This study of 3 individuals with linear porokeratosis examines whether the disease is associated with germline and somatic mutations in the PMVK and MVD genes using whole-exome sequencing.
Key Points
Question
What genetic characteristics are associated with linear porokeratosis?
Finding
This study of 3 participants with linear porokeratosis used whole-exome sequencing of unaffected and affected tissue and finds a combination of germline mutations and novel somatic mutations or loss of heterozygosity within the lesional skin in all cases. While 2 participants had a germline PMVK mutation and somatic loss of heterozygosity or a second mutation, the third had a germline splice site mutation in MVD and somatic frameshift deletion in MVD.
Meaning
Our findings suggest that linear porokeratosis is a mosaic disorder associated with mutations in the genes that encode enzymes within the mevalonate biosynthesis pathway.
Abstract
Importance
Linear porokeratosis features linear and whorled configurations of keratotic papules and plaques, with coronoid lamellae present on histologic examination. Because linear porokeratosis manifests in the lines of Blaschko representing the dorsoventral migration patterns of keratinocyte precursors, it has been suggested that postzygotic somatic mutation underlies the disease. However, no genetic evidence has supported this hypothesis to date.
Objective
To identify genetic mutations associated with linear porokeratosis.
Design, Setting, and Participants
Paired whole-exome sequencing of affected skin and blood/saliva samples from 3 participants from 3 academic medical centers with clinical and histologic diagnoses of linear porokeratosis.
Interventions or Exposures
Whole-exome sequencing of paired blood/saliva and affected tissue samples isolated from linear porokeratosis lesions.
Main Outcomes and Measures
Germline and somatic genomic characteristics of participants with linear porokeratosis.
Results
Of the 3 participants, 2 were male. Participant ages ranged from 5 to 20 years old. We found a combination of a novel germline mutation and a novel somatic mutation within affected tissue in all cases. One participant had a germline heterozygous PMVK c.329G>A mutation and a somatic copy-neutral loss of heterozygosity confined to the lesional skin, while a second had a germline heterozygous PMVK c.79G>T mutation and an additional PMVK c.379C>T mutation in the lesional skin. In a third participant, there was a germline splice-site mutation in MVD (c.70 + 5G>A) and a somatic deletion in MVD causing frameshift and premature codon termination within the lesional skin (c.811_815del, p.F271Afs*33 frameshift).
Conclusions and Relevance
Our findings suggest that linear porokeratosis is associated with the presence of second-hit postzygotic mutations in the genes that encode enzymes within the mevalonate biosynthesis pathway, and provide further evidence that the mevalonate pathway may be a potential target for therapeutic intervention in porokeratosis.
Introduction
Porokeratosis is a disorder of keratinization traditionally classified into 5 main variants based on clinical appearance: porokeratosis of Mibelli, disseminated superficial actinic porokeratosis (DSAP), disseminated superficial porokeratosis (DSP), porokeratosis palmaris et plantaris disseminata, and linear porokeratosis (LP).1 Other rarer types of presentation such as porokeratosis ptychotropica, solar facial porokeratosis, and porokeratoma have been described as single presentation or part of the main clinical variants. All variants share the histopathological feature of a cornoid lamella, a vertical column of parakeratosis situated above dyskeratotic cells within the granular layer of the skin.2
The most common variant of porokeratosis, DSAP, presents in adults as small atrophic plaques with a keratotic rim over sun-exposed areas.1,3 Solar facial porokeratosis could be considered as a forme fruste of this variant. Similar lesions localized to exposed and unexposed areas are seen in DSP. Porokeratosis palmaris et plantaris disseminata appears on palms and soles as small punctate papules, and gradually progresses to involve other areas of the skin with DSP-like plaques.3 The classic form of porokeratosis, porokeratosis of Mibelli, presents as large atrophic plaques with a prominent keratotic rim, typically appears at an early age, and can be localized to all areas of skin and mucous membranes.1 The characteristics of LP present during infancy as stripes of porokeratotic plaques. The plaques associated with LP can be either small and superficial or large and thick. Involvement of the palmar skin appears as stripes of punctate papules. Fingernails and toenails may also be involved. Recently, heterozygous germline mutations of the mevalonate pathway genes MVK (MIM 251170), PMVK (MIM 607622), MVD (MIM 603236), and FDPS (MIM 134629) were identified in familial and sporadic porokeratosis, while 1 study identified germline mutations in SLC17A9 (MIM 612107) in familial DSAP.4,5,6,7
Mosaicism refers to the presence of genetically distinct cells within an organism that result from postzygotic mutation.8 The patterning and appearance of cutaneous mosaic disorders are largely determined by mutation timing, its pathophysiological effects, and by the affected cutaneous progenitor cells. In the constitutional state, autosomal dominant and autosomal recessive mutations are present in all cells, and are transmitted through the germline to affected offspring. Mosaicism manifests in cutaneous disease via 2 primary mechanisms. In the first mechanism, a dominant, heterozygous, postzygotic, somatic mutation is confined to a subpopulation of precursor cells in an otherwise wild-type individual, and manifests as a stripe or patch of affected skin. In the second mechanism, an independent somatic mutation arises in a subpopulation of precursor cells in an individual already carrying a germline heterozygous mutation, generating areas of more severely affected skin.9 In recessive disorders, somatic mosaicism can also occur via a 2-hit mechanism; for example, an inherited heterozygous loss-of-function mutation may be paired with a somatic mutation of the remaining wild-type allele, generating segmental disease.
In LP, porokeratotic plaques are distributed in a Blaschko-linear pattern corresponding to the dorsoventral migration path of keratinocyte precursors during embryogenesis, suggesting an underlying postzygotic somatic mutation.10 While it has been speculated that LP may result from second-hit postzygotic mutation,9 no genetic evidence has proven this hypothesis to our knowledge. To identify the genetic characteristics associated with LP, we used paired whole-exome sequencing (WES) of affected skin and blood or saliva in 3 cases.
Methods
The study was approved by the Yale Human Investigation Committee and complied with the Declaration of Helsinki principles. Participants were referred by dermatologists with a suspected diagnosis of LP. Individual consent or parental permission was obtained in writing for each case. Genomic DNA from lesional skin was obtained from fresh full-thickness skin biopsies or 1-mm cores from affected epidermis of formalin-fixed paraffin-embedded specimens, using the DNeasy Micro Kit (Qiagen) with added deparaffinization performed for formalin-fixed paraffin-embedded tissue. Genomic DNA from blood was isolated via a standard phenol-chloroform protocol, and genomic DNA from saliva was isolated using the Saliva DNA Isolation Reagent Kit (Norgen).
Whole-Exome Sequencing
Genomic DNA of blood or saliva and affected tissue from all 3 participants, as well as the DNA from participant 3’s mother, were sheared and bar coded. Whole-exome capture was performed (IDT xGen Exome Research Panel V1.0) by the Yale Center for Genome Analysis. Illumina HiSeq4000 instruments were used for high-throughput sequencing, with blood samples pooled at 16 per lane, and tissue samples pooled at 4 per lane with 100-bp paired-end reads. Resulting reads were aligned to the human reference genome (University of California Santa Cruz Genome browser, hg19) using the Burrows-Wheeler Aligner (BWA-MEM).11 Reads were then trimmed, and polymerase chain reaction (PCR) duplicates were removed using Picard (Broad Institute). Resulting BAM files were calibrated using the Genome Analysis Toolkit (Broad Institute).12 Germline single-nucleotide variants (SNVs) and insertions/deletions were identified using HaplotypeCaller,13 and somatic SNVs and insertions/deletions were identified using Mutect2.14 A Fisher exact test was also used to compare mutant and wild-type read numbers in tissue and blood with P = .001 used as a cutoff for somatic variants. Subsequently, all variants were annotated for functional impact with AnnoVar.15 The resultant SNVs and insertions/deletions were filtered in Microsoft Excel to exclude those with a somatic mutant allele frequency of 5% or less, a 1 or more mutant read in the blood sample, or a prevalence of greater than 1% in the exome Aggregation Consortium repository. Aligned reads were then examined with the Broad Institute Integrative Genomics Viewer to exclude variants resulting from miscalls and alignment error.16
Copy Number Variation and Loss of Heterozygosity
Loss of heterozygosity (LOH) data was plotted by dividing the number of B-allele (nonreference) reads by the total number of reads, independently for both tissue and blood at each SNV position using Python and R scripts. The difference in B-allele frequency values between tissue and blood was plotted against the genomic location. For participant 1, copy number variations were evaluated from whole-exome data using CoNIFER.17 In addition, we used quantitative real-time PCR to quantify genomic PMVK copy number using the SYBR Green master mix (Thermo Fisher Scientific) on a 7500 Real Time PCR system (Applied Biosystems). Quantitative PCR was performed in triplicate, with ACTB as a control. Relative difference in the copy number of PMVK in affected skin compared to blood was determined using the relative standard curve method. The fold change of the copy number of PMVK in affected skin was compared with blood and normalized to ACTB using relative quantification.
Mutation Verification
Verification of mutations identified by exome sequencing of affected tissue and blood was performed via PCR using Kapa 2G Fast polymerase (Kapa Biosystems) followed by Sanger sequencing. For participant 3, PCR was used to amplify MVD in DNA isolated from skin and blood, and digestion with the restriction endonuclease HpyAV (NEB Biolabs) was used to test for the MVD c.811_815del restriction fragment length polymorphism (RFLP).
Splice-Site Mutation Analysis
Total RNA was isolated from wild-type and mutated MVD human keratinocyte cell lines with DNase on column digestion (Allprep, Qiagen) and subjected to reverse transcriptase (RT)-PCR (SuperScript III RT, Invitrogen), followed by PCR with MVD exon–specific primers (eTable 1 in the Supplement). Products were visualized via agarose gel electrophoresis, and Sanger sequencing was performed. To analyze the expression levels of the wild-type MVD isoform in the mutated keratinocytes, quantitative RT-PCR was performed and compared with ACTB as an expression control. Results are expressed as a relative quantification.
Results
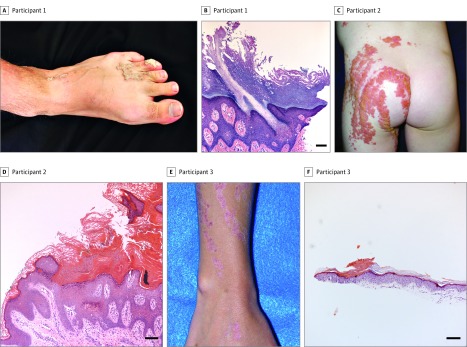
Of the 3 participants, 2 were male. Participant ages ranged from 5 to 20 years old. Participant 1 was a 20-year-old man presenting with whorls of linear pink verrucous papules and plaques on his upper extremities and left lower extremity that appeared at birth and became thicker over time (Figure 1A). Participant 2, a 5-year-old girl, presented with slightly pruritic extensive whorled-linear scaly pink plaques over the left side of her body which had been present since birth (Figure 1C). Participant 3 was a 13-year-old boy who had asymptomatic linear erythematous depressed plaques with a keratotic collar over his right upper and lower extremities, as well as central longitudinal dystrophy of the right first toenail (Figure 1E). His mother had bilateral photodistributed small, thin depressed pink papules with a collarette of scale. In all participants, a cornoid lamella was evident on histopathologic examination of affected tissue, which varied in width (Figure 1B, D, and F).
Figure 1. Clinical and Histologic Features of Linear Porokeratosis.
A, Participant 1 presented with whorls of pink verrucous papules and plaques. B, Histopathologic examination of affected skin revealed striking columns of parakeratosis overlying hypogranulosis and dyskeratotic cells (corresponding to cornoid lamellae) alternating with hyperorthokeratosis, acanthosis, and papillomatosis. C, Participant 2 presented with extensive whorls of pink plaques, some covered with yellowish crusts, clinically thought to be inflammatory linear verrucous epidermal nevus. D, Histopathologic evaluation showed alternate parakeratosis overlying hypogranulosis (corresponding to wide cornoid lamellae) and orthokeratosis, acanthosis, and papillomatosis with perivascular and interstitial lymphocytic infiltrate in the superficial dermis. E, Participant 3 had linear erythematous depressed plaques with prominent keratotic collar at lesional borders. F, Histologic examination of affected skin showed subtle columns of parakeratosis overlying hypogranulosis alternating with orthokeratosis and mild acanthosis. Histologic slides stained with hematoxylin-eosin. Scale bar = 100 μm.
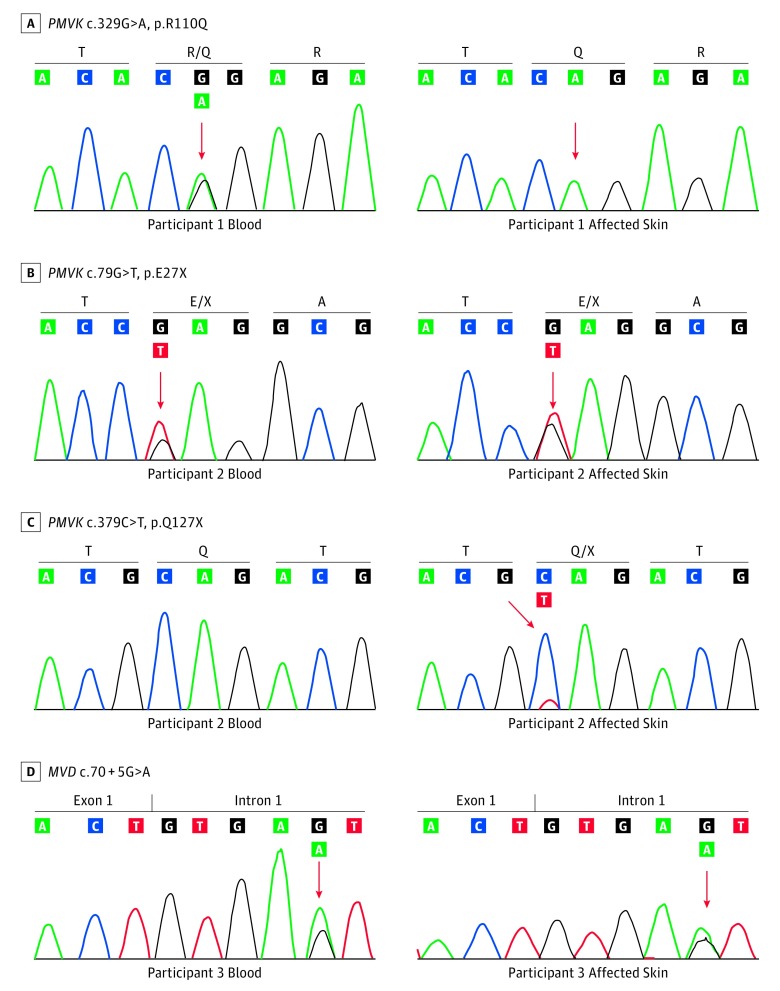
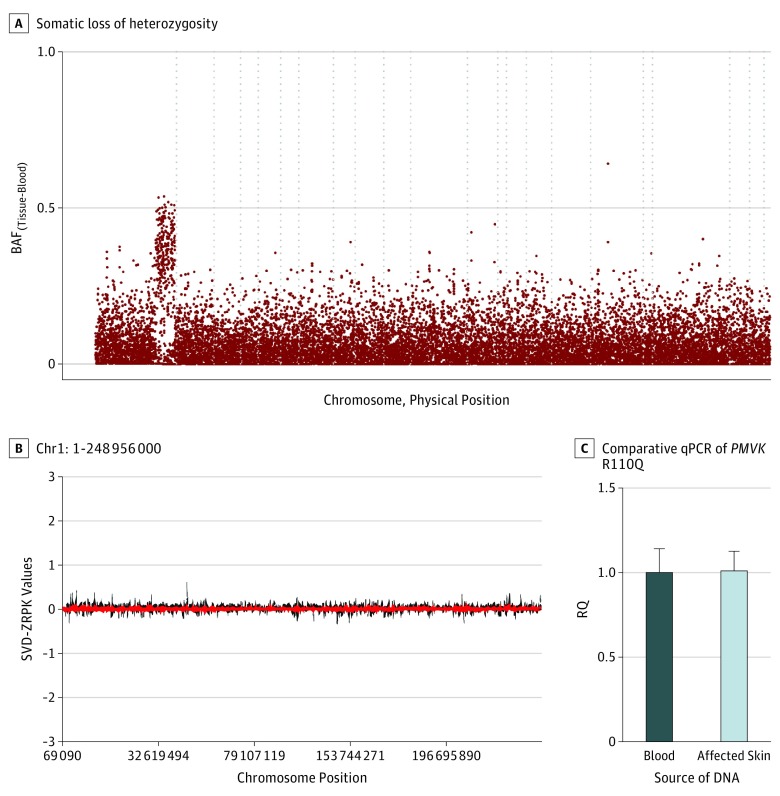
Following paired WES, germline and somatic variants were identified (Table; eTable 2 in the Supplement).14 Candidate mutations were confirmed via Sanger sequencing or RFLP analysis. Participant 1 had a germline-heterozygous PMVK c.329G>A, p.R110Q mutation in the blood as well as in the lesional skin. A higher mutant allele fraction in the skin suggested somatic LOH (Figure 2A), and this was confirmed by plotting B-allele frequency across the genome to demonstrate a large segment of LOH on chromosome 1q extending from 14.66 megabases to the telomere at 24.86 megabases (Figure 3A). Further analysis of copy number variation including comparative quantitative PCR of the target region confirmed copy-neutral LOH (Figure 3B and C). The PMVK gene catalyzes the reaction of mevalonate 5-phosphate with ATP to form mevalonate 5-diphosphate and ADP (Figure 4). The arginine at position 110, which is conserved in 76 vertebrate species, is located within the acceptor substrate binding region and is assumed to contribute to the neutralization of the negatively charged pentacoordinate phosphate reaction intermediate.18 Mutagenesis of this residue was found to diminish the specific activity of the enzyme by over 10 000-fold,19 suggesting that the p.R110Q mutation in participant 1 reduced PMVK enzymatic activity.
Table. Second-Hit Postzygotic PMVK and MVD Mutations in Linear Porokeratosis.
| Patient | Germline Mutation | Reads in Blood, No. | Reads in Tissue, No. | Somatic Mutation | Reads in Blood, No. | Reads in Tissue, No. | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ref | Non-Ref | Ref | Non-Ref | Ref | Non-Ref | Ref | Non-Ref | |||
| 1 | PMVK c.329G>A, p.R110Q | 21 | 15 | 16 | 61 | CN-LOH Chr 1:146-248Mba | NA | NA | NA | NA |
| 2 | PMVK c.79G>T, p.E27X | 77 | 63 | 86 | 88 | PMVK c.379C>T, p.Q127X | 113 | 0 | 119 | 34 |
| 3 | MVD c.70 + 5G>A | 18 | 8 | 45 | 44 | MVD c.811_815del, p.F271A fs*33 | 68 | 0 | 83 | 10 |
Abbreviations: Chr, chromosome; CN-LOH, copy-neutral loss of heterozygosity; Mb, megabases; NA, not applicable; Ref, reference.
PMVK spans Chr 1:154 897 208-154 909 484.
Figure 2. Second-Hit Somatic Mosaicism for PMVK and MVD Mutations Associated With Linear Porokeratosis.
Germline and somatic mutations were identified via whole-exome sequencing of DNA isolated from tissue and blood/saliva of all 3 participants. Sanger sequencing was used to confirm mutations found in exome sequencing data. A, In participant 1 there was a germline heterozygous PMVK p.R110Q mutation. This mutation was enriched in affected skin due to loss of heterozygosity. B, In participant 2 there was a germline heterozygous PMVK p.E27X mutation, as observed in blood and affected skin. C, In participant 2 there was also a somatic PMVK p.Q127X mutation in the affected skin leading to a compound heterozygous state. D, In participant 3 there was a germline heterozygous MVD c.70 + 5G>A mutation, as observed in the blood and affected skin. The same mutation was found in his mother (data not shown).
Figure 3. Copy Neutral Loss of Heterozygosity Is Evident in Affected Tissue From Participant 1.
Abbreviations: BAF, B-allele frequency; RQ, relative quantification; SVD, singular value decomposition; ZRPK, z-scores of reads per kilobase. A, B-allele frequency differences between the affected tissue and blood are plotted across the genome for participant 1 by chromosome and physical position. Dashed vertical lines separate individual chromosomes. Somatic loss of heterozygosity on chromosome 1q of participant 1 extends from 14.66 megabases to the telomere, and contains the PMVK gene. B, The CoNIFER program was used to detect copy number variations on chromosome 1. Singular value decomposition of standardized z-scores of reads per thousand bases, per million reads is plotted against positions on chromosome 1 and shows no evidence for copy number variation. C, This is further corroborated by comparative qPCR assessing the PMVK R110Q location. The fold change of the copy number of genomic PMVK from affected skin is calculated using relative quantification (RQ): RQ, 1.01 (range, 0.91-1.13) for affected skin when the blood of participant 1 was used as the reference sample.
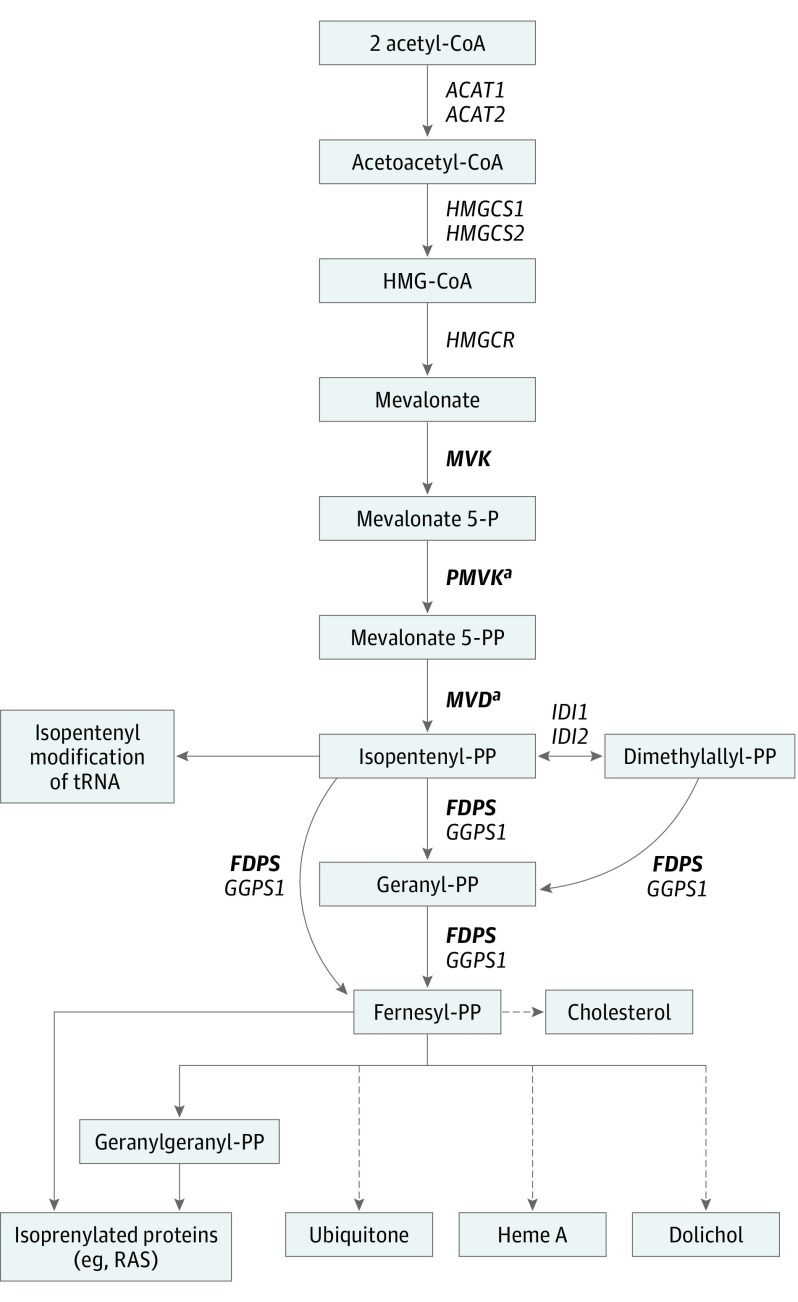
Figure 4. The Mevalonate Pathway.
The mevalonate pathway is an essential metabolic pathway that uses acetyl–Coenzyme A (CoA) to produce sterols and isoprenoid metabolites that are essential for a broad range of metabolic processes. Genes previously found to be involved in familial porokeratosis are in bold. Dashed arrows indicate multiple processes. tRNA refers to transfer RNA.
aGenes in which second-hit postzygotic mutations were identified.
Participant 2 had germline heterozygous PMVK c.79G>T p.E27X and somatic PMVK c.379C>T, p. Q127X mutations (Figure 2B and C), previously suggested to attenuate PMVK enzyme activity and ATP-binding capacity, respectively.18,19 There was no evidence for somatic LOH (eFigure 1 in the Supplement). A recent study of a PMVK p.P138X nonsense mutation found reduced solubility and disrupted cellular localization of the enzyme in transfected HaCaT cells, as well as abnormal apoptosis and differentiation of keratinocytes in the lesional skin of affected individuals.7
Participant 3 and his mother shared a novel germline heterozygous MVD c.70 + 5G>A mutation (Figure 2D). In silico analysis predicted this variant to alter splicing with a dbscSNV11 score of 0.99.20 To assess the consequence of this mutation on MVD splicing, reverse transcription and amplification of a portion of MVD RNA spanning exons 1 through 5 from both wild-type and heterozygous MVD c.70 + 5G>A human keratinocyte cell lines was performed. In both cell lines, only a wild-type splice product was identified, suggesting that the aberrant splice variant undergoes degradation. Indeed, quantitative RT-PCR demonstrated that the expression of the wild-type allele was largely reduced in mutated keratinocytes compared with normal human keratinocytes (eFigure 2 in the Supplement). The affected skin of participant 3 harbored somatic MVD c.811_815del, p.F271Afs*33 frameshift with no evidence for LOH (eFigure 1, eFigure 3 in the Supplement). Germline MVD frameshift heterozygous deletions distal to this mutation have been previously reported to be associated with DSAP.6
Discussion
Our findings suggest that LP is a segmental mosaic disorder due to second-hit postzygotic mutations in genes encoding enzymes within the mevalonate biosynthesis pathway. All participants had germline heterozygous PMVK or MVD pathogenic mutations with a somatic second mutation or allelic loss, which clinically manifested as segmentally distributed skin lesions on the background of normal-appearing skin. While germline heterozygous PMVK and MVD mutations are known to cause generalized forms of porokeratosis,6 lesions appear later in life, presumably via a 2-hit mechanism. The more severe presentation of our LP cases is associated with segmental loss of function in these genes, and it is expected that affected individuals will develop generalized DSAP lesions in sun-exposed skin over time. As has been observed in heritable generalized porokeratosis,6 the participants with LP in the present study with PMVK mutations had large and thickened plaques, while the participant with MVD mutations had thinner plaques (Figure 1).
Importantly, although we considered participant 2 to have LP based on her histologic evaluation, her clinical appearance was not typical of porokeratosis (keratotic edge and atrophic center), but rather of inflammatory linear verrucous epidermal nevus (ILVEN).21 Cornoid lamellae are not pathognomonic to porokeratosis, as these can be found in a range of inflammatory, hyperplastic, and neoplastic skin conditions.2 Indeed, coronoid lamellae have been found in ILVEN and in 1.2% of 167 epidermal nevi in studies that did not include genetic evaluation.22,23 The present case suggests that a fraction of individuals thought to have ILVEN may, in fact, have LP, and emphasizes the utility of histopathologic evaluation of Blaschkoid lesions. If pathogenesis-based therapy for porokeratosis is developed based on targeting the mevalonate pathway, genetic analysis should also be considered.
While our findings show that recessive, loss-of-function mutations in PMVK and MVD were associated with LP, the mechanism by which solitary lesions arise in heritable, nonmosaic forms of porokeratosis remains unclear. Prior reports using allelic expression imbalance assays showed decreased wild-type allele DNA and RNA expression in lesions from participants with MVK and MVD heterozygous mutations, but no copy-number variation or methylation of the promotor region was observed.6 In a single lesion of the participant with heterozygous MVK mutation, RNA editing of the wild-type allele was found,6 and in another study, no second somatic mutations were discovered via direct sequencing of MVK in microdissected lesional keratinocytes from patients with DSAP bearing heterozygous MVK mutations.4 While admixture with wild-type cells could contribute, our data suggest that further investigation using a broader array of screening methodologies will reveal loss-of-function mutations affecting the mevalonate kinase pathway.
In both LP and DSAP, a germline heterozygous mutation in mevalonate pathway genes is present, but while LP is congenital or recognized during childhood, DSAP lesions develop during adulthood. The timing of second-hit somatic mutation could explain the difference in skin lesion distribution and onset. Since in LP lesions the second-hit somatic mutation in mevalonate pathway genes appears in utero, skin lesions are congenital and segmentally distributed, deriving from a clonal expansion of mutated keratinocyte progenitor cells that migrate during embryogenesis along the lines of Blaschko. In DSAP, however, it is suggested that since skin lesions are photodistributed and appear in adulthood, second-hit somatic mutations leading to loss of function in mevalonate pathway genes may occur over time due to UV-induced DNA damage.
In the mevalonate pathway, MVK, PMVK, and MVD catalyze 3 rate-limiting steps sequentially to produce isopentenyl diphosphate, a fundamental building block for the biosynthesis of isoprenoid compounds such as sterols, including cholesterol, ubiquinone, dolichols, carotenoids, and some classes of isoprenylated proteins (Figure 4).18 The mevalonate pathway’s products are essential for the regulation of gene expression, cell growth and differentiation, cytoskeleton assembly, and posttranslational modification of proteins involved in intracellular signaling.24 Cholesterol is 1 of the components of the extracellular lipid matrix in the stratum corneum, playing an essential role in providing and maintaining the skin barrier. Depletion of cholesterol has been reported to result in increased sensitivity of keratinocytes to stimuli driving apoptosis.25 Premature apoptosis and dysregulated differentiation of keratinocytes have been identified in several types of porokeratosis.26 Increased apoptosis beneath and at the cornoid lamella has been found in PMVK-mutated individuals with disseminated superficial porokeratosis,7 and human keratinocytes overexpressing MVK were more resilient to UV-A radiation compared with MVK-depleted cells.4 Although the link between increased keratinocyte apoptosis and disrupted keratinocyte differentiation seen in lesions of porokeratosis4,7,26 and anomalies in isoprenoid metabolism is incompletely understood, our findings provide further evidence that the mevalonate pathway may be a potential target for therapeutic intervention in LP and DSAP.
Limitations
For the present study, we were able to enroll a limited cohort of 3 participants with linear porokeratosis in which we could perform genetic analysis.
Conclusions
The present findings indicate that LP is associated with the combination of germline and second-hit postzygotic mutations in the genes encoding enzymes within the mevalonate pathway, and suggest that the mevalonate pathway may be a potential target for therapeutic interventions in porokeratosis.
eTable 1. Primers List
eTable 2. Exome Sequencing Statistics
eFigure 1. There is no evidence for somatic LOH in participants 2 and 3. B- allele frequency differences between affected tissue and blood are plotted across the genome for participant 2 (a) and participant 3 (c) by chromosome and physical position. Dashed vertical lines separate individual chromosomes.
eFigure 2. Expression of wild type MVD transcript is significantly reduced in human keratinocytes with heterozygous MVD c.70 + 5G>A mutation suggesting that MVD c.70 + 5G>A transcript undergoes degradation. Comparative quantitative RT-PCR was employed to assess the expression of wild-type MVD transcript in heterozygous MVD c.70 + 5G>A mutated human keratinocytes. Wild-type human keratinocytes were used as a reference sample. The fold change in the expression level of wild-type MVD transcript from mutated keratinocytes is calculated using relative quantification (RQ). RQ = 0.25; P < .001.
eFigure 3. Restriction fragment length polymorphism (RFLP) analysis confirms MVD c.811_815del p.F2.71fs in affected skin of participant 3. Agarose gel electrophoresis of digested and undigested PCR amplicons of affected tissue (473 bp) and normal (478 bp)of participant 3. Digestion with HpyAV of mutated amplicon showing specific band of 254 bp length that is absent in normal tissue.
References
- 1.Chernosky ME. Porokeratosis. Arch Dermatol. 1986;122(8):869-870. doi: 10.1001/archderm.1986.01660200041009 [DOI] [PubMed] [Google Scholar]
- 2.Biswas A. Cornoid lamellation revisited: apropos of porokeratosis with emphasis on unusual clinicopathological variants. Am J Dermatopathol. 2015;37(2):145-155. doi: 10.1097/DAD.0000000000000039 [DOI] [PubMed] [Google Scholar]
- 3.Sertznig P, von Felbert V, Megahed M. Porokeratosis: present concepts. J Eur Acad Dermatol Venereol. 2012;26(4):404-412. doi: 10.1111/j.1468-3083.2011.04275.x [DOI] [PubMed] [Google Scholar]
- 4.Zhang S-Q, Jiang T, Li M, et al. Exome sequencing identifies MVK mutations in disseminated superficial actinic porokeratosis. Nat Genet. 2012;44(10):1156-1160. doi: 10.1038/ng.2409 [DOI] [PubMed] [Google Scholar]
- 5.Cui H, Li L, Wang W, et al. Exome sequencing identifies SLC17A9 pathogenic gene in two Chinese pedigrees with disseminated superficial actinic porokeratosis. J Med Genet. 2014;51(10):699-704. doi: 10.1136/jmedgenet-2014-102486 [DOI] [PubMed] [Google Scholar]
- 6.Zhang Z, Li C, Wu F, et al. Genomic variations of the mevalonate pathway in porokeratosis. Elife. 2015;4:e06322. doi: 10.7554/eLife.06322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J, Liu Y, Liu F, et al. Loss-of-function mutation in PMVK causes autosomal dominant disseminated superficial porokeratosis. Sci Rep. 2016;6:24226. doi: 10.1038/srep24226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strachan T, Read A. Human Molecular Genetics. 4th ed New York, NY: Garland Science; 2010. [Google Scholar]
- 9.Happle R. The categories of cutaneous mosaicism: a proposed classification. Am J Med Genet A. 2016;170A(2):452-459. doi: 10.1002/ajmg.a.37439 [DOI] [PubMed] [Google Scholar]
- 10.Moss C, Larkins S, Stacey M, Blight A, Farndon PA, Davison EV. Epidermal mosaicism and Blaschko’s lines. J Med Genet. 1993;30(9):752-755. doi: 10.1136/jmg.30.9.752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li H, Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics. 2010;26(5):589-595. doi: 10.1093/bioinformatics/btp698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van der Auwera GA, Carneiro MO, Hartl C, et al. From FastQ data to high confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Curr Protoc Bioinformatics. 2013;43(11):1-33. doi: 10.1002/0471250953.bi1110s43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poplin R, Ruano-Rubio V, DePristo MA, et al. Scaling accurate genetic variant discovery to tens of thousands of samples. bioRxiv. 2017;201178(November). doi: 10.1101/201178 [DOI] [Google Scholar]
- 14.Cibulskis K, Lawrence MS, Carter SL, et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol. 2013;31(3):213-219. doi: 10.1038/nbt.2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38(16):e164. doi: 10.1093/nar/gkq603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinson JT, Thorvaldsdóttir H, Winckler W, et al. Integrative genomics viewer. Nat Biotechnol. 2011;29(1):24-26. doi: 10.1038/nbt.1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krumm N, Sudmant PH, Ko A, et al. ; NHLBI Exome Sequencing Project . Copy number variation detection and genotyping from exome sequence data. Genome Res. 2012;22(8):1525-1532. doi: 10.1101/gr.138115.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang Q, Yan X-X, Gu S-Y, Liu J-F, Liang D-C. Crystal structure of human phosphomevalonate kinase at 1.8 A resolution. Proteins. 2008;73(1):254-258. doi: 10.1002/prot.22151 [DOI] [PubMed] [Google Scholar]
- 19.Herdendorf TJ, Miziorko HM. Functional evaluation of conserved basic residues in human phosphomevalonate kinase. Biochemistry. 2007;46(42):11780-11788. doi: 10.1021/bi701408t [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jian X, Boerwinkle E, Liu X. In silico prediction of splice-altering single nucleotide variants in the human genome. Nucleic Acids Res. 2014;42(22):13534-13544. doi: 10.1093/nar/gku1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morag C, Metzker A. Inflammatory linear verrucous epidermal nevus: report of seven new cases and review of the literature. Pediatr Dermatol. 1985;3(1):15-18. doi: 10.1111/j.1525-1470.1985.tb00479.x [DOI] [PubMed] [Google Scholar]
- 22.Su WP. Histopathologic varieties of epidermal nevus. A study of 160 cases. Am J Dermatopathol. 1982;4(2):161-170. doi: 10.1097/00000372-198204000-00011 [DOI] [PubMed] [Google Scholar]
- 23.Tiwary A, Mishra D. A unique porokeratotic variant of inflammatory linear verrucous epidermal nevus. Indian J Paediatr Dermatol. 2017;18(3):237-240. doi: 10.4103/2319-7250.206088 [DOI] [Google Scholar]
- 24.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343(6257):425-430. doi: 10.1038/343425a0 [DOI] [PubMed] [Google Scholar]
- 25.Calay D, Vind-Kezunovic D, Frankart A, Lambert S, Poumay Y, Gniadecki R. Inhibition of Akt signaling by exclusion from lipid rafts in normal and transformed epidermal keratinocytes. J Invest Dermatol. 2010;130(4):1136-1145. doi: 10.1038/jid.2009.415 [DOI] [PubMed] [Google Scholar]
- 26.Shen C-S, Tabata K, Matsuki M, Goto T, Yokochi T, Yamanishi K. Premature apoptosis of keratinocytes and the dysregulation of keratinization in porokeratosis. Br J Dermatol. 2002;147(3):498-502. doi: 10.1046/j.1365-2133.2002.04853.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Primers List
eTable 2. Exome Sequencing Statistics
eFigure 1. There is no evidence for somatic LOH in participants 2 and 3. B- allele frequency differences between affected tissue and blood are plotted across the genome for participant 2 (a) and participant 3 (c) by chromosome and physical position. Dashed vertical lines separate individual chromosomes.
eFigure 2. Expression of wild type MVD transcript is significantly reduced in human keratinocytes with heterozygous MVD c.70 + 5G>A mutation suggesting that MVD c.70 + 5G>A transcript undergoes degradation. Comparative quantitative RT-PCR was employed to assess the expression of wild-type MVD transcript in heterozygous MVD c.70 + 5G>A mutated human keratinocytes. Wild-type human keratinocytes were used as a reference sample. The fold change in the expression level of wild-type MVD transcript from mutated keratinocytes is calculated using relative quantification (RQ). RQ = 0.25; P < .001.
eFigure 3. Restriction fragment length polymorphism (RFLP) analysis confirms MVD c.811_815del p.F2.71fs in affected skin of participant 3. Agarose gel electrophoresis of digested and undigested PCR amplicons of affected tissue (473 bp) and normal (478 bp)of participant 3. Digestion with HpyAV of mutated amplicon showing specific band of 254 bp length that is absent in normal tissue.