Abstract
Importance
It is challenging to differentiate melanoma from melanocytic nevus on the volar skin in the absence of typical dermoscopic patterns.
Objective
To identify the frequency and clinical and dermoscopic characteristics of melanocytic lesions on the volar skin not displaying a parallel furrow pattern, lattice-like pattern, fibrillar pattern, or parallel ridge pattern on results of dermoscopy.
Design, Setting, and Participants
In this retrospective cohort study, a total of 504 melanocytic lesions on the volar skin were evaluated in the Shinshu University Hospital department of dermatology between January 1, 2000, and December 31, 2012. Dermoscopic images were independently assessed by 3 dermoscopists for the presence of established dermoscopic criteria. Statistical analysis was performed from October 1, 2017, to April 30, 2018.
Main Outcomes and Measures
Frequency of dermoscopic criteria and corresponding clinical (patient age and size and location of lesion) and histopathologic features.
Results
Of 504 lesions, 110 (21.8%) (melanocytic nevus, 97; melanoma, 8; and equivocal melanocytic lesion, 5) from 108 patients (68 female and 40 male patients; mean age, 40.1 years [range, 1-86 years]) did not show a parallel furrow pattern, lattice-like pattern, fibrillar pattern, or parallel ridge pattern. Among them, the mean patient age was significantly higher for melanoma than for melanocytic nevus (65.3 vs 38.0 years; P < .001), as was mean maximum lesion diameter (11.8 vs 5.7 mm; P < .001). Melanomas and equivocal melanocytic lesions tended to be distributed on weight-bearing areas of the foot sole, such as the heel, while nevi were spread over non–weight-bearing regions. Dermoscopically, 95 melanocytic nevi (97.9%) were symmetrical in 1 or 2 axes while melanomas were not. A total of 91 melanocytic nevi (93.8%) had 1 or 2 colors per lesion, and 4 melanomas (50.0%) had more than 2 colors. Vascular structures were seen in 3 melanocytic nevi (3.1%) and 3 melanomas (37.5%). Blue-white structures were seen in 18 melanocytic nevi (18.6%) and 3 melanomas (37.5%). Dots and globules were seen in 22 melanocytic nevi (22.7%) and 4 melanomas (50.0%). Vascular structures, blue-white structures, and dots and globules were irregularly distributed in the melanomas. Ulcer, hyperkeratosis, and irregular streaks were observed only in melanomas.
Conclusions and Relevance
More than one-fifth of melanocytic lesions on the volar skin did not display typical dermoscopic patterns. Asymmetry, numerous colors (≥3), and other melanoma-specific dermoscopic findings were more frequently observed for melanomas. Clinical information, including patient age and lesion size and location, was helpful in differentiating melanoma from melanocytic nevus. Further prospective clinical studies are warranted to clarify the diagnostic accuracy of dermoscopy combined with clinical information.
This cohort study examines the frequency and clinical and dermoscopic characteristics of melanocytic lesions on the volar skin not displaying a parallel furrow pattern, lattice-like pattern, fibrillar pattern, or parallel ridge pattern on results of dermoscopy.
Key Points
Question
What are the frequency and clinical and dermoscopic features of melanocytic lesions on the volar skin not exhibiting typical dermoscopic patterns?
Findings
In this cohort study, 110 of 504 melanocytic lesions (21.8%) on the volar skin showed no typical benign or malignant dermoscopic patterns. Among them, melanoma was significantly associated with higher age, larger lesion size, weight-bearing areas, asymmetry, numerous colors (≥3), vascular structures, irregularly distributed dots and globules, and blue-white structures.
Meanings
For melanocytic lesions on the volar skin without typical dermoscopic patterns, clinical information and other melanoma-specific dermoscopic findings may help diagnosis.
Introduction
Dermoscopy is an established technique that enhances the ability to diagnose both melanocytic and nonmelanocytic skin lesions. It is especially useful for identifying melanocytic lesions on the acral volar skin. In those areas, melanoma shows dominant pigmentation in ridges under dermoscopy, while melanocytic nevus exhibits linear pigmentation in furrows. The characteristic dermoscopic findings for melanoma and melanocytic nevus are parallel ridge patterns (PRP), parallel furrow patterns (PFP), lattice-like patterns (LLP), and fibrillar patterns (FP),1,2 with a sensitivity of PRP for melanoma of 99% and specificity of 86%.3 Furthermore, a revised 3-step algorithm for the management of acquired acral melanocytic lesions has enabled the diagnosis of most melanocytic lesions on the acral volar skin as nevus or melanoma, regardless of size, when 1 PFP, LLP, PF, or PRP is present.4 However, the frequency of melanocytic lesions that do not show typical patterns is reportedly between 5% and 17%,5,6,7,8 and precise details on such lesions are lacking. This study sought to identify the frequency and clinical and dermoscopic characteristics of melanocytic lesions on the volar skin not displaying typical patterns on dermoscopy.
Methods
Lesion Selection
We retrospectively collected the data of patients who were seen in the Shinshu University Hospital department of dermatology between January 1, 2000, and December 31, 2012, for evaluation of melanocytic lesions on the volar skin. Patients with congenital lesions were excluded. The process of differential diagnosis at our hospital was based largely on a revised 3-step algorithm. Lesions showing typical PFP, LLP, and/or FP were diagnosed as melanocytic nevus without follow-up. Lesions displaying complete or partial PRP were biopsied for histopathologic diagnosis. Lesions with no PFP, LLP, FP, or PRP were monitored for clinical course and/or biopsied for histopathologic evaluation. This study was approved by the ethics board of Shinshu University School of Medicine. A waiver of patient consent was granted by the ethics board because this study was retrospective and noninterventional.
Dermoscopic Evaluation
Dermoscopic images were retrospectively and independently evaluated by 3 dermoscopists (Y.M., A.M., and H.K.) to classify their pattern as PFP, LLP, FP, or PRP. The evaluators were blinded to the diagnosis of the lesions when evaluating dermoscopic findings. In the case of disagreement, a consensus was reached by discussion among the evaluators. Lesions showing none of those characteristics were followed up for further clinical and dermoscopic analyses.
In the dermoscopic images, the number of colors (black, light brown, dark brown, red, white, and blue) per lesion, distribution of dermoscopic structures and/or color (symmetry in 0, 1, or 2 axes), and frequency of dermoscopic structures outlined in a consensus of the International Society of Dermoscopy were evaluated.2,9,10 We divided the melanocytic nevus lesions showing none of the 3 benign patterns into the following groups: (1) hyperpigmentation indicating a black or dark-brown lesion with an ill-defined ridge or furrow, (2) hypopigmentation indicating a light-brown or gray-blue lesion without clear demarcation, and (3) elevation indicating a lesion with dilation of the furrow lines.
Clinical and Histopathologic Analysis
Patients’ clinical background and lesion size were obtained from medical records. The precise location of each lesion was identified according to clinical pictures and then plotted on a diagram of the foot sole and palm. For cases that included tissue biopsy, histopathologic correlations to dermoscopic findings were evaluated as well.
Statistical Analysis
Statistical analyses were carried out from October 1, 2017, to April 30, 2018, using R, version 3.4.1, software (R Foundation for Statistical Computing). Mean comparisons using t tests were used for the quantitative study of age. Mean comparisons with the Mann-Whitney test were adopted for the quantitative study of size. All P values were from 2-sided tests, and results were deemed statistically significant at P < .01 after adjustment with Bonferroni correction.
Results
A total of 504 acral melanocytic lesions were included in this study. Of those, 394 lesions exhibited the typical PFP, LLP, FP, or PRP, including 331 nevi from 331 patients (94 male and 237 female patients; mean age, 31 years [range, 1-83 years]) and 63 melanomas from 63 patients (31 males and 32 females; mean age, 76 years [range, 38-94 years]; median Breslow tumor thickness, 2.12 mm [range, in situ to 25.00 mm]). All lesions showing PRP on dermoscopy were histopathologically diagnosed as malignant melanoma. A total of 110 lesions (21.8%) from 108 patients did not exhibit PFP, LLP, FP, or PRP and were subjected to further analyses. Those lesions included 97 melanocytic nevi from 95 patients, 8 melanomas from 8 patients, and 5 equivocal melanocytic lesions from 5 patients. Patient age and sex and lesion location and size of the 110 lesions are summarized in Table 1. The mean age of patients with melanoma was significantly higher than that of patients with melanocytic nevus (65.3 vs 38.0 years; P < .001). Melanocytic nevi either with or without PFP, LLP, and/or FP were predominant among females (65 [67.0%]), while most melanomas without typical PRP developed in males (7 [87.5%]). The mean maximum diameters of melanomas without PRP were significantly larger than those of melanocytic nevi without PFP, LLP, or FP (11.8 vs 5.7 mm; P < .001), as were the minimum diameters. The maximum diameter of melanomas was greater than 7 mm in all cases compared with less than 7 mm in 68 of 97 melanocytic nevi (70.1%).
Table 1. Demographic Characteristics of Melanocytic Nevi, Melanomas, and Equivocal Lesions Not Showing Typical Dermoscopic Patterns.
| Clinical Subtype | Nevi | Melanomas (n = 8) | Equivocal Lesions (n = 5) | |||
|---|---|---|---|---|---|---|
| Hyperpigmentation (n = 29) | Hypopigmentation (n = 48) | Elevated (n = 20) | Total (n = 97) | |||
| Frequency per disease, No./No. (%) | 29/428 (6.8) | 48/428 (11.2) | 20/428 (4.7) | 97/428 (22.7) | 8/71 (11.3) | NA |
| Hand, No. | 5 | 3 | 3 | 11 | 1 | 0 |
| Foot, No. | 24 | 45 | 17 | 86 | 7 | 5 |
| Males, No. | 9 | 17 | 6 | 32 | 7 | 1 |
| Females, No. | 20 | 31 | 14 | 65 | 1 | 4 |
| Age, mean (range), y | 31.3 (1-71) | 41.2 (16-83) | 40.6 (2-79) | 38.0 (1-83) | 65.3 (39-86) | 38.8 (21-55) |
| Maximum diameter, mean (range), mm | 4.0 (1.0-10.2) | 6.5 (1.9-18.0) | 6.2 (1.2-11.0) | 5.7 (1.0-18.0) | 11.8 (7.5-17.0) | 8.8 (6.8-10.0) |
Abbreviation: NA, not applicable.
For melanocytic nevi, the largest number of lesions was categorized as hypopigmentation (48 [49.5%]), followed by hyperpigmentation (29 [29.9%]) and elevated (20 [20.6%]) (Table 1). With regard to patient age, all melanocytic nevi in patients 15 years or younger were hyperpigmentation (n = 10). On the contrary, 13 of 16 melanocytic nevi (81.3%) were categorized as hypopigmentation in patients older than 60 years, with 2 nevi classified as hyperpigmentation and 1 as elevated. In patients aged 16 to 59 years, the largest number of nevi (38 of 71 [53.5%]) were categorized as hypopigmentation, followed by hyperpigmentation (23 of 71 [32.4%]) and elevated (10 of 71 [14.1%]).
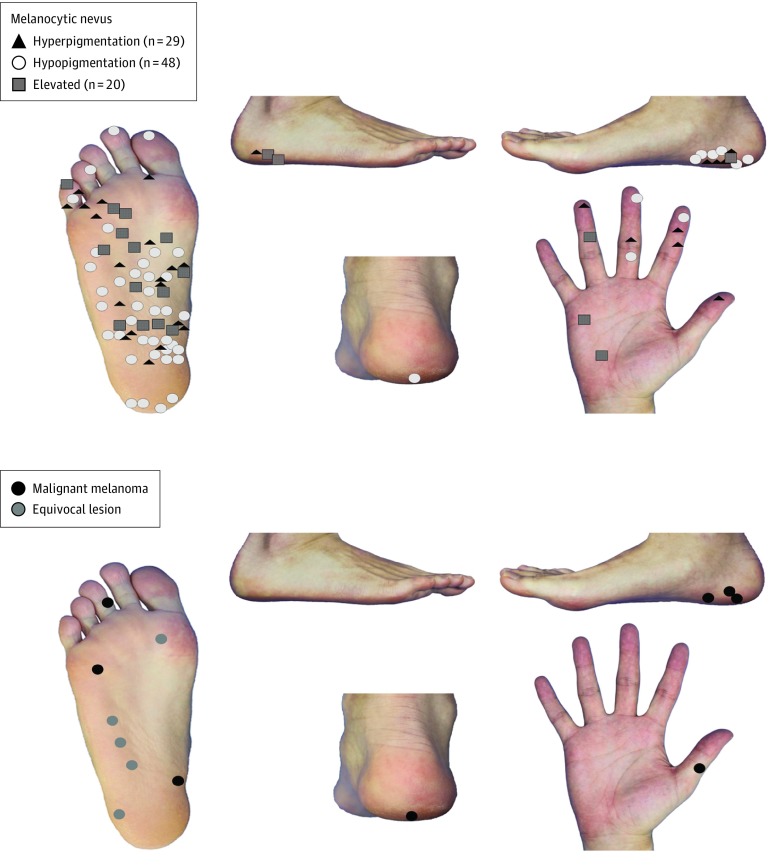
The anatomical prevalence of nevi; melanomas showing no PFP, LLP, FP, or PRP; and equivocal melanocytic lesions is shown in Figure 1. Melanomas and equivocal melanocytic lesions were localized predominantly on the sole of the foot, such as the heel and thenar area, while melanocytic nevi tended to be localized on non–weight-bearing areas.
Figure 1. Distribution of Melanocytic Nevi, Melanomas, and Equivocal Lesions on the Palm and Sole of the Foot .
Melanocytic nevi (n = 97) are widely distributed on the sole of the foot apart from weight-bearing areas, such as the heel, while melanomas (n = 8) and equivocal melanocytic lesions (n = 5) are preferentially located on weight-bearing regions.
The frequencies of dermoscopic findings are summarized in Table 2. No melanocytic nevi presented with benign, minor dermoscopic patterns such as globular, reticular, homogeneous, transition, globulostreak-like, and crista dotted. Ninety-five of 97 melanocytic nevi (97.9%) were symmetrical in at least 1 axis, with the remaining 2 and all melanomas displaying asymmetry. Most of the melanocytic nevi (91 [93.8%]) had 1 or 2 colors per lesion, and half of the melanomas (4 [50.0%]) had more than 2 colors. The areas of each color were symmetrically distributed on 5 of the 6 melanocytic nevi that had more than 2 colors. Vascular structures were observed in 3 melanocytic nevi (3.1%) and 3 melanomas (37.5%). Blue-white structures were seen in 18 melanocytic nevi (18.6%) and 3 melanomas (37.5%), and dots and globules were seen in 22 melanocytic nevi (22.7%) and 4 melanomas (50.0%); however, the findings were irregularly distributed in the melanomas. Ulcer, hyperkeratosis, diffuse pigmentation, abrupt ending at the periphery, and irregular streaks were observed only in melanomas.
Table 2. Dermoscopic Findings of Melanocytic Nevi, Melanomas, and Equivocal Lesions Not Showing Typical Dermoscopic Patterns.
| Dermoscopic Feature | Nevi, No. (%) | Melanomas, No. (%) (n = 8) | Equivocal Lesions, No. (%) (n = 5) | |||
|---|---|---|---|---|---|---|
| Hyperpigmentation (n = 29) | Hypopigmentation (n = 48) | Elevated (n = 20) | Total (n = 97) | |||
| Symmetry | ||||||
| In 1 axis | 6 (20.7) | 5 (10.4) | 6 (30.0) | 17 (17.5) | 0 | 3 (60.0) |
| In 2 axes | 23 (79.3) | 42 (87.5) | 13 (65.0) | 78 (80.4) | 0 | 0 |
| None | 0 | 1 (2.1) | 1 (5.0) | 2 (2.1) | 8 (100) | 2 (40.0) |
| Vessels | ||||||
| Dotted | 0 | 0 | 1 (5.0) | 1 (1.0) | 3 (37.5) | 0 |
| Comma | 0 | 0 | 2 (10.0) | 2 (2.1) | 0 | 0 |
| Glomerular | 0 | 0 | 0 | 0 | 1 (12.5) | 0 |
| No. of colors | ||||||
| 1 | 21 (72.4) | 35 (72.9) | 1 (5.0) | 57 (58.8) | 0 | 4 (80.0) |
| 2 | 8 (27.6) | 12 (25.0) | 14 (70.0) | 34 (35.1) | 4 (50.0) | 1 (20.0) |
| 3 | 0 | 1 (2.1) | 5 (25.0) | 6 (6.2) | 3 (37.5) | 0 |
| 4 | 0 | 0 | 0 | 0 | 1 (12.5) | 0 |
| Blue-white structures | ||||||
| Central | 3 (10.3) | 9 (18.8) | 2 (10.0) | 14 (14.4) | 0 | 0 |
| Peripheral | 0 | 0 | 0 | 0 | 0 | 0 |
| Broad | 0 | 4 (8.3) | 0 | 4 (4.1) | 3 (37.5) | 0 |
| Dots and globules | ||||||
| Regular | 5 (17.2) | 12 (25.0) | 5 (25.0) | 22 (22.7) | 0 | 0 |
| Peripheral | 0 | 0 | 0 | 0 | 4 (50.0) | 1 (20.0) |
| Ulcer | 0 | 0 | 0 | 0 | 1 (12.5) | 0 |
| Hyperkeratosis | 0 | 0 | 0 | 0 | 1 (12.5) | 0 |
| Diffuse pigmentation | 0 | 0 | 0 | 0 | 1 (12.5) | 0 |
| Ends abruptly at periphery | 0 | 0 | 0 | 0 | 1 (12.5) | 0 |
| Milky red areas | 0 | 0 | 0 | 0 | 3 (37.5) | 0 |
| Irregular streaks | 0 | 0 | 0 | 0 | 1 (12.5) | 0 |
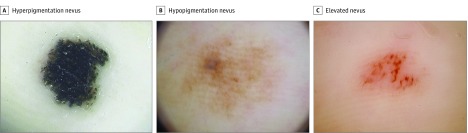
Nineteen of 97 melanocytic nevi underwent biopsy and were distributed as 4 with hyperpigmentation, 9 with hypopigmentation, and 6 with elevation. All hyperpigmented nevi were histopathologically compound nevi. Several epidermal nevus cells had proliferated mostly in a large nest formation (30-50 nevus cells), and melanin granule aggregation was frequently observed in the corner layer (Figure 2A and eFigure 1A in the Supplement). On the contrary, 7 of 9 hypopigmented nevi were a junctional nevus: 4 cases showed predominant individual proliferation of nevus cells in the epidermis rather than in a nest formation, and 3 cases presented a small, nested proliferation of 15 to 30 nevus cells in the epidermis. The remaining 2 cases with hypopigmentation featured a compound nevus with the predominant dermal nevus component corresponding to the areas of blue-whitish veil under dermoscopy (Figure 2B and eFigure 1B in the Supplement). In the elevated nevi, 5 of 6 lesions were compound nevi and 1 was a dermal nevus (Figure 2C and eFigure 1C in the Supplement). The dermal components of nevus cells were predominant in all compound nevi.
Figure 2. Dermoscopic Images of Representative Melanocytic Nevus Cases Not Showing Typical Benign Dermoscopic Patterns.
A, A black macule (4.9 × 4.3 mm) on the heel of a 68-year-old woman. Dark black pigmentation is observed on the entire lesion. B, A light-brown macule (11.0 × 10.0 mm) on the middle of the foot of a 77-year-old man. Linear light-brown pigmentation is detected without clear predominance on the furrows. C, A brown macule (5.1 × 4.4 mm) on the middle of the foot of a 31-year-old man. Enlarged pink ridges and dotted vessels are observed in the center of the lesion. Although the pigmentation appears to be in a fibrillar fashion, the pigmentation filaments are varied in size and distributed irregularly (original magnification ×10).
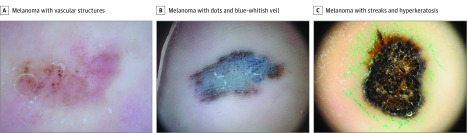
All 8 melanoma cases that did not present the typical PRP were biopsied. The median Breslow tumor thickness of the melanomas was 0.96 mm (range, in situ to 3.00 mm), and notable dermoscopic findings were recorded. In cases 1 to 3, dermoscopic clues in 1 hypomelanotic macule on the right heel were glomerular and dotted vessels associated with irregular black dots (Figure 3A and eFigure 2A in the Supplement). The other hypomelanotic melanoma presented a combination of dotted vessels and milky red areas, and 1 amelanotic melanoma presented a combination of ulcer and milky red areas. In cases 4 to 6, a blue to brown macule on the right heel exhibited irregular black dots covered with a blue-whitish veil on dermoscopy (Figure 3B and eFigure 2B in the Supplement). This combination was also observed in the other 2 melanomas. Histopathologically, the number of aggregations of atypical melanocytes and melanin granules in the stratum corneum corresponded to the black dots and globules under dermoscopy. In cases 7 and 8, streaks were irregularly distributed on the periphery of a black nodule on the left heel on dermoscopy (Figure 3C and eFigure 2C in the Supplement). Other diagnostic findings suggestive of melanoma were not apparent owing to surface hyperkeratosis. Results of histopathologic examination revealed a spitzoid melanoma with hyperkeratosis. The other melanoma of a brown macule on the left third toe exhibited diffuse pigmentation with abrupt edges.
Figure 3. Dermoscopic Images of Representative Melanoma Cases Not Showing Typical Parallel Ridge Pattern by Dermoscopy.
A, A light-brown macule (7.5 × 3.0 mm) on the heel of a 62-year-old woman. Results of dermoscopy show clusters of glomerular and dotted vessels on the right side of the image. Focal melanin deposition is detected on the left side of the image. B, A blue to brown macule (17.0 × 9.0 mm) on the heel of a 39-year-old man. Black dots and globules of various sizes are irregularly distributed on the lesion. C, A black macule with hyperkeratosis (11.0 × 8.9 mm) on the heel of a 77-year-old woman. Irregularly distributed streaks and hyperkeratosis are observed on the lesion surface (original magnification ×10).
Discussion
This study revealed that more than one-fifth of melanocytic lesions (21.8%) in the acral volar skin did not display typical PFP, LLP, FP, or PRP on dermoscopy. The profile of the 110 lesions was 97 of 428 melanocytic nevi (22.7%), 8 of 71 melanomas (11.3%), and 5 equivocal lesions. The frequency of melanocytic nevus without typical benign patterns was slightly higher than that of previous reports (5%-17%),5,6,7,8 likely because more patients in our series were referred to dermoscopy specialists by clinic doctors.
In the volar skin region, it is challenging to differentiate melanoma from melanocytic nevus, especially in the absence of typical PFP, LLP, FP, or PRP dermoscopic findings. The clinical management of these lesions basically followed the revised 3-step algorithm that recommended biopsy for lesions with a maximum diameter larger than 7 mm.4 Our descriptive data added that asymmetry, higher number of colors (≥3), irregular distribution of blue-white structures, dots and globules, and vascular structures including milky red areas were more frequently observed in melanomas and that ulcer, diffuse pigmentation, and irregular streaks were detected in melanomas only by dermoscopy. As a high prevalence of diffuse pigmentation (20.5%10 and 60%11), blue-white veil (42%11 and 32.8%12), peripheral dots and globules (12.1%,10 41%,11 and 46.6%12), and milky red areas (19.4%,10 42%,11 and 14.5%12) has already been reported in 3 melanoma case series on the volar skin,10,11,12 those dermoscopic findings appear to be clinically useful to identify melanoma even without PRP.
This study revealed that lesion size, patient age, and anatomical location of the lesion differed between melanomas and melanocytic nevi not showing typical dermoscopic patterns in the volar skin area. First, melanomas were significantly larger than melanocytic nevi; the maximum diameter of melanomas was greater than 7 mm in all cases compared with less than 7 mm in 68 of 97 melanocytic nevi (70.1%). This finding confirmed the validity of the revised 3-step algorithm in that melanocytic lesions on the palmoplantar area should be more carefully examined at larger sizes. Second, the age of patients was significantly higher for those with melanomas than for those with melanocytic nevi. We detected no patients with melanoma in this series who were younger than 20 years. The incidence of acral lentiginous melanoma in patients younger than 20 years was 0.1 per 1 000 000 US patients in a past report,13 indicating that melanocytic lesions without a typical dermoscopic pattern required more attention when appearing in individuals older than 20 years. Third, our data showed that melanocytic nevi presenting no PFP, LLP, or FP were widely distributed in areas of the sole apart from weight-bearing regions, such as the heel, which suggested that dermoscopic pattern was not influenced by mechanical load. In the heel and other weight-bearing plantar regions, dermoscopic patterns can be altered by the incline of the cornified layer, such as from PFP to FP.1 Indeed, anatomical location on the foot sole may be helpful to differentiate melanoma from melanocytic nevus because melanoma develops much more frequently on the heel than on the arch.14 Although further study is required to clarify the diagnostic accuracy of dermoscopy for melanocytic lesions in the volar skin area in association with anatomical location, our findings suggest that lesions on weight-bearing areas of the foot sole should be more strongly considered for biopsy than those on non–weight-bearing areas in the absence of a typical PFP, LLP, or FP.
We divided the cases of melanocytic nevus without PFP, LLP, or FP into 3 subgroups (hyperpigmentation, hypopigmentation, and elevated) for descriptive purposes. Regarding age, most nevi with hyperpigmentation were found in young patients (≤15 years), while nevi with hypopigmentation were more frequently detected in patients older than 60 years. Histopathologically, the hyperpigmented nevi were compound nevi composed of large nevi nests and melanin granules in the epidermis. The hypopigmented nevi, on the other hand, tended to be junctional nevi predominantly with individual nevus-cell proliferation. Moreover, the hypopigmented nevi featured capillary hyperplasia and melanophages in the upper dermis that have been described as the histopathologic features of regressed melanocytic nevi.15,16 Although we did not confirm a secular change, the dermoscopic characteristics of nevi by patient age reportedly reflect the regression process of melanocytic nevi during the lifetime.17 Last, the elevated nevi had an abundant dermal nevus component. The surface skin of the nevi was expanded by the protuberance of the dermal component to cause widening of the ridges, a finding previously termed central enlarged pink ridges.18
Limitations
This study has some limitations. First, this study was carried out in a single center and all the participants were Japanese. Second, this study included a small number of melanoma cases that did not show the typical PRP.
Conclusions
In this study, more than one-fifth of melanocytic lesions on the volar skin did not show typical dermoscopic patterns. Asymmetry, higher number of colors (≥3), and dermoscopic melanoma clues of blue-white structures, vascular structures, irregular dots, irregular streaks, and abrupt edges were more frequently observed in melanomas. Clinical information, such as patient age and lesion size and location, was helpful for clinicians to differentiate melanoma from melanocytic nevus. Prospective, multicenter clinical studies are warranted to clarify the diagnostic accuracy of dermoscopy combined with clinical information.
eFigure 1. Histopathologic Images of Melanocytic Nevus Cases in Figure 2
eFigure 2. Histopathologic Images of Melanoma Cases in Figure 3
References
- 1.Miyazaki A, Saida T, Koga H, Oguchi S, Suzuki T, Tsuchida T. Anatomical and histopathological correlates of the dermoscopic patterns seen in melanocytic nevi on the sole: a retrospective study. J Am Acad Dermatol. 2005;53(2):230-236. doi: 10.1016/j.jaad.2005.04.045 [DOI] [PubMed] [Google Scholar]
- 2.Minagawa A, Koga H, Saida T. Dermoscopic characteristics of congenital melanocytic nevi affecting acral volar skin. Arch Dermatol. 2011;147(7):809-813. doi: 10.1001/archdermatol.2011.150 [DOI] [PubMed] [Google Scholar]
- 3.Saida T, Miyazaki A, Oguchi S, et al. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol. 2004;140(10):1233-1238. doi: 10.1001/archderm.140.10.1233 [DOI] [PubMed] [Google Scholar]
- 4.Koga H, Saida T. Revised 3-step dermoscopic algorithm for the management of acral melanocytic lesions. Arch Dermatol. 2011;147(6):741-743. doi: 10.1001/archdermatol.2011.136 [DOI] [PubMed] [Google Scholar]
- 5.Saida T, Oguchi S, Ishihara Y. In vivo observation of magnified features of pigmented lesions on volar skin using video macroscope: usefulness of epiluminescence techniques in clinical diagnosis. Arch Dermatol. 1995;131(3):298-304. doi: 10.1001/archderm.1995.01690150062013 [DOI] [PubMed] [Google Scholar]
- 6.Malvehy J, Puig S. Dermoscopic patterns of benign volar melanocytic lesions in patients with atypical mole syndrome. Arch Dermatol. 2004;140(5):538-544. doi: 10.1001/archderm.140.5.538 [DOI] [PubMed] [Google Scholar]
- 7.Altamura D, Altobelli E, Micantonio T, Piccolo D, Fargnoli MC, Peris K. Dermoscopic patterns of acral melanocytic nevi and melanomas in a white population in central Italy. Arch Dermatol. 2006;142(9):1123-1128. doi: 10.1001/archderm.142.9.1123 [DOI] [PubMed] [Google Scholar]
- 8.Ozdemir F, Karaarslan IK, Akalin T. Variations in the dermoscopic features of acquired acral melanocytic nevi. Arch Dermatol. 2007;143(11):1378-1384. doi: 10.1001/archderm.143.11.1378 [DOI] [PubMed] [Google Scholar]
- 9.Kittler H, Marghoob AA, Argenziano G, et al. Standardization of terminology in dermoscopy/dermatoscopy: results of the third consensus conference of the International Society of Dermoscopy. J Am Acad Dermatol. 2016;74(6):1093-1106. doi: 10.1016/j.jaad.2015.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braun RP, Thomas L, Dusza SW, et al. Dermoscopy of acral melanoma: a multicenter study on behalf of the International Dermoscopy Society. Dermatology. 2013;227(4):373-380. doi: 10.1159/000356178 [DOI] [PubMed] [Google Scholar]
- 11.Phan A, Dalle S, Touzet S, Ronger-Savlé S, Balme B, Thomas L. Dermoscopic features of acral lentiginous melanoma in a large series of 110 cases in a white population. Br J Dermatol. 2010;162(4):765-771. doi: 10.1111/j.1365-2133.2009.09594.x [DOI] [PubMed] [Google Scholar]
- 12.Lallas A, Kyrgidis A, Koga H, et al. The BRAAFF checklist: a new dermoscopic algorithm for diagnosing acral melanoma. Br J Dermatol. 2015;173(4):1041-1049. doi: 10.1111/bjd.14045 [DOI] [PubMed] [Google Scholar]
- 13.Bradford PT, Goldstein AM, McMaster ML, Tucker MA. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145(4):427-434. doi: 10.1001/archdermatol.2008.609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minagawa A, Omodaka T, Okuyama R. Melanomas and mechanical stress points on the plantar surface of the foot. N Engl J Med. 2016;374(24):2404-2406. doi: 10.1056/NEJMc1512354 [DOI] [PubMed] [Google Scholar]
- 15.McCardle TW, Messina JL, Sondak VK. Completely regressed cutaneous melanocytic lesion revisited. Semin Oncol. 2009;36(6):498-503. doi: 10.1053/j.seminoncol.2009.10.003 [DOI] [PubMed] [Google Scholar]
- 16.Martín JM, Rubio M, Bella R, Jordá E, Monteagudo C. Complete regression of melanocytic nevi: correlation between clinical, dermoscopic, and histopathologic findings in 13 patients [in Spanish]. Actas Dermosifiliogr. 2012;103(5):401-410. doi: 10.1016/j.ad.2011.11.004 [DOI] [PubMed] [Google Scholar]
- 17.Zalaudek I, Argenziano G, Ferrara G, et al. Clinically equivocal melanocytic skin lesions with features of regression: a dermoscopic-pathological study. Br J Dermatol. 2004;150(1):64-71. doi: 10.1111/j.1365-2133.2004.05657.x [DOI] [PubMed] [Google Scholar]
- 18.Chuah SY, Tsilika K, Chiaverini C, et al. Dermoscopic features of congenital acral melanocytic naevi in children: a prospective comparative and follow-up study. Br J Dermatol. 2015;172(1):88-93. doi: 10.1111/bjd.13187 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Histopathologic Images of Melanocytic Nevus Cases in Figure 2
eFigure 2. Histopathologic Images of Melanoma Cases in Figure 3