Abstract
Background:
Dimethyl fumarate (DMF) is licensed for treatment of relapsing–remitting multiple sclerosis (RRMS). DMF can induce lymphopenia, which is assumed to increase the risk for opportunistic infections like progressive multifocal leukoencephalopathy. Our goal for this work was to estimate the frequency of grade 3 lymphopenia in DMF-treated patients with RRMS and to characterize patient-sided factors influencing the time course of lymphocyte repopulation after DMF withdrawal.
Material and methods:
A single-center retrospective data analysis was performed at University Hospital Bern, Switzerland. Patients with DMF treatment were analyzed for lymphocyte counts. Demographic factors were statistically analyzed in grade 3 lymphopenic patients.
Results:
We estimated a grade 3 lymphopenia frequency of 11/246 (4.5%), corroborating previous studies. In all patients, lymphocytes recovered to values ⩾800/µl within 0.5 years. Multivariate linear regression analysis unmasked older age as being associated with a longer duration of repopulation.
Conclusion:
Considering the aging population, our findings warrant further investigations of DMF-induced lymphopenia.
Keywords: adverse event, age, fumarate, multiple sclerosis, lymphocytes, white blood cells
Introduction
Dimethyl fumarate (DMF; Tecfidera®, Biogen Idec, Cambridge, MA, USA) was labelled to treat relapsing–remitting multiple sclerosis (RRMS), after two randomized placebo-controlled phase III clinical trials had demonstrated a favorable risk–benefit ratio.1,2 In a recent review, 19 cases of progressive multifocal leukoencephalopathy (PML) were reported in patients treated with DMF or other oral fumarate formulations (5 RRMS; 14 psoriasis).3 Since 13 of these patients showed grade 3 lymphopenia prior to PML diagnosis, high-grade lymphopenia was considered as a risk factor for PML development during fumarate therapy.3 Consequently, recommendations for monitoring DMF in RRMS were changed and medication withdrawal is recommended in patients with an absolute lymphocyte count below 500/µl.4 The frequency of ⩾ grade 1 lymphopenia was reported to occur in approximately 16.5% of DMF-treated patients5 and between 2.4–7% of the patients develop grade 3 lymphopenia (200 to <500/µl).1,2,6,7 Recently, a small case series of five patients with RRMS showed that even after DMF withdrawal, severe lymphopenia can persist for at least half a year.8 Thus, studies focusing on lymphocyte repopulation after DMF withdrawal are urgently needed. In addition, patient-related factors predisposing for a longer duration of post-DMF lymphopenia are not yet described. We therefore aimed to characterize the time course and patient-related factors for lymphocyte repopulation in patients with RRMS after DMF withdrawal due to grade 3 lymphopenia.
Materials and methods
We performed a single-center (Inselspital, Bern University Hospital, Switzerland) retrospective data analysis, which was approved by the cantonal ethics committee (KEK-BE 2017-01369).
Electronic patient records were searched for patients with RRMS treated with DMF for at least 1 month using the following search terms: ‘dimethyl fumarate’, ‘Tecfidera’ and ‘relapsing–remitting multiple sclerosis’. We identified 246 patients with RRMS with DMF treatment. The ‘Common Terminology Criteria for Adverse Events (version 3.0)’ definition of grade 3 lymphopenia (200 to <500 per µl) was used in this study.9 Lymphocyte counts of patients with grade 3 lymphopenia were evaluated from DMF withdrawal until reaching lymphocyte counts ⩾800/µl, which was defined as lymphocyte repopulation. We did not include patients with an infection around the time point of blood sampling.
Statistical analysis included comparative statistic as well as multiple linear regression analysis and was performed using SPSS (IBM, Armonk, NY, USA).
Results
A total of 11 of 246 DMF-treated (240 mg twice daily) patients with RRMS developed grade 3 lymphopenia after a mean of 501.9 days of treatment [95% confidence interval (CI) 288.4–715.4; range 172–1064]. No grade 4 lymphopenia was observed. There was no statistically significant difference between sex distribution in the grade 3 lymphopenic group compared with the whole DMF-treated population (female-to-male ratios: grade 3 lymphopenia: 2.8:1 versus whole population 2.2:1, Chi-square: p = 0.81).
Of the 11 patients, 4 developed lymphopenia within the first year of treatment, 5 within the second year and 2 after the second year. None of the patients received pretreatment with cell-depleting immunotherapies; the most frequently used drug before DMF was interferon-β (4/11; Table 1). Overall, two patients had co-medication potentially alleviating leuko- or lymphopenia (one clozapine, one carbamazepine; Supplementary Table 1). Lymphocytes recovered after DMF withdrawal in all of the 11 patients within half a year (mean 71.2 days, 95% CI 24.5–117.8, range 9–180; Figure 1). Multiple linear regression analysis adjusted for sex and duration of DMF treatment identified age at withdrawal of DMF as an independent predictor for a longer duration until reaching lymphocyte repopulation (regression coefficient: 4.07 (95% CI 1.26–6.87), p = 0.01). After lymphocyte recovery, 9 of the 11 patients were re-exposed to an immunotherapy (see Supplementary Table 2). All patients (n = 2) directly re-exposed with DMF 240 mg twice daily re-experienced lymphopenia (Supplementary Table 2).
Table 1.
Characteristics of DMF-treated and lymphopenic (grade 3) patient cohort (n = 11). Multiple linear regression analysis (MVreg) for the outcome variable ‘duration until reaching lymphocyte recovery in days’ was run. Lymphocyte recovery was defined as lymphocyte count ⩾800/µl. MVreg was adjusted for sex and duration of DMF treatment (days). R2 (MVreg): 0.69, n = 11.
| Mean (95% CI) | Range | |
|---|---|---|
| Age (years) | 49.8 (40.4–59.3) | 22.7–71.8 |
| Duration of DMF treatment (days) | 501.9 (288.4–715.4) | 172–1064 |
| Dosage of DMF (mg) | 480 (480–480) | 480–480 |
| Time until lymphocyte counts ⩾800 µl (days) | 71.2 (24.5–117.8) | 9–180 |
| N | % | |
| Diagnosis (RRMS) | 11/11 | 100 |
| Sex (female) | 8/11 | 72.7 |
| Immunotherapy prior to DMF | ||
| None | 6/11 | 54.5 |
| Interferon | 4/11 | 36.4 |
| Natalizumab | 1/11 | 9.1 |
| Coefficient (95% CI) | p value | |
| Multiple Linear Regression Analysis | ||
| Age at DMF withdrawal (years) | 4.07 (1.26–6.87) | 0.01 |
CI, confidence interval; DMF, dimethyl fumarate; MVreg, multiple linear regression analysis; N, number of observations; RRMS, relapsing–remitting multiple sclerosis.
Figure 1.
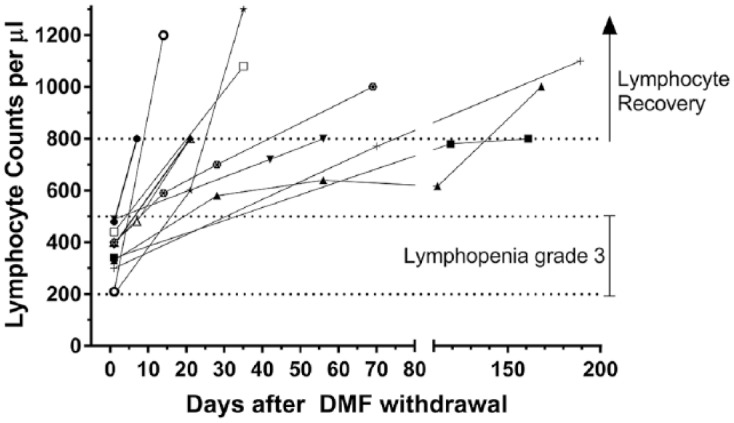
Time course of lymphocyte counts/μl after DMF withdrawal. Each line represents an individual patient with DMF-induced grade 3 lymphopenia (n = 11). Definition of grade 3 lymphopenia followed CTCAE recommendations.9 Individual lines start at the date of DMF withdrawal of each patient (set as day 0).
CTCAE, Common Terminology Criteria for Adverse Events; DMF, dimethyl fumarate.
Discussion
In our retrospective analysis, the frequency of grade 3 lymphopenia in DMF-treated patients with RRMS (4.5%) corroborates previous findings.1,2,6,7 In contrast with phase III clinical trials, where lymphocytes especially declined during the first year of treatment and remained stable thereafter,1,2 we demonstrated that, compared with the first treatment year, grade 3 lymphopenia occurred more frequently afterwards (first year 4/11 versus ⩾second year 7/11). This highlights the importance of regular full blood counts independent of DMF treatment duration. For lymphocyte recovery in our retrospective analysis, we demonstrated that older age was associated with a longer recovery phase. Only a small case series investigated recovery of lymphopenia after DMF withdrawal. Here, applying our definition of lymphocyte recovery, none of five patients recovered over a period of at least 6 months.8 In our study, lymphocytes did recover after DMF withdrawal in all of the 11 patients within half a year (Figure 1). After recovery of lymphopenia, five patients were re-exposed to DMF. Of these, two patients re-exposed with DMF 240 mg twice daily re-experienced lymphopenia whereas three patients continuing with reduced DMF dosage did not (one of the three patients was switched to a full dose after 12 months on reduced dosage; Supplementary Table 2).
Reasons for a longer recovery in older patients are speculative. However, immunosenescence leading to profound changes in the immune system is likely to be causative.10 In our aging population, there is thus an unmet need for future research to focus on age-associated treatment effects in this vulnerable patient cohort. Limitations of our data analysis are the monocentric and retrospective nature of our observational study. Therefore, findings should be re-evaluated prospectively with a larger patient population.
Supplementary Material
Acknowledgments
Robert Hoepner and Anke Salmen contributed equally to this work.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: MB received travel grants from Merck and Biogen.
MB and AM report no disclosures.
CF received speaker’s honoraria and travel grants from Biogen
LD received travel grants from Merck, Biogen, Roche and Bayer Schweiz.
AC has received personal compensation for activities with Bayer, Biogen, Genzyme, Merck, Novartis, Roche, Teva. He received research support from the Swiss National Fonds (SNF, No. 310030_172952), Genzyme and UCB.
RH received research and travel grants from Novartis and Biogen Idec. He also received speaker’s honoraria from Biogen, Novartis, Merk and Almirall.
AH received speaker honoraria or travel compensation for activities with Almirall Hermal GmbH, Biogen, Merck, Novartis, Roche and Sanofi Genzyme, none related to this work.
ORCID iD: Robert Hoepner  https://orcid.org/0000-0002-0115-7021
https://orcid.org/0000-0002-0115-7021
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Myriam Briner, Department of Neurology, Inselspital, Bern University Hospital and University of Bern, Freiburgstrasse, CH-3010 Bern, Switzerland.
Maud Bagnoud, Department of Neurology, Inselspital, Bern University Hospital and University of Bern, Bern, Switzerland.
Andrei Miclea, Department of Neurology, Inselspital, Bern University Hospital and University of Bern, Bern, Switzerland.
Christoph Friedli, Department of Neurology, Inselspital, Bern University Hospital and University of Bern, Bern, Switzerland.
Lara Diem, Department of Neurology, Inselspital, Bern University Hospital and University of Bern, Bern, Switzerland.
Andrew Chan, Department of Neurology, Inselspital, Bern University Hospital and University of Bern, Bern, Switzerland.
Robert Hoepner, Department of Neurology, Inselspital, Bern University Hospital and University of Bern, Bern, Switzerland.
Anke Salmen, Department of Neurology, Inselspital, Bern University Hospital and University of Bern, Bern, Switzerland.
References
- 1. Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012; 367: 1098–1107. [DOI] [PubMed] [Google Scholar]
- 2. Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012; 367: 1087–1097. [DOI] [PubMed] [Google Scholar]
- 3. Mills EA, Ogrodnik MA, Plave A, et al. Emerging understanding of the mechanism of action for dimethyl fumarate in the treatment of multiple sclerosis. Front Neurol 2018; 9: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. European Medicine Agency. Tecfidera: EPAR - product information, www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002601/WC500162069.pdf (2014, accessed 28 August 2018).
- 5. Mirabella M, Prosperini L, Lucchini M, et al. Safety and efficacy of dimethyl fumarate in multiple sclerosis: an Italian, multicenter, real-world study. CNS Drugs. 2018; 32: 963–970. [DOI] [PubMed] [Google Scholar]
- 6. Sejbaek T, Nybo M, Petersen T, et al. Real-life persistence and tolerability with dimethyl fumarate. Mult Scler Relat Disord 2018; 24: 42–46. [DOI] [PubMed] [Google Scholar]
- 7. Fox RJ, Chan A, Gold R, et al. Characterizing absolute lymphocyte count profiles in dimethyl fumarate–treated patients with MS Patient management considerations. Neurol Clin Pract 2016; 6: 220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zecca C, Antozzi CG, Torri Clerici V, et al. Severe multiple sclerosis reactivation during prolonged lymphopenia after dimethyl fumarate discontinuation. Acta Neurol Scand 2018; 137: 623–625. [DOI] [PubMed] [Google Scholar]
- 9. Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events v3.0 (CTCAE), ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf#search=%22Common Terminology Criteria for Adverse Events Version 3.0 %22 (2006, accessed 28 August 2018).
- 10. Nikolich-Zugich J. The twilight of immunity: emerging concepts in aging of the immune system. Nat Immunol 2018; 19: 10–19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


