Key Points
Question
Is low-dose, low-frequency oral psoralen–UV-A effective, and does maintenance therapy extend disease-free remission in patients with early-stage mycosis fungoides?
Findings
In this randomized clinical trial of 27 patients with mycosis fungoides, 70% of patients had complete response after 12 to 24 weeks of psoralen–UV-A treatment, maintenance therapy prolonged the median disease-free remission, and certain inflammatory mediators of the serum were associated with clinical outcome. High density of histologic infiltrate and percentage of clonal T-cell receptor sequences in skin were inversely associated with complete response.
Meaning
Low-dose, low-frequency oral psoralen–UV-A treatment, followed by maintenance, appeared to be safe and effective and can be used to treat early-stage mycosis fungoides; potential biomarkers for therapeutic response to PUVA in mycosis fungoides were identified.
Abstract
Importance
Psoralen–UV-A (PUVA) photochemotherapy is standard first-line treatment for skin-limited, early-stage mycosis fungoides capable of producing high initial complete response (CR) rates. However, much remains unknown about PUVA’s therapeutic mechanisms, optimal duration and frequency of treatment, dose escalation, or use as maintenance therapy.
Objectives
To evaluate low-dose, low-frequency PUVA, and whether maintenance treatment extends disease-free remission in patients with mycosis fungoides.
Design, Setting, and Participants
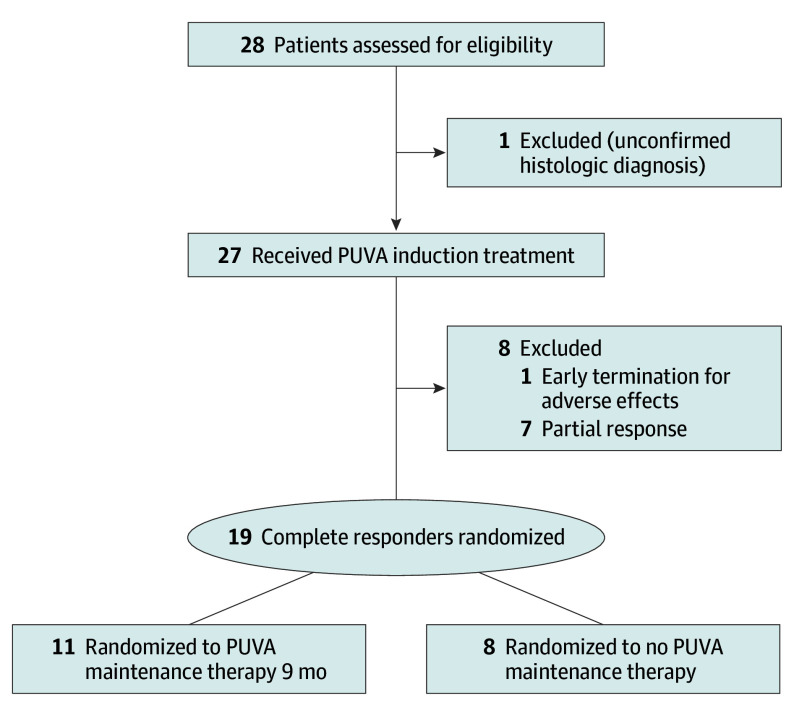
This prospective randomized clinical trial with defined PUVA dosing regimen was carried out in 5 centers (Graz, Vienna, Hietzing, Innsbruck, and Salzburg) across Austria. Patients with stage IA to IIA mycosis fungoides (n = 27) were enrolled in the study beginning March 13, 2013, with the last patient enrolled March 21, 2016. These patients were treated with oral 8-methoxypsoralen followed by UV-A exposure 2 times per week for 12 to 24 weeks until CR. Patients with CR were randomized to PUVA maintenance for 9 months (14 total exposures) or no maintenance. The study was conducted from April 27, 2012, to July 27, 2018. Data analysis of the primary end point was of the intention-to-treat population, and the secondary end point analysis was of the evaluable population.
Main Outcomes and Measures
Efficacy of the PUVA regimen was determined by the rate of CR as defined by a modified severity-weighted assessment tool (mSWAT) score reduction to 0. Levels of proinflammatory molecules in serum and histologic features and percentage of clonal T cells in skin were assessed to search for biomarkers of clinical response.
Results
In 27 patients with mycosis fungoides, 19 (70%) were male with mean (range) age 61 (30-80) years. At baseline, patients with CR had a mean (range) mSWAT score of 18.6 (1-66) compared with 16.8 (3-46) in patients with partial response. The 12- to 24-week PUVA induction regimen reduced the mSWAT score in all patients and led to CR in 19 (70%) of 27 patients and a low mean cumulative UV-A dose of 78.5 J/cm2. The subsequent standardized 9-month PUVA maintenance phase prolonged median (range) disease-free remission from 4 (1-20) months to 15 (1-54) months (P = .02). High density of histologic infiltrate and high percentage of clonal TCR sequences in skin biopsy specimens at baseline were inversely associated with therapeutic response. No severe adverse effects were seen during the PUVA induction or maintenance phase.
Conclusions and Relevance
This proof-of-concept study identifies potential biomarkers for therapeutic response to PUVA in mycosis fungoides; it also demonstrates that low-dose, low-frequency PUVA appears to be highly effective, and maintenance treatment may extend disease-free remission.
Trial Registration
ClinicalTrials.gov identifier: NCT01686594
This randomized clinical trial examines whether the use of psoralen–UV-A with maintenance therapy can prolong clinical response and disease remission in patients with early-stage mycosis fungoides.
Introduction
Mycosis fungoides (MF) is the most common type of cutaneous T-cell lymphoma, accounting for about two-thirds of all cutaneous T-cell lymphomas and almost 50% of all primary cutaneous lymphomas.1 It is characterized by the progressive evolution of erythematous patches, plaques, and tumors over years, eventually leading to metastasis and death.
In early MF disease stages, malignant T cells represent a minority of infiltrating T cells within the inflammatory cellular environment.2 Lesional skin is characterized by the presence of small- to medium-sized T cells displaying the memory immunophenotype of CD3+, CD4+, and CD45RO+ and, in most cases, clonal rearrangement of the T-cell receptor (TCR) gene.3,4 High-throughput TCR sequencing studies have shown that neoplastic cells arise from mature T lymphocytes and have helped to identify malignant clones, thereby facilitating their distinction from benign T-cell inflammatory diseases.5 T cells that are isolated from MF lesions express CCR4 and the skin homing receptor CLA but lack CCR7/L-selectin and the differentiation marker CD27, a phenotype suggestive of skin-resident effector memory T cells.6 Reports on the role of regulatory T-cells (Tregs) in MF are conflicting; in some cases, MF malignant clones share features with Tregs.7 However, a Treg phenotype in malignant clones does not necessarily involve suppressive functionality.8
Early-stage MF is initially treated with skin-directed therapies that may include corticosteroids, chemotherapy, bexarotene, radiotherapy, and UV-B and psoralen–UV-A (PUVA) phototherapy.9,10,11 More aggressive treatments are reserved for patients whose MF is more advanced or does not respond to skin-directed therapies.9,12 Introduced in 1976,13 PUVA is one of the most effective treatments for MF and is the standard first-line treatment for skin-limited MF (stages IA to IIA), with a complete response (CR) rate of 65% to 85%, depending on staging.14 However, little is known about PUVA’s therapeutic mechanisms or optimal dosing, duration and frequency of treatment (2, 3, or 4 times weekly), dose escalation, and maintenance therapy.12,15,16,17,18,19,20,21,22,23 Whether PUVA maintenance therapy can prolong clinical response in MF on initial complete clearance is controversial.24,25 An online survey of 30 members of the International Society for Cutaneous Lymphoma revealed that 88% of respondents used some sort of PUVA maintenance therapy at frequencies ranging from weekly to monthly.15 Despite this finding, the European Organisation for Research and Treatment of Cancer issued the recommendation to avoid maintenance because of unproven efficacy and safety concerns.9
We carried out a prospective randomized clinical trial with low-dose, low-frequency oral PUVA with or without maintenance for patients with stage IA to IIA MF to evaluate its effectiveness and determine if maintenance extends disease-free remission. During this study, we also analyzed skin and blood samples to identify potential biomarkers for treatment response.
Methods
Study Design and Setup
The main aims of this randomized clinical trial were to determine the clinical response of patients with MF to oral PUVA (2 times/wk; start dose depending on minimal phototoxic dose [MPD] testing; weekly dose increments) and to investigate whether a maintenance regimen would prolong disease-free remission after CR. This study was carried out in accordance with the International Conference on Harmonization Good Clinical Practice guideline.26 It received approval from the ethics committee of the Medical University of Graz and was in full compliance with the Austrian Medicinal Products Act. All participants gave written informed consent in accordance with the principles of the Declaration of Helsinki.27 The trial protocol is provided in Supplement 1.
During the PUVA induction phase, treatment was administered for 12 to 24 weeks until a modified severity-weighted assessment tool (mSWAT, a quantification of the body surface area of each lesion multiplied by a weighting factor for lesion type)28 score was reduced to 0 and (whenever consented by the patient) confirmed by biopsy. Patients with CR were randomly assigned to receive either PUVA maintenance therapy for 9 months (arm A) or no maintenance (arm B) (Figure 1). Patients with partial response (PR) were not randomized to either arm but were switched instead to an alternative therapeutic regimen. Randomized patients were evaluated (as long as relapse had not occurred) at 4, 12, 24, and 36 weeks after randomization and thereafter 4 times a year (first year), twice a year (second year), and then once a year (third to fifth year) (eFigure 1 in Supplement 2). Relapse was defined as an mSWAT score of 1 or higher. When the clinical presentation was ambiguous, a skin biopsy specimen was taken for histologic examination.
Figure 1. Patient Flowchart.
PUVA indicates psoralen–UV-A.
Details on patient inclusion and exclusion criteria, MPD determination, PUVA treatment, tissue sample collection and processing, histologic assessment, immunobead assay, quantitative polymerase chain reaction, flow cytometry, and CD3/CD28 stimulation are provided in eMethods in Supplement 2. The study was conducted from April 27, 2012, to July 27, 2018.
Statistical Analysis
Data analysis of the primary end point was of the intention-to-treat population, and the secondary end point analysis was of the evaluable population. The primary end point was the median time to relapse in patients with CR randomized to PUVA maintenance or no maintenance. A sample size of 82 (including a dropout rate of 10%) was calculated using nQuery Advisor, version 4.0 (Statsols), assuming α = .05 and power = 0.8 for an effect size of 12-month prolongation of the disease-free interval after CR. An accrual time of 24 months was assumed. Minimum follow-up was set to 12 months and the maximum follow-up to 60 months after CR and PUVA treatment completion. Secondary end points included the expression of Treg-related molecules in lesional tissue, the proliferative capacity of blood circulating T cells and serum cytokine levels to identify biomarkers that are associated with the response to PUVA treatment.
Kaplan-Meier analysis and log-rank tests were performed to determine disease-free survival. Unpaired or paired, 2-tailed t test, Mann-Whitney test, and χ2 or Fisher exact test, whichever was appropriate, was used to analyze certain clinical, histologic, and biomarker data. Hierarchical cluster analysis was conducted to determine the association coefficient for chemotactic mediator levels. The threshold for statistical significance was set to P < .05.
Results
Patients
Twenty-seven patients were enrolled in the study at the centers in Graz, Vienna, Hietzing, Innsbruck, or Salzburg, Austria, beginning March 13, 2013, and the last patient was enrolled on March 21, 2016 (Figure 1). Enrollment was closed in August 2016 because of low accrual (after extension of the accrual period from 24 to 40 months), and the study was terminated in July 2018, when the last patient recruited had completed the minimum of 12 months of follow-up according to the trial protocol. Of the 27 patients, 19 (70%) were male and the mean (range) age was 61 (30-80) years. Baseline demographic and diagnostic details are listed in the Table.
Table. Patient Characteristics and Outcome.
| Variable | No. (%) |
|---|---|
| All patients | 27 |
| Age, mean (range), y | 61 (30-80) |
| Sex | |
| Male | 19 (70) |
| Female | 8 (30) |
| Lesion type | |
| Patch | 27 (100) |
| Plaque | 9 (33) |
| Stage | |
| IA | 13 (48) |
| IB | 13 (48) |
| IIA | 1 (4) |
| Previous treatments | |
| Narrow band (311 nm) UV-B | 6 (22) |
| PUVA | 4 (15) |
| Narrow band (311 nm) UV-B/PUVA | 4 (15) |
| Clinical response, rate (%) | |
| Complete response | 19 (70) |
| Partial response | 8 (30) |
| Initial mSWAT score, mean (range) | |
| Complete response | 18.6 (1-66) |
| Partial response | 16.8 (3-46) |
| Clonality (HT-TCR sequencing), rate (%)a | |
| Patients with complete response (n = 17) | 11 (65) |
| Patients with partial response (n = 4) | 4 (100) |
| Patients in remission (12 mo after CR), rate (%) | |
| Arm A (PUVA maintenance) (n = 11) | 7 (64) |
| Arm B (no maintenance) (n = 8) | 1 (13) |
| Disease-free remission after randomization, median (range), mo | |
| Arm A (PUVA maintenance) | 15 (1-54) |
| Arm B (no maintenance) | 4 (1-20) |
Abbreviations: HT-TCR, high-throughput T-cell receptor; mSWAT, modified severity-weighted assessment tool; PUVA, psoralen–UV-A.
Only 21 patients were tested for clonality by HT-TCR sequencing.
Previous treatments included phototherapy in 14 patients (UV-B: 6 [22%]; PUVA: 4 [15%]; and PUVA/UV-B: 4 [15%]). The patients received PUVA treatment twice a week for 12 to 24 weeks, depending on clinical response (eFigure 1 in Supplement 2). The phototherapeutic characteristics of the 26 patients who completed the minimum 12 weeks of the PUVA induction phase are depicted in eTable 1 in Supplement 2.
Clinical Treatment Response
Nineteen patients (70%) had CR, and 8 patients (30%) had PR (Table), as defined by mSWAT score reduction of more than 50% (objective response rate of 100%). Patients received a maximum of 48 treatment sessions during the PUVA induction phase, with a similar cumulative mean dose of 78.5 J/cm2 for patients with CR and 94.4 J/cm2 for patients with PR. Photographs of treatment outcome and histologic associations are shown in Figure 2. One patient (4%) with PR (patient 20) had an early termination of participation (after 7 sessions) owing to recurring nausea and vomiting associated with 8-methoxypsoralen. No severe adverse effects were observed, but minor adverse effects occurred in 23 patients (85%), including nausea (7), vertigo (1), vomiting (1; patient switched to 5-methoxypsoralen), cephalea (1), burning and itch (2), urticaria (2), high serum creatinine level (1), and phototoxic erythema (12 during the induction phase, and 1 in both induction and maintenance phases). Three patients (11%; 2 CR and 1 PR) necessitated transient dose reduction owing to phototoxic effects, and 1 of the 3 received topical steroids for a week. Four other patients (15%; 3 CR and 1 PR) received topical steroids for 1 to 6 weeks for circumscribed, irritated skin lesions.
Figure 2. Clinical, Histologic, and Clonal Response of Patients With Psoralen–UV-A (PUVA) Treatment.
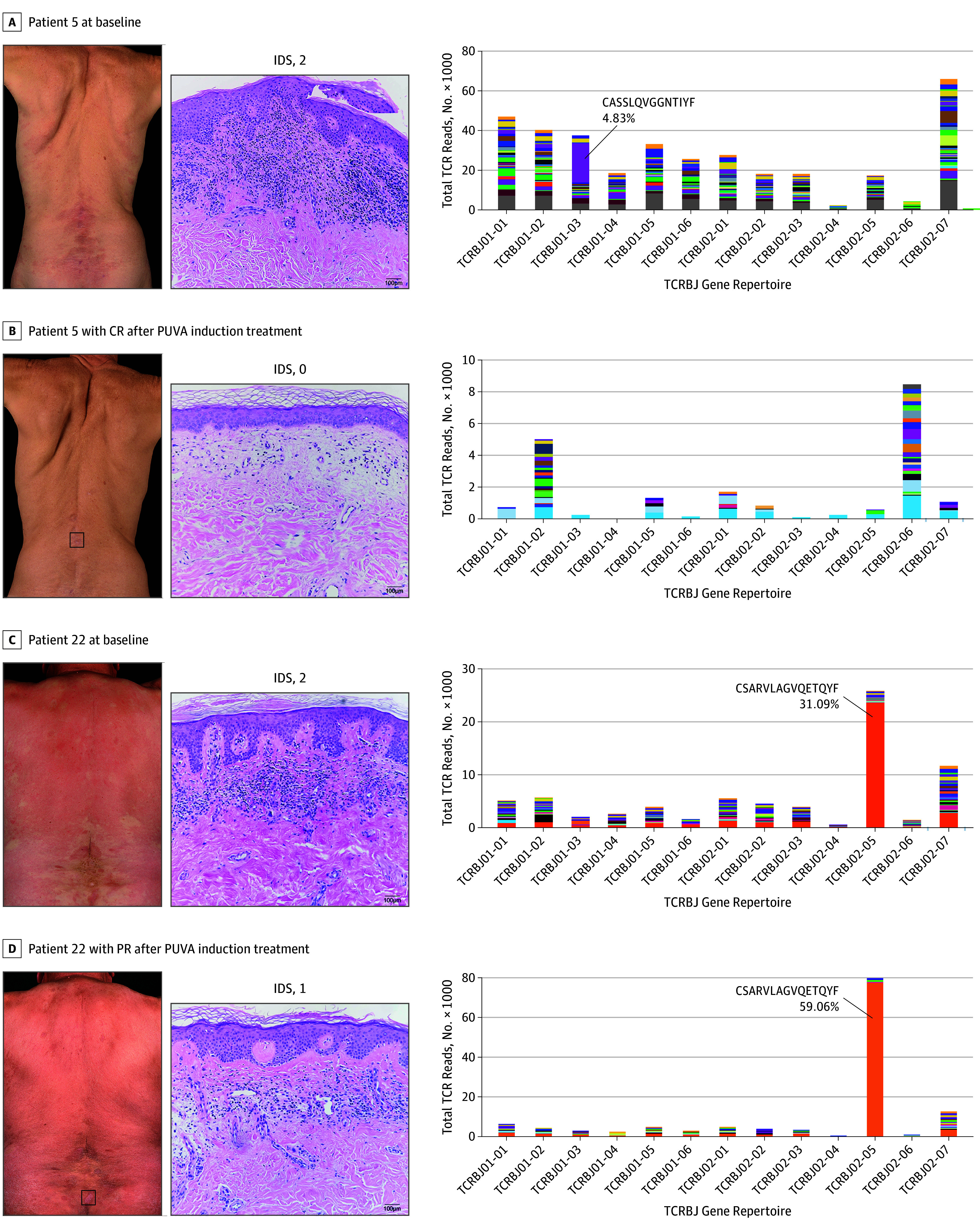
Representative cases of patients with complete response (CR) and partial response (PR). Baseline evaluation of clinical presentation, histologic features and high-throughput T-cell receptor sequencing results from skin biopsy specimens of patients 5 (A) and 22 (C) are shown. Respective reevaluation of these parameters after 16 (B) or 24 (D) weeks of PUVA induction treatment are depicted. The box in the clinical photographs indicates area of biopsy. Imaging of hematoxylin-eosin staining (original magnification ×20) was performed, and the infiltration density score (IDS) for each patient is shown. Plots of clonality analyses show the total count of T-cell receptor (TCR) reads per TCRBJ family. The malignant clone is annotated, and associated amino acid sequence and percentage of clonal TCR reads are shown.
Overall, phototherapy characteristics were similar in patients with CR and PR, with an accumulated UVA dose below 95.0 J/cm2 (eTable 1 in Supplement 2). The degree of visible skin involvement at baseline did not influence treatment response; patients with CR had a mean (range) mSWAT score of 18.6 (1-66), compared with 16.8 (3-46) in patients with PR at baseline (Table; eFigure 2A in Supplement 2). The PUVA induction phase reduced the mSWAT score in all patients (Figure 3A). Patients with CR decreased their mSWAT score by 100% within 12 weeks (5 [26%]), 16 weeks (4 [21%]), 20 weeks (5 [26%]), and 24 weeks (5 [26%]) of treatment (Figure 3B). The response rate increased along the length of the PUVA induction phase: By 12 weeks, the response rate was 19% (5 of 27 patients); after 14 to 16 weeks (9 of 27 patients), 33%; and by 20 weeks, 52% (14 of 27 patients). Among patients with PR, the mean mSWAT decreased 63%, from 16.8 to 3.9.
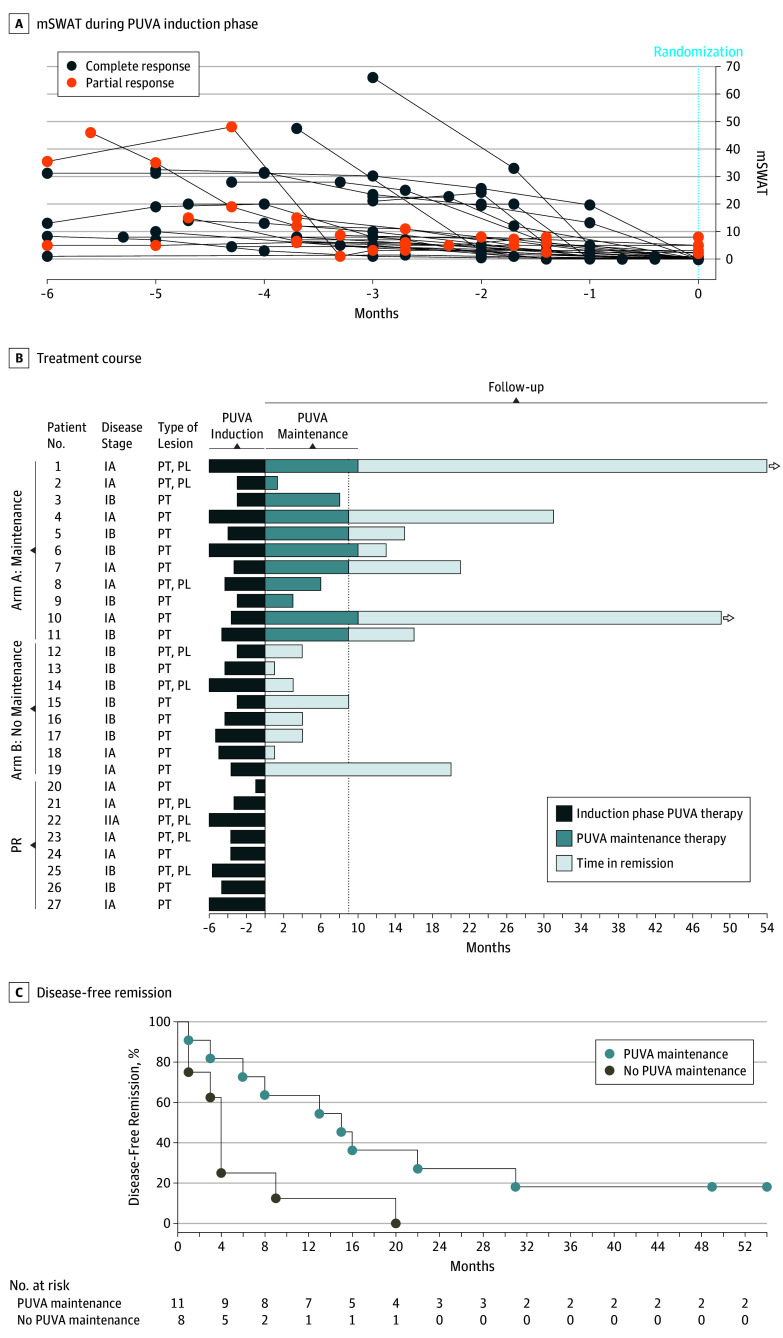
Figure 3. Effect of Psoralen–UV-A (PUVA) Maintenance on Disease-Free Remission Time .
A, Score on the modified severity-weighted assessment tool (mSWAT) was monitored throughout the duration (3-6 months) of the PUVA induction phase until randomization (month 0). Reduction of the mSWAT score to a value of 0 was defined in the trial protocol as complete response (n = 19). A substantial reduction of mSWAT values occurred also in patients who showed partial response (n = 8). B, This swimmer plot of the treatment course of each patient shows the length of the PUVA induction phase (dark blue), maintenance treatment (medium blue), and time in remission (light blue). The arrowheads indicate patients who had not yet relapsed (n = 2) at study termination. C, This Kaplan-Meier plot of disease-free remission shows a statistically significant (P = .02; calculated using log-rank test) difference between patients randomized to either PUVA maintenance or to no PUVA maintenance. PL indicates plaque; PT, patch.
All patients with CR were randomized to either PUVA maintenance or no maintenance. The mean percentages of body surface involvement and mSWAT values were similar at baseline (eFigure 2A in Supplement 2). Patients in arm A received a total UV-A dose (induction and maintenance phases) of 130.3 J/cm2. After the first 12 months from randomization, 7 (64%) of 11 patients in maintenance were in remission. In comparison, only 1 (13%) of 8 patients with no maintenance remained cleared (and within 20 months after randomization, all of them relapsed; Table). In the maintenance group, 4 patients (36%) relapsed during maintenance; 5 patients (45%) relapsed after completing maintenance (months 13 to 31); and, at the closing date of the study, 2 patients (18%) were still in remission (1 for 49 months, and 1 for 54 months). The median (range) duration of disease-free remission (Table) was 15 (1-54) months in patients with maintenance compared with 4 (1-20) months in those without it (15 vs 4 months; P = .02; Figure 3C). The hazard ratio for recurrence was 3.25 (95% CI, 1.14-9.29), comparing PUVA maintenance with no maintenance. These results indicate that PUVA maintenance extends the duration of disease-free remission in MF.
Histopathologic Evaluation, Clonality, and Response
Blinded histopathologic reevaluation of all biopsy samples after study completion revealed an overall reduction in pathognomonic MF features scores (eFigure 2B in Supplement 2). At baseline, 18 patients (67%) had a score of 2 (high), and 9 patients (33%) had a score of 1 (low). The PUVA induction phase reduced this score to 0 (absent) in 17 patients (65%) and to 1 (low) in 6 patients (23%), but it did not reduce the score at all in 3 patients (12%). For infiltration density at baseline (eFigure 2C in Supplement 2), 15 patients (56%) had a score of 2 (high), and 12 patients (44%) had a score of 1 (medium).
After 12 to 24 weeks of PUVA therapy, infiltration density score was reduced to 0 (no/low) in 14 patients (54%) and to 1 (medium) in 6 patients (23%), but it was not reduced at all in 6 patients (23%). Low cellular infiltrate density at baseline was associated with CR to PUVA treatment. At baseline, 11 (58%) of 19 patients with CR had a density score of 1, compared with 1 (13%) of 8 patients with PR (11 of 19 vs 1 of 8; P = .04). However, after the PUVA induction phase, 15 (79%) of 19 patients with CR had a pathognomonic MF score of 0, compared with 2 (29%) of 7 patients with PR (15 of 19 vs 2 of 7; P = .03). The infiltration score was also reduced at least by 1 grade in 13 (69%) of 19 patients with CR after the PUVA induction phase, compared with 1 (14%) of 7 patients with PR (13 of 19 vs 1 of 7; P = .02).
Lesional biopsy specimens taken at baseline from 21 (78%) of 27 patients provided DNA of sufficient quality and quantity with which to perform high-throughput TCR sequencing, as previously described.29 A predominant T-cell clone (a clonotype with a minimum 3-fold higher frequency of reads over the second most frequent TCR sequence) was detected in 15 (71%) of 21 patients. We found a predominant clone in 11 (65%) of 17 cases with CR and in all 4 (100%) of 4 patients with PR (Table). Representative examples of TCR clonal analysis are shown in Figure 2. A case of malignant clone depletion and decreased total T-cell infiltrate after the PUVA induction phase is shown in Figure 2A, and a case of failed depletion in a patient with a high percentage of malignant cells and high density of cell infiltrate is given in Figure 2B. At baseline, patients with pathognomonic MF score of 2 (eFigure 2D in Supplement 2) and an infiltration density score of 2 (eFigure 2E in Supplement 2) had a higher mean percentage of clonal TCR sequence of total reads that was inversely associated with therapeutic response (eFigure 2F in Supplement 2), suggesting that detectable levels of malignant clones may be associated with clinical outcome.
Effect of PUVA on T Cells
To assess the effects of PUVA on T cells and immune function, we evaluated skin biopsy specimens and blood samples taken at baseline and throughout treatment. In particular, we evaluated the expression of Treg-related markers in lesional skin and the proliferative capacity of T cells together with the percentage of Tregs in blood. Quantitative polymerase chain reaction analysis of CD3 and CD4 expression in lesional skin confirmed the decrease of lymphocytic infiltrate observed in hematoxylin-eosin (original magnification ×20) slides (eFigure 2B and C in Supplement 2). After 12 to 24 weeks of PUVA treatment, expression of CD3 and CD4 mRNA was statistically significantly lower than at baseline, reaching levels similar to those in normal skin controls (eFigure 3A in Supplement 2).
We quantified the expression of CTLA-4, Foxp3, GITR, and TGF-β to characterize Tregs in skin and their change throughout PUVA treatment (see eTable 3 in Supplement 2). In general, the expression of each of these markers was higher in lesional skin at baseline than in normal skin from donors. After 12 to 24 weeks, PUVA treatment statistically significantly reduced the expression of these markers to levels seen in normal skin (eFigure 3A in Supplement 2). Biopsy specimens from patients with PR showed higher levels of CD3, CTLA-4, Foxp3, and GITR after PUVA treatment, indicating that residual infiltrating cells after therapy had a Treg phenotype (eFigure 3B in Supplement 2). Analysis of T-cell proliferative capacity in cells from blood at baseline and throughout treatment showed that PUVA therapy reduced the response to CD3/CD28 stimulation, reaching statistical significance after 12 to 24 weeks of treatment (eFigure 4A in Supplement 2). Moreover, determination of Treg frequency in blood showed that PUVA treatment increased the percentage of Tregs from 6 weeks after start of treatment until termination of the PUVA induction phase (12-24 weeks; eFigure 4B in Supplement 2).
Biomarker Assessment
Using an immunobead array, we screened 65 proinflammatory analytes (eTable 2 in Supplement 2) in serum samples taken from patients at baseline. Nineteen markers were present at detectable levels (eTable 2 in Supplement 2) and selected for quantification throughout PUVA treatment (eFigure 5 in Supplement 2) to identify the association of serum biomarkers with clinical response. Multivariate analysis of samples taken from patients with CR and PR at baseline (Figure 4) revealed a set of 6 cytokines and chemokines associated with clinical response. High levels of CXCL9, CXCL11, CXCL12, TNFSF13, and CXCL13 were negatively associated with clinical response (Figure 4A), with elevated absolute values in patients with PR (Figure 4B). The expression of TWEAK was directly associated with clinical response, with high levels of TWEAK in patients with CR. We detected high levels of soluble CD30 and CXCL13 in serum at baseline; however, both levels were statistically significantly decreased after 1 year of PUVA treatment (eFigure 5 in Supplement 2).
Figure 4. Baseline Serum Levels of Proinflammatory Mediators and Clinical Outcome After Psoralen–UV-A (PUVA) Treatment.

Multivariate comparison of serum levels of proinflammatory mediators at baseline between patients with partial response (PR) and patients with complete response (CR). A, Hierarchical cluster analysis is shown with association coefficient (ibs) of chemotactic mediator levels. Mediators negatively associated with CR are shown in dark blue; mediators positively associated with CR are shown in light blue. Dashed line indicates a threshold of P < .05. B, Comparison of baseline levels of CXCL9, CXCL11, CXCL12, CXCL13, TNFSF13, and TWEAK in patients with CR and those with PR is shown. Mean (SEM) is plotted, Mann-Whitney test was used to evaluate statistical significance (P values are shown).
Discussion
In this study, twice-weekly PUVA treatment (starting with a relatively low dose of approximately 30% MPD) given for 12 to 24 weeks resulted in low cumulative UV-A doses (eTable 1 in Supplement 2) and led to a high CR rate of 70% (Table). Subsequent use of standardized low frequency maintenance therapy prolonged disease-free remission. At the 12-month follow-up, 64% of patients with PUVA maintenance were in remission and only 13% of those with no maintenance remained clinically cleared (7 of 11 vs 1 of 8; P = .03; Figure 3). In the short and intermediate term, this PUVA induction-maintenance regimen was safe and tolerated with minor adverse effects in a few cases. Patients received a maximum of 48 PUVA treatment sessions and a mean cumulative UV-A dose of 78.5 J/cm2 in patients with CR and 94.4 J/cm2 in patients with PR during the induction phase. The mean cumulative UV-A dose (induction and maintenance phases) of patients in arm A was 130.3 J/cm2 (eTable 1 in Supplement 2). This was well below the 150 to 200 sessions and the 1200 J/cm2 cumulative dose defined by current guidelines11,30 as the risk threshold for PUVA-associated squamous cell carcinoma31 and melanoma.32
Consistent with a previous report in psoriasis,33 twice-weekly PUVA treatment may be as effective as more frequent treatment in MF as well. In the present study, we had the theoretical concern of a decay in adaptive tolerance (achieved by steady UV-A dose increments in the induction phase) during the maintenance phase, considering the low frequency of sessions (intervals up to 4 weeks). However, this was not the case: Treatment-associated phototoxic erythema did occur in 12 (46%) of 28 patients during the induction phase and in 1 patient during both induction and maintenance. Compared with the results of other studies, the twice-weekly PUVA regimen of 12 to 24 weeks followed by 9-month maintenance resulted in a cumulative UV-A dose similar to a PUVA regimen of 3 or more sessions per week with no maintenance. Reports of other study protocols required UV-A doses higher than 180 J/cm2 for treatment with no maintenance.18,34
To date, this study is the first prospective randomized clinical trial that addresses the efficacy of low-dose, low-frequency oral PUVA and maintenance.25 No clear evidence of the potential advantages of PUVA maintenance has been presented so far.25 The reason may have been the unintentional skewing of results by nonrandomized treatment allocation by physicians, who often prescribe prolonged treatment with slow withdrawal (similar to PUVA maintenance) in cases of more severe disease. In a prospective study,34 the CR rate after a maximum of 16 weeks of PUVA monotherapy (3 times/wk; 70% MPD starting dose) was 22.2% (10 of 45 patients) and the median time to relapse after CR was 9.7 months without maintenance. In comparison, this study’s low-dose, low-frequency PUVA regimen for up to 24 weeks (2 times/wk; <50% MPD starting dose) had a superior CR rate of 70% (19 of 27 patients), and maintenance extended the median time to relapse from 4 to 15 months.
Moreover, patients in this study received on average a substantially lower accumulated UV-A dose of 82.8 J/cm2, compared with the 107.0 J/cm2 dose that patients received in the aforementioned study.34 Whittaker et al34 pointed out that the CR rate they observed for PUVA alone (and the combination of bexarotene and PUVA) was lower than the rates given in previous reports.18 They suggested that this difference may have been the result of the use of rigorous assessment tools and shorter duration of PUVA therapy in their study (ie, median duration of 12 weeks). In the present study, the CR rate after 12 weeks was 19% (5 of 27 patients), after 14 to 16 weeks was 33% (9 of 27 patients), and after 20 weeks was 52% (14 of 27 patients) (Figure 3B), indicating that the length of PUVA treatment is crucial for the outcome.
High infiltration density score at baseline associated with high frequency of malignant cells (eFigure 2E in Supplement 2) and subsequent clinical outcome (eFigure 2F in Supplement 2). Patients with a low proportion of clonal cells in lesional skin (Figure 2A) tended to have better responses to PUVA than did those with a high proportion of clonal cells (Figure 2B). Moreover, the percentage of clonal TCR sequences associated with high pathognomonic MF features score (eFigure 2D in Supplement 2). All cases with PR but only 65% of cases with CR who were tested for high-throughput TCR sequencing showed clonality (Table). Furthermore, patients with PR had a higher clonal read frequency (eFigure 2F in Supplement 2), indicating that the presence of malignant clones at detectable and high levels may be a marker of disease severity and associated with clinical failure, as previously suggested.5,35
Previous reports have shown that Tregs inhabit lesional MF skin in early stages at similar levels to inflammatory skin diseases such as psoriasis and eczematous dermatitis.36 In the present study, the residual infiltrate after treatment in patients with CR had low levels of Treg markers CTLA-4, Foxp3, and GITR, similar to healthy controls, whereas the residual infiltrate in patients with PR had a clear Treg phenotype (eFigure 3B in Supplement 2), going in line with the concept that Tregs in lesional skin promote an environment in which malignant cells can escape immune surveillance.37
In MF, it is unclear whether the presence and proliferation of nonmalignant T cells with a diverse TCR repertoire suppress the overgrowth of malignant cells as observed in other T-cell malignant neoplasms38 and whether PUVA treatment affects T cells in the periphery. Analysis of blood-circulating T cells throughout treatment showed that PUVA therapy reduced the response to CD3/CD28 stimulation of effector T cells (eFigure 4B in Supplement 2) and that the percentage of Tregs increased as early as 6 weeks after start of treatment and remained high up to 12 to 24 weeks (for as long as monitored; eFigure 4B in Supplement 2). Despite this finding, Treg suppression capacity (measured by cocultures of Tregs with effector T cells) did not increase after treatment.
Few alterations have been reported in the serum of early-stage MF. One report suggests that patients with MF with no signs of blood involvement have alterations in serum lipid composition, including constituents such as phosphatidylcholines and sphingomyelin, that have potential diagnostic value.39 Although all patients in the present study had normal leukocyte levels in peripheral blood, most of them had a large number of soluble chemotactic factors with measurable levels in serum (eFigure 5 in Supplement 2). High levels of CXCL9, CXCL11, CXCL12, TNFSF13, and CXCL13 at baseline were negatively associated with clinical response (Figure 4). In contrast, the expression of TWEAK was directly associated with clinical response (ie, high levels of TWEAK at baseline associated with CR). The role of these mediators remains elusive; however, their chemotactic properties may have a role in counteracting the therapeutic effect of PUVA treatment. For instance, CXCL9 is a PU.1-regulated chemokine that was associated with therapeutic failure (ie, PR, Figure 4B). In previous reports, this chemokine has been implicated in short-term relapses of follicular lymphoma.40 CXCL9 may form heterocomplexes with CXCL12 (also associated with therapeutic failure) in tumor vasculature and enhance its chemotactic effect.41 TNFSF13 had a negative predictive value in this study, perhaps associated with its capacity to promote accumulation of proinflammatory cells in tissue and activate antiapoptotic BCL-2.42 Patients with CR showed high levels of TWEAK (Figure 4B), suggesting that this factor may counteract the dysregulation of cytokine secretion in patients with MF, as seen in muscular tissue.43 CD30+ cells are commonly found in MF skin lesions, and CD30 positivity is a marker of proliferating cells and often associated with aggressive disease.7
Our monitoring throughout treatment and follow-up showed that PUVA therapy substantially diminished the level of soluble CD30 in the serum (eFigure 5 in Supplement 2). Although this result does not indicate directly that the number of CD30+ cells decreased after treatment, it does suggest that PUVA therapy impairs CD30 shedding of T cells activated by CCR3-eotaxin engagement,44 causing lower CD30 serum levels. Eotaxins and CCR3 interactions have been previously implicated in the predominant TH2 environment of MF lesional skin.45 Lactate dehydrogenase serum levels at baseline were within normal limits in all but 2 patients of the study; overall, they were not associated with clinical outcome as this has been previously observed only in patients with advanced disease stages.46,47
Limitations
The major limitations of this study are the small overall sample size and the uneven distribution of the patients in different disease stages randomized to either PUVA maintenance or no maintenance (Figure 3B). The mean mSWAT score and percentage of body surface involvement, however, were similar in both arms of the study (eFigure 2A in Supplement 2).
Conclusions
Low-dose, low-frequency PUVA treatment resulted in a high rate of CR in MF, as assessed by clinical outcomes, histologic criteria, and high-throughput TCR sequencing. Certain soluble chemotactic factors (CXCL9, CXCL11-13, TNFSF13, and TWEAK) are potential biomarkers for therapeutic response to PUVA. Finally, PUVA maintenance treatment appears to be safe (at least at short and intermediate term) in MF, and patients benefit from extended disease-free intervals.
Trial Protocol
eTable 1. PUVA Characteristics of Patients Who Completed the Minimum of 12 Weeks of Treatment
eTable 2. Proinflammatory Mediators Prescreened
eTable 3. Primer List
eFigure 1. Study Design
eFigure 2. Effect of PUVA Induction Treatment on mSWAT and Histologic Features
eFigure 3. Expression of Treg-related Markers in Lesional Skin
eFigure 4. Proliferation Capacity of Peripheral T-cells and Treg Levels in Blood of MF Patients
eFigure 5. Assessment of Serum Levels of Proinflammatory Mediators
eMethods. Patient Inclusion and Exclusion Criteria
Data Sharing Statement
References
- 1.Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105(10):3768-3785. doi: 10.1182/blood-2004-09-3502 [DOI] [PubMed] [Google Scholar]
- 2.Fivenson DP, Hanson CA, Nickoloff BJ. Localization of clonal T cells to the epidermis in cutaneous T-cell lymphoma. J Am Acad Dermatol. 1994;31(5, pt 1):717-723. doi: 10.1016/S0190-9622(94)70231-4 [DOI] [PubMed] [Google Scholar]
- 3.Girardi M, Heald PW, Wilson LD. The pathogenesis of mycosis fungoides. N Engl J Med. 2004;350(19):1978-1988. doi: 10.1056/NEJMra032810 [DOI] [PubMed] [Google Scholar]
- 4.Hwang ST, Janik JE, Jaffe ES, Wilson WH. Mycosis fungoides and Sézary syndrome. Lancet. 2008;371(9616):945-957. doi: 10.1016/S0140-6736(08)60420-1 [DOI] [PubMed] [Google Scholar]
- 5.Kirsch IR, Watanabe R, O’Malley JT, et al. TCR sequencing facilitates diagnosis and identifies mature T cells as the cell of origin in CTCL. Sci Transl Med. 2015;7(308):308ra158. doi: 10.1126/scitranslmed.aaa9122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell JJ, Clark RA, Watanabe R, Kupper TS. Sezary syndrome and mycosis fungoides arise from distinct T-cell subsets: a biologic rationale for their distinct clinical behaviors. Blood. 2010;116(5):767-771. doi: 10.1182/blood-2009-11-251926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edinger JT, Clark BZ, Pucevich BE, Geskin LJ, Swerdlow SH. CD30 expression and proliferative fraction in nontransformed mycosis fungoides. Am J Surg Pathol. 2009;33(12):1860-1868. doi: 10.1097/PAS.0b013e3181bf677d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wada DA, Pittelkow MR, Comfere NI, Gibson LE, Ansell SM, Wilcox RA. CD4+CD25+FOXP3+ malignant T cells in Sézary syndrome are not necessarily functional regulatory T cells. J Am Acad Dermatol. 2013;69(3):485-489. doi: 10.1016/j.jaad.2010.01.013 [DOI] [PubMed] [Google Scholar]
- 9.Trautinger F, Knobler R, Willemze R, et al. EORTC consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome. Eur J Cancer. 2006;42(8):1014-1030. doi: 10.1016/j.ejca.2006.01.025 [DOI] [PubMed] [Google Scholar]
- 10.Trautinger F, Eder J, Assaf C, et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome: Update 2017. Eur J Cancer. 2017;77:57-74. doi: 10.1016/j.ejca.2017.02.027 [DOI] [PubMed] [Google Scholar]
- 11.Ling TC, Clayton TH, Crawley J, et al. British Association of Dermatologists and British Photodermatology Group guidelines for the safe and effective use of psoralen-ultraviolet A therapy 2015. Br J Dermatol. 2016;174(1):24-55. doi: 10.1111/bjd.14317 [DOI] [PubMed] [Google Scholar]
- 12.Trautinger F. Phototherapy of mycosis fungoides. Photodermatol Photoimmunol Photomed. 2011;27(2):68-74. doi: 10.1111/j.1600-0781.2011.00559.x [DOI] [PubMed] [Google Scholar]
- 13.Gilchrest BA, Parrish JA, Tanenbaum L, Haynes HA, Fitzpatrick TB. Oral methoxsalen photochemotherapy of mycosis fungoides. Cancer. 1976;38(2):683-689. doi: [DOI] [PubMed] [Google Scholar]
- 14.Olsen EA, Hodak E, Anderson T, et al. Guidelines for phototherapy of mycosis fungoides and Sézary syndrome: a consensus statement of the United States Cutaneous Lymphoma Consortium. J Am Acad Dermatol. 2016;74(1):27-58. doi: 10.1016/j.jaad.2015.09.033 [DOI] [PubMed] [Google Scholar]
- 15.Carter J, Zug KA. Phototherapy for cutaneous T-cell lymphoma: online survey and literature review. J Am Acad Dermatol. 2009;60(1):39-50. doi: 10.1016/j.jaad.2008.08.043 [DOI] [PubMed] [Google Scholar]
- 16.Dummer R, Assaf C, Bagot M, et al. Maintenance therapy in cutaneous T-cell lymphoma: who, when, what? Eur J Cancer. 2007;43(16):2321-2329. doi: 10.1016/j.ejca.2007.06.015 [DOI] [PubMed] [Google Scholar]
- 17.Pothiawala SZ, Baldwin BT, Cherpelis BS, Glass LF, Fenske NA. The role of maintenance phototherapy in cutaneous T-cell lymphoma. J Drugs Dermatol. 2010;9(7):800-803. [PubMed] [Google Scholar]
- 18.Querfeld C, Rosen ST, Kuzel TM, et al. Long-term follow-up of patients with early-stage cutaneous T-cell lymphoma who achieved complete remission with psoralen plus UV-A monotherapy. Arch Dermatol. 2005;141(3):305-311. doi: 10.1001/archderm.141.3.305 [DOI] [PubMed] [Google Scholar]
- 19.Roenigk HH Jr. Photochemotherapy for mycosis fungoides. Arch Dermatol. 1977;113(8):1047-1051. doi: 10.1001/archderm.1977.01640080049003 [DOI] [PubMed] [Google Scholar]
- 20.Roenigk HH Jr. Photochemotherapy for mycosis fungoides: long-term followup study. Cancer Treat Rep. 1979;63(4):669-673. [PubMed] [Google Scholar]
- 21.Sánchez MA, González T, Gaitán MF, Zuluaga A, Jiménez SB, de Galvis YT. Is PUVA maintenance therapy necessary in patients with early-stage mycosis fungoides? evaluation of a treatment guideline over a 28-month follow-up. Int J Dermatol. 2011;50(9):1086-1093. doi: 10.1111/j.1365-4632.2010.04833.x [DOI] [PubMed] [Google Scholar]
- 22.Wackernagel A, Hofer A, Legat F, Kerl H, Wolf P. Efficacy of 8-methoxypsoralen vs. 5-methoxypsoralen plus ultraviolet A therapy in patients with mycosis fungoides. Br J Dermatol. 2006;154(3):519-523. doi: 10.1111/j.1365-2133.2005.07008.x [DOI] [PubMed] [Google Scholar]
- 23.Hönigsmann H, Tanew A. Photo(chemo)therapy for Cutaneous T-Cell Lymphoma. In: Hönigsmann H, Elmets C, Krutmann J, eds. Dermatological Phototherapy and Photodiagnostic Methods. Berlin/Heidelberg, Germany: Springer; 2009:135-149. [Google Scholar]
- 24.Vieyra-Garcia P, Wolf P. Psoralen-ultraviolet A maintenance in mycosis fungoides: the underlying question. Br J Dermatol. 2017;177(2):336-337. doi: 10.1111/bjd.15670 [DOI] [PubMed] [Google Scholar]
- 25.Grandi V, Delfino C, Pileri A, Pimpinelli N. Maintenance phase in psoralen-ultraviolet A phototherapy of early-stage mycosis fungoides. A critically appraised topic. Br J Dermatol. 2017;177(2):406-410. doi: 10.1111/bjd.15302 [DOI] [PubMed] [Google Scholar]
- 26.Dixon JR. The International Conference on Harmonization Good Clinical Practice guideline. Qual Assur. 1998;6(2):65-74. doi: 10.1080/105294199277860 [DOI] [PubMed] [Google Scholar]
- 27.Williams JR. The Declaration of Helsinki and public health. Bull World Health Organ. 2008;86(8):650-652. doi: 10.2471/BLT.08.050955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olsen EA, Whittaker S, Kim YH, et al. ; International Society for Cutaneous Lymphomas; United States Cutaneous Lymphoma Consortium; Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer . Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29(18):2598-2607. doi: 10.1200/JCO.2010.32.0630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vieyra-Garcia PA, Wei T, Naym DG, et al. STAT3/5-Dependent IL9 overexpression contributes to neoplastic cell survival in mycosis fungoides. Clin Cancer Res. 2016;22(13):3328-3339. doi: 10.1158/1078-0432.CCR-15-1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whittaker SJ, Marsden JR, Spittle M, Russell Jones R; British Association of Dermatologists; U.K. Cutaneous Lymphoma Group . Joint British Association of Dermatologists and U.K. Cutaneous Lymphoma Group guidelines for the management of primary cutaneous T-cell lymphomas. Br J Dermatol. 2003;149(6):1095-1107. doi: 10.1111/j.1365-2133.2003.05698.x [DOI] [PubMed] [Google Scholar]
- 31.Stern RS; PUVA Follow-Up Study . The risk of squamous cell and basal cell cancer associated with psoralen and ultraviolet A therapy: a 30-year prospective study. J Am Acad Dermatol. 2012;66(4):553-562. doi: 10.1016/j.jaad.2011.04.004 [DOI] [PubMed] [Google Scholar]
- 32.Stern RS; PUVA Follow up Study . The risk of melanoma in association with long-term exposure to PUVA. J Am Acad Dermatol. 2001;44(5):755-761. doi: 10.1067/mjd.2001.114576 [DOI] [PubMed] [Google Scholar]
- 33.Legat FJ, Hofer A, Quehenberger F, Kahofer P, Kerl H, Wolf P. Reduction of treatment frequency and UVA dose does not substantially compromise the antipsoriatic effect of oral psoralen-UVA. J Am Acad Dermatol. 2004;51(5):746-754. doi: 10.1016/j.jaad.2004.04.029 [DOI] [PubMed] [Google Scholar]
- 34.Whittaker S, Ortiz P, Dummer R, et al. Efficacy and safety of bexarotene combined with psoralen-ultraviolet A (PUVA) compared with PUVA treatment alone in stage IB-IIA mycosis fungoides: final results from the EORTC Cutaneous Lymphoma Task Force phase III randomized clinical trial (NCT00056056). Br J Dermatol. 2012;167(3):678-687. doi: 10.1111/j.1365-2133.2012.11156.x [DOI] [PubMed] [Google Scholar]
- 35.de Masson A, O’Malley JT, Elco CP, et al. High-throughput sequencing of the T cell receptor β gene identifies aggressive early-stage mycosis fungoides. Sci Transl Med. 2018;10(440):eaar5894. doi: 10.1126/scitranslmed.aar5894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujimura T, Okuyama R, Ito Y, Aiba S. Profiles of Foxp3+ regulatory T cells in eczematous dermatitis, psoriasis vulgaris and mycosis fungoides. Br J Dermatol. 2008;158(6):1256-1263. doi: 10.1111/j.1365-2133.2008.08504.x [DOI] [PubMed] [Google Scholar]
- 37.Ishida T, Ishii T, Inagaki A, et al. Specific recruitment of CC chemokine receptor 4-positive regulatory T cells in Hodgkin lymphoma fosters immune privilege. Cancer Res. 2006;66(11):5716-5722. doi: 10.1158/0008-5472.CAN-06-0261 [DOI] [PubMed] [Google Scholar]
- 38.Newrzela S, Al-Ghaili N, Heinrich T, et al. T-cell receptor diversity prevents T-cell lymphoma development. Leukemia. 2012;26(12):2499-2507. doi: 10.1038/leu.2012.142 [DOI] [PubMed] [Google Scholar]
- 39.Xu C, Zhou D, Luo Y, et al. Tissue and serum lipidome shows altered lipid composition with diagnostic potential in mycosis fungoides. Oncotarget. 2017;8(29):48041-48050. doi: 10.18632/oncotarget.18228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mir MA, Maurer MJ, Ziesmer SC, et al. Elevated serum levels of IL-2R, IL-1RA, and CXCL9 are associated with a poor prognosis in follicular lymphoma. Blood. 2015;125(6):992-998. doi: 10.1182/blood-2014-06-583369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Venetz D, Ponzoni M, Schiraldi M, et al. Perivascular expression of CXCL9 and CXCL12 in primary central nervous system lymphoma: T-cell infiltration and positioning of malignant B cells. Int J Cancer. 2010;127(10):2300-2312. doi: 10.1002/ijc.25236 [DOI] [PubMed] [Google Scholar]
- 42.Moreaux J, Legouffe E, Jourdan E, et al. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood. 2004;103(8):3148-3157. doi: 10.1182/blood-2003-06-1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Enwere EK, Lacasse EC, Adam NJ, Korneluk RG. Role of the TWEAK-Fn14-cIAP1-NF-κB signaling axis in the regulation of myogenesis and muscle homeostasis. Front Immunol. 2014;5:34. doi: 10.3389/fimmu.2014.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kleinhans M, Tun-Kyi A, Gilliet M, et al. Functional expression of the eotaxin receptor CCR3 in CD30+ cutaneous T-cell lymphoma. Blood. 2003;101(4):1487-1493. doi: 10.1182/blood-2002-02-0475 [DOI] [PubMed] [Google Scholar]
- 45.Miyagaki T, Sugaya M, Fujita H, et al. Eotaxins and CCR3 interaction regulates the Th2 environment of cutaneous T-cell lymphoma. J Invest Dermatol. 2010;130(9):2304-2311. doi: 10.1038/jid.2010.128 [DOI] [PubMed] [Google Scholar]
- 46.Diamandidou E, Colome M, Fayad L, Duvic M, Kurzrock R. Prognostic factor analysis in mycosis fungoides/Sézary syndrome. J Am Acad Dermatol. 1999;40(6, pt 1):914-924. doi: 10.1016/S0190-9622(99)70079-4 [DOI] [PubMed] [Google Scholar]
- 47.Vonderheid EC, Bernengo MG, Burg G, et al. ; ISCL . Update on erythrodermic cutaneous T-cell lymphoma: report of the International Society for Cutaneous Lymphomas. J Am Acad Dermatol. 2002;46(1):95-106. doi: 10.1067/mjd.2002.118538 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. PUVA Characteristics of Patients Who Completed the Minimum of 12 Weeks of Treatment
eTable 2. Proinflammatory Mediators Prescreened
eTable 3. Primer List
eFigure 1. Study Design
eFigure 2. Effect of PUVA Induction Treatment on mSWAT and Histologic Features
eFigure 3. Expression of Treg-related Markers in Lesional Skin
eFigure 4. Proliferation Capacity of Peripheral T-cells and Treg Levels in Blood of MF Patients
eFigure 5. Assessment of Serum Levels of Proinflammatory Mediators
eMethods. Patient Inclusion and Exclusion Criteria
Data Sharing Statement