Abstract
We have previously shown that protein kinase Cε (PKCε) induces neurite outgrowth via its regulatory domain and independently of its kinase activity. This study aimed at identifying mechanisms regulating PKCε-mediated neurite induction. We show an increased association of PKCε to the cytoskeleton during neuronal differentiation. Furthermore, neurite induction by overexpression of full-length PKCε is suppressed if serum is removed from the cultures or if an actin-binding site is deleted from the protein. A peptide corresponding to the PKCε actin-binding site suppresses neurite outgrowth during neuronal differentiation and outgrowth elicited by PKCε overexpression. Neither serum removal, deletion of the actin-binding site, nor introduction of the peptide affects neurite induction by the isolated regulatory domain. Membrane targeting by myristoylation renders full-length PKCε independent of both serum and the actin-binding site, and PKCε colocalized with F-actin at the cortical cytoskeleton during neurite outgrowth. These results demonstrate that the actin-binding site is of importance for signals acting on PKCε in a pathway leading to neurite outgrowth. Localization of PKCε to the plasma membrane and/or the cortical cytoskeleton is conceivably important for its effect on neurite outgrowth.
INTRODUCTION
The members of the protein kinase C (PKC) family are implicated in the regulation of a wide range of cellular processes. Based on structural similarities and requirement for activators, this family of serine/threonine kinases can be subgrouped into classical (α, βI, βII, and γ), novel (δ, ε, η, and θ), and atypical (ι/λ and ζ) PKC isoforms (Nishizuka, 1992; Newton, 1995; Liu, 1996).
The outgrowth of neurites that accompanies neuronal differentiation is one cellular process that has been suggested to be regulated by PKC. Based on experiments with cell lines of various origin, both PKCδ (O'Driscoll et al., 1995; Corbit et al., 1999) and PKCε (Hundle et al., 1995; Fagerström et al., 1996; Hundle et al., 1997; Brodie et al., 1999; Zeidman et al., 1999) have been proposed to be the PKC isoform that positively regulates neurite outgrowth. This could suggest that these isoforms have redundant functions, but in several other cell systems PKCδ and ε have unique and sometimes opposite effects (Mischak et al., 1993; Lehel et al., 1994; Fleming et al., 1998).
We have previously demonstrated that in neuroblastoma cells, overexpression of PKCε, but not PKCα, βII, or δ leads to neurite outgrowth (Zeidman et al., 1999). The effect is mediated by the regulatory domain (RD) and independent of the catalytic activity of the kinase. We also identified a dominant negative construct that suppresses both PKCε-mediated neurite induction and the outgrowth of neurites that accompanies neuronal differentiation. This suppression was observed when using two established differentiation protocols of neuroblastoma cells: treatment with retinoic acid (RA) of SK-N-BE(2) cells (Helson and Helson, 1985; Hanada et al., 1993) and with nerve growth factor (NGF) of SH-SY5Y cells stably transfected with TrkA (Lavenius et al., 1995). This provides evidence for the involvement of PKCε in regulation of neurite outgrowth during differentiation of neuroblastoma cells.
The fact that increasing the levels of PKCε is sufficient to induce neurites could imply that elevation of endogenous levels of PKCε may be a mechanism through which neurite outgrowth is induced during neuronal differentiation. Another putative mechanism leading to PKCε-mediated neurite outgrowth may be a shift toward a neurite-inducing state of PKCε, which may involve either a change in localization and/or conformation of the PKCε molecule. Such alterations have been shown to take place when regulators interact with the PKC molecule (reviewed in Newton, 1997). Overexpression of PKCε would in this case, by increasing the total amount of molecules, lead to an increase in the absolute number of PKCε molecules that spontaneously adopt the conformation and/or localization that mediates neurite outgrowth. Because PKCε is the only isoform that induces neurite outgrowth in neuroblastoma cells, there are likely unique structures in PKCε that would be of importance for the acquisition of a neurite-inducing state of this isoform.
Studies comparing PKCδ and ε have shown that structures responsible for isoform-specific effects may reside both in the regulatory and the catalytic domain, depending on the effect that is elicited by PKC (Ács et al., 1997a,b; Wang et al., 1997, 1998). The C2 domain, in the RD, is crucial for the binding of PKCε to its receptor for activated C-kinase (Mochly-Rosen and Gordon, 1998) and this domain has been used to specifically block the translocation and function of PKCε (Johnson et al., 1996; Hundle et al., 1997). Furthermore, there is an actin-binding site between the C1 domains, unique for PKCε, which is of importance for the localization of PKCε and also can mediate an F-actin–induced activation of the enzyme (Prekeris et al., 1996, 1998). This is of interest because there is an increased association of PKCε to the cytoskeleton during neuronal differentiation of PC12 cells (Brodie et al., 1999) and because PKCε is enriched in the F-actin–rich growth cones of differentiating neuroblastoma cells (Fagerström et al., 1996). The aim of this study was to investigate whether the actin-binding site is important for PKCε-mediated neurite outgrowth and to analyze whether this involves an altered conformation and/or localization of the protein during this process.
MATERIALS AND METHODS
Plasmids
Plasmids containing cDNA encoding full-length PKCη and PKCθ were generated by polymerase chain reaction (PCR) with cDNA from human placenta and SH-SY5Y cells, respectively. Other plasmids encoding full-length or RD of human PKC isoforms fused to enhanced green fluorescent protein (EGFP) cDNA have been described previously (Zeidman et al., 1999).
Plasmid encoding full-length PKCε with deleted actin-binding site (ABS), i.e., amino acids (aa) 223–228, called εFLΔABS, was generated by PCR amplifying cDNA encoding aa 1–222 and 229–737 from PKCε, respectively, introducing an MluI site in the primers. The two fragments were cleaved, ligated, and subjected to a second PCR amplifying the combination of the two cDNAs (Figure 3A). A similar approach was used when constructing plasmids encoding PKCε with alanine 159 changed for a glutamate when a SalI site was introduced in the primers by modification of nucleotides encoding arginines 161–163 (Figure 5A). The DNA fragments were introduced into the pEGFP-N1 vector (CLONTECH, Palo Alto, CA), thereby fusing the PKCε cDNA with EGFP cDNA. Plasmid encoding the RD of PKCε with deleted actin-binding site, called εRDΔABS, was generated with PCR amplifying cDNA encoding aa 1–373 by using εFLΔABS as template. As before, the cDNA was cloned in the pEGFP-N1 vector.
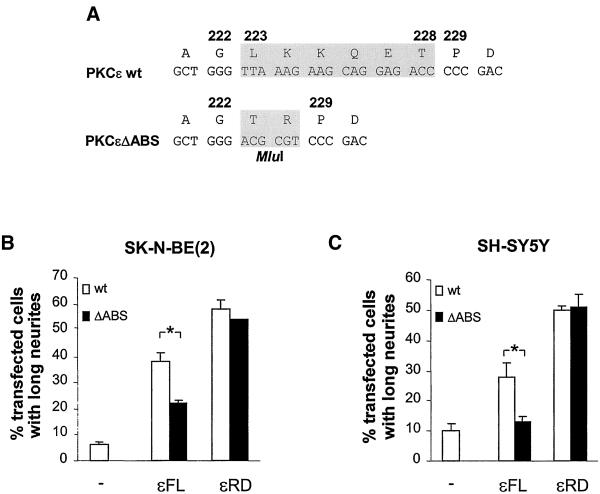
Figure 3.
Actin-binding site is of importance for neurite induction by full-length PKCε. (A) PKCε was modified by removing nucleotides encoding amino acids 223–228, i.e., the ABS, from wild-type PKCε cDNA (wt) and replacing it with an MluI recognition sequence, coding for amino acids T and R. This mutated PKCε (ΔABS) was also used to generate the new construct εRDΔABS. Neurite outgrowth was examined in SK-N-BE(2) (B) and SH-SY5Y cells (C) transfected with vectors encoding wild-type εFL and εRD (wt) and corresponding proteins with deleted actin-binding site (ΔABS), all fused to EGFP. The cells were fixed and mounted 16 h after transfection and transfected cells with long neurites were counted. Data (mean ± SEM, n = 3–6) are presented as percentage transfected cells with long neurites. ∗, statistically significant differences with analysis of variance followed by Duncan's multiple range test.
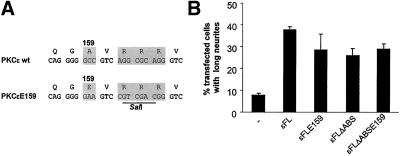
Figure 5.
Pseudosubstrate mutation does not compensate for deletion of the actin-binding site. (A) Nucleotide sequence encoding the pseudosubstrate sequence of PKCε with and without actin-binding site was mutated so that alanine 159 was changed to glutamate. (B) SK-N-BE(2) cells were transfected with vectors encoding EGFP fusions of PKCε, PKCε E159, PKCεΔABS, and PKCεΔABS E159, and the number of cells with neurites longer than two cell bodies was counted. Data (mean ± SEM, n = 4) are presented as percentage of transfected cells with neurites longer than two cell bodies.
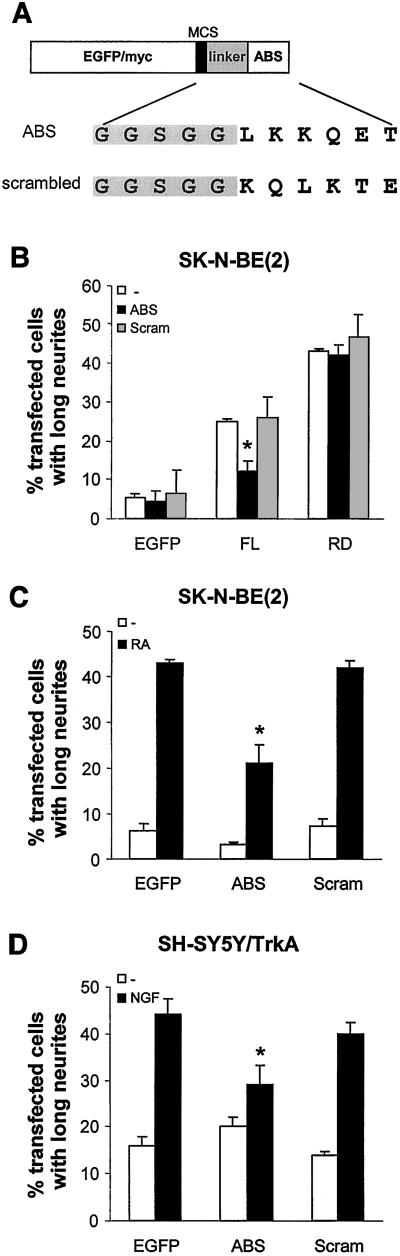
A double-stranded oligonucleotide encoding the actin-binding site from PKCε fused to a linker sequence was cloned into pEGFP-C1 (CLONTECH) so that, upon expression, a fusion protein consisting of EGFP-linker-ABS, would be produced. A construct encoding the scrambled version of the actin-binding site was produced in the same way. Both constructs were also cloned into a vector containing a C-terminal myc-tag under the control of a cytomegalovirus promoter. The constructs are described in Figure 4A.
Figure 4.
Isolated actin-binding site from PKCε inhibits neurite outgrowth. (A) Expression vectors encoding the actin-binding site from PKCε, or a scrambled version of this site, fused to either EGFP or a myc-tag via a linker sequence was constructed. (B) SK-N-BE(2) cells were cotransfected in a 1:7 ratio with vectors encoding EGFP, εFL-EGFP, or εRD-EGFP and myc-tagged ABS (ABS), scrambled ABS (Scram), or empty myc-vector (−). Cells were fixed 16 h after transfection and transfected cells, identified with EGFP fluorescence, were counted and the number of cells with long neurites was determined. Data (mean ± SEM, n = 3) are presented as percentage of transfected cells with long neurites. EGFP-tagged ABS, scrambled actin-binding site (Scram), and EGFP alone (EGFP) were expressed in SK-N-BE(2) cells (C) and SH-SY5Y/TrkA cells (D). The cells were incubated in regular medium (−) or treated with 10 μM RA for 2 d or with 100 ng/ml NGF for 4 d (RA, NGF). The cells were fixed and the number of transfected cells bearing long neurites was assessed. Data (mean ± SEM, n = 3) are presented as percentage of transfected cells with long neurites. ∗, statistically significant differences with analysis of variance followed by Duncan's multiple range test compared with similar conditions by using control vector instead of ABS vector.
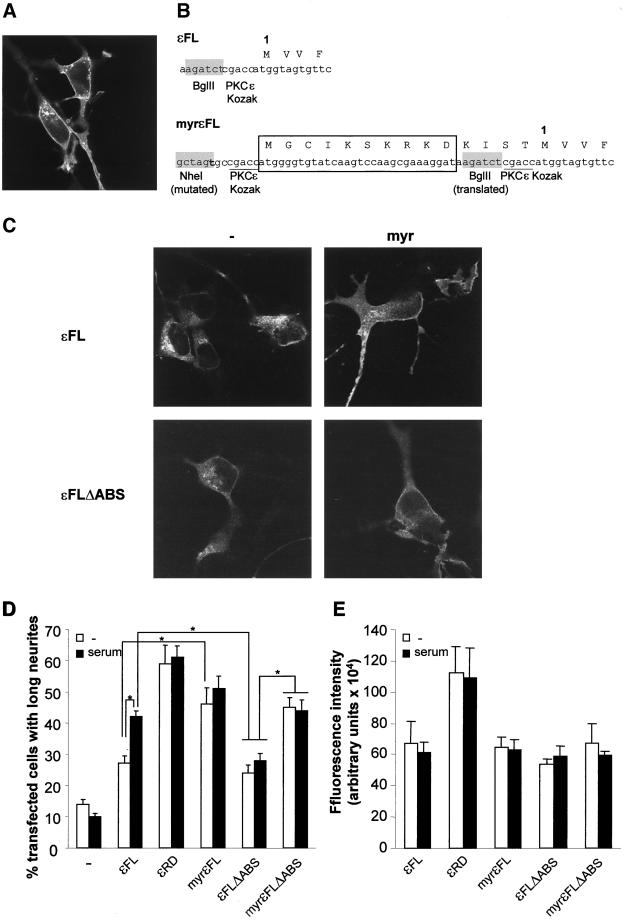
Vector encoding myristoylated PKCε (myrεFL) was created by insertion of a double-stranded oligonucleotide encoding the myristoylation sequence from Lyn (Resh, 1999) into the NheI and BglII sites N terminally to the start of the PKCε coding sequence in the pEGFP-N1 expression vector. The construct is described in Figure 7B. To create myrεFLΔABS, the εFL cDNA was exchanged for cDNA encoding εFLΔABS in the myrεFL vector.
Figure 7.
Negative effects of deletion of the actin-binding site on neurite induction are reversed by myristoylation of PKCε. (A) Localization to the plasma membrane of the isolated RD from PKCε fused to EGFP expressed in SK-N-BE(2) cells was shown with confocal microscopy. (B) cDNA encoding a myristoylation sequence derived from Lyn was fused to cDNA encoding εFL and εFLΔABS. In the schematic representation of the PKCε-EGFP vector, the BglII site used for cloning and the PKCε Kozak sequence precede the first codon, labeled 1. In the myristoylated PKCε (myrεFL) a PKCε Kozak sequence and a sequence encoding the first 10 aa from Lyn (boxed) are inserted into the NheI and BglII sites. The original Kozak sequence and BglII site are now translated. The original starting methionine is labeled 1. (C) SK-N-BE(2) cells were transfected with expression vectors encoding εFL, εFLΔABS, and the corresponding myristoylated variants (myr), all fused to EGFP. The cells were grown for 16 h and thereafter fixed, mounted, and examined with confocal microscopy. (D) Percentage of transfected cells with long neurites was quantified in cells expressing EGFP alone, PKCε RD (εRD), full-length PKCε (εFL), myristoylated PKCε (myrεFL), PKCε without the actin-binding site (εFLΔABS), and myristoylated PKCε without the actin-binding site (myrεFLΔABS). After transfection, the cells were cultured for 16 h with or without 10% serum. Data (mean ± SEM, n = 5–6) are presented as percentage of transfected cells with long neurites. (E) Expression levels in single cells of the EGFP fusion proteins in D were quantified with laser scanning cytometry. Data (mean ± SEM, n = 3, 50–100 cells analyzed in each experiment) are arbitrary units of fluorescence intensity. ∗, statistically significant differences with analysis of variance followed by Duncan's multiple range test.
PCRs were performed with Pfu polymerase (Promega, Madison, WI) to minimize introduction of mutations and all PCR-generated fragments were sequenced. For all EGFP constructs expression of proteins of the anticipated size was confirmed with Western blot analysis.
Cell Culture and Transfections
Human neuroblastoma SH-SY5Y, SH-SY5Y/TrkA (Lavenius et al., 1995), and SK-N-BE(2) cells were maintained in minimal essential medium supplemented with 10% fetal bovine serum, 100 IU/ml penicillin, and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA). For transfection experiments, SH-SY5Y and SH-SY5Y/TrkA cells were trypsinized and seeded at a density of either 350,000 or 100,000 cells/35-mm cell culture dish on glass coverslips as previously described (Zeidman et al., 1999). Seeding at the lower cell density was done for differentiation of SH-SY5Y/TrkA cells by using NGF (100 ng/ml; Promega) for 4 d. SK-N-BE(2) cells were seeded on glass coverslips (300,000 cells/dish). For differentiation experiments SK-N-BE(2) cells were seeded at a density of 150,000 cells/35-mm dish and treated for 48 h with 10 μM RA (Sigma, St. Louis, MO). Ethanol (final concentration 0.25%) was added to the control to obtain the same solvent concentration. Transfections were initiated 24 h after seeding and were done with 2 μg of DNA and 4 μl of Lipofectin (Invitrogen) for SH-SY5Y cells and 4 μl of LipofectAMINE (Invitrogen) for SK-N-BE(2) cell. When indicated, 12-O-tetradecanoylphorbol-13-acetate (TPA; Sigma) was used at a concentration of 16 nM and latrunculin B (Calbiochem, San Diego, CA) at 0.6 μM.
Morphology Studies
Sixteen hours after the end of transfections, unless otherwise indicated, cells were fixed and mounted (Zeidman et al., 1999). Transfected cells were identified by the fluorescence of EGFP and examined with a fluorescence microscope. A transfected cell was considered to have long neurites if the length of the process exceeded that of two cell bodies. Two hundred transfected cells per experiment were counted.
Laser Scanning Cytometry
The amount of PKC-EGFP fusion proteins in individual cells was estimated with a laser scanning cytometer (CompuCyte) by using 488-nm excitation and a 530/30 emission filter with a 20× lens. The levels of laser strength and detection gain were set so that no pixel of PKC-EGFP–expressing cells reached maximal levels and these settings were the same for all experiments. After the scan each cell was relocated to check that only single cells were used for quantification.
Cytoskeletal Preparation
SK-N-BE(2) cells (seeded at a density of 500,000 cells/100-mm dish) were treated for 4 d with 10 μM RA (with ethanol added to the control). Crude cytoskeletal preparations were done essentially as previously described (Särndahl et al., 1993). Cells were scraped off the culture dishes in cold phosphate-buffered saline (PBS), pelleted, and lysed for 15 min on ice in a buffer containing 25 mM HEPES, pH 7.4, 2 mM MnCl2, 4 mM iodoactetic acid, 10 μM Na3VO4, 1 mM EDTA, 1% Triton X-100, and Complete protease inhibitor cocktail (Roche Molecular Biochemicals, Indianapolis, IN). The lysates were centrifuged for 10 min at 500 × g to remove debris and nuclei, followed by a centrifugation for 10 min at 5000 × g. The pellet, containing the crude cytoskeleton, was washed once with the lysis buffer. The protein concentration in the supernatant was determined and used to normalize the protein amount in the cytoskeletal pellets from differently treated cells. The pellets were thereafter subjected to Western blot analysis.
Western Blot Analysis
Samples were separated with SDS-PAGE and transferred to Hybond-C extra nitrocellulose filter (Amersham Biosciences, Piscataway, NJ) as previously described (Zeidman et al., 1999). Proteins were detected with primary antibodies toward EGFP (CLONTECH), PKCε (Santa Cruz Biotechnology, Santa Cruz, CA), and actin (clone C4; ICN, Costa Mesa, CA), and visualized with horseradish peroxidase-labeled secondary antibody (Amersham Biosciences) by using the SuperSignal system (Pierce Chemical, Rockford, IL) as substrate. The chemoluminescence was detected with a charge-coupled device camera (Fujifilm; Fiji Photo Film, Tokyo, Japan). Band intensities were analyzed with Lab Science software (Fujifilm).
Subcellular Fractionations
For subcellular fractionations, 3 × 106 SK-N-BE(2) cells were seeded and transfected with 6.4 μg of DNA and 20 μl of LipofectAMINE 2000 (Invitrogen). Cells were washed in PBS, suspended in homogenization buffer (20 mM Tris-HCl, pH 7.5, 10 mM EGTA, 2 mM EDTA, Complete protease inhibitor cocktail), and homogenized using a Dounce homogenizer, which was followed by centrifugation for 10 min at 500 × g to remove cell debris and nuclei. Lysates were centrifuged for 1 h at 100,000 × g and the supernatant, the cytosolic fraction, was collected and the pellet was treated for 3 h with homogenization buffer containing 1% Triton X-100 followed by a 1-h centrifugation at 100,000 × g. The resulting supernatant (the Triton-soluble membrane fraction), the pellet (the Triton-insoluble cytoskeletal fraction), and the cytosolic fraction were subjected to Western blot analysis.
Immunofluorescence and Staining of F-Actin
Cells were fixed with 4% paraformaldehyde in PBS for 4 min, permeabilized, and blocked with 5% normal goat serum and 0.3% Triton X-100 in Tris-buffered saline (TBS) for 30 min. F-Actin was stained for 20 min with Alexa Fluor 546-conjugated phalloidin (Molecular Probes, Eugene, OR) diluted 1:40 in TBS. Endogenous PKCε was detected with a primary polyclonal rabbit anti-PKCε antibody (Santa Cruz Biotechnology) diluted 1:100 in TBS followed by the secondary antibody Alexa Fluor 488-conjugated goat anti-rabbit IgG (Molecular Probes) diluted 1:400 in TBS. Both incubations were 1 h in length. Extensive washing with TBS was done between all steps and the coverslips were mounted on object slides (Zeidman et al., 1999).
Confocal Microscopy
Cells were examined using a Bio-Rad Radiance 2000 confocal system fitted on a Nikon microscope. A 60×/numerical aperture 1.40 oil lens was used and excitation wavelengths were 488 nm (EGFP and Alexa Fluor 488) and 543 nm (Alexa Fluor 546) and the emission filters used were HQ515/30 (EGFP and Alexa Fluor 488) and 600LP (Alexa Fluor 546). Colocalization was analyzed with the LaserPix software (Bio-Rad, Hercules, CA).
RESULTS
Induction of Neurites by PKCε
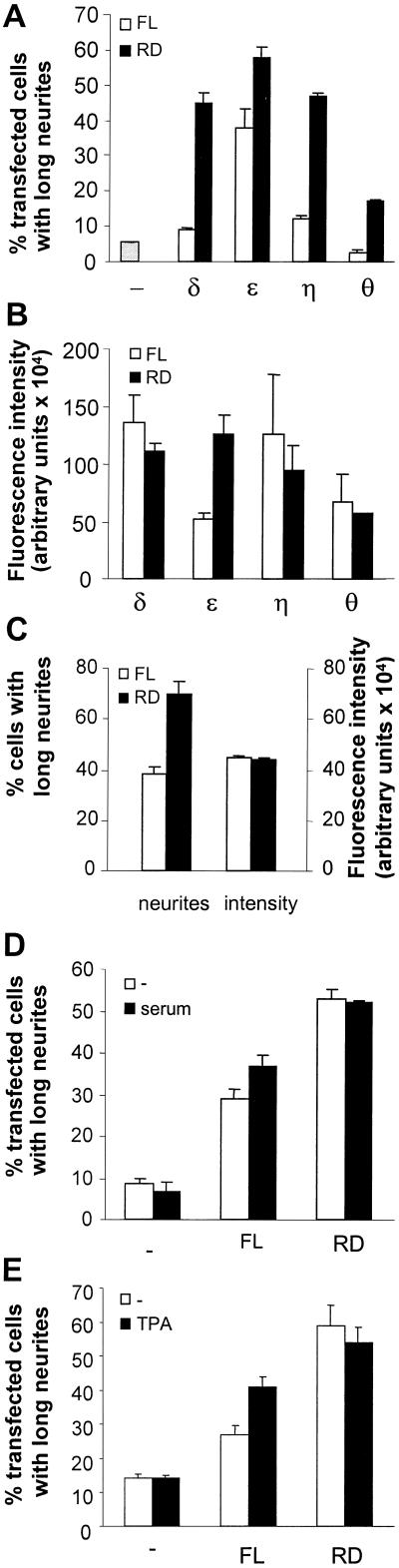
In a previous study, we demonstrated that overexpression of PKCε, but not α, βII, or δ resulted in neurite outgrowth in neuroblastoma cells. The effect was mediated by the RD and similar results were obtained by the RDs of other novel PKC isoforms (Zeidman et al., 1999). To establish whether the neurite induction is specific for full-length PKCε, expression vectors encoding full-length PKCη and θ fused to EGFP were created. SK-N-BE(2) neuroblastoma cells were thereafter transfected with expression vectors coding for full-length or RD of all novel PKC isoforms, fused to EGFP (Figure 1A). PKCε-transfected cells (38%) had long neurites, compared with 2.5–12% of cells expressing the other novel isoforms or EGFP alone, demonstrating that PKCε is the only novel PKC isoform that induces neurites upon overexpression. Supporting our previous finding, neurites were induced upon overexpression of the RD from all novel PKC isoforms, except PKCθ, which only had a minor effect.
Figure 1.
Induction of neurite outgrowth by overexpression of PKCε. (A) SK-N-BE(2) neuroblastoma cells were transfected with expression vectors encoding full-length (FL) and RD from PKCδ, ε, η, and θ, fused to EGFP. Empty EGFP vector (−) was used as control. Cells were fixed 16 h after transfection and transfected cells with neurites longer than the length of two cell bodies were counted. Data (mean ± SEM, n = 2) are presented as percentage of transfected cells with long neurites. (B) Amount of EGFP fusion proteins in individual cells was analyzed with laser scanning cytometry. For each experiment, 50–150 cells were analyzed and the average of these cells was used as the observation value from that experiment. Data (mean ± SEM) are presented as arbitrary fluorescence intensity units from two separate experiments. (C) Cells expressing full-length PKCε or the PKCε RD fused to EGFP with fluorescence intensity of 350,000–550,000 U were scored for the presence of neurites. Twenty cells were scored in each experiment and data are presented as percentage of cells with neurites (mean ± SEM, five separate experiments) and arbitrary fluorescence intensity units (mean ± SEM, 100 different cells). SK-N-BE(2) cells were grown with or without 10% serum (D) or in serum-free medium with or without 16 nM TPA (E) for 16 h after transfection with vectors encoding EGFP (−), full-length PKCε (FL), or the PKCε RD (RD). Transfected cells were scored for neurites longer than two cell bodies and data, mean ± SEM, n = 3 (D) or 5 (E), are presented as percentage of transfected cells with neurites.
The fact that only full-length PKCε induces neurites may be due to a higher degree of overexpression of this isoform. We therefore analyzed the concentration of the different PKC-EGFP proteins in individual cells with laser scanning cytometry (Figure 1B). The levels of PKCε-EGFP were actually lower than those of full-length PKCδ and η EGFP fusion proteins, demonstrating that the selective neurite induction by PKCε is not due to higher levels of this isoform. However, the isolated RD of PKCε displayed higher expression levels than full-length PKCε, which could explain why overexpression of the PKCε RD leads to more cells with neurites than overexpression of the full-length protein. We therefore selected cells with similar levels of RD PKCε and full-length PKCε and examined whether these cells had neurites (Figure 1C). This analysis revealed that when the same amounts of EGFP fusion proteins were present in a cell, it was more probable that cells expressing the isolated RD would have neurites.
The fact that the isolated RD of PKCε was more potent than the full-length protein in terms of inducing neurite outgrowth may suggest that the RD mimics a state of PKCε that is favorable for neurite induction and that full-length PKCε can acquire this state upon stimulation. The requirement for stimulus of the RD and the full-length PKCε for efficient neurite induction was therefore investigated. SK-N-BE(2) neuroblastoma cells were transfected with expression vectors encoding RD or full-length PKCε fused to EGFP and grown in the absence or presence of 10% serum or 16 nM TPA for 16 h (Figure 1, D and E). This demonstrated that the neurite-inducing effect of full-length PKCε was sensitive to removal of extracellular stimulus because 37% PKCε-EGFP–expressing cells grown in the presence of serum had neurites (Figure 1D), whereas the corresponding number for cells grown in the absence of serum was 29%. Potent neurite induction by full-length PKCε in serum-free medium was restored by inclusion of TPA in the medium (Figure 1E). Unlike full-length PKCε, neither serum nor TPA influenced the neurite-inducing capacity of PKCε RD.
Increased PKCε Association with Cytoskeleton during Neuronal Differentiation
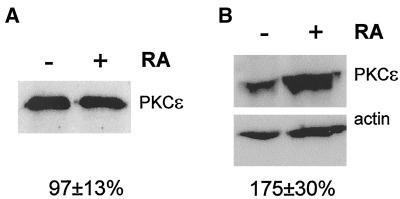
The fact that overexpression of PKCε leads to neurite outgrowth could imply that increased levels of PKCε are required for neurite outgrowth during neuronal differentiation. SK-N-BE(2) cells were differentiated with 10 μM RA for 4 d and the content of PKCε was analyzed (Figure 2A). The results demonstrate that PKCε levels were unaltered during neuronal differentiation. Hence, increased amounts of PKCε are not required for neurite outgrowth during neuronal differentiation, a conclusion which is further supported by the fact that PKCε is not up-regulated during NGF-induced differentiation of SH-SY5Y/TrkA cells (Fagerström et al., 1996).
Figure 2.
Association of PKCε with the cytoskeleton during neuronal differentiation. SK-N-BE(2) cells were induced to differentiate with 10 μM RA for 4 d. (A) Total cell lysates, normalized for total protein content, were analyzed for PKCε immunoreactivity and band intensities were quantified. Data (mean ± SEM, n = 4) represent PKCε levels in differentiated cells in percentage of values obtained in control cell lysates. (B) Lysed cells were divided into a Triton X-100 soluble cytosolic/membrane and a particulate cytoskeletal fraction. Cytoskeletal fractions, normalized to the Triton X-100-soluble fractions for protein content, were subjected to Western blot analysis by using anti-PKCε (top) and anti-actin (bottom) antibodies. Data (mean ± SEM, n = 10) are PKCε/actin intensity ratios from differentiated cells in percentage of corresponding values obtained from control cells. The increase is statistically significant (p < 0.05) using Student's t test.
It has previously been shown that there is an increased association of PKCε with the cytoskeleton during NGF-induced neuronal differentiation of PC12 cells (Brodie et al., 1999) and also an enrichment of PKCε in the actin-rich growth cones of differentiated neuroblastoma cells (Fagerström et al., 1996). Interaction with the cytoskeleton could be one way whereby PKCε acquires a neurite-inducing capacity. We examined whether such an association takes place during RA-induced differentiation of neuroblastoma cells (Figure 2B). PKCε levels in a crude cytoskeletal preparation were elevated with 75 ± 30% (n = 10) in SK-N-BE(2) cells treated with 10 μM RA for 4 d.
Actin-binding Site Is Necessary for Neurite Induction by PKCε
The finding that PKCε associates with the cytoskeleton during neuronal differentiation suggested that the actin-binding site, which is only found in PKCε and not in other PKC isoforms, may be of importance for neurite induction. This structure is located between the C1 domains in the RD of PKCε (Prekeris et al., 1996). Removal of the actin-binding site from PKCε decreases its binding to F-actin in vitro but does not seem to alter other properties of the protein (Prekeris et al., 1998). To investigate the importance of this motif for the effect of PKCε on neurite outgrowth, we created expression vectors encoding both EGFP-fused full-length PKCε and PKCε RD lacking the ABS, εFLΔABS and εRDΔABS (Figure 3A).
The constructs were introduced into SK-N-BE(2) and SH-SY5Y cells and the effect on neurite outgrowth was examined (Figure 3, B and C). Neurite induction by full-length PKCε was markedly reduced if the actin-binding site had been deleted. In SK-N-BE(2) cells (Figure 3B), 38% of cells overexpressing normal full-length PKCε had long neurites, whereas the corresponding number for cells expressing PKCε lacking the actin-binding site was 22%. In contrast, the neurite-inducing capacity of the RD was not affected by removal of the actin-binding site, because both the complete and the mutated RD caused neurite outgrowth in 50–60% of the transfected SK-N-BE(2) cells. The same effect of the removal of the actin-binding site from PKCε was seen in SH-SY5Y cells (Figure 3C). The neurite induction by full-length PKCε constructs were generally lower in this cell line, perhaps due to lower levels of endogenous factors that signal to PKCε, and thereby render it in a neurite-inducing state.
Isolated Actin-binding Site Suppresses Neurite Outgrowth
Removal of the actin-binding site from PKCε clearly reduced its ability to induce neurites. The PKCε binding to and activation by F-actin have been shown to be blocked by the peptide LKKQET (Prekeris et al., 1998), which is identical to the structure that was removed in PKCε without actin-binding site. We investigated whether introduction of this peptide would interfere with the PKCε pathway leading to neurite outgrowth. Expression vectors encoding the isolated actin-binding site, or a scrambled actin-binding site, fused either to EGFP (EGFP-ABS and EGFP-scrambled) or to a myc-tag (myc-ABS and myc-scrambled) via a linker were created (Figure 4A).
The expression vector coding for myc-tagged actin-binding site was cotransfected with vectors encoding εFL and εRD in a 7:1 ratio (Figure 4B). Because immunofluorescence staining of the myc-tag with tetramethylrhodamine B isothiocyanate-conjugated secondary antibodies was weak (our unpublished data), the EGFP fluorescence was used to identify transfected cells. When myc-ABS and myc-scrambled were expressed on their own, immunofluorescence staining by using fluorescein isothiocyanate-conjugated secondary antibody showed that the proteins are expressed in neuroblastoma cells (our unpublished data). Coexpression of the actin-binding site reduced the number of full-length PKCε-expressing cells having long neurites, whereas the scrambled actin-binding site had no effect. In contrast, expression of the actin-binding site together with the PKCε RD did not decrease the percentage of RD-expressing cells with long neurites, further supporting the finding presented in Figure 3, B and C, that the actin-binding site is only important for the neurite-inducing capacity of full-length PKCε.
If the interaction of PKCε with F-actin through the actin-binding site is part of a general mechanism involved in neurite outgrowth, it would be expected that the actin-binding site peptide would suppress outgrowth during neuronal differentiation. To explore this possibility, EGFP-tagged peptides (EGFP-ABS and EGFP-scrambled) were expressed in neuroblastoma cells that were induced to differentiate with either RA or NGF. Expression of the isolated actin-binding site led to a marked suppression of SK-N-BE(2) cells with long neurites after 48 h of RA treatment (Figure 4C). A similar effect was seen in SH-SY5Y/TrkA cells treated with NGF for 4 d (Figure 4D).
Deletion of Actin-binding Site Does not Reduce Neurite-inducing Capacity of PKCε with Mutated Pseudosubstrate
The interaction with F-actin was shown to stabilize PKCε in an open conformation in vitro (Prekeris et al., 1998). The decreased neurite-inducing capacity of PKCε upon deletion of the actin-binding site may therefore be due to the fact that a closed conformation of PKCε will be favored and the neurite-inducing domains may consequently be hidden by the catalytic domain. One way to render PKC in an open and active conformation is to mutate the alanine residue in the pseudosubstrate to a glutamate (Pears et al., 1990). To investigate whether an open conformation of PKCε could compensate for the effects of the deletion of the actin-binding site, this modification of PKCε and PKCε with deleted actin-binding site was done (Figure 5A). When these proteins were overexpressed in SK-N-BE(2) cells, it was found that deletion of the actin-binding site of PKCε with a mutated pseudosubstrate did not have an effect on neurite induction by this protein (Figure 5B). However, mutation of the pseudosubstrate did not restore the neurite-inducing capacity of PKCε with deleted actin-binding site to the effect observed with wild-type PKCε.
PKCε Localizes Primarily to Cortical Cytoskeleton during Neurite Outgrowth
Another reported effect of deleting the actin-binding site is a decreased colocalization of PKCε and F-actin in fibroblasts (Prekeris et al., 1998). The colocalization pattern of PKCε and F-actin in neurites was therefore examined (Figure 6). On overexpression, PKCε-EGFP was present in growth cones where a marked colocalization of PKCε and cortical F-actin was seen (Figure 6, A and B). There was a similar pattern of colocalization in growth cones of SK-N-BE(2) cells, which had been induced to differentiate by RA treatment (Figure 6, C and D). Both endogenous PKCε and F-actin primarily localized along the edges of the growth cone. In cells with high levels of PKCε-EGFP, this fusion protein was also detected in the interior of the growth cone, perhaps due to a saturation of the binding sites in the cortical cytoskeleton. Such a distribution all over the growth cone was regularly observed for PKCε without actin-binding site.
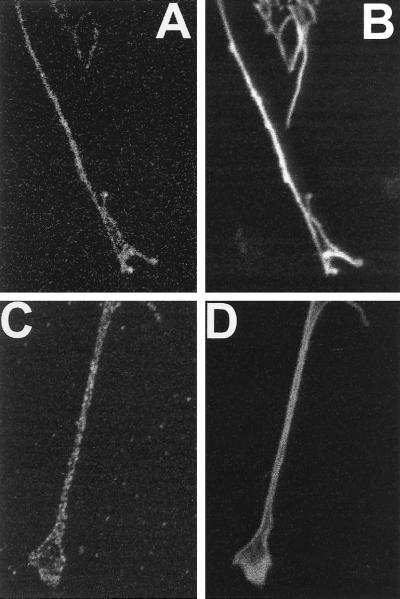
Figure 6.
Colocalization of PKCε and F-actin in growth cones. SK-N-BE(2) cells were transfected with vector encoding PKCε fused to EGFP (A and B) or treated with 10 μM RA for 3 d (C and D). Cells were fixed and F-actin was visualized with Alexa Fluor 546-conjugated phalloidin (B and D) and PKCε was visualized using EGFP fluorescence (A) or by immunofluorescence with Alexa Fluor 488-conjugated antibodies (C).
Membrane Targeting of PKCε Overcomes Requirement for Actin-binding Site
The neurite induction by PKCε RD is independent of extracellular stimulus, insensitive to ablation of the actin-binding site, and is not influenced by coexpression of the actin-binding site peptide. Furthermore, the RD to a large extent localizes to the plasma membrane (Figure 7A). This, together with the fact that PKCε localized to the cortical cytoskeleton in growth cones, suggested to us that targeting to the plasma membrane might overcome the requirement for the actin-binding site for optimal neurite induction by PKCε.
To target PKCε to the plasma membrane, this isoform was tagged with a myristoylation sequence by generating expression vectors encoding myristoylated PKCε (myrεFL) and PKCε with deleted actin-binding site (myrεFLΔABS). These were created by addition of nucleotides encoding the myristoylation sequence from Lyn before the translation start of PKCε (Figure 7B). SK-N-BE(2) cells were transfected with vectors encoding the myristoylation variants myrεFL and myrεFLΔABS along with their nonmyristoylated counterparts εFL and εFLΔABS and the subcellular localization of the proteins was analyzed with confocal microscopy (Figure 7C). The results show that myristoylation causes an increased association with the plasma membrane of both wild-type PKCε and of PKCε with deleted actin-binding site. The effects of these modifications on the neurite-inducing capacity of the PKCε variants were thereafter examined (Figure 7D). Cells were grown in the absence or presence of serum to investigate whether the requirement of extracellular stimulation was affected by the modifications of PKCε. As seen before, εFL was not a potent inducer of neurite outgrowth in the absence of serum (27% cells with long neurites). Serum stimulation of εFL-expressing cells enhanced this effect to around 40% cells with long neurites. The myristoylated PKC variant (myrεFL), as well as the RD, was independent of serum. The percentage of transfected cells with long neurites was enhanced from 27% for nonmyristoylated to 46% for myristoylated PKCε in the absence of serum.
Serum did not cause a marked potentiation of neurite outgrowth by εFLΔABS (Figure 7D). In the absence of serum, deletion of the actin-binding site actually had no effect on the neurite-inducing capacity of PKCε, which indicates that serum stimulation influences PKCε through the actin-binding site. Myristoylation of PKCε with deleted actin-binding site restored the neurite-inducing capacity of this protein. The effect of myrεFLΔABS was not further enhanced by treatment with serum. The levels of neurite induction reached by myristoylation of εFLΔABS were comparable to the effects of myrεFL under serum-free conditions and of εFL when cells were grown with serum. Thus, targeting of PKCε to the plasma membrane overcomes the dependence on extracellular stimulus and the effect of removal of the actin-binding site.
To certify that the different effects of the various PKCε constructs were not due to differences in expression levels, the amount of EGFP fluorescence in single cells was analyzed by laser scanning cytometry (Figure 7E). This demonstrated that the full-length variants were expressed at similar levels, whereas, as observed in Figure 1, the amount of the RD-EGFP protein per cell was higher. Serum did not influence the expression levels of the PKCε variants.
Effects of Deletion of Actin-binding Site on Subcellular Localization of PKCε
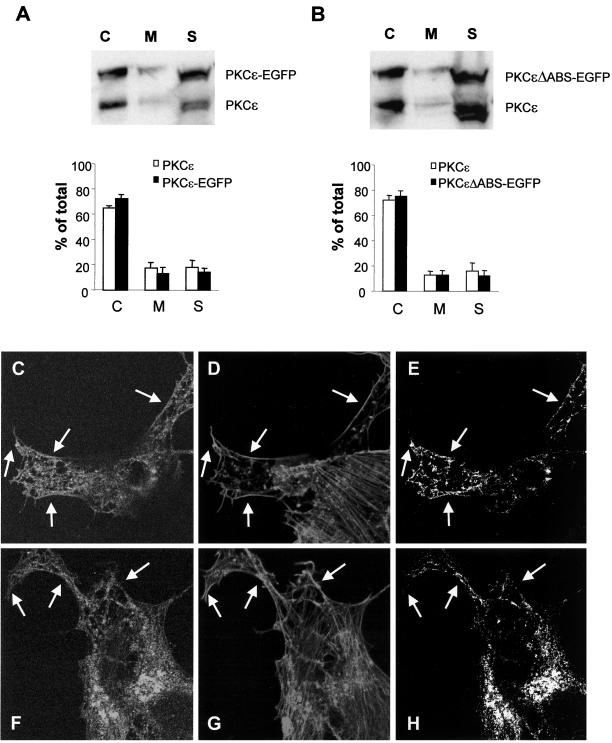
Although no apparent difference in localization between wild-type PKCε and PKCε without actin-binding site could be detected (Figure 7C), targeting of the latter protein to the plasma membrane overcame the attenuation of neurite-inducing capacity. To further analyze whether deletion of the actin-binding site resulted in aberrant localization of PKCε, we performed a subcellular fractionation analysis comparing the distribution of full-length PKCε and PKCεΔABS to endogenous PKCε (Figure 8, A and B). This demonstrated that both proteins had the same distribution pattern as endogenous PKCε and there was no effect by deletion of the actin-binding site.
Figure 8.
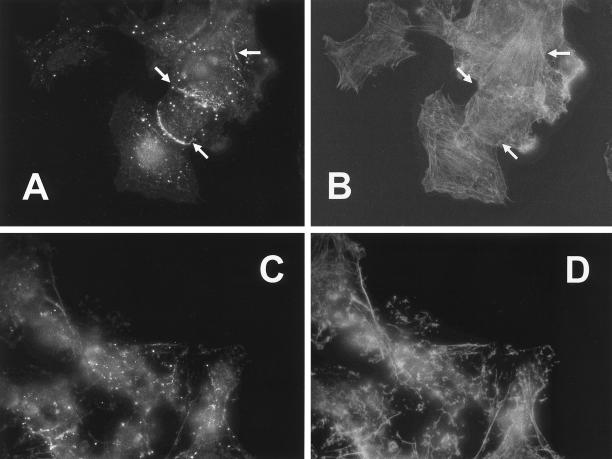
Effects on the subcellular localization of PKCε by deletion of the actin-binding site. SK-N-BE(2) cells transfected with vector encoding full-length PKCε (A and C–E) and PKCε without the actin-binding site (B and F–H), both fused to EGFP. Transfected cells were subjected to subcellular fractionation (A and B) and divided into a cytosolic fraction (C), a membrane fraction (M), and a cytoskeletal fraction (S), which were analyzed with Western blotting with antibodies directed toward PKCε. Immunoblots from three individual experiments were quantified and data are presented as percentage of PKCε in each fraction out of total PKCε content. White bars represent endogenous PKCε and black bars represent EGFP-tagged PKCε variants. Cells expressing wild-type PKCε (C–E) and PKCε without actin-binding site (F-H) were fixed, and F-actin was stained with Alexa Fluor 546-conjugated phalloidin and by using confocal microscopy, a colocalization analysis was done. The images depict PKCε-EGFP (C and F), F-actin (D and G), and pixels that represent colocalization of PKCε and F-actin (E and H). Arrows highlight areas with cortical F-actin. The amount of phalloidin-positive pixels in the cortical cytoskeleton that was also EGFP-positive was 24% (wild-type PKCε) and 14% (PKCε with deleted actin-binding site) by using LaserPix software.
The same issue was also addressed by confocal microscopy and colocalization analysis (Figure 8, C–H). SK-N-BE(2) cells were transfected with vectors encoding EGFP fusion proteins of PKCε with the actin-binding site intact or deleted. The actin cytoskeleton was visualized with Alexa Fluor 546-conjugated phalloidin. For wild-type PKCε, a colocalization with F-actin could be detected both in the interior of the cells and, perhaps more predominantly, along the cortical cytoskeleton. For PKCε with deleted actin-binding site, the colocalization was most striking in the interior of the cell. However, this PKCε variant to some extent also localized to the cortical cytoskeleton, demonstrating that deletion of the actin-binding site does not abolish PKCε localization to the cortical cytoskeleton. A putative role for the actin-binding site may be to strengthen the binding of PKCε to this structure.
Disruption of F-Actin Results in Loss of PKCε Localized at Cortical Cytoskeleton
To further explore whether the localization of PKCε to cortical areas of the cell involves interaction with F-actin, SK-N-BE(2) cells were treated with latrunculin B to disrupt the microfilaments (Figure 9). In untreated cells, a substantial amount of the endogenous PKCε appeared not to be present in the plasma membrane. However, a localization to the cortical cytoskeleton was also observed in several cells, invariably at cell-cell contacts, as exemplified in Figure 9, A and B. Treatment with latrunculin B (Figure 9, C and D) leads to a severe disruption of the F-actin network and upon this treatment the enrichment of PKCε to cortical areas was lost. However, a few F-actin structures remained in the latrunculin B-treated cells and PKCε was in several cases found to localize to these remaining structures.
Figure 9.
Disruption of F-actin leads to altered PKCε localization. SK-N-BE(2) cells were treated with 0.6 μM latrunculin B for 20 min whereafter PKCε was visualized with immunofluorescence (A and C) with Alexa Fluor 488-conjugated secondary antibodies, and F-actin was detected with Alexa Fluor 546-conjugated phalloidin (B and D). Cells were analyzed with a fluorescence microscope and images show control cells (A and B) and latrunculin B-treated cells (C and D). Arrows in A and B indicate cortical areas enriched in PKCε. These were invariably found in cortical areas where the cell had contact with another cell.
DISCUSSION
The outgrowth of neurites is a complex process involving a number of regulatory proteins. In a recent study we found that PKCε, via its RD, induces neurites in neuroblastoma cells (Zeidman et al., 1999). We also found that a dominant negative construct derived from PKCε, where the second C1 domain was deleted, suppresses neurite outgrowth during neuronal differentiation, clearly indicating a crucial role for PKCε in this process. This conclusion is further supported by the finding in this study that a peptide, derived from the actin-binding site of PKCε, attenuates neurite outgrowth during neuronal differentiation.
The actin-binding site peptide does not suppress neurite outgrowth in general, for instance, by abolishing the interaction of several crucial proteins with F-actin, as shown by the fact that it has no effect on the induction by the isolated PKCε RD. This result also demonstrates that the actin-binding site has no role for the downstream effect of PKCε, which leads to neurite outgrowth. This conclusion is further supported by the findings that RDs from other novel PKC isoforms, which lack actin-binding site, and that the RD from PKCε with deleted actin-binding site, efficiently induce neurites. Previously we found that a structure encompassing the C1 domains is necessary and sufficient for PKCε-induced neurite outgrowth (Zeidman et al., 1999). It is therefore likely that the downstream effects of PKCε are mediated by the C1 domains. These structures have in several studies been shown to exert different biological effects (Lehel et al., 1995; Pawelczyk et al., 1998; Kiss et al., 1999; Aroca et al., 2000) and to interact with other proteins (Matto-Yelin et al., 1997; Yao et al., 1997; Pawelczyk et al., 1998; Hausser et al., 1999; Johannes et al., 1999).
Instead of mediating downstream PKCε effects, it is conceivable that the actin-binding site is of importance for upstream signaling to PKCε in a pathway leading to neurite outgrowth. This site has been shown to be of importance both for the conformation and the subcellular localization of PKCε (Prekeris et al., 1998). Mutation of the pseudosubstrate, a modification that has been demonstrated to render PKC in an open conformation and thus constitutively active (Pears et al., 1990), did not reverse the effect of the actin-binding site deletion. However, deletion of the actin-binding site did not influence the neurite-inducing capacity of PKCε with mutated pseudosubstrate. This indicates that an effect on the conformation of PKCε may be one mechanism through which signaling via the actin-binding site causes PKCε to induce neurites.
We also found evidence supporting the importance of a proper subcellular localization of PKCε during neuronal differentiation. There was an increased association of PKCε with the cytoskeleton during neurite outgrowth observed both in this study using neuroblastoma cells and in PC12 cells (Brodie et al., 1999). This is in accordance with the importance of the actin-binding site that we describe herein. However, by using subcellular fractionation no differences in the proportion of PKCε bound to the cytoskeleton were observed as a result of deleting the actin-binding site. The PKCε actin-binding site is therefore not necessarily crucial for the interaction of PKC with F-actin in neuroblastoma cells and there are several reports demonstrating that other PKC isoforms, which lack the actin-binding site, can bind F-actin both in vivo and in vitro (Blobe et al., 1996; Nakhost et al., 1998; Slater et al., 2000). There may thus be other actin-interacting sites in the PKC molecule that could possibly be the reason why no difference in the subcellular fractionation assay was observed between wild-type PKCε and PKCε without actin-binding site. If the actin-binding site is not important for proper localization of PKCε for neurite outgrowth, it may instead be to target PKCε to specific regions of the cytoskeleton. We speculate that PKCε needs to be localized to the cortical cytoskeleton and/or to the plasma membrane to exert the neurite-inducing effect. This speculation is based on the following observations: 1) PKCε localized to the cortical cytoskeleton and the leading edge in the growth cone. 2) Targeting of PKCε to the plasma membrane by myristoylation overcame the reduction in neurite-inducing capacity by deletion of the actin-binding site. And 3) The RD of PKCε, a potent inducer of neurite outgrowth, localized to a large extent to the plasma membrane.
An enhanced concentration of PKCε in the plasma membrane or the cortical cytoskeleton may thus be a trigger for neurite induction. The leading edge in the growth cone is one area where there was a prominent colocalization of PKCε and F-actin. This highly dynamic area could possibly be where the C1 domains exert their effect, for instance, by inducing a conformational change of a protein already present in the leading edge or by recruiting other proteins to this site.
The dependence on extracellular stimulation for potent neurite induction by full-length PKCε indicates that the effect of PKCε is sensitive to signaling. Because the PKCε with deleted actin-binding site was not influenced by serum, it is likely that this signaling pathway targets the actin-binding site of PKCε. There are several pathways that could transduce a neuronal differentiation signal to PKCε. For instance, in both differentiation protocols used in this study it is conceivable that there are increased levels of small molecules that can interact with PKC. Stimulation of growth factor receptors frequently leads to activation of phospholipase Cγ with subsequent formation of diacylglycerol, which can induce changes in both conformation and localization of PKC. There are recent reports demonstrating that retinoids can directly bind to the PKC molecule (Hoyos et al., 2000; Radominska-Pandya et al., 2000). Alternative pathways leading to F-actin–mediated effects of PKCε could include activation of the small GTPases of the Rho family that have been shown to modulate the actin cytoskeleton and to be involved in the regulation of neurite outgrowth (reviewed in Hall, 1998).
In conclusion, this study demonstrates that the PKCε actin-binding site is crucial for the upstream signaling pathway acting on PKCε during neurite outgrowth. We propose a model where localization to the cortical cytoskeleton and/or the plasma membrane is a necessary event for PKCε to exert its neurite-inducing effect.
ACKNOWLEDGMENTS
This work was supported by the Swedish Cancer Society; the Swedish Medical Research Council; the Children's Cancer Foundation of Sweden; the Swedish Society for Medical Research; the Royal Physiographic Society of Lund; the Crafoord; Magnus Bergvall; Gunnar, Arvid, and Elisabeth Nilsson; Greta and Johan Kock; Ollie and Elof Ericsson; John and Augusta Persson Foundations; and Malmö University Hospital Research Funds.
Abbreviations used:
- ABS
actin-binding site
- EGFP
enhanced green florescent protein
- NGF
nerve growth factor
- PKC
protein kinase C
- RA
retinoic acid
- RD
regulatory domain
- TPA
12-O-tetradecanoylphorbol-13-acetate
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–04-0210. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.01–04-0210.
REFERENCES
- Ács P, Bögi K, Lorenzo PS, Marquez AM, Bíró T, Szállási Z, Blumberg PM. The catalytic domain of protein kinase C chimeras modulates the affinity and targeting of phorbol ester-induced translocation. J Biol Chem. 1997a;272:22148–22153. doi: 10.1074/jbc.272.35.22148. [DOI] [PubMed] [Google Scholar]
- Ács P, Wang QJ, Bögi K, Marquez AM, Lorenzo PS, Bíró T, Szállási Z, Mushinski JF, Blumberg PM. Both the catalytic and regulatory domains of protein kinase C chimeras modulate the proliferative properties of NIH 3T3 cells. J Biol Chem. 1997b;272:28793–28799. doi: 10.1074/jbc.272.45.28793. [DOI] [PubMed] [Google Scholar]
- Aroca P, Santos E, Kazanietz MG. Recombinant C1b domain of PKCδ triggers meiotic maturation upon microinjection in Xenopus laevisoocytes. FEBS Lett. 2000;483:27–32. doi: 10.1016/s0014-5793(00)02075-5. [DOI] [PubMed] [Google Scholar]
- Blobe GC, Stribling DS, Fabbro D, Stabel S, Hannun YA. Protein kinase C βII specifically binds to and is activated by F-actin. J Biol Chem. 1996;271:15823–15830. doi: 10.1074/jbc.271.26.15823. [DOI] [PubMed] [Google Scholar]
- Brodie C, Bogi K, Ács P, Lazarovici P, Petrovics G, Anderson WB, Blumberg PM. Protein kinase C-ε plays a role in neurite outgrowth in response to epidermal growth factor and nerve growth factor in PC12 cells. Cell Growth Differ. 1999;10:183–191. [PubMed] [Google Scholar]
- Corbit KC, Foster DA, Rosner MR. Protein kinase Cδ mediates neurogenic but not mitogenic activation of mitogen-activated protein kinase in neuronal cells. Mol Cell Biol. 1999;19:4209–4218. doi: 10.1128/mcb.19.6.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerström S, Påhlman S, Gestblom C, Nånberg E. Protein kinase C-ε is implicated in neurite outgrowth in differentiating human neuroblastoma cells. Cell Growth Differ. 1996;7:775–785. [PubMed] [Google Scholar]
- Fleming I, MacKenzie SJ, Vernon RG, Anderson NG, Houslay MD, Kilgour E. Protein kinase C isoforms play differential roles in the regulation of adipocyte differentiation. Biochem J. 1998;333:719–727. doi: 10.1042/bj3330719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hanada M, Krajewski S, Tanaka S, Cazals-Hatem D, Spengler BA, Ross RA, Biedler JL, Reed JC. Regulation of Bcl-2 oncoprotein levels with differentiation of human neuroblastoma cells. Cancer Res. 1993;53:4978–4986. [PubMed] [Google Scholar]
- Hausser A, Storz P, Link G, Stoll H, Liu YC, Altman A, Pfizenmaier K, Johannes FJ. Protein kinase C μ is negatively regulated by 14-3-3 signal transduction proteins. J Biol Chem. 1999;274:9258–9264. doi: 10.1074/jbc.274.14.9258. [DOI] [PubMed] [Google Scholar]
- Helson L, Helson C. Human neuroblastoma cells and 13-cis-retinoic acid. J Neurooncol. 1985;3:39–41. doi: 10.1007/BF00165170. [DOI] [PubMed] [Google Scholar]
- Hoyos B, Imam A, Chua R, Swenson C, Tong GX, Levi E, Noy N, Hämmerling U. The cysteine-rich regions of the regulatory domains of raf and protein kinase C as retinoid receptors. J Exp Med. 2000;192:835–846. doi: 10.1084/jem.192.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundle B, McMahon T, Dadgar J, Chen CH, Mochly-Rosen D, Messing RO. An inhibitory fragment derived from protein kinase Cε prevents enhancement of nerve growth factor responses by ethanol and phorbol esters. J Biol Chem. 1997;272:15028–15035. doi: 10.1074/jbc.272.23.15028. [DOI] [PubMed] [Google Scholar]
- Hundle B, McMahon T, Dadgar J, Messing RO. Overexpression of ε-protein kinase C enhances nerve growth factor-induced phosphorylation of mitogen-activated protein kinases and neurite outgrowth. J Biol Chem. 1995;270:30134–30140. doi: 10.1074/jbc.270.50.30134. [DOI] [PubMed] [Google Scholar]
- Johannes F, Hausser A, Storz P, Truckenmüller L, Link G, Kawakami T, Pfizenmaier K. Bruton's tyrosine kinase (Btk) associates with protein kinase C μ. FEBS Lett. 1999;461:68–72. doi: 10.1016/s0014-5793(99)01424-6. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Gray MO, Chen CH, Mochly-Rosen D. A protein kinase C translocation inhibitor as an isozyme-selective antagonist of cardiac function. J Biol Chem. 1996;271:24962–24966. doi: 10.1074/jbc.271.40.24962. [DOI] [PubMed] [Google Scholar]
- Kiss Z, Petrovics G, Olah Z, Lehel C, Anderson WB. Overexpression of protein kinase C-ε and its regulatory domains in fibroblasts inhibits phorbol ester-induced phospholipase D. activity. Arch Biochem Biophys. 1999;363:121–128. doi: 10.1006/abbi.1998.1066. [DOI] [PubMed] [Google Scholar]
- Lavenius E, Gestblom C, Johansson I, Nånberg E, Påhlman S. Transfection of TRK-Ainto human neuroblastoma cells restores their ability to differentiate in response to nerve growth factor. Cell Growth Differ. 1995;6:727–736. [PubMed] [Google Scholar]
- Lehel C, Olah Z, Jakab G, Anderson WB. Protein kinase Cε is localized to the Golgi via its zinc-finger domain and modulates Golgi function. Proc Natl Acad Sci USA. 1995;92:1406–1410. doi: 10.1073/pnas.92.5.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehel C, Olah Z, Mishak H, Mushinski JF, Anderson WB. Overexpressed protein kinase C-δ and -ε subtypes in NIH 3T3 cells exhibit differential subcellular localization and differential regulation of sodium-dependent phosphate uptake. J Biol Chem. 1994;269:4761–4766. [PubMed] [Google Scholar]
- Liu J-P. Protein kinase C and its substrates. Mol Cell Endocrinol. 1996;116:1–29. doi: 10.1016/0303-7207(95)03706-3. [DOI] [PubMed] [Google Scholar]
- Matto-Yelin M, Aitken A, Ravid S. 14-3-3 inhibits the Dictyosteliummyosin II heavy-chain-specific protein kinase C activity by a direct interaction: identification of the 14-3-3 binding domain. Mol Biol Cell. 1997;8:1889–1899. doi: 10.1091/mbc.8.10.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischak H, Goodnight JA, Kolch W, Martiny BG, Schaechtle C, Kazanietz MG, Blumberg PM, Pierce JH, Mushinski JF. Overexpression of protein kinase C-δ and -ε in NIH 3T3 cells induces opposite effects on growth, morphology, anchorage dependence, and tumorigenicity. J Biol Chem. 1993;268:6090–6096. [PubMed] [Google Scholar]
- Mochly-Rosen D, Gordon AS. Anchoring proteins for protein kinase C: a means for isozyme selectivity. FASEB J. 1998;12:35–42. [PubMed] [Google Scholar]
- Nakhost A, Forscher P, Sossin WS. Binding of protein kinase C isoforms to actin in Aplysia. J Neurochem. 1998;71:1221–1231. doi: 10.1046/j.1471-4159.1998.71031221.x. [DOI] [PubMed] [Google Scholar]
- Newton AC. Protein kinase C: structure, function and regulation. J Biol Chem. 1995;270:28495–28498. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- Newton AC. Regulation of protein kinase C. Curr Opin Cell Biol. 1997;9:161–167. doi: 10.1016/s0955-0674(97)80058-0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- O'Driscoll KR, Teng KK, Fabbro D, Greene LA, Weinstein IB. Selective translocation of protein kinase C-δ in PC12 cells during nerve growth factor-induced neuritogenesis. Mol Cell Biol. 1995;6:449–458. doi: 10.1091/mbc.6.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelczyk T, Matecki A, Dettlaff A. Recombinant protein kinase C-γ phorbol binding domain upon microinjection blocked insulin-induced maturation of Xenopus laevisoocytes. FEBS Lett. 1998;423:31–34. doi: 10.1016/s0014-5793(98)00054-4. [DOI] [PubMed] [Google Scholar]
- Pears CJ, Kour G, House C, Kemp BE, Parker PJ. Mutagenesis of the pseudosubstrate site of protein kinase C leads to activation. Eur J Biochem. 1990;194:89–94. doi: 10.1111/j.1432-1033.1990.tb19431.x. [DOI] [PubMed] [Google Scholar]
- Prekeris R, Hernandez RM, Mayhew M, White MK, Terrian DM. Molecular analysis of the interactions between protein kinase C-ε and filamentous actin. J Biol Chem. 1998;273:26790–26978. doi: 10.1074/jbc.273.41.26790. [DOI] [PubMed] [Google Scholar]
- Prekeris R, Mayhew MW, Cooper JB, Terrian DM. Identification and localization of an actin-binding motif that is unique to the epsilon isoform of protein kinase C. and participates in the regulation of synaptic function. J Cell Biol. 1996;132:77–90. doi: 10.1083/jcb.132.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radominska-Pandya A, Chen G, Czernik PJ, Little JM, Samokyszyn VM, Carter CA, Nowak G. Direct interaction of all trans-retinoic acid with protein kinase C: implications for PKC signaling and cancer therapy. J Biol Chem. 2000;275:22324–22320. doi: 10.1074/jbc.M907722199. [DOI] [PubMed] [Google Scholar]
- Resh MD. Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim Biophys Acta. 1999;1451:1–16. doi: 10.1016/s0167-4889(99)00075-0. [DOI] [PubMed] [Google Scholar]
- Slater SJ, Milano SK, Stagliano BA, Gergich KJ, Curry JP, Taddeo FJ, Stubbs CD. Interaction of protein kinase C with filamentous actin: isozyme specificity resulting from divergent phorbol ester and calcium dependencies. Biochemistry. 2000;39:271–280. doi: 10.1021/bi9916527. [DOI] [PubMed] [Google Scholar]
- Särndahl E, Bokoch GM, Stendahl O, Andersson T. Stimulus-induced dissociation of alpha subunits of heterotrimeric GTP-binding proteins from the cytoskeleton of human neutrophils. Proc Natl Acad Sci USA. 1993;90:6552–6556. doi: 10.1073/pnas.90.14.6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QJ, Ács P, Goodnight J, Blumberg PM, Mischak H, Mushinski JF. The catalytic domain of PKC-ε, in reciprocal PKC-δ and -ε chimeras, is responsible for conferring tumorgenicity to NIH3T3 cells, whereas both regulatory and catalytic domains of PKC-ε contribute to in vitro transformation. Oncogene. 1998;16:53–60. doi: 10.1038/sj.onc.1201507. [DOI] [PubMed] [Google Scholar]
- Wang QJ, Ács P, Goodnight J, Giese T, Blumberg PM, Mischak H, Mushinski JF. The catalytic domain of protein kinase C-δ in reciprocal δ and ε chimeras mediates phorbol ester-induced macrophage differentiation of mouse promyelocytes. J Biol Chem. 1997;272:76–82. doi: 10.1074/jbc.272.1.76. [DOI] [PubMed] [Google Scholar]
- Yao L, Suzuki H, Ozawa K, Deng J, Lehel C, Fukamachi H, Anderson WB, Kawakami Y, Kawakami T. Interactions between protein kinase C and pleckstrin homology domains. Inhibition by phosphatidylinositol 4,5-bisphosphate and phorbol 12-myristate 13-acetate. J Biol Chem. 1997;272:13033–13039. doi: 10.1074/jbc.272.20.13033. [DOI] [PubMed] [Google Scholar]
- Zeidman R, Löfgren B, Påhlman S, Larsson C. PKCε, via its regulatory domain and independently of its catalytic domain, induces neurite-like processes in neuroblastoma cells. J Cell Biol. 1999;145:713–726. doi: 10.1083/jcb.145.4.713. [DOI] [PMC free article] [PubMed] [Google Scholar]