This study uses Irish national registry data to characterize variation in nonmelanoma skin cancer incidence by treatment modality among patients receiving multiple kidney transplants.
Key Points
Question
Is kidney transplant failure and return to dialysis treatment associated with risk of nonmelanoma skin cancer (NMSC) that is different from the risk of the general population?
Findings
This study of Irish national registry data suggests that periods of transplant allograft failure are associated with reduced risk of NMSC, and receipt of a subsequent transplant is associated with increased risk. However, the lower risk associated with the period of graft failure is still substantially higher than that of the general population.
Meaning
These findings may help physicians in counseling transplant recipients about the risks of skin cancer and also serve as a reminder that cancer surveillance should continue during periods of graft failure.
Abstract
Importance
Existing data suggest that nonmelanoma skin cancer (NMSC) is more common in renal transplant recipients than in maintenance dialysis patients. However, whether the risk of NMSC varies as the treatment modality for end-stage kidney disease (ESKD) changes between dialysis and transplantation is not well described.
Objective
To determine whether the incidence of NMSC is attenuated during periods of graft loss with a return to dialysis in those who receive multiple kidney transplants.
Design, Setting, and Participants
Retrospective analysis of data from recipients of kidney transplants from the Irish National Kidney Transplant Service database, linked with the Irish Cancer Registry, from 1994 to 2014. All analysis took place between January 10, 2018 and March 31, 2018. Standardized incidence ratios (SIRs) were calculated for NMSC incidence in comparison with the general population using Irish census data as the denominator. Incidence of NMSC was calculated with modality of treatment for ESKD varying over time; incidence rates and rate ratios associated with dialysis intervals were calculated using Poisson regression; and disease was defined according to International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes for cancer diagnosis.
Exposures
Kidney transplantation.
Main Outcomes and Measures
Incidence rates per 1000 patient-years and incident rate ratios of NMSC after kidney transplant.
Results
Data from the records of 3821 deceased or living donor kidney transplant recipients were assessed; 2399 (62.8%) male and 1422 (37.2%) female recipients; mean (SD) age at time of first data recorded, 41.9 (16.0) years. A total of 3433 recipients were included who had a functioning transplant on January 1, 1994, or received a transplant after that date up to December 31, 2014: 3215 received 1 transplant, 522 a second kidney transplant, and 84 had 3 or more kidney transplants. Periods of treatment with a functioning transplant were associated with a higher incidence of NMSC diagnosis than periods of graft failure: adjusted incidence rate ratio (aIRR), 2.19 (95% CI, 1.56-3.07), P < .001. The aIRRs of NMSC fell from 41.7 (95% CI, 39.38-44.15) per 1000 patient-years in the first transplant to 19.29 (95% CI, 13.41-27.76) in the dialysis period following the first allograft failure. Incidence similarly rose and fell following each subsequent consecutive transplant.
Conclusions and Relevance
In recipients of multiple kidney transplants, while the incidence of NMSC fell during periods defined by transplant failure, there was residual elevated risk. While ascertainment bias may have contributed to the observed trends, the stagnant incidence of invasive cancer overall highlights the need for continued cancer surveillance during graft failure.
Introduction
Nonmelanoma skin cancer (NMSC) is the most common form of cancer following solid organ transplantation and is reportedly more common in renal transplant recipients than in maintenance dialysis patients.1,2,3,4,5,6,7 However, individuals undergoing maintenance dialysis are also thought to be at increased risk for NMSC, and not only patients with poorer health and higher comorbidity but also those on the active transplant waitlist.7,8,9
Whether this NMSC risk varies by treatment modality for kidney failure is not well described. We evaluated whether the incidence of NMSC changes during periods of graft loss of function and patient return to dialysis vs during periods of functioning grafts in recipients of multiple consecutive kidney transplants.
Methods
The Irish National Kidney Transplant Service database was accessed for the years 1994 through 2014, and all kidney transplant recipients with available data were included in this study. This registry is 98.9% complete in terms of long-term follow-up of recipients (P.O. and P.J.C.). These data were then linked with the Irish National Cancer Registry to capture episodes of cancer during follow-up.
In this study we define NMSC as International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) code C44, including basal cell carcinoma and squamous cell carcinoma. All cancers are coded using ICD-10 codes in the National Cancer Registry database. Any cancers previously coded using International Classification of Diseases, Ninth Revision have been translated into ICD-10 codes. In this study, graft failure is defined by the date of first maintenance dialysis following allograft failure (ie, excluding periods of delayed graft function) and extends until the date of the subsequent kidney transplant, death, or date of censoring.
Incidence rates and rate ratios were calculated using Poisson regression. In addition, standardized incidence ratios (SIRs) of cancer were calculated in comparison with the general population using Irish national census data as the denominator, and using ICD-10 coding for cancer diagnosis. Transplant recipients were considered at risk beginning at transplantation or the start of cancer registry coverage on January 1, 1994 (whichever came last). Follow-up ended at death or at the cancer registry censor date, December 31, 2015 (whichever came first). SIRs were determined by comparing the rate of cancer diagnosis within the follow-up period for each transplant separately. Incidence rates for NMSC were calculated with end-stage kidney disease (ESKD) treatment modality varying with time, fluctuating between periods of functioning allografts, and periods of nonfunction necessitating dialysis treatment. To assess whether time with a functioning transplant as a covariate was associated with NMSC risk, we used a generalized estimating equation Poisson model with random effects conditional on the individual and treatment modality. This model also included age, sex, treatment modality (dialysis or transplant), number of kidney transplants, biopsy-proven rejection, and calcineurin inhibitor subtype. Statistical analysis was conducted using STATA software, version 13. The National Kidney Transplant Service, Beaumont Hospital institutional research ethics board waived approval and written informed consent for this study.
Results
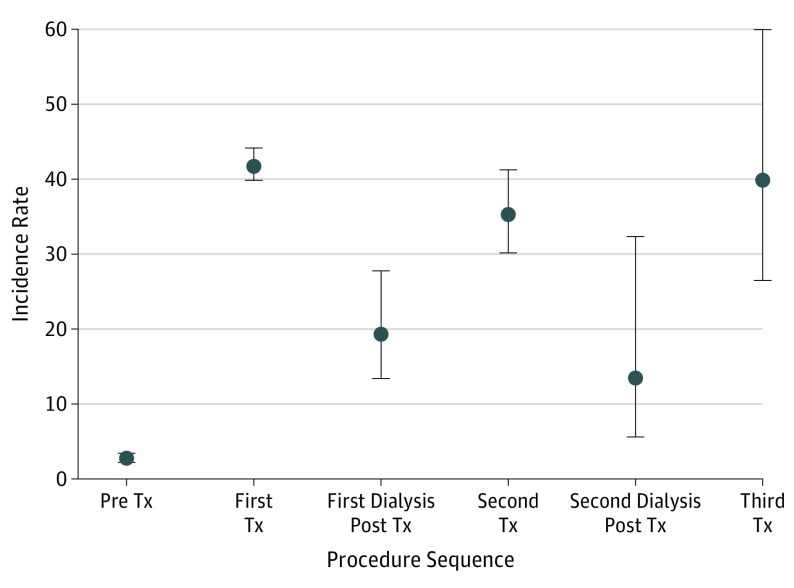
A total of 3821 individual deceased and living kidney transplant recipients were included for analysis. All included patients had a functioning transplanted kidney on January 1, 1994, or received a transplant after that date up to December 31, 2014. This cohort included 1422 (37.2%) female recipients; 3215 recipients had 1 transplant, 522 recipients a second; and 84 recipients had 3 kidney transplants. The total exposure time of observation was 35 297 years. With 1401 reported NMSC cases occurring posttransplant (808 squamous cell carcinoma, 569 basal cell carcinoma, and 24 other/unspecified NMSC), the incidence rate was 39.69 per 1000 patient-years overall. The Table and Figure 1 detail the incidence rates of NMSC by treatment period stratified by the first, second, and third kidney transplants and intervening periods.
Table. Incidence Rates of Nonmelanoma Skin Cancer by Treatment Period.
| Modality of Renal Replacement Therapy | Incidence Rate per 1000 Patient-Years (95% CI) |
|---|---|
| Pretransplant (dialysis) | 2.75 (2.2-3.4) |
| During the first transplant | 41.7 (39.38-44.15) |
| For dialysis following first transplant | 19.29 (13.41-27.76) |
| During the second transplant | 35.27 (30.17-41.25) |
| For dialysis following second transplant | 13.46 (5.60-32.34) |
| During the third transplant | 39.85 (26.48-59.97) |
| For dialysis following third transplant | 21.16 (2.98-150.22) |
Figure 1. Rates (95%CIs) of Nonmelanoma Skin Cancer per 1000 Patient-Years of Observation by Sequential Kidney Transplantation.
Tx indicates transplant. Pre Tx refers to the period before the first kidney transplant, while the patient was wait-listed. First Tx represents the period of successful treatment by the first renal transplant. First dialysis post Tx represents the period defined by graft failure of the first transplant and return to dialysis. Second Tx represents the period defined by a second transplant following a period of treatment with dialysis. Second dialysis post Tx period is defined by failure of the second renal transplant and return to dialysis. Third Tx defines the period of subsequent transplant of a third kidney transplant.
Periods of treatment with a functioning transplant had a higher incidence of NMSC diagnosis than intervening periods of dialysis (adjusted incidence rate ratio [aIRR], 2.19; 95% CI, 1.56-3.07; P < .001). Other risk factors for NMSC from the fully adjusted model included male sex (aIRR, 2.34; 95% CI, 2.05-2.67; P < .001), the number of transplants (aIRR, 1.17; 95% CI, 1.04-1.32; P = .01), and patient age (graduated aIRR with increasing age; data not reported). Tacrolimus use was associated with a lower incidence of NMSC (aIRR, 0.38; 95% CI, 0.34-0.43; P < .001) compared with cyclosporin. The median duration (interquartile range [IQR]) of the dialysis period between the first and second transplants was 2.2 (1.1-3.9) years, and between the second and third transplants was 2.77 (1.69-4.30) years. Median (IQR) exposure time (ie, exposure to a functioning transplant and the accompanying immunosuppression) for the period of the first transplant was 7.49 (3.86-12.75) years; for the second transplant, 7.47 (2.79-13.08) years; and for the third transplant, 7.37 (3.83-11.11) years.
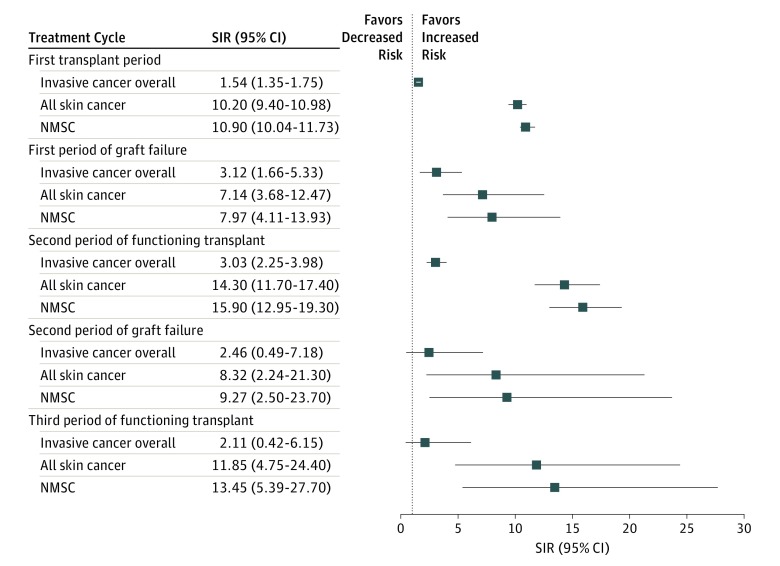
As assessed by the mixed effects model, time with a functioning transplant as a covariate was independently associated with NMSC development in the fully adjusted model (coefficient, 0.77; 95% CI, 0.68-0.86; P < .001). SIRs of invasive cancer excluding NMSC (ICD-10 codes for all invasive cancers, C00-C96, excluding C44) and of different skin cancer subtypes following successive kidney transplants compared with the general population are detailed in Figure 2.
Figure 2. Cancer Incidence Following Multiple Kidney Transplants.
Standardized incidence ratios (SIRs) of invasive cancers overall, all skin cancers, and nonmelanoma skin cancer (NMSC) in patients after consecutive kidney transplantation compared with the general population. Invasive cancer overall refers to a composite group formed by International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, codes C00 through C96, excluding C44.
Discussion
In this study using national data for cancer incidence following consecutive kidney transplants, there appeared to be a variation in the incidence of NMSC between periods defined by a functioning transplant and periods defined by graft failure. In the realm of solid-organ transplantation, kidney transplant provides a unique opportunity to study the association of transplantation with cancer incidence, since dialysis constitutes an extracorporeal form of maintenance treatment following allograft failure.
Apprehension regarding infection while the patient is undergoing dialysis usually stimulates a reduction in or withdrawal of immunosuppression following graft failure.10 This decision depends on the likelihood of receiving another transplant and the likely duration of the waiting period.10 Therefore, in the present study, periods of dialysis likely represent intervals of reduced immunosuppressive burden.
Previous studies have suggested higher skin cancer rates in recipients of kidney transplants compared with dialysis patients overall.6,11 However, the present study focused on an intracluster comparison, which likely represents a more comprehensive analysis of cancer risk over multiple kidney transplants in the same individuals.
The average duration of each dialysis period was similar, as was each period of functioning transplant. However, given the data suggesting prolonged alterations in DNA repair mechanisms associated with calcineurin inhibitors such as cyclosporin, it is likely that immunosuppression has a lasting effect on cancer development even after withdrawal.12 As to whether the duration of the functioning transplant period has an association with NMSC risk, it appears from our data that this exposure time does have a positive association with NMSC development. This may suggest that recipients with shorter time to graft failure may be at lower risk of NMSC.
Tacrolimus-based regimens were associated with a lower incidence of NMSC in the present study compared with cyclosporin. This may be a specific effect but could also be owing to a period effect, with longer follow-up and therefore higher ascertainment of NMSC in the cyclosporin group. In the Irish national kidney transplant program, tacrolimus use in preference to cyclosporin as the initial calcineurin inhibitor in kidney transplantation began in 1996 and rose progressively to complete replacement of cyclosporin for this purpose by 2003 onward. A very similar pattern is seen for the introduction of mycophenolate in preference to azathioprine as the initial antimetabolite in the Irish national kidney transplant program.
Some recent studies have suggested a lower risk of squamous cell carcinoma with mycophenolate-based regimens vs azathioprine in maintenance transplant immunosuppression, which may coincide with a switch to tacrolimus from cyclosporin.13,14 This is also substantiated by in vitro evidence suggesting an attenuation of UV radiation–induced DNA damage with a switch from azathioprine to mycophenolate in kidney transplant recipients.15
Limitations
Limitations of our study include the retrospective nature of the analysis, which makes it difficult to capture the effect of the lag between exposure, cancer development, presentation, and diagnosis, and which may vary by treatment period. Our incidence calculations may be affected by an element of ascertainment bias, since kidney transplant clinics tend to have a greater focus on skin cancer surveillance.3 However, patients on maintenance dialysis are also followed closely clinically to the extent that one might reasonably expect clinically significant lesions to be detected. Balanced against the likelihood of ascertainment bias is also the fact that the standardized incidence of invasive cancer overall (excluding NMSC) remained elevated throughout the first 3 treatment intervals (Figure 1).
One previous US-based registry study looked at cancer incidence variability by interval of transplant function and similarly found higher cancer incidence in functioning transplant periods as well as elevated risk compared with that of the general population during nonfunctioning periods.16 Of interest, these investigators found a variation in cancer incidence patterns by interval based on whether cancers were defined as immune related, infection related, or ESRD related, but they did not address NMSC specifically.16 This US-based study also did not assess the incidence of overall invasive cancer by interval, which might represent an approach less susceptible to ascertainment bias, than individual cancer subtypes.
There are a number of possible explanations for the discrepancy between trends in overall invasive cancer and that of NMSC in the present study. One explanation might be ascertainment bias influencing toward the null during periods of graft failure, with lower NMSC incidence due to lower clinical surveillance during periods of graft failure. This could mean that true NMSC incidence during periods of graft failure is actually higher. An alternative explanation might be that incidence patterns vary by cancer subtype, for instance rising progressively over intervals for some cancers so that the trends in overall cancer incidence are more difficult to interpret.16
Conclusions
While the risk of NMSC varies between intervals of graft function and failure, the risk remains high during periods of failure. In addition, in the present study, the elevated incidence of invasive cancer overall in comparison with that of the general population persisted throughout the initial kidney transplant intervals and did not display the sawtooth pattern observed with NMSC incidence. These observations serve to highlight the importance of continued cancer surveillance during periods of graft failure in kidney transplant recipients.
References
- 1.Vajdic CM, McDonald SP, McCredie MR, et al. Cancer incidence before and after kidney transplantation. JAMA. 2006;296(23):2823-2831. doi: 10.1001/jama.296.23.2823 [DOI] [PubMed] [Google Scholar]
- 2.Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. Am J Transplant. 2004;4(6):905-913. doi: 10.1111/j.1600-6143.2004.00450.x [DOI] [PubMed] [Google Scholar]
- 3.Harwood CA, Mesher D, McGregor JM, et al. A surveillance model for skin cancer in organ transplant recipients: a 22-year prospective study in an ethnically diverse population. Am J Transplant. 2013;13(1):119-129. doi: 10.1111/j.1600-6143.2012.04292.x [DOI] [PubMed] [Google Scholar]
- 4.Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med. 2003;348(17):1681-1691. doi: 10.1056/NEJMra022137 [DOI] [PubMed] [Google Scholar]
- 5.Walder BK, Robertson MR, Jeremy D. Skin cancer and immunosuppression. Lancet. 1971;2(7737):1282-1283. doi: 10.1016/S0140-6736(71)90602-7 [DOI] [PubMed] [Google Scholar]
- 6.Sułowicz J, Wojas-Pelc A, Ignacak E, Krzanowska K, Kuźniewski M, Sułowicz W. Comparison of the incidence of skin cancers in patients on dialysis and after kidney transplantation. Postepy Dermatol Alergol. 2017;34(2):138-142. doi: 10.5114/ada.2017.67078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang CC, Tang CH, Huang SY, Huang KC, Sue YM. Risk of non-melanoma skin cancer in patients with chronic kidney disease and its relationship to uraemic pruritus. Acta Derm Venereol. 2017;97(10):1230-1234. doi: 10.2340/00015555-2762 [DOI] [PubMed] [Google Scholar]
- 8.Fischereder M. Cancer in patients on dialysis and after renal transplantation. Nephrol Dial Transplant. 2008;23(8):2457-2460. doi: 10.1093/ndt/gfn183 [DOI] [PubMed] [Google Scholar]
- 9.Maisonneuve P, Agodoa L, Gellert R, et al. Cancer in patients on dialysis for end-stage renal disease: an international collaborative study. Lancet. 1999;354(9173):93-99. doi: 10.1016/S0140-6736(99)06154-1 [DOI] [PubMed] [Google Scholar]
- 10.Kassakian CT, Ajmal S, Gohh RY, Morrissey PE, Bayliss GP. Immunosuppression in the failing and failed transplant kidney: optimizing outcomes. Nephrol Dial Transplant. 2016;31(8):1261-1269. doi: 10.1093/ndt/gfv256 [DOI] [PubMed] [Google Scholar]
- 11.Birkeland SA, Løkkegaard H, Storm HH. Cancer risk in patients on dialysis and after renal transplantation. Lancet. 2000;355(9218):1886-1887. doi: 10.1016/S0140-6736(00)02298-4 [DOI] [PubMed] [Google Scholar]
- 12.Kuschal C, Thoms KM, Schubert S, et al. Skin cancer in organ transplant recipients: effects of immunosuppressive medications on DNA repair. Exp Dermatol. 2012;21(1):2-6. doi: 10.1111/j.1600-0625.2011.01413.x [DOI] [PubMed] [Google Scholar]
- 13.Coghill AE, Johnson LG, Berg D, Resler AJ, Leca N, Madeleine MM. Immunosuppressive medications and squamous cell skin carcinoma: nested case-control study within the Skin Cancer after Organ Transplant (SCOT) cohort. Am J Transplant. 2016;16(2):565-573. doi: 10.1111/ajt.13596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vos M, Plasmeijer EI, van Bemmel BC, et al. Azathioprine to mycophenolate mofetil transition and risk of squamous cell carcinoma after lung transplantation. J Heart Lung Transplant. 2018;37(7):853-859. doi: 10.1016/j.healun.2018.03.012 [DOI] [PubMed] [Google Scholar]
- 15.Hofbauer GF, Attard NR, Harwood CA, et al. Reversal of UVA skin photosensitivity and DNA damage in kidney transplant recipients by replacing azathioprine. Am J Transplant. 2012;12(1):218-225. doi: 10.1111/j.1600-6143.2011.03751.x [DOI] [PubMed] [Google Scholar]
- 16.Yanik EL, Clarke CA, Snyder JJ, Pfeiffer RM, Engels EA. Variation in cancer incidence among patients with ESRD during kidney function and nonfunction intervals. J Am Soc Nephrol. 2016;27(5):1495-1504. doi: 10.1681/ASN.2015040373 [DOI] [PMC free article] [PubMed] [Google Scholar]