Key Points
Question
Which histologic features in lentigo maligna diagnosed by an incisional partial biopsy are associated with an invasive component?
Findings
In this cross-sectional study of 96 patients with a lentigo maligna or lentigo maligna melanoma diagnosis, the presence of melanocytes arranged in rows, subepidermal clefts, nests, and a decreased degree of solar elastosis was associated with an invasive component.
Meaning
In patients with lentigo maligna diagnosed through an incisional biopsy and who are candidates for nonsurgical management, knowledge of the condition’s histologic characteristics may help in identifying the most appropriate management.
Abstract
Importance
Lentigo maligna (LM) presents an invasive component in up to 20% of biopsied cases, but to date the histologic features useful in detecting this invasive component have not been described. Some histologic characteristics are hypothesized to contribute to the progression of LM invasion.
Objective
To identify the histologic characteristics associated with lentigo maligna melanoma (LMM) in patients with LM diagnosed by a partial diagnostic biopsy.
Design, Setting, and Participants
A retrospective cross-sectional study of patients treated between January 1, 2000, and December 31, 2017, was conducted in a referral oncology center in València, Spain. Data and specimens of patients (n = 96) with a diagnosis of primary cutaneous melanoma in the form of either LM or LMM who had undergone surgical treatment, a complete histologic examination of the whole tumor, and an initial diagnostic partial biopsy of LM were included in the study. Histologic assessment was blinded to the presence of an invasive component.
Interventions
All biopsy specimens were evaluated for the presence of certain histologic characteristics.
Main Outcomes and Measures
Comparisons between invasive samples and samples without an invasive component were performed. The differences in the distribution of variables between the groups were assessed using the χ2 and Fisher exact tests, and the degree of association of the relevant variables was quantified by logistic regression models. A classification and regression tree analysis was performed to rank the variables by importance.
Results
In total, 96 patients had sufficient histologic material that could be evaluated. The patients were predominantly male (56 [58.3%]) and had a mean (SD) age at diagnosis of 72 (12) years. Of these patients, 63 (65.6%) had an LM diagnosis and 33 (34.4%) had an LMM diagnosis (an invasive component). The histologic variables associated with the presence of an invasive component were melanocytes forming rows (odds ratio [OR], 11.5; 95% CI, 1.4-94.1; P = .02), subepidermal clefts (OR, 2.8; 95% CI, 1.0-7.9; P = .049), nests (OR, 3.0; 95% CI, 1.1-8.6; P = .04), and a lesser degree of solar elastosis (OR, 0.4; 95% CI, 0.1-1.1; P = .07). A classification and regression tree analysis of the relevant histologic features was able to accurately identify lentigo maligna with an invasive component (LMM) in more than 60% of patients.
Conclusions and Relevance
These findings may be useful in classifying early LM specimens at higher risk of invasion, which may eventually be relevant in identifying the most appropriate management for LM.
This cross-sectional study examines the histopathological features found with a partial biopsy of lentigo maligna tumors that are associated with an invasive component.
Introduction
Cutaneous melanoma is a malignant tumor developed from the melanocytes of the epithelium of the skin and its appendages. It accounts for 3% of all cancers, and it is responsible for 80% of deaths from skin cancer worldwide.1 Lentigo maligna (LM) or lentigo maligna melanoma (LMM) is one of the classic clinicopathological types of melanoma. This type of melanoma has a long-standing in situ radial growth phase, accounting for 80% of all in situ melanomas in white populations.2
If untreated, LM can become invasive (LMM). Invasion occurs in 5% to 50% of LM, although the actual risk for progression remains unknown.3 Lentigo maligna melanoma represents 4% to 15% of all invasive cutaneous melanomas.4 It has the same prognosis as the other invasive melanoma types when adjusted for common prognostic factors, such as Breslow thickness, ulceration, or tumor mitotic rate.5 Patients with invasive lesions may require more complex management with sentinel lymph node examination and wider excision.
Lentigo maligna develops on chronic sun-damaged skin and most often in older adults between 65 and 80 years of age.6,7,8 Therefore, LM appears more frequently on sites chronically exposed to the sun, mostly on the head9 and particularly on the cheeks in women and the scalp or the nose in men.10 To a lesser extent, it can develop on the upper back and on the dorsum of the forearm.11
Clinically, LM presents as an irregularly pigmented and shaped macule that grows slowly for months or years. The appearance of a plaque or nodule, which can happen at any time during its evolution, indicates dermal invasion or the development of a desmoplastic melanoma.12 Histologically, LM is characterized by a basal irregular proliferation of atypical melanocytes (not equidistant to one another), with scarce pagetoid spread, constant adnexal involvement, poorly defined borders, and a variable degree of solar elastosis.6,12,13 Cytologically, 2 patterns are distinguished: the first is a continuous proliferation of uniformly atypical nevoid to epithelioid melanocytes along the dermoepidermal junction, and the second is a tendency toward nest formation.6
Previous studies suggest that certain histologic findings might indicate sequential phases of LM evolution from the time of tumor onset to the final in situ stage before the tumor penetrates the dermis.12,13 Initially, subtle changes are found, with epidermal atypical melanocytes distributed at an unequal distance above a dermis with solar elastosis. After these subtle changes, melanocytes increase in number and become more spindled. Subsequently, they appear irregularly distributed along the basal membrane and occasionally are confluent, forming rows and involving the adnexal epithelia. In more advanced stages, melanocytes can aggregate and form nests.13,14 To our knowledge, this hypothetical histologic evolution has not been previously evaluated.13
Because LM can eventually invade and evolve into LMM, the treatment of choice is a surgical procedure, preferably one that allows complete margin assessment such as slow Mohs.15 However, the lesion characteristics, such as anatomic location, and the patient performance status may preclude the operation, and nonsurgical treatments (imiquimod or radiotherapy) or even surveillance16,17 may be advised. In cases in which a nonsurgical approach is considered, ruling out the presence of an invasive component is crucial.
Thus far, the presence of an invasive component has not been detected when an initial diagnosis of LM is made by a partial biopsy, a finding that occurs in a maximum of 20% of such lesions.18 We hypothesize that certain histologic features found in partial diagnostic biopsies are associated with an invasive component in the whole lesion. In this study, we aimed to identify the histologic characteristics associated with LMM in a series of 96 patients with LM diagnosed by a partial diagnostic biopsy.
Methods
This retrospective cross-sectional study was approved by the internal review board of the Instituto Valenciano de Oncología in València, Spain. Verbal informed consent was obtained from all patients who were alive at the time of the study.
Patient data were obtained from the melanoma database of the Instituto Valenciano de Oncología, an oncology center in which patients with cancer are diagnosed and treated; specifically, the department of dermatology at the center is a referral unit for melanoma management and Mohs surgery in the Valencia region. For the purpose of this study, we selected patients with a diagnosis of primary cutaneous melanoma in the form of either LM or LMM who had undergone surgical treatment, a complete histologic examination of the whole tumor, and an initial diagnostic partial biopsy of LM between January 1, 2000, and December 31, 2017. This initial diagnostic partial biopsy is commonly done by deep shave biopsy (saucerization) or, to a lesser extent, by incisional biopsy. We included for review only those specimens with adequate available histologic material, which allowed the assessment of both the tumor area and surrounding healthy skin in the partial biopsy. Recurrent tumors were excluded.
Sequential conventional sectioning of the samples was performed, and all of the slides were reviewed independently by 2 dermatopathologists (O.S and V.T.), who were blinded to the presence or absence of an invasive component. The level of agreement between the observers varied, ranging from κ = 0.4 to κ = 0.8 (eTable in the Supplement). All discrepancies in the readings were resolved after a conjunct review of all cases in which an agreement on the final value for each variable for the analysis was reached.
Two patient groups were defined for the study: those with an LM diagnosis and those with an LMM diagnosis. The LM diagnosis was defined according to previously described characteristics,12 including an increased number of atypical and enlarged melanocytes irregularly disposed along the basal layer of the epidermis, with occasional agglomeration in files, and involvement of the adnexal epithelia, overlying a dermis with at least moderate solar elastosis. Cases with a predominance of smaller cells and with an absence of solar elastosis or presence of only single elastic fibers were considered as having a lentiginous pattern of superficial spreading melanomas and were, therefore, excluded.
To evaluate all characteristics, we collected histologic variables after revision of the original slides of those specimens with sufficient material. For each specimen of the diagnostic partial biopsy, we assessed the following features: pagetoid extension (none or rare, <25%, 25%-50%, or >50% of intraepidermal melanocytes), nest formation (none or rare, <25%, 25%-50%, or >50% of intraepidermal cells), solar elastosis in surrounding healthy skin (classified as chronic sun damage [CSD] or non-CSD according to previously described criteria19]), epidermal contour (atrophic, thinned, normal, thickened, or hyperplastic), lateral circumscription (discontinuous, gradual but continuous, or abrupt), melanocyte confluence along the junction that forms rows (yes or no), extensive or deep involvement of adnexal epithelium (yes or no), presence of multinucleated melanocytes (yes or no), presence of melanophages (yes or no), and presence of subepidermal clefts. The descriptions of these features are detailed in the eAppendix in the Supplement. For epidemiologic variables, we included age (categorized into 2 groups), sex (male or female), and anatomic location of the melanoma (head and neck or other locations).
Because of the recent changes to the World Health Organization classification of skin tumors, in which cases with a lentiginous pattern and with low levels of solar elastosis (non-CSD) are included in the general category of low-CSD melanoma (superficial spreading melanomas), we decided to repeat our analyses. We included only the cases with a CSD degree of solar elastosis as a post hoc analysis.
Statistical Analysis
The differences in the distributions of the variables were assessed using the χ2 and Fisher exact tests. Only 2-sided P < .05 were considered statistically significant. We used univariable and multivariable logistic regression models to calculate the odds ratios (ORs) and 95% CIs, which were used as measures of the association between the most important variables and the presence of an invasive component. Only variables with a P < .10 in the univariable model were included in the multivariable analyses. Furthermore, a classification and regression tree analysis was performed to arrange in order of importance the associations between the relevant variables and the presence of an invasive component. All analyses were carried out with the IBM SPSS, version 20.0 statistical software package (IBM).
Results
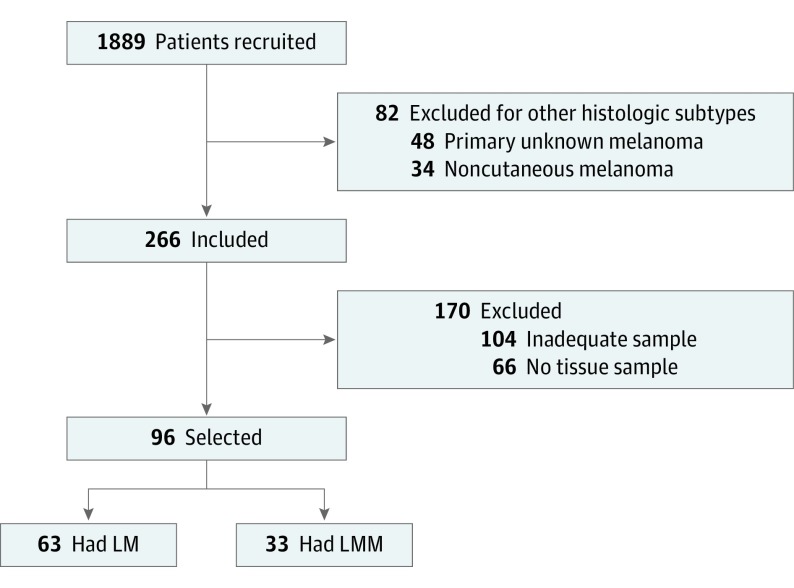
Of the 1889 patients recruited, 266 (14.0%) had an LM or LMM diagnosis. Of these 266 patients, 96 (36.1%) had sufficient histologic material that could be evaluated for the proposed features in the histologic study. The study group included 56 male patients (58.3%) and had a mean (SD) age at diagnosis of 72 (12) years. In total, 63 patients (65.6%) had an LM diagnosis and 33 (34.4%) had an LMM diagnosis (Figure 1).
Figure 1. Flow Diagram of Patient Selection Process.
LM indicates lentigo maligna; LMM, lentigo maligna melanoma.
The face or scalp was the most common site of presentation (23 [69.7%]), and a histologic pattern of chronic sun exposure was predominant (76% [73 of 96]) (Table 1). Furthermore, when the clinical and histologic features were compared between LM and LMM, we found that subepidermal clefts (OR, 2.8; 95% CI, 1.0-7.9; P = .049), melanocytes forming rows (OR, 11.5; 95% CI, 1.4-94.1; P = .02) or nests (OR, 3.0; 95% CI, 1.1-8.6; P = .04), and a non-CSD degree of elastosis (OR for CSD, 0.4; 95% CI, 0.1-1.1; P = .07) were statistically significantly associated with the presence of an invasive component (Table 2).
Table 1. Characteristics of Study Variables.
| Variable | No. (%) | P Value | ||
|---|---|---|---|---|
| Total (N = 96) | LM or In Situ (N = 63) | LMM or Invasive (N = 33) | ||
| Age at diagnosis, mean (SD), y | 72 (12) | 70 (11) | 72 (13) | .29 |
| <70 | 42 (43.8) | 30 (47.6) | 12 (36.4) | |
| ≥70 | 54 (56.2) | 33 (52.4) | 21 (63.6) | |
| Sex | .23 | |||
| Male | 56 (58.3) | 34 (54.0) | 22 (58.3) | |
| Female | 40 (41.7) | 29 (46.0) | 11 (41.7) | |
| Location | .29 | |||
| Face or scalp | 73 (76.0) | 50 (79.4) | 23 (69.7) | |
| Other | 23 (24.0) | 13 (20.6) | 10 (30.3) | |
| Solar elastosis degreea | .04 | |||
| Non-CSD | 23 (24.0) | 11 (17.5) | 12 (36.4) | |
| CSD | 73 (76.0) | 52 (82.5) | 21 (63.6) | |
| Pagetoid extension | .74 | |||
| No | 31 (32.3) | 21 (33.3) | 10 (32.3) | |
| <50% | 50 (52.1) | 32 (50.8) | 18 (52.1) | |
| 50% | 13 (13.5) | 8 (12.7) | 5 (13.5) | |
| >50% | 2 (2.1) | 2 (3.2) | 0 (2.1) | |
| Nests formation | .002 | |||
| No | 29 (30.2) | 23 (36.5) | 6 (18.2) | |
| <25% | 40 (41.7) | 29 (46.0) | 11 (33.3) | |
| 25%-50% | 16 (16.7) | 9 (14.3) | 7 (21.2) | |
| >50% | 11 (11.5) | 2 (3.2) | 9 (27.3) | |
| Nests 2 categories | .001 | |||
| No, <25% | 69 (71.9) | 52 (82.5) | 17 (51.5) | |
| ≥25% | 27 (28.1) | 11 (17.5) | 16 (48.5) | |
| Epidermal contour | .99 | |||
| Atrophic | 11 (11.5) | 7 (11.1) | 4 (12.1) | |
| Thinned | 21 (21.9) | 13 (20.6) | 8 (24.2) | |
| Normal | 35 (36.5) | 23 (36.5) | 12 (36.4) | |
| Thickened | 12 (12.5) | 8 (12.7) | 4 (12.1) | |
| Hyperplastic | 17 (17.7) | 12 (19.0) | 5 (15.2) | |
| Melanocytes forming rows | <.001 | |||
| No | 24 (25.0) | 23 (36.5) | 1 (3.0) | |
| Yes | 72 (75.0) | 40 (63.5) | 32 (97.0) | |
| Extensive adnexal epithelium involvement | .26 | |||
| No | 77 (80.2) | 53 (84.1) | 24 (80.2) | |
| Yes | 19 (19.8) | 10 (15.9) | 9 (19.8) | |
| Multinucleated melanocytes | .23 | |||
| No | 85 (88.5) | 54 (85.7) | 31 (93.9) | |
| Yes | 11 (11.5) | 9 (14.3) | 2 (6.1) | |
| Melanophages | .25 | |||
| No | 21 (21.9) | 16 (25.4) | 5 (15.2) | |
| Yes | 75 (78.1) | 47 (74.6) | 28 (84.8) | |
| Subepidermal clefts | .005 | |||
| No | 67 (69.8) | 50 (79.4) | 17 (51.5) | |
| Yes | 29 (30.2) | 13 (20.6) | 16 (48.5) | |
Abbreviations: CSD, chronic sun damage; LM, lentigo maligna; LMM, lentigo maligna melanoma.
Solar elastosis in CSD degree according to previously described criteria.17
Table 2. Associations of Histologic Features With the Presence of an Invasive Component .
| Variable | OR (95% CI) | P Value |
|---|---|---|
| Melanocytes forming rows | 11.5 (1.4-94.1) | .02 |
| ≥25% Melanocytes forming nests | 3.0 (1.1-8.6) | .04 |
| Subepidermal clefts | 2.8 (1.0-7.9) | .049 |
| Chronic sun damagea | 0.4 (0.1-1.1) | .07 |
Abbreviation: OR, odds ratio.
Solar elastosis in the degree of sun damage according to previously described criteria.17
Accordingly, we observed that melanocytes forming rows were more frequently present in LMM samples than in LM samples (97% vs 63.5%; P < .001); only 1 patient (3.0%) with LMM did not present this feature. In addition, the presence of subepidermal clefts was more frequently associated with an invasive component compared with in situ stage (16 [48.5%] vs 13 [20.6%]; P = .005), and the presence of at least 25% of the intraepidermal melanocytes arranged in nests was more frequent in LMM than in LM (16 [48.5%] vs 11 [17.5%]; P = .001). Lesions with an invasive component had solar elastosis in non-CSD degree more frequently than those with LM (12 [36.4%] vs 11 [17.5%]; P = .04) (Table 1). All of these variables were included in the final multivariate regression model (Table 2). No statistically significant differences were found in the remaining variables (age, sex, previous history of sun exposure, presence of melanophages, pagetoid extension, extent of adnexal implication, multinucleated melanocytes, and epidermal thickness) between the LM and LMM groups.
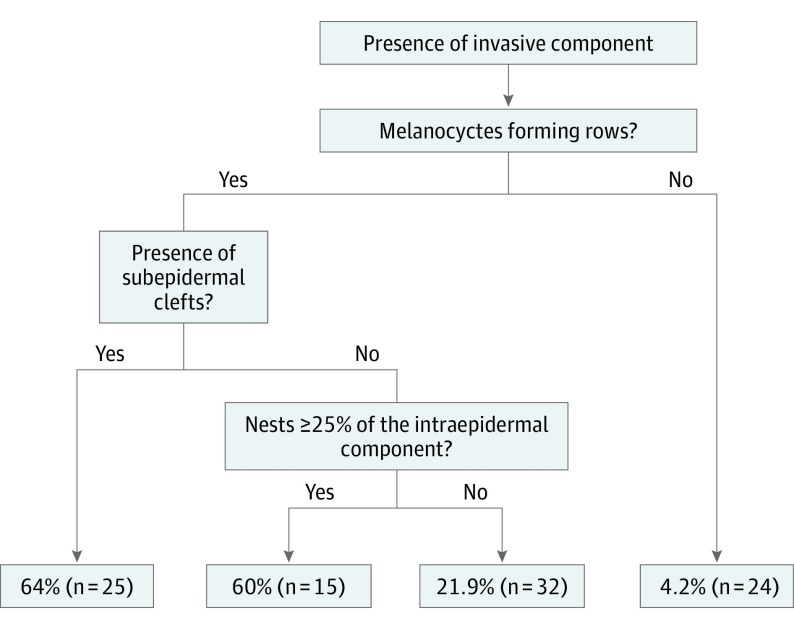
When we performed the classification and regression tree analysis (Figure 2), we found that the most relevant variable associated with invasion was melanocytes forming rows. The second most important variable highlighted by the classification and regression tree analysis was the subepidermal clefts. In the absence of both variables, the presence of 25% or more melanocytes forming nests located in the epidermis was the most relevant. In repeating our analyses to include only the 73 cases with a CSD degree of solar elastosis in a post hoc analysis, we found that melanocytes forming rows (OR, 8.0; 95% CI, 1.0-9.8) and 25% or more melanocytes forming nests (OR, 3.1; 95% CI, 1.0-67.0) were associated with LMM.
Figure 2. Classification and Regression Tree Analysis for the Probability of Invasive Component.
Discussion
In this study, we observed that melanocytes forming rows, nests, subepidermal clefts, and a lesser degree of elastosis were all statistically significant histologic features, found in a partial biopsy of LM lesions, frequently associated with an invasive component (Figure 2). The first 3 findings may reflect an increased proliferative process in which melanocytes in the dermal-epidermal junction are abundant.
To our knowledge, no previous studies have evaluated whether the histologic findings of a partial biopsy of a clinically suspicious LM are associated with the presence of an invasive component. Massi and LeBoit12 describe the presence of melanocyte nests in LM as a previous step for invasion, although this description is based on the experience of those authors rather than on published studies. In the current study, the presence of nests (specifically when they formed 25% or more of the intraepidermal melanocyte component) was statistically significantly associated with the presence of an invasive component, although this association was most valuable in the cases of melanocytes forming rows without subepidermal clefts (Figure 3).
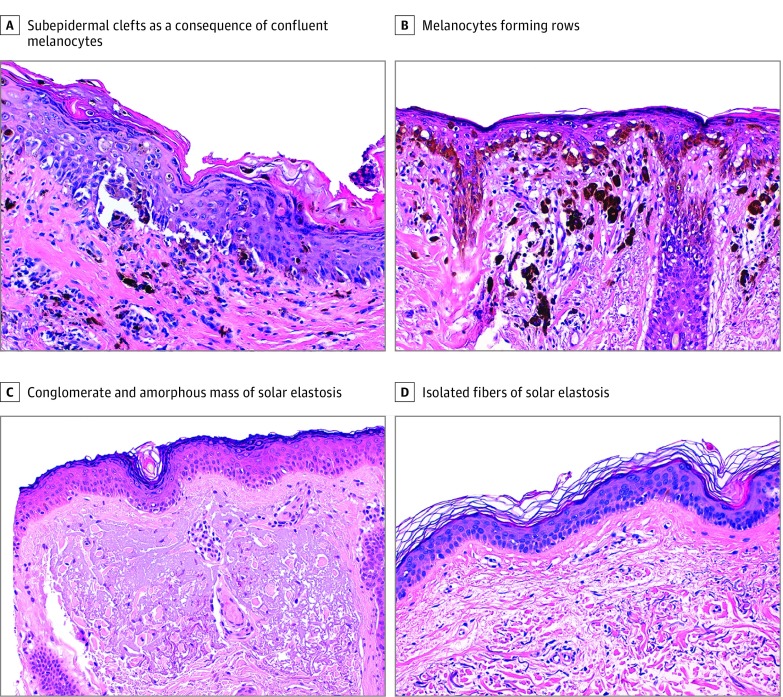
Figure 3. Representative Examples of the Most Relevant Histologic Features.
A, Subepidermal clefts as a consequence of confluent melanocytes along the junction (hematoxylin-eosin, original magnification ×200). B, Melanocytes forming rows along the basal layer (hematoxylin-eosin, original magnification ×200). C, Conglomerate and amorphous mass of solar elastosis in degree of chronic sun damage (CSD) (hematoxylin-eosin, original magnification ×200). D, Isolated fibers of solar elastosis in dermis in degree of non–CSD (hematoxylin-eosin, original magnification ×200).
Although no consistent criteria for distinguishing the evolutionary stages between LM and LMM have been proposed, the association of melanocytes forming rows, nests, and subepidermal clefts with the presence of an invasive component suggests that these histologic characteristics are probably associated with increased melanocytic proliferation, which represents a previous step of the invasive phase of LMM. Likewise, the association between a lesser degree of elastosis and an invasive component suggests that the peritumoral stroma could play a role in LM progression. Alternatively, this association may be because of a different mutational profile, which justifies the most recent inclusion of non-CSD melanomas with a lentiginous pattern of growing within superficial spreading melanomas.6 Aside from the degree of solar elastosis, subtle differences (such as a predominance of smaller cells) are described to differentiate those cases from LM.6 In the current study, when repeating the analysis but excluding non-CSD lesions, all the same histologic characteristics remained statistically significantly associated with the presence of an invasive component.
When LM is diagnosed through a partial biopsy, complete excision of the tumor and its posterior histopathological analysis will indicate the presence of an invasive component in up to 20% of cases.18 This fact can offer 1 explanation, among others, for the subsequent development of LMM in patients with a previous diagnosis of LM who underwent nonsurgical treatment (eg, topical imiquimod).20 In daily clinical practice, some patients are not candidates for surgical treatment, owing to their physical status and/or the location of their tumor. For these patients, a nonsurgical approach is selected (eg, imiquimod, radiotherapy, even clinical follow-up).17,21 The selection of an adequate second-line treatment depends mainly on the presence of an invasive component in the biopsy result, in which case radiotherapy is typically selected as the most adequate treatment.
The diagnosis of LM is often made through a partial biopsy (the specimen for which is usually taken from the most atypical area), with clinical and/or dermatoscopic features that are compatible with the diagnosis and (if LM is present) with the highest probability of presenting an invasive component. An invasive component could also be detected by using noninvasive imaging methods, such as dermatoscopy, hyperspectral imaging, or confocal microscopy. Multiple colors, pigmented rhomboidal structures, follicle obliteration, and erythematous rhomboidal structures are all dermatoscopic signs associated with an invasive component.22 Although hyperspectral images have demonstrated a sensitivity of 90% and a specificity of 86.3% in detecting LMM,23 we do not have enough experience with this technology to validate its use for the early diagnosis of LMM.24 Confocal microscopy makes it possible to completely evaluate the lesion in vivo and to detect areas suggestive of an invasive component.25 In addition, confocal microscopy may be used to identify a persistently invasive component in patients previously treated with radiotherapy; in this way, the real efficacy of the treatment could be determined.25 However, confocal microscopy, which would make our findings clinically relevant, is currently not available in all dermatology clinics.
These findings appear to warrant larger, multicenter prospective studies that could validate their utility. If corroborated, these results may be helpful in identifying the most appropriate management strategy for LM, especially when nonsurgical therapy is selected.
Strengths and Limitations
The strengths of this study include the rigorous data collection and analysis processes we used. In addition, we performed a reevaluation of all histologic samples using standardized criteria. Limitations of this study include its retrospective review of cases. For this reason, the method for selecting biopsy areas was not standardized and the clinical or dermoscopic information of the whole lesion and the biopsied area was not available. Moreover, the histopathological samples for some eligible patients were not available for review.
Conclusions
Results of this study seem to indicate that melanocytes forming rows, nests, subepidermal clefts, and a lesser degree of elastosis are histologic features in incisional LM samples that are associated with the presence of an invasive component. A prospective study may be warranted to validate the utility of these findings.
eAppendix. Histopathological Feature Descriptions
eTable. Interobserver Agreement for Each Studied Variable
References
- 1.Piérard-Franchimont C, Hermanns-Lê T, Delvenne P, Piérard GE. Dormancy of growth-stunted malignant melanoma: sustainable and smoldering patterns. Oncol Rev. 2014;8(2):252. doi: 10.4081/oncol.2014.252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gardner KH, Hill DE, Wright AC, et al. Upstaging from melanoma in situ to invasive melanoma on the head and neck after complete surgical resection. Dermatol Surg. 2015;41(10):1122-1125. doi: 10.1097/DSS.0000000000000466 [DOI] [PubMed] [Google Scholar]
- 3.Patel AN, Perkins W, Leach IH, Varma S. Johnson square procedure for lentigo maligna and lentigo maligna melanoma. Clin Exp Dermatol. 2014;39(5):570-576. doi: 10.1111/ced.12363 [DOI] [PubMed] [Google Scholar]
- 4.Elsner P, Diepgen TL, Schliemann S. Lentigo maligna and lentigo maligna melanoma as occupational skin diseases in a forestry worker with long-standing occupational UV-exposure. J Dtsch Dermatol Ges. 2014;12(10):915-917. [DOI] [PubMed] [Google Scholar]
- 5.Mora AN, Karia PS, Nguyen BM. A quantitative systematic review of the efficacy of imiquimod monotherapy for lentigo maligna and an analysis of factors that affect tumor clearance. J Am Acad Dermatol. 2015;73(2):205-212. doi: 10.1016/j.jaad.2015.05.022 [DOI] [PubMed] [Google Scholar]
- 6.Elder DE, Bastian BC, Kim J, et al. Lentigo maligna melanoma In: Elder DE, Massi D, Scolyer RA, Willemze R, eds. WHO Classification of Skin Tumours. 4th ed Lyon, France: International Agency for Research on Cancer; 2018:102-104. [Google Scholar]
- 7.Samaniego E, Redondo P. Lentigo maligna. Actas Dermosifiliogr. 2013;104(9):757-775. doi: 10.1016/j.ad.2012.05.006 [DOI] [PubMed] [Google Scholar]
- 8.Stadelmeyer E, Heitzer E, Resel M, Cerroni L, Wolf P, Dandachi N. The BRAF V600K mutation is more frequent than the BRAF V600E mutation in melanoma in situ of lentigo maligna type. J Invest Dermatol. 2014;134(2):548-550. doi: 10.1038/jid.2013.338 [DOI] [PubMed] [Google Scholar]
- 9.Star P, Guitera P. Lentigo maligna, macules of the face, and lesions on sun-damaged skin: confocal makes the difference. Dermatol Clin. 2016;34(4):421-429. doi: 10.1016/j.det.2016.05.005 [DOI] [PubMed] [Google Scholar]
- 10.Tiodorovic-Zivkovic D, Argenziano G, Lallas A, et al. Age, gender, and topography influence the clinical and dermoscopic appearance of lentigo maligna. J Am Acad Dermatol. 2015;72(5):801-808. doi: 10.1016/j.jaad.2015.01.030 [DOI] [PubMed] [Google Scholar]
- 11.Jaimes N, Marghoob AA, Rabinovitz H, et al. Clinical and dermoscopic characteristics of melanomas on nonfacial chronically sun-damaged skin. J Am Acad Dermatol. 2015;72(6):1027-1035. doi: 10.1016/j.jaad.2015.02.1117 [DOI] [PubMed] [Google Scholar]
- 12.Massi G, LeBoit PE. Histological Diagnosis of Nevi and Melanoma. 2nd ed. Berlin, Germany: Springer-Verlag; 2014. [Google Scholar]
- 13.Clark WH Jr, Mihm MC Jr. Lentigo maligna and lentigo-maligna melanoma. Am J Pathol. 1969;55(1):39-67. [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins HW II, Lee KC, Galan A, Leffell DJ. Melanoma in situ: part II. histopathology, treatment, and clinical management. J Am Acad Dermatol. 2015;73(2):193-203. doi: 10.1016/j.jaad.2015.03.057 [DOI] [PubMed] [Google Scholar]
- 15.Hou JL, Reed KB, Knudson RM, et al. Five-year outcomes of wide excision and Mohs micrographic surgery for primary lentigo maligna in an academic practice cohort. Dermatol Surg. 2015;41(2):211-218. doi: 10.1097/DSS.0000000000000248 [DOI] [PubMed] [Google Scholar]
- 16.Kai AC, Richards T, Coleman A, Mallipeddi R, Barlow R, Craythorne EE. Five-year recurrence rate of lentigo maligna after treatment with imiquimod. Br J Dermatol. 2016;174(1):165-168. doi: 10.1111/bjd.14311 [DOI] [PubMed] [Google Scholar]
- 17.Nagore E, Botella-Estrada R. Imiquimod in the treatment of lentigo maligna [in Spanish]. Actas Dermosifiliogr. 2011;102(8):559-562. doi: 10.1016/j.ad.2011.03.003 [DOI] [PubMed] [Google Scholar]
- 18.Erickson C, Miller SJ. Treatment options in melanoma in situ: topical and radiation therapy, excision and Mohs surgery. Int J Dermatol. 2010;49(5):482-491. doi: 10.1111/j.1365-4632.2010.04423.x [DOI] [PubMed] [Google Scholar]
- 19.Landi MT, Bauer J, Pfeiffer RM, et al. MC1R germline variants confer risk for BRAF-mutant melanoma. Science. 2006;313(5786):521-522. doi: 10.1126/science.1127515 [DOI] [PubMed] [Google Scholar]
- 20.Tio D, van der Woude J, Prinsen CAC, Jansma EP, Hoekzema R, van Montfrans C. A systematic review on the role of imiquimod in lentigo maligna and lentigo maligna melanoma: need for standardization of treatment schedule and outcome measures. J Eur Acad Dermatol Venereol. 2017;31(4):616-624. doi: 10.1111/jdv.14085 [DOI] [PubMed] [Google Scholar]
- 21.Marsden JR, Newton-Bishop JA, Burrows L, et al. ; British Association of Dermatologists Clinical Standards Unit . Revised U.K. guidelines for the management of cutaneous melanoma 2010. Br J Dermatol. 2010;163(2):238-256. doi: 10.1111/j.1365-2133.2010.09883.x [DOI] [PubMed] [Google Scholar]
- 22.Pralong P, Bathelier E, Dalle S, Poulalhon N, Debarbieux S, Thomas L. Dermoscopy of lentigo maligna melanoma: report of 125 cases. Br J Dermatol. 2012;167(2):280-287. doi: 10.1111/j.1365-2133.2012.10932.x [DOI] [PubMed] [Google Scholar]
- 23.Neittaanmäki N, Salmivuori M, Pölönen I, et al. Hyperspectral imaging in detecting dermal invasion in lentigo maligna melanoma. Br J Dermatol. 2017;177(6):1742-1744. doi: 10.1111/bjd.15267 [DOI] [PubMed] [Google Scholar]
- 24.Smith L, Macneil S. State of the art in non-invasive imaging of cutaneous melanoma. Skin Res Technol. 2011;17(3):257-269. doi: 10.1111/j.1600-0846.2011.00503.x [DOI] [PubMed] [Google Scholar]
- 25.Richtig E, Arzberger E, Hofmann-Wellenhof R, Fink-Puches R. Assessment of changes in lentigo maligna during radiotherapy by in-vivo reflectance confocal microscopy: a pilot study. Br J Dermatol. 2015;172(1):81-87. doi: 10.1111/bjd.13141 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Histopathological Feature Descriptions
eTable. Interobserver Agreement for Each Studied Variable