug Administration has concluded that current data do not support an increased risk of stroke, myocardial infarction, or death associated with tiotropium in patients with COPD.7 This discrepancy can be explained in part because patients with significant cardiovascular disease are typically excluded from clinical trials such as the UPLIFT study.6
Short-term studies of aclidinium have demonstrated decreased annual rates of exacerbation compared with placebo after 3 to 6 months of treatment,13 but this has not been established beyond 6 months. Given this background, the dual objectives of the current trial were to evaluate the effects of aclidinium bromide, 400 μg twice daily, on MACE (noninferiority to placebo) and COPD exacerbations (superiority to placebo) in patients with COPD and a history of cardiovascular disease or cardiac risk factors.14
Methods
Study Design
The study design and methods of the ASCENT-COPD trial are available in Supplement 1.14 In brief, this was a phase IV, multicenter, double-blind, randomized, placebo-controlled, parallel-group noninferiority study conducted at 522 sites in the United States and Canada (eFigure 1 in Supplement 1).14 The study comprised a 2-week washout/run-in period followed by a double-blind treatment phase, during which patients were randomized 1:1 to receive aclidinium, 400 μg, or matching placebo twice daily from a multidose dry-powder inhaler (Genuair/Pressair; AstraZeneca) for up to 36 months. This was an event-driven trial that was planned to terminate on the date that at least 122 patients were projected to have experienced an adjudicated MACE. Each patient was exposed to aclidinium or matching placebo for a maximum of 3 years. The trial protocol (see Supplement 2 and statistical analysis plan in Supplement 3) and informed consent procedures were approved by the institutional review board with controlling authority at each study site. All patients provided written informed consent prior to the conduct of any study-specific procedures. The study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines.
Study Population
Eligible patients were male or female, were aged 40 years or older, had stable moderate to very severe COPD (forced expiratory volume in 1 second [FEV1]/forced vital capacity <0.70 and FEV1 <70% predicted1), and had a smoking history of at least 10 pack-years. Predicted values of lung function were based on sex, height, and self-reported race and ethnicity (based on fixed categories).15 Patients were required to have at least 1 of the following: cerebrovascular disease (eg, stroke or transient ischemic attack, carotid stenosis), coronary artery disease (myocardial infarction, angina, angioplasty/stent/coronary artery bypass graft), or peripheral vascular disease (history of claudication). Alternatively, patients could have at least 2 atherothrombotic risk factors, including age 65 years or older for men and 70 years or older for women, waist circumference 40 inches or more for men and 38 inches or more for women, estimated glomerular filtration rate less than 60 mL/min and microalbuminuria (30-300 mg albumin per 24 hours or 30-300 μg albumin per 1 mg creatinine on a spot urine test), dyslipidemia, diabetes, or hypertension. Patients could continue background treatment, with the exception of anticholinergics (LAMAs and SAMAs), which were not permitted.
Patients were excluded if they were taking triple therapy (inhaled corticosteroid [ICS], LAMA, and long-acting β2-agonist [LABA]), had unstable or life-threatening COPD or cardiovascular disease, had a lung disease other than COPD, had planned lung transplantation or lung surgery, or had a malignancy treated within 5 years before screening. Initially, patients were required to have had at least 1 treated COPD exacerbation in the year prior to screening but were excluded if an exacerbation occurred within 4 weeks of screening.14 To enhance recruitment, the requirement of a prior exacerbation was removed after approximately 50% of patients (n = 1734) were enrolled to increase accrual and allow for a broader patient population.14 At the same time, the upper limit of FEV1 was increased from 70% to 80% predicted.
Outcome Measures
The primary safety outcome was “on-study” time to first adjudicated MACE (ie, irrespective of treatment exposure; with MACE defined as cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke). The primary efficacy outcome measure was the annual rate of moderate to severe COPD exacerbations during the first year of the study while taking the study drug (“on-treatment” time). This time frame was selected because it was expected that almost all nondiscontinued patients would have completed 1 year of treatment by the time that at least 122 patients experienced an adjudicated MACE.14 Exacerbations were defined as increased COPD symptoms lasting 2 days or longer and required treatment with antibiotics and/or systemic corticosteroids or led to hospitalization or death. The study was not designed with co–primary end points; therefore, a positive result on risk of MACE would be interpreted as such regardless of the outcome on exacerbations. However, following a negative result for MACE, the results for efficacy would be exploratory only.
A secondary safety analysis was performed of risk of MACE that also included other serious cardiovascular events (eg, acute heart failure or life-threatening arrhythmias). Exacerbations that required hospitalization were analyzed as a secondary efficacy outcome.
Sensitivity analyses were performed to include exacerbations that occurred during the first year of follow-up, regardless of whether patients were taking the study treatment. In addition, the time to first MACE was analyzed with an “on-treatment” approach (ie, among patients actively taking study treatment) as a sensitivity analysis of the primary safety analysis.
Exploratory efficacy analyses included subgroup annual rates of moderate to severe COPD exacerbations, including patients taking an ICS (including ICS-LABA treatment) at baseline. Individual MACE components were also examined.
An exploratory safety outcome measure was all-cause mortality, analyzed at the time of the common closeout date or 3-year follow-up (vital status was ascertained approximately every 3 months for the first 2 years and once every 6 months during year 3).
Treatment-emergent adverse events, serious adverse events, and adverse events leading to discontinuation were recorded.
To account for the multisite design of the study, a frailty analysis incorporating site as a covariate was performed as a post hoc sensitivity analysis to determine whether 1 or more sites had an undue influence on the results.
Adjudication of MACE
Members of a clinical event committee, consisting of 2 cardiologists and 1 pulmonologist, provided an independent, blinded medical review and adjudication of all MACE and other cardiovascular events of interest. Potential events were adjudicated using a consistent and prespecified set of criteria defined in the clinical event committee’s charter, which was based on the 2014 draft Clinical Data Interchange Standards Consortium guidance16; discrepancies among reviewers were resolved by a consensus of the committee.
Statistical Analysis
All analyses were prespecified. The trial was powered on the 3-year safety outcome, with the 1-year efficacy outcome nested as a separate end point within the overall trial. The study was designed such that at least 122 patients having a MACE would achieve 90% power to rule out a hazard ratio (HR) of 1.8 comparing time to first MACE for aclidinium vs placebo. Statistical noninferiority was concluded if the 1-sided 97.5% CI was less than 1.8. The noninferiority margin of 1.8 was based on the US Food and Drug Administration’s guidance for establishing the cardiovascular safety for marketing of diabetes drugs.17 The initially projected accrual of 4000 patients needed to acquire 122 patients with MACE provided 89% power to detect a 1-year on-treatment 14% reduction in exacerbation rate at α=.05, assuming an exacerbation rate of 0.81 per patient-year and 30% discontinuation rate of study treatment. The 14% reduction in exacerbation rate was based on data from the UPLIFT trial.6
Time to first MACE was analyzed using a Cox proportional hazards model with baseline cardiovascular risk group (prior events and risk factors), smoking status, and treatment group as covariates. The assumption of proportional hazards was supported by inspection of log-cumulative hazard plots. Chronic obstructive pulmonary disease exacerbation rates were analyzed using negative binomial regression with treatment group, and baseline ICS use, baseline COPD severity, smoking status, and 1-year exacerbation history as factors. The study was powered for overall MACE but not for the individual components, which were analyzed in an exploratory fashion only. The frailty model included site as a frailty term with a log-normal distribution.
Safety and efficacy analyses were conducted on the full analysis set (all patients who took at least 1 dose of treatment). The primary safety analysis was based on randomized treatment assignment irrespective of treatment discontinuation (“on-study” analysis). A sensitivity analysis of MACE that occurred while patients were taking study treatment (until the time of study drug discontinuation) used windows of 0 and 15 days after stopping treatment.
The primary efficacy analysis was an on-treatment analysis during the first year of treatment, excluding events that occurred after treatment discontinuation. An on-study sensitivity analysis included exacerbations that occurred during the first year of the study regardless of treatment discontinuation. Prespecified subgroups were analyzed based on age, sex, race, smoking status, baseline ICS use (including ICS-LABA treatment), baseline COPD severity, and history of COPD exacerbations in the year prior to baseline visit. Subgroup differences were inferred based on tests of treatment × subgroup interaction. Efficacy end points were tested sequentially in a hierarchical manner; ie, the secondary analysis of the primary efficacy end point (rate of moderate to severe COPD exacerbations; on-study analysis) was tested only when the primary analysis (rate of moderate to severe COPD exacerbations; on-treatment analysis) achieved statistical significance and the secondary efficacy end point (exacerbations that required hospitalization; on-treatment analysis) was tested only when both primary and secondary analyses of the primary efficacy end point (rate of moderate to severe COPD exacerbations; on-treatment and on-study analyses) achieved statistical significance. Otherwise, nominal P values were to be provided and interpreted descriptively. To assess the robustness to variations of the data assumptions underlying the primary analysis of the primary efficacy end point, several sensitivity analyses were conducted using jump-to-reference and copy reference approaches and tipping-point analysis.18
Various multiple imputation methods were used to address the effect of missing data assumptions in the analysis of the primary safety and efficacy variables (eAppendix in Supplement 1). All reported outputs were produced using SAS version 9.3 (SAS Institute Inc) in a secure, validated environment. The significance threshold was P < .05 for all end points.
Protocol Amendment
Approximately halfway through the study, the protocol was amended to increase accrual rates (eTables 1 and 2 in Supplement 1). As the study progressed, it was determined that many potential participants who met the requirement for prior COPD exacerbation were taking triple therapy; however, patients taking triple therapy were excluded from the trial to avoid switching patients from triple therapy to dual therapy should they be randomized to placebo. The protocol was subsequently amended to permit enrollment of high-risk patients who did not have an exacerbation history as well as other minor changes while maintaining the exclusion of patients taking triple therapy.14
Results
Patient Characteristics
The first patient was enrolled on October 16, 2013, and the last on August 22, 2016. The final patient completed follow-up on September 21, 2017. At study closeout, 3630 patients had been randomized; 1791 patients randomized to aclidinium and 1798 randomized to placebo were included in analyses and 41 patients overall were excluded (Figure 1). The treatment groups were well matched for clinical and demographic characteristics (Table); the patient population (mean age, 67.2 years) comprised mostly male patients (58.7%); former smokers (56.5%); and those with symptomatic disease (mean COPD assessment test score, 20.7 of 40, with higher scores indicating more symptoms), moderate to severe airflow limitation (85.3%), and 1 or more COPD exacerbations in the preceding year (60.1%). Inhaled corticosteroids were used by 56.8% of patients prior to the study, usually in combination with a LABA (51.2%) (Table). During the study, 64.2% of patients used an ICS, most frequently in combination with a LABA (59.4%) (eTable 3 in Supplement 1). At least 2 atherothrombotic risk factors were present in 95.9% of patients; 47.7% had a history of cardiovascular disease (eTable 4 in Supplement 1).
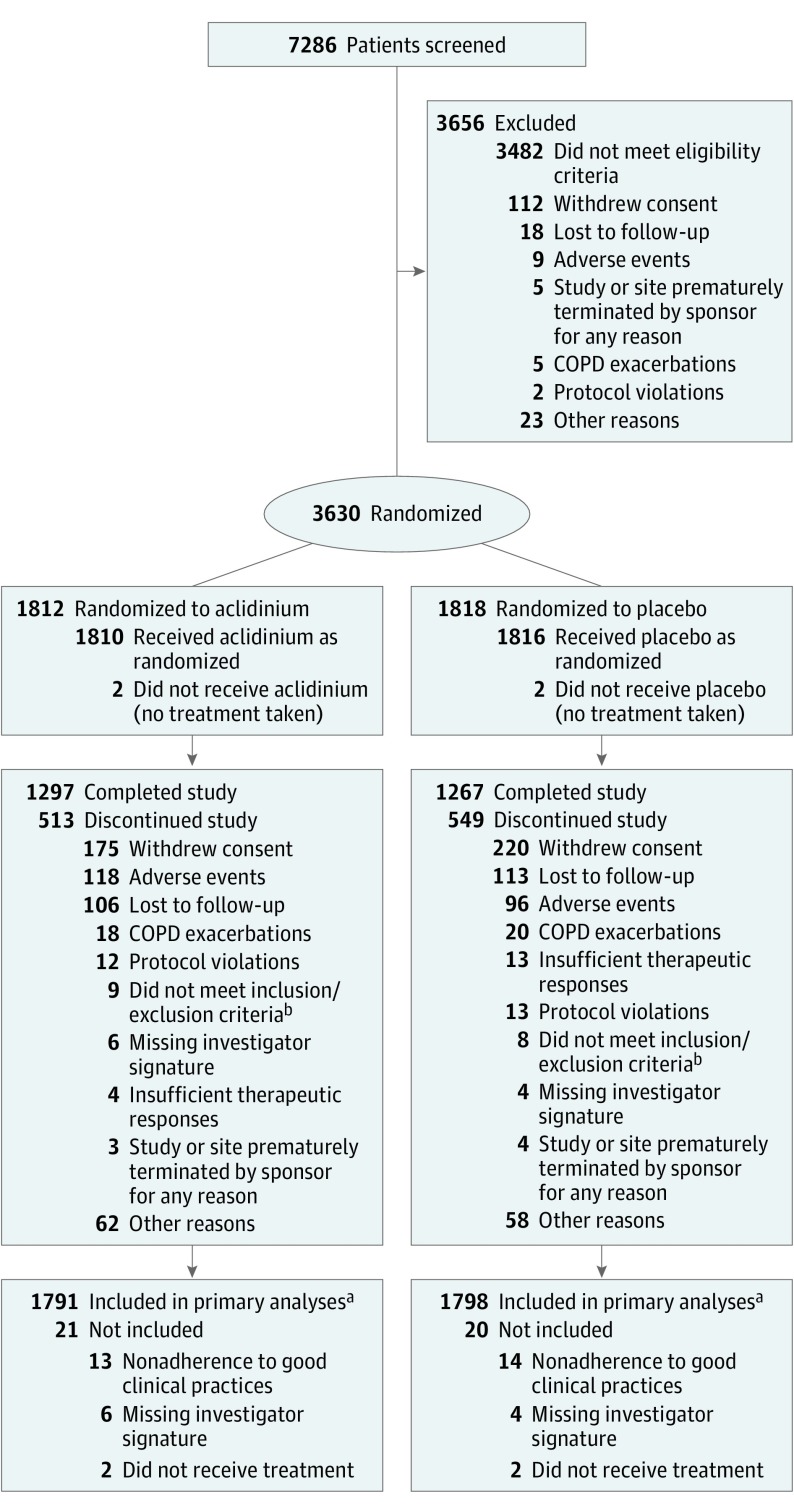
Figure 1. Patient Flow in the ASCENT-COPD Randomized Clinical Trial.
COPD indicates chronic obstructive pulmonary disease.
aFor safety outcomes, 70.7% of patients had completed the 3-year study or were currently enrolled in the study at the time the study was stopped; for efficacy outcomes, 67.3% of patients had completed 12 months of treatment or were in their first year of treatment when the study was stopped.
bPatients were randomized in error.
Table. Patient Demographics and Characteristics by Treatment Group.
| Characteristics | Aclidinium (n = 1791) | Placebo (n = 1798) |
|---|---|---|
| Age, mean (SD), y | 67.1 (8.5) | 67.2 (8.3) |
| Sex, No. (%) | ||
| Male | 1059 (59.1) | 1046 (58.2) |
| Female | 732 (40.9) | 752 (41.8) |
| Race, No. (%)a | ||
| White | 1603 (89.5) | 1650 (91.8) |
| Black or African American | 165 (9.2) | 138 (7.7) |
| Otherb | 23 (1.3) | 10 (0.6) |
| Body mass index, mean (SD)c | 29.7 (6.8) | 29.5 (6.9) |
| Obese (≥30), No. (%) | 771 (43.1) | 739 (41.1) |
| Current smoker, No. (%) | 784 (43.8) | 778 (43.3) |
| COPD assessment test score, mean (SD)d | 20.7 (7.3) | 20.7 (7.2) |
| Postbronchodilator FEV1 % predicted, mean (SD) | 47.7 (14.9) | 47.8 (15.2) |
| COPD severity, No. (%) | ||
| Moderate (FEV1 ≥50% to <80% predicted) | 796 (44.4) | 808 (44.9) |
| Severe (FEV1 ≥30% to <50% predicted) | 742 (41.4) | 714 (39.7) |
| Very severe (FEV1 <30% predicted) | 220 (12.3) | 244 (13.6) |
| COPD exacerbations in previous year, No. (%)e | 1068 (59.6) | 1088 (60.5) |
| No. of prior exacerbations, No. (%) | ||
| 0 | 723 (40.4) | 710 (39.5) |
| 1 | 786 (43.9) | 810 (45.1) |
| ≥2 | 282 (15.8) | 278 (15.5) |
| COPD exacerbation rate in previous year, mean (SD) | 0.8 (0.9) | 0.8 (1.0) |
| Prior COPD-related medication, No. (%)f | 1650 (92.1) | 1678 (93.3) |
| SABA | 1217 (68.0) | 1249 (69.5) |
| LABA + ICS (fixed combination) | 838 (46.8) | 800 (44.5) |
| LAMA | 217 (12.1) | 247 (13.7) |
| Leukotriene inhibitor | 150 (8.4) | 127 (7.1) |
| SABA + SAMA | 144 (8.0) | 135 (7.5) |
| Oxygen | 124 (6.9) | 125 (7.0) |
| SAMA | 89 (5.0) | 89 (5.0) |
| Systemic corticosteroids | 70 (3.9) | 77 (4.3) |
| ICS | 69 (3.9) | 69 (3.8) |
| LABA + LAMA + ICS (free combination) | 45 (2.5) | 64 (3.6) |
| LABA + ICS (free combination) | 36 (2.0) | 52 (2.9) |
| LAMA + ICS (free combination) | 33 (1.8) | 31 (1.7) |
| LABA + LAMA (fixed combination) | 29 (1.6) | 30 (1.7) |
| Xanthines | 29 (1.6) | 30 (1.7) |
| LABA + LAMA (free combination) | 24 (1.3) | 14 (0.8) |
| LABA | 19 (1.1) | 26 (1.5) |
| Oral PDE4 inhibitor | 19 (1.1) | 24 (1.3) |
| Vaccines | 5 (0.3) | 10 (0.6) |
| Cromones | 1 (0.1) | 1 (0.1) |
| LABA + ICS fixed and LABA + LAMA fixed, separately | 1 (0.1) | 1 (0.1) |
| Monoclonal antibody | 1 (0.1) | 0 |
Abbreviations: COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; PDE4, phosphodiesterase type 4; SABA, short-acting β2-agonist; SAMA, short-acting muscarinic antagonist.
Self-reported.
Includes Asian, Native Hawaiian or other Pacific Islander, American Indian or Alaska Native, and other races.
Calculated as weight in kilograms divided by height in meters squared.
Score range of 0 to 40 with higher scores indicating more symptoms; a score >10 indicates symptomatic COPD and >20 indicates a high disease impact.
Defined as worsening of COPD symptoms (dyspnea, cough, sputum) for ≥2 consecutive days that required treatment with antibiotics and/or systemic corticosteroids or resulted in hospitalization or led to death.
Prior treatments were not mutually exclusive. Fixed combinations were defined as >1 drug in a single device; free combinations used multiple individual devices. Data included in fixed or free combinations do not appear in monotherapy categories. For prior medications that were stopped before randomization and concomitant medications that were taken before randomization and continued beyond the first dose of treatment, see eTable 3 in Supplement 1.
Overall, 67.3% of patients completed 12 months of treatment or were in their first year of treatment when the study was stopped; the median exposure time during the first year (on-treatment analysis) was 365 days for both aclidinium and placebo. In addition, 70.7% of patients had completed the 3-year study or were currently enrolled in the study at the time the study was stopped (Figure 1); the median exposure time (including time after treatment discontinuation) was 495 days for aclidinium and 478 days for placebo. Vital status at the end of the study was available for 1732 patients (96.7%) in the aclidinium group and 1725 (95.9%) in the placebo group. Patients whose vital status was missing were censored at their last study contact.
Primary Outcomes
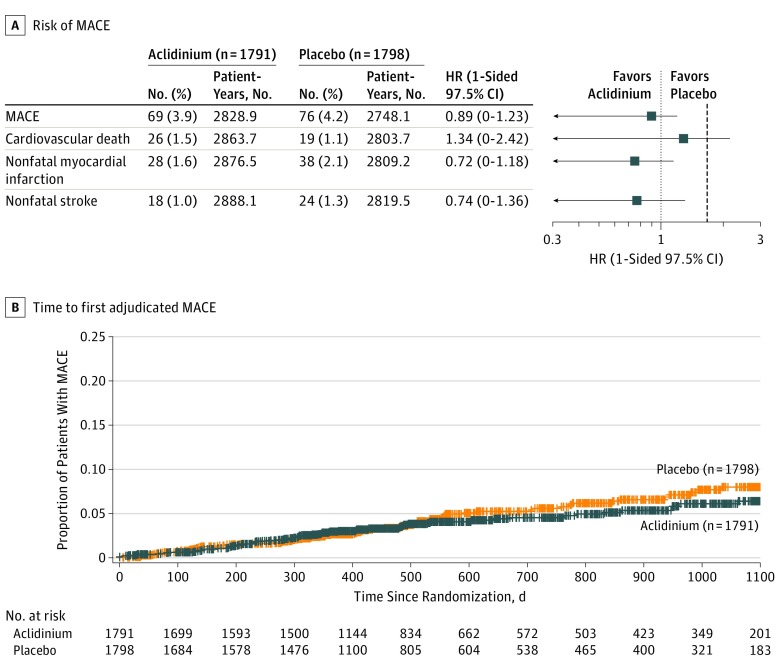
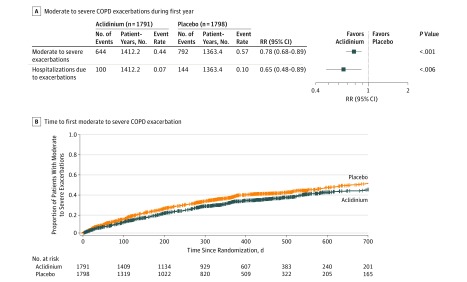
The number of patients who experienced an adjudicated composite MACE was 69 (3.9%) in the aclidinium group vs 76 (4.2%) in the placebo group. The Cox regression HR was 0.89 (1-sided 97.5% CI, 0-1.23), which did not cross the prespecified noninferiority margin of 1.8 (Figure 2). The annual rate of moderate to severe exacerbations during the first year of treatment was significantly lower in patients treated with aclidinium vs placebo by on-treatment analysis (aclidinium, 0.44; placebo, 0.57; rate ratio, 0.78; 2-sided 95% CI, 0.68-0.89; P < .001) (Figure 3A).
Figure 2. Risk of MACE and Time to First Adjudicated MACE (On-Study Analysis).
HR indicates hazard ratio; MACE, major adverse cardiovascular event. Estimates of HRs and 1-sided 97.5% CIs comparing aclidinium with placebo were derived using the Cox proportional hazard model with treatment group, baseline cardiovascular severity, and smoking status as factors. Observations were censored for Kaplan-Meier curve. An HR >1.0 indicates higher risk of MACE with aclidinium and an HR <1.0, lower risk of MACE with aclidinium. The vertical dashed line indicates the margin of noninferiority, 1.8. The median exposure time (including time after treatment discontinuation) was 495 days (interquartile range, 369-893 days) for aclidinium and 478 days (interquartile range, 357-869 days) for placebo.
Figure 3. Moderate to Severe COPD Exacerbations During the First Year and Time to First Moderate to Severe Exacerbation (On-Treatment Analysis).
COPD indicates chronic obstructive pulmonary disease; RR, rate ratio. A, Least-squares means from a negative binomial regression model, with treatment group, baseline inhaled corticosteroid use, baseline COPD severity, history of ≥1 exacerbation in the past 12 months, and smoking status as factors; the log of the exposure time adjusted for the time the patients experienced exacerbations was an offset variable. B, Cox regression analysis. Observations were censored for Kaplan-Meier curve. Log-rank P = .001 comparing aclidinium with placebo, stratified by baseline COPD severity and smoking status. There were 574 patients (32.5%) with events in the aclidinium group and 631 (35.6%) in the placebo group (hazard ratio, 0.82; 95% CI, 0.73-0.92; P<.001). Median observation time was 321 days (interquartile range, 121.5-365 days) for aclidinium and 283 days (interquartile range, 96-365 days) for placebo. A unique exacerbation was one that occurred 7 or more days after completion of treatment with corticosteroids or antibiotics for a previous event.
Secondary Outcomes
Because the primary objective was met, the secondary analyses were valid. The rate of COPD exacerbations requiring hospitalization was significantly reduced with aclidinium vs placebo in the on-treatment analysis (0.07 vs 0.10, respectively; rate ratio, 0.65; 2-sided 95% CI, 0.48-0.89; P = .006) (Figure 3A).
The secondary safety analysis, using an expanded definition of MACE comprising serious events of heart failure, arrhythmias, or cerebrovascular disease, yielded similar results, with events reported in 168 patients (9.4%) receiving aclidinium vs 160 (8.9%) receiving placebo (HR, 1.03; 1-sided 97.5% CI, 0-1.28) (eTable 5 in Supplement 1).
Sensitivity Analyses
The prespecified sensitivity analysis of time to first MACE using the on-treatment approach showed similar results to analysis using the on-study approach (eFigure 2 in Supplement 1). Sensitivity analyses of the annual rate of moderate to severe COPD exacerbations during the first year of treatment showed similar results using the on-study population (regardless of treatment status) (eFigure 3 in Supplement 1). An additional on-treatment analysis of time to first moderate to severe exacerbation in 574 patients in the aclidinium group (32.5%) and 631 (35.6%) in the placebo group who had events also supported this finding (HR, 0.82; 2-sided 95% CI, 0.73-0.92; P < .001) (Figure 3B). Subgroup analyses based on demographic and clinical characteristics showed a consistent treatment effect across all subgroups except among patients with FEV1 greater than 50% predicted (comprising 45% of patients), who had fewer exacerbations and less relative benefit (P=.01 for interaction) (eFigure 4 in Supplement 1). The subgroup analysis also showed improvement in exacerbations both with and without baseline ICS use (including ICS-LABA use).
All-cause mortality was not significantly different between treatment groups (HR, 0.99; 2-sided 95% CI, 0.76-1.28) (eFigure 5 in Supplement 1).
Exploratory Outcomes
Among the components of MACE, nonfatal myocardial infarction (HR, 0.72; 1-sided 97.5% CI, 0-1.18), nonfatal stroke (HR, 0.74; 95% CI, 0-1.36), and cardiovascular death (HR, 1.34; 95% CI, 0-2.42) were also analyzed (Figure 2).
Adverse Events
Rates of treatment-emergent adverse events and serious adverse events were comparable between treatment groups (eTable 6 in Supplement 1). The most common treatment-emergent adverse events (occurring in ≥5% of patients) were pneumonia, urinary tract infection, and upper respiratory tract infection. The anticholinergic adverse effect of dry mouth was uncommon, occurring in 5 patients (0.3%) receiving aclidinium and 10 (0.6%) receiving placebo; urinary retention occurred in 7 patients (0.4%) in each treatment group. The most common serious adverse events (occurring in ≥1% of patients) were pneumonia, atrial fibrillation, congestive heart failure, and coronary artery disease.
Post Hoc Analysis
Hazard ratio estimates from the on-study analysis of time to first MACE with site included as a frailty term did not differ substantially from the primary analysis (eFigure 6 in Supplement 1).
Discussion
Among patients with COPD and increased cardiovascular risk, aclidinium compared with placebo did not result in an inferior risk of major cardiovascular events over 3 years, indicating no evidence of increased cardiovascular risk among patients receiving aclidinium vs placebo. The rate of moderate to severe COPD exacerbations was reduced over the first year.
No patient subgroup demonstrated a difference in efficacy except when analyzed by baseline COPD severity, in which the treatment benefit was observed only in patients with FEV1 of 50% predicted or less. This may be explained by the lower exacerbation rate seen in the placebo group in patients with moderate airway obstruction vs severe or very severe obstruction. In addition, outcomes were similar regardless of on-study or on-treatment analysis. Patients who at baseline were taking an ICS or dual ICS-LABA treatment had a lower exacerbation rate with aclidinium vs placebo, and similar benefit was also present in those not taking an ICS or ICS-LABA. Thus, aclidinium decreased the exacerbation rate when added to background ICS or ICS-LABA treatment.
Outcomes of this trial add data to the long-standing controversy over the safety of LAMAs in COPD.5,6,7,8,9,10,11 In general, observational studies have shown increased cardiovascular risk whereas placebo-controlled clinical trials have not shown increased cardiovascular risk.7,19 This discrepancy has been attributed to the exclusion of patients with cardiovascular comorbidities and renal impairment in COPD clinical trials of LAMA therapy.20,21 Consequently, this trial specifically enrolled patients with elevated cardiovascular risk and found no increase in cardiovascular risk with aclidinium. Furthermore, aclidinium was well tolerated, and the incidences of anticholinergic adverse events and atrial fibrillation were not increased vs placebo, although the study was not powered to evaluate these events. These findings extend the results of a pooled analysis (n = 2781) of 6 short-term randomized clinical trials in patients with moderate to severe COPD that found no evidence of increased cardiovascular risk with aclidinium vs placebo and a subanalysis of 1607 patients with cardiovascular risk factors that found no difference in the incidence of cardiovascular or cerebrovascular adverse events.22 In contrast, 2 large case-control studies showed similar increases in risk for both LAMAs and LABAs within 30 days of treatment initiation, with a reduction in cardiovascular risk after 30 days.19,23 One possibility is that the early observation of cardiac events with both classes of bronchodilators was the result of patients with new onset of chest symptoms receiving an incorrect diagnosis of worsening COPD symptoms, when the correct diagnosis was worsening cardiac symptoms. This trial cannot specifically address the concern regarding new-onset treatment because many patients were using a LAMA or LABA prior to screening.
Because patients with COPD exacerbations are at higher risk of subsequent MACE and all-cause mortality,3,4,24,25,26,27 it might be hypothesized that the reduction in exacerbations seen with aclidinium might be reflected in a reduction in cardiovascular events or the exploratory outcome of all-cause mortality. However, consistent with a recent study of ICS-LABA treatment in patients with mild to moderate COPD and cardiovascular risk factors,28 this was not seen in this trial. In addition, a recent post hoc study of tiotropium found no increased risk in patients with recent cardiovascular events; however, patients with unstable or acute cardiovascular disease were not enrolled in that study.12
This trial had several strengths. By enrolling patients with increased cardiovascular risk and continuing the study until an adequate number of adjudicated MACE had occurred, the study demonstrated that aclidinium was noninferior to placebo with respect to the risk of a new MACE. Furthermore, this trial is unique insofar as it was designed with an efficacy outcome nested within it, allowing for risk-benefit comparisons within the same study population.
Limitations
The study has several limitations. It was not powered for cause-specific mortality, and a numerically increased point estimate for cardiac death with aclidinium was observed. However, the number of events was small and the confidence intervals were wide. Accordingly, the possibility that there was an increase in cardiac deaths cannot be excluded. However, there was no difference between treatments in the more reliable exploratory outcome of all-cause mortality, and the point estimates of the other MACE components tended to be lower with aclidinium. In addition, the study used a LAMA with a low potential for systemic effect,29 as reflected by low reported rates of dry mouth and urinary retention; this finding should therefore be extrapolated cautiously.
Conclusions
Among patients with COPD and increased cardiovascular risk, aclidinium was noninferior to placebo for risk of MACE over 3 years. The rate of moderate to severe exacerbations was reduced over the first year.
eAppendix. Imputation of Missing Data
eTable 1. Major Protocol Amendments and Other Significant Changes to Study Conduct
eTable 2. Major Changes to Planned Analyses
eTable 3. Chronic Pulmonary Disease-Related Prior Medications That Were Stopped Prior to Randomization and Concomitant Medications (Full Analysis Set)
eTable 4. Cardiovascular Risk Factors by Treatment Group
eTable 5. Major Adverse Cardiovascular Events and Other Serious Cardiovascular Events of Interest (On-Study)
eTable 6. Treatment-Emergent Adverse Events (Full Analysis Set)
eFigure 1. Study Design
eFigure 2. Risk of Major Adverse Cardiovascular Events With Aclidinium Versus Placebo (On-Treatment)
eFigure 3. Chronic Obstructive Pulmonary Disease Moderate/Severe Exacerbations and Hospitalizations Due to Exacerbations During the First Year of Treatment (On-Study)
eFigure 4. Moderate/Severe Chronic Obstructive Pulmonary Disease Exacerbations During the First Year by Patient Subgroup (On-Treatment)
eFigure 5. All-Cause Mortality (Full Analysis Set)
eFigure 6. Frailty Analysis of Risk of Major Adverse Cardiovascular Events (On-Study)
eReferences
Trial Protocol
Statistical Analysis Plan
Data Sharing Statement
References
- 1.Global Initiative for Chronic Obstructive Lung Disease Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: 2019 https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf. Accessed February 7, 2019.
- 2.Curkendall SM, DeLuise C, Jones JK, et al. Cardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada: cardiovascular disease in COPD patients. Ann Epidemiol. 2006;16(1):63-70. doi: 10.1016/j.annepidem.2005.04.008 [DOI] [PubMed] [Google Scholar]
- 3.Sin DD, Anthonisen NR, Soriano JB, Agusti AG. Mortality in COPD: role of comorbidities. Eur Respir J. 2006;28(6):1245-1257. doi: 10.1183/09031936.00133805 [DOI] [PubMed] [Google Scholar]
- 4.Donaldson GC, Hurst JR, Smith CJ, Hubbard RB, Wedzicha JA. Increased risk of myocardial infarction and stroke following exacerbation of COPD. Chest. 2010;137(5):1091-1097. doi: 10.1378/chest.09-2029 [DOI] [PubMed] [Google Scholar]
- 5.Singh S, Loke YK, Furberg CD. Inhaled anticholinergics and risk of major adverse cardiovascular events in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2008;300(12):1439-1450. doi: 10.1001/jama.300.12.1439 [DOI] [PubMed] [Google Scholar]
- 6.Tashkin DP, Celli B, Senn S, et al. ; UPLIFT Study Investigators . A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543-1554. doi: 10.1056/NEJMoa0805800 [DOI] [PubMed] [Google Scholar]
- 7.Michele TM, Pinheiro S, Iyasu S. The safety of tiotropium—the FDA’s conclusions. N Engl J Med. 2010;363(12):1097-1099. doi: 10.1056/NEJMp1008502 [DOI] [PubMed] [Google Scholar]
- 8.Singh S, Loke YK, Enright PL, Furberg CD. Mortality associated with tiotropium mist inhaler in patients with chronic obstructive pulmonary disease: systematic review and meta-analysis of randomised controlled trials. BMJ. 2011;342:d3215. doi: 10.1136/bmj.d3215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verhamme KM, Afonso A, Romio S, Stricker BC, Brusselle GG, Sturkenboom MC. Use of tiotropium Respimat soft mist inhaler versus HandiHaler and mortality in patients with COPD. Eur Respir J. 2013;42(3):606-615. doi: 10.1183/09031936.00005813 [DOI] [PubMed] [Google Scholar]
- 10.Dong YH, Lin HH, Shau WY, Wu YC, Chang CH, Lai MS. Comparative safety of inhaled medications in patients with chronic obstructive pulmonary disease: systematic review and mixed treatment comparison meta-analysis of randomised controlled trials. Thorax. 2013;68(1):48-56. doi: 10.1136/thoraxjnl-2012-201926 [DOI] [PubMed] [Google Scholar]
- 11.Celli B, Decramer M, Kesten S, Liu D, Mehra S, Tashkin DP; UPLIFT Study Investigators . Mortality in the 4-year trial of tiotropium (UPLIFT) in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180(10):948-955. doi: 10.1164/rccm.200906-0876OC [DOI] [PubMed] [Google Scholar]
- 12.Tashkin DP, Leimer I, Metzdorf N, Decramer M. Cardiac safety of tiotropium in patients with cardiac events: a retrospective analysis of the UPLIFT trial. Respir Res. 2015;16:65. doi: 10.1186/s12931-015-0216-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wedzicha JA, Agusti A, Donaldson G, Chuecos F, Lamarca R, Garcia Gil E. Effect of aclidinium bromide on exacerbations in patients with moderate-to-severe COPD: a pooled analysis of five phase III, randomized, placebo-controlled studies. COPD. 2016;13(6):669-676. doi: 10.3109/15412555.2016.1170111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wise RA, Chapman KR, Scirica BM, et al. Long-term evaluation of the effects of aclidinium bromide on major adverse cardiovascular events and COPD exacerbations in patients with moderate to very severe COPD: rationale and design of the ASCENT COPD study. Chronic Obstr Pulm Dis. 2018;5(1):5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med. 1999;159(1):179-187. doi: 10.1164/ajrccm.159.1.9712108 [DOI] [PubMed] [Google Scholar]
- 16.Hicks KA, Hung HMJ, Mahaffey KW, et al. Standardized Definitions for Cardiovascular and Stroke Endpoint Events in Clinical Trials August 20, 2014:1-33. https://www.cdisc.org/system/files/all/standard/Draft%20Definitions%20for%20CDISC%20August%2020%2C%202014.pdf. Accessed August 28, 2018.
- 17.US Food and Drug Administration Guidance for Industry: Diabetes Mellitus—Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes December 2008. https://www.fda.gov/downloads/Drugs/Guidances/ucm071627.pdf. Accessed September 5, 2018.
- 18.Keene ON, Roger JH, Hartley BF, Kenward MG. Missing data sensitivity analysis for recurrent event data using controlled imputation. Pharm Stat. 2014;13(4):258-264. doi: 10.1002/pst.1624 [DOI] [PubMed] [Google Scholar]
- 19.Gershon A, Croxford R, Calzavara A, et al. Cardiovascular safety of inhaled long-acting bronchodilators in individuals with chronic obstructive pulmonary disease. JAMA Intern Med. 2013;173(13):1175-1185. doi: 10.1001/jamainternmed.2013.1016 [DOI] [PubMed] [Google Scholar]
- 20.Schmiedl S, Fischer R, Ibanez L, et al. Tiotropium Respimat vs HandiHaler: real-life usage and TIOSPIR trial generalizability. Br J Clin Pharmacol. 2016;81(2):379-388. doi: 10.1111/bcp.12808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker S, Fingleton J, Weatherall M, Beasley R. Limited generalisability of UPLIFT findings to clinical practice. Thorax. 2013;68(11):1066-1067. doi: 10.1136/thoraxjnl-2013-203724 [DOI] [PubMed] [Google Scholar]
- 22.Chapman KR, Beck E, Alcaide D, Garcia Gil E. Overall and cardiovascular safety of aclidinium bromide in patients with COPD: a pooled analysis of six phase III, placebo-controlled, randomized studies. Chronic Obstr Pulm Dis. 2015;3(1):435-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang MT, Liou JT, Lin CW, et al. Association of cardiovascular risk with inhaled long-acting bronchodilators in patients with chronic obstructive pulmonary disease: a nested case-control study. JAMA Intern Med. 2018;178(2):229-238. doi: 10.1001/jamainternmed.2017.7720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selvaraj CL, Gurm HS, Gupta R, Ellis SG, Bhatt DL. Chronic obstructive pulmonary disease as a predictor of mortality in patients undergoing percutaneous coronary intervention. Am J Cardiol. 2005;96(6):756-759. doi: 10.1016/j.amjcard.2005.05.016 [DOI] [PubMed] [Google Scholar]
- 25.Rothnie KJ, Connell O, Müllerová H, et al. Myocardial infarction and ischemic stroke after exacerbations of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2018;15(8):935-946. doi: 10.1513/AnnalsATS.201710-815OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halpin DM, Decramer M, Celli B, Kesten S, Leimer I, Tashkin DP. Risk of nonlower respiratory serious adverse events following COPD exacerbations in the 4-year UPLIFT trial. Lung. 2011;189(4):261-268. doi: 10.1007/s00408-011-9301-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothnie KJ, Yan R, Smeeth L, Quint JK. Risk of myocardial infarction (MI) and death following MI in people with chronic obstructive pulmonary disease (COPD): a systematic review and meta-analysis. BMJ Open. 2015;5(9):e007824. doi: 10.1136/bmjopen-2015-007824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vestbo J, Anderson JA, Brook RD, et al. ; SUMMIT Investigators . Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet. 2016;387(10030):1817-1826. doi: 10.1016/S0140-6736(16)30069-1 [DOI] [PubMed] [Google Scholar]
- 29.Cazzola M. Aclidinium bromide, a novel long-acting muscarinic M3 antagonist for the treatment of COPD. Curr Opin Investig Drugs. 2009;10(5):482-490. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Imputation of Missing Data
eTable 1. Major Protocol Amendments and Other Significant Changes to Study Conduct
eTable 2. Major Changes to Planned Analyses
eTable 3. Chronic Pulmonary Disease-Related Prior Medications That Were Stopped Prior to Randomization and Concomitant Medications (Full Analysis Set)
eTable 4. Cardiovascular Risk Factors by Treatment Group
eTable 5. Major Adverse Cardiovascular Events and Other Serious Cardiovascular Events of Interest (On-Study)
eTable 6. Treatment-Emergent Adverse Events (Full Analysis Set)
eFigure 1. Study Design
eFigure 2. Risk of Major Adverse Cardiovascular Events With Aclidinium Versus Placebo (On-Treatment)
eFigure 3. Chronic Obstructive Pulmonary Disease Moderate/Severe Exacerbations and Hospitalizations Due to Exacerbations During the First Year of Treatment (On-Study)
eFigure 4. Moderate/Severe Chronic Obstructive Pulmonary Disease Exacerbations During the First Year by Patient Subgroup (On-Treatment)
eFigure 5. All-Cause Mortality (Full Analysis Set)
eFigure 6. Frailty Analysis of Risk of Major Adverse Cardiovascular Events (On-Study)
eReferences
Trial Protocol
Statistical Analysis Plan
Data Sharing Statement