Short abstract
Introduction
Aortic calcification as detected by computed tomography is associated with arterial stiffening and is an important predictor of cardiovascular morbidity and mortality. Uptake of 18F-sodium fluoride (18F-NaF) in the aortic wall reflects metabolically active areas of calcification. The aim of this study was to determine if 18F-NaF uptake in the aorta is associated with calcification and progression of calcification as detected by computed tomography.
Methods
Twenty-one postmenopausal women (mean age 62 ± 6 years) underwent assessment of aortic 18F-NaF uptake using positron emission tomography/computer tomography at baseline and a repeat computed tomography scan after a mean follow-up of 3.8 ± 1.3 years. Tracer uptake was quantified by calculating the target-to-background (TBR) ratios at baseline and follow-up. Calcification was assessed at baseline and follow-up using computed tomography.
Results
Over the follow-up period, aortic calcium volume increased from 0.46 ± 0.62 to 0.71 ± 0.93 cm3 (P < 0.05). However, the change in calcium volume did not correlate with baseline TBR either unadjusted (r = 0.00, P = 1.00) or adjusted for age and baseline calcium volume (beta coefficient = −0.18, P = 0.42). TBR at baseline did not differ between participants with (n = 16) compared to those without (n = 5) progression in calcium volume (2.43 ± 0.46 vs. 2.31 ± 0.38, P = 0.58). In aortic segments identified to have the highest tracer uptake at baseline, calcium volume did not significantly change over the follow-up period (P = 0.41).
Conclusion
In a cohort of postmenopausal women, 18F-NaF uptake as measured by TBR in the lumbar aorta did not predict progression of aortic calcification as detected by computed tomography over a four-year follow-up.
Keywords: Aortic calcification, progression, positron emission tomography, 18F-sodium fluoride
Introduction
Aortic calcification is an independent predictor of cardiovascular morbidity and mortality,1–6 improving risk classification for cardiovascular events by 14–15%.1,7,8 Within the aorta, the prevalence of aortic calcification increases with age and can occur in both the intimal and medial layers of the aortic wall. Intimal calcification occurs in association with atherosclerosis and may affect plaque rupture.9 Medial calcification occurs in association with elastin fragmentation10 and is associated with stiffening of large arteries independently of atherosclerosis.11–13 Large artery stiffness predisposes to the development of isolated systolic hypertension and is an independent predictor of cardiovascular morbidity and mortality.14 Despite the negative impact of vascular calcification and its association with cardiovascular outcomes there are currently no therapies that target aortic calcification.
Aortic calcification is now recognised to be an active process that resembles osteogenesis.15 The current gold standard measure of calcification is computed tomography (CT). However, this technique is limited to detecting existing macro-calcification and is insensitive to tissue undergoing novel mineralisation. Recent data suggest that 18F-sodium fluoride (18F-NaF) position emission tomography combined with computed tomography (PET/CT) has the potential to detect areas of biologically active calcification, which may be more susceptible to treatment.16 In bone, 18F-NaF is incorporated into exposed hydroxyapatite. In the vasculature, 18F-NaF absorbs to areas of micro-calcification and calcified deposits within plaque and localises adjacent to areas of existing calcification.16 This technique therefore, represents a potentially important tool for non-invasive in vivo imaging of arterial calcification and its impact on plaque vulnerability and large artery function. The aim of this study was to determine if 18F-NaF uptake in the aorta is associated with calcification and whether it predicts progression of calcification as detected by CT.
Methods
Twenty-one women who had previously undergone 18F-NaF PET/CT imaging for the assessment of bone mineralisation were studied and invited for a follow-up scan. Dates of the first and last scans in this prospective study were May 2009 to March 2015. Exclusion criteria included previous research X-ray exposure that exceeded 10 mSv, any contraindication to PET/CT and poor quality of baseline PET/CT image. The study was approved by St Thomas’ Hospital Research Ethics Committee, and written informed consent was obtained from all subjects. The study was conducted according to the principles of the 1975 Declaration of Helsinki.
PET CT scan acquisition
Baseline hybrid PET/CT with 180 MBq of 18F-NaF were performed as part of separate research studies conducted at the Osteoporosis Unit, Guy’s Hospital, London, UK.17–19 All scans were performed on a GE Discovery PET/CT scanner (General Electric Medical Systems, Waukesha, WI, USA). Subjects had an intravenous injection of 180 MBq 18F-NaF (three participants had 90 MBq) approximately 60 min prior to static PET/CT scan of the abdominal aorta referenced to lumbar spine region L1 to L4. Low-dose CT images were acquired for attenuation correction and quantification of calcium within the aorta. PET images were reconstructed by filtered back-projection using a Hanning 6.3-mm filter. This resulted in 47 × 3.27 mm2 slice for each frame with pixel size of 2.73 mm for PET and 0.98 mm for CT in the transaxial plane. Analysis of the static scans provided quantitative information of bone turnover using standardised uptake values (SUV).
A follow-up CT scan was performed after an average follow-up of 3.8 ± 1.3 years later using the same CT scanner with patients in a supine position with arms raised. A non-enhanced CT scan of the lumbar region (140 kV, 80 mA) was used to acquire transverse slices of the abdominal aorta over the same region as on the first visit.
Quantification of tracer uptake in the abdominal aorta
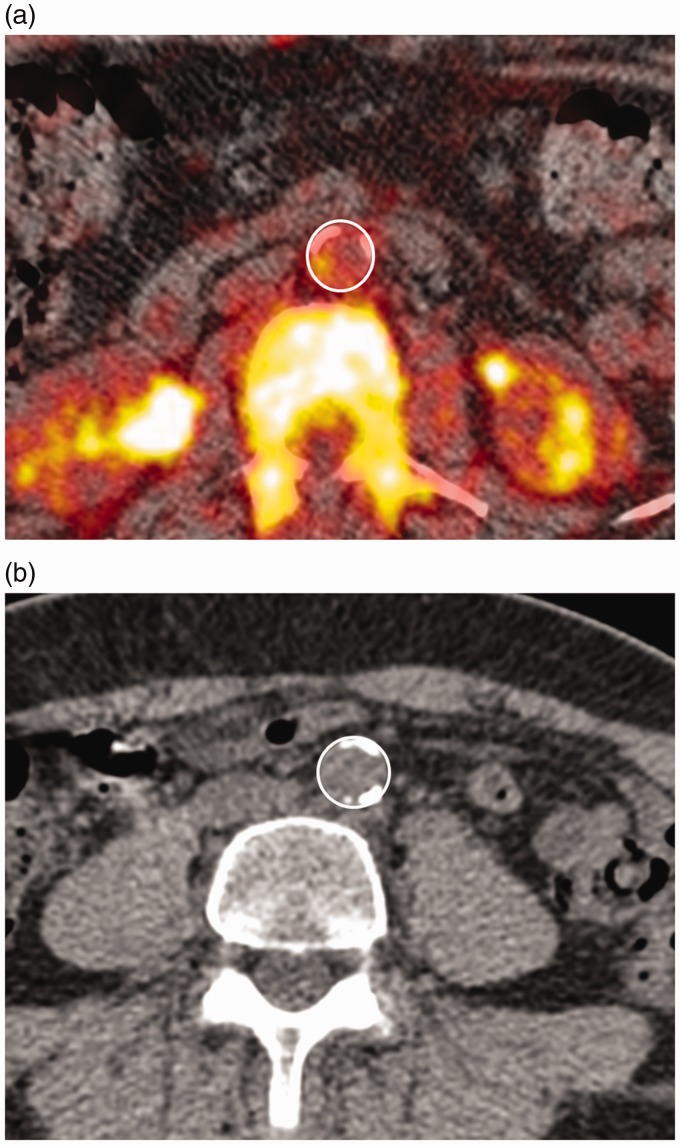
Retrospective and follow-up PET and CT images were viewed using the open-source DICOM viewer OsirixX (Osirix Imaging Software, Geneva, Switzerland). For quantification of aortic tracer uptake PET and CT images were fused together. Tracer uptake was quantified by a single reader using previously published methods.20 For each 3.27 mm image slice, mean standardised uptake value (SUVmean) and maximum standardised uptake value (SUVmax) of 18F-NaF was obtained by placing a region of interest around the wall of the aorta (Figure 1). The SUV measure is a well-recognised semi-quantitative measure of uptake corrected for radioactive decay and normalised to the amount of injected activity and body weight.21 To get the background blood activity of sodium fluoride tracer, SUVmean was measured in the vena cava and averaged for at least 8 consecutive slices. For this a region of interest (ROI) was placed within the centre of the vena cava in an area devoid of significant spill over activity. Tracer uptake was then quantified by calculating the mean target-to-background ratio (TBRmean) and maximum target-to-background ratio (TBRmax) as ratios of SUVmean and SUVmax in aorta and vena cava, respectively. In areas where tracer uptake in the vertebrae appeared to spill into the abdominal aorta a region of interest was drawn to include as much of the aorta as possible while avoiding activity in the vertebrae. Baseline and follow-up images were matched using anatomical landmarks on the CT scans and only matched images were analysed. For active vessel segment analysis, the slice with the greatest tracer uptake at baseline was identified and three consecutive slices were used for analysis centred on the slice with the maximum uptake. On the follow-up scan the same three slices were analysed for calcification.
Figure 1.
Example of fused 18F-NaF Pet and CT scan of the abdominal aorta at baseline (a) and a CT scan at follow-up (b) in separate individuals with the region of interest indicated.
Quantification of calcium score from CT imaging
The CT scan was used to produce the CT attenuation correction for the PET imaging and also as a diagnostic scan for aortic calcification. Calcification was defined as any region over 1 mm2 within the aorta with attenuation ≥130 Hounsfield units and quantified in cubic millimetres to give the calcium volume score (voxel volume × number of voxels ≥130 Hounsfield units).22
Statistical analysis
All analysis was performed using Stata (version 14). Subject characteristics are presented as mean ± standard deviation if they approximated a normal distribution. Categorical data were presented as n (%). Continuous variables were compared between the two time points using Student’s paired t-test and categorical variables using Wilcoxon-signed-rank test. Correlations between calcium score and radiotracer uptake were assessed using Spearman’s rank correlation coefficient because calcium score was not normally distributed. The present study had 90% power to detect a significant (P < 0.05) correlation of 0.65 between tracer uptake and progression of calcification, previously reported for tracer uptake in the aortic valve.23 Additionally, participants were split into those with and without progression according to whether there was an increase or no change in calcium volume change over the follow-up period. Progression in calcium volume was quantified as the change in in calcium volume between the baseline and follow-up visit. Association between progression in calcium volume score and baseline calcium and radiotracer uptake values were assessed using multivariable regression analysis.
Reproducibility study
To determine the intra-observer reproducibility, a PET/CT scan was performed in four additional participants at two time points 12 weeks apart. The scan protocol was identical to that of the second visit. Reproducibility was assessed by calculating intra-observer variability, defined as the absolute difference between measurements divided by the mean of the two measurements,24 for TBRmax, TBRmean and calcium score.
Results
Characteristics of participants at baseline and follow-up are listed in Table 1. At baseline, the average age of participants was 62.6 ± 6.0 years, two participants (10%) were current smokers, two (10%) were on treatment for hypertension and four (19%) were on treatment for hypercholesterolemia. Average aortic calcium volume was 0.46 ± 0.62 cm3 and radiotracer uptake measured as TBRmax and TBRmean were 2.40 ± 0.44 and 1.23 ± 1.34, respectively. After an average follow-up of 3.8 ± 1.3 years, the number of participants on statin treatment increased to 5 (24%) participants and the number on bisphosphonate therapy increased to 6 (29%). Aortic calcium volume progressed to 0.71 ± 0.93 cm3 (P < 0.05).
Table 1.
Demographic and clinical characteristics.
| Baseline | Follow-up | P-value | |
|---|---|---|---|
| Age (years) | 62.6 ± 6.0 | 66.3 ± 6.1 | <0.0001 |
| Height (cm) | 160.3 ± 7.1 | 160.0 ± 7.6 | 0.41 |
| Weight (kg) | 65.7 ± 7.5 | 65.4 ± 7.8 | 0.72 |
| Current smoker, n (%) | 2 (10) | 2 (10) | 1.00 |
| Hypertension, n (%) | 2 (10) | 2 (10) | 1.00 |
| Statins, n (%) | 4 (19) | 5 (24) | 0.31 |
| Bisphosphonates, n (%) | 0 (0) | 6 (29) | <0.05 |
| TBRmax | 2.40 ± 0.44 | – | – |
| TBRmean | 1.23 ± 0.12 | – | – |
| Calcium volume (cm3) | 0.46 ± 0.62 | 0.71 ± 0.93 | <0.05 |
Correlation between radiotracer uptake, calcium score and progression in calcium score
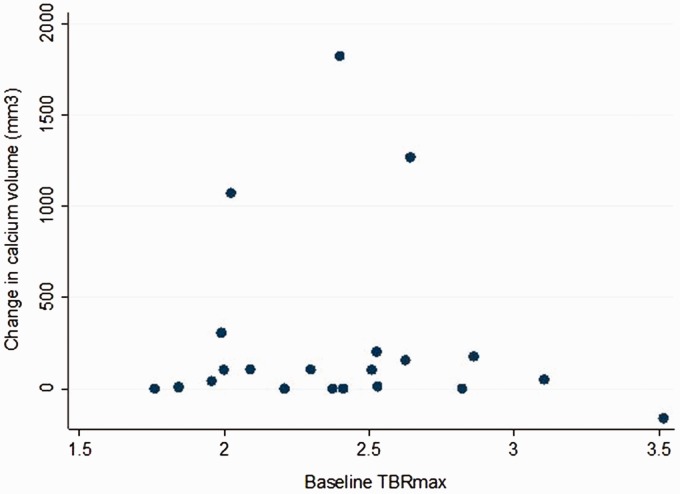
Even though there was a significant increase in aortic calcium volume over the four-year follow-up period, with average aortic calcium volume increasing from 0.46 ± 0.62 to 0.71 ± 0.93 cm3 (P < 0.05), there was no correlation between change in calcium volume with baseline radiotracer uptake values (r = 0.00 (95% confidence interval −0.46–0.46), P = 1.00 for TBRmax and r = −0.12 (95% confidence interval −0.54–0.31), P = 0.61 for TBRmean, Figure 2). The results did not change when analysis was repeated excluding women on bisphosphonate treatment or excluding women on statin treatment. In multivariate regression analysis (incorporating age and baseline aortic calcium volume as covariates), progression of aortic calcium volume score did not significantly correlate with baseline TBRmax (beta coefficient = −0.18, P = 0.42).
Figure 2.
Correlation between change in aortic calcium volume (mm3) over a four-year follow-up and baseline aortic 18F-NaF tracer uptake (TBRmax).
Progression in aortic calcium volume over the four-year follow-up was observed in 15/21 participants. Participants with no progression in aortic calcium volume did not have any calcium at baseline as detected by CT. TBRmax and TBRmean did not differ between participants with progression (n = 15) compared to non-progressors (n = 6) in aortic calcium volume (TBRmax 2.36 ± 0.37 vs. 2.51 ± 0.60, P = 0.48 and TBRmean 1.23 ± 0.12 vs. 1.24 ± 0.13, P = 0.86). In aortic segments identified to have the highest radiotracer uptake at baseline, there was a trend towards an increase in calcium volume however this did not reach statistical significance (calcium volume at baseline 0.08 ± 0.15 cm3 vs. 0.12 ± 0.26 cm3 at follow-up, P = 0.41).
Reproducibility study
There was good reproducibility for the quantification of aortic TBRmax, TBRmean and calcium score in the four participants with repeat PET-CT scans. The mean absolute difference between repeat measurements on the same participant for aortic TBRmax, TBRmean and calcium score was 0.12 (range: 0.01–0.33), 0.16 (range: 0.06–0.33) and 0.05 cm3 (range: 0–0.17 cm3), respectively. The relative intra-observer variability for the absolute difference was 9.2% (95% confidence interval (CI): −6.9–25%) for TBRmax and 12.7% (95% CI: 1.0–25%) for TBRmean. The intra-observer variability for aortic calcium score was 6.2% (95% CI: −14–26%).
Discussion
Aortic calcification, detected using CT or X-rays, is associated with increased cardiovascular morbidity and mortality.1–6 The mechanism by which the presence of aortic calcification is predictive of cardiovascular outcome is unknown but is likely to involve negative effects of calcification on plaque rupture, arterial stiffening and predisposition to isolated systolic hypertension.9–14 Despite the prognostic importance of vascular calcification there are currently no interventions available to reduce or prevent vascular calcification. Developing novel therapeutic interventions targeting vascular calcification may further be impeded by current imaging techniques, which are limited to detecting macro-calcification rather than biologically active areas of novel mineralisation, which may be more susceptible to therapy.
PET/CT imaging using 18F-NaF is a possible new approach to detect metabolically active calcification non-invasively. The 18F-NaF radiotracer has been shown to absorb to calcified deposits within plaque using electron microscopy, autoradiography, histology, and preclinical PET/CT.16 Furthermore, Derlin et al.25,26 showed radiotracer uptake, as detected by PET, to co-localise with regions of macro-calcification detected by CT in carotid arteries of 269 oncology patients. In patients with aortic valve disease, imaged radiotracer uptake correlates with aortic valve disease severity, Framingham risk score and prior cardiovascular events.20,27 In the present study, average aortic TBRmax was 2.40 ± 0.44, which is similar to that previously reported from histological studies16 and in the aorta and large arteries (range 2.20–2.40),20,26,28–30 but higher than reported in the aortic valve of asymptomatic individuals (1.56; range 1.41–1.64).27 The average TBRmean of 1.23 ± 1.34, which was similar to that observed in the aortic valve (1.23; range 1.2–1.59).27 We found limited evidence of a correlation between baseline calcium volume and radiotracer uptake score. This is similar to findings from a recent study by Oliveira-Santos et al.31 that showed 18F-NaF tracer uptake in the coronary, aortic and carotid arteries but found no correlation between tracer uptake and calcium score. Similarly, Li et al.28 and Ishiwata et al.30 showed no correlation between calcium density and radiotracer uptake in the aorta and large arteries. A direct correlation between NaF vascular uptake and CV risk score factors was demonstrated by Morbelli et al.32 However, the same study did not find any correlation between TBR and calcium load (similarly to the present study).32 In contrast, Dweck et al.20,27 found a strong positive correlation between tracer uptake and calcification in both the aortic valve and coronary artery in patients with aortic stenosis and controls. However, the authors also noted that a large proportion of participants with extensive calcification had normal radiotracer uptake.20 These inconsistent findings may be due to the correlation between available surface area to the isotope and radiotracer uptake, which may be greater in the valve compared to the aorta.16 Another study has demonstrated that only plaques with a lower density display a relevant tracer uptake in cross-sectional analysis.33 This phenomenon was attributed to the different ‘active’ and ‘passive’ calcification patterns that prevail in initial and consolidated plaques, and this might explain the inconsistent findings reported.
The ability to predict future calcification cannot be inferred from cross-sectional observations and is of importance since a surrogate marker for interventions to prevent and/or reverse calcification that may have to be sustained over the longer term would be invaluable. In the present study, over an average follow-up of 3.8 years, even though there was a significant increase in aortic calcification as detected by CT, radiotracer uptake within the aorta did not correlate with progression in calcification. Additionally, aortic radiotracer uptake did not differ between individuals with and without progression in aortic calcification and areas identified to have the highest radiotracer uptake did not have a significant change in calcium volume.
Previous studies that assessed the relationship between radiotracer uptake and progression of macro-calcification have reported inconsistent findings. Dweck et al.23 investigated the progression of aortic valve calcification in 18 patients with aortic sclerosis and stenosis. After one year, baseline radiotracer uptake correlated with progression of valvular calcification. However, the authors noted areas of high tracer uptake that did not develop into detectable change in calcification. Li et al.28 showed that, despite significant radiotracer uptake and radiotracer progression in the aorta, carotid and iliac arteries, there was limited evidence that this was associated with the progression of macro-calcification detected by CT in 19 myeloma patients (P = 0.07). Ishiwati et al.30 found that out of 96 identified hotspots of radiotracer uptake, only 19 developed calcification as detected by CT. In areas of existing calcification tracer uptake did predict progression of calcification as detected by CT, but this analysis was limited to areas of existing macro-calcification and did not include the whole of the aorta. The discrepancy between these findings may be due to differences in the distribution of intimal and medial calcification between the aorta and other vascular beds where 18F-NaF tracer uptake may not be able to penetrate calcification in the media of the aorta.16
Conclusion
In a cohort of 21 postmenopausal women, 18F-NaF uptake as measured by TBR in the lumbar aorta did not predict progression of aortic calcification as detected by CT over a four-year follow-up period and is unlikely to be a useful marker of calcification in small-scale interventional studies of aortic calcification confined to the lumbar region.
Limitations
We recognise several limitations of the present study. This study is limited to female participants and may not be generalisable to men or other populations, in particular high-risk patients. The proximity of the aorta to the vertebrae may mean that our results may have been influenced by overspill from tracer uptake in the vertebrae and we cannot necessarily extrapolate our results to the thoracic aorta or to other areas of vascular calcification. However, care was taken to exclude these sections of the aorta and the observed aortic tracer uptake was similar to other studies that have reported tracer uptake in large arteries distant from the vertebrae.25,27 In addition, we found good repeatability of radiotracer uptake from scans performed on two separate occasions with the limits similar to that previously reported in patients with aortic sclerosis, stenosis and controls.27 The CT scans were performed at different radiation doses. However, previous studies have found that low-dose scans have produced equivalent calcium scores compared to higher dose scans and this is thus unlikely to impact the present study.34 In addition, the CT scan settings did not vary between subjects and thus any systematic effect is unlikely to contribute to between subject variability. Furthermore, the calcium volume score does not take into account any changes in calcium density. The sample size of the present study was relatively small. However, the upper 95% CI for the correlation between 18F-NaF TBRmax and progression of calcium score accounted for 21% of the variability in the progression of calcification. This suggests that, even if a positive correlation were demonstrated in a larger sample size, 18F-NaF tracer uptake would not be a useful marker in small-scale interventional studies. In addition, this is the largest study to date to investigate the association between tracer uptake and progression in calcification.
Acknowledgement
The authors are thankful to the PET Centre at St Thomas’ Hospital, London, UK.
Contributorship
MC, AM, MF were responsible for data collection. PC, MC, AM, MF and IF were responsible for study design. MC drafted the manuscript. PC, GB were responsible for critical revisions of manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval
The study was approved by St Thomas’ Hospital Research Ethics Committee, and written informed consent was obtained from all subjects. The study was conducted according to the principles of the 1975 Declaration of Helsinki. Ethical approval for this study was obtained from London-Westminster Research Ethics Committee) (REC 10/H0802/89).
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: M Frost, G Blake and I Fogelman received grants from the Wellcome Trust and Novartis, which included funding for the baseline 18F-fluoride PET scans. This work was also supported by a British Heart Foundation Centre for Research Excellence Career Development Fellowship. The authors acknowledge financial support from the Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre and Clinical Research Facilities awards to Guy’s and St Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust.
Guarantor
Dr Marina Cecelja
References
- 1.Wilson PW, Kauppila LI, O'Donnell CJ, et al. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation 2001; 103: 1529–1534. [DOI] [PubMed] [Google Scholar]
- 2.Walsh CR, Cupples LA, Levy D, et al. Abdominal aortic calcific deposits are associated with increased risk for congestive heart failure: the Framingham Heart Study. Am Heart J 2002; 144: 733–739. [DOI] [PubMed] [Google Scholar]
- 3.Hollander M, Hak AE, Koudstaal PJ, et al. Comparison between measures of atherosclerosis and risk of stroke: the Rotterdam Study. Stroke 2003; 34: 2367–2372. [DOI] [PubMed] [Google Scholar]
- 4.van der Meer IM, Bots ML, Hofman A, et al. Predictive value of noninvasive measures of atherosclerosis for incident myocardial infarction: the Rotterdam Study. Circulation 2004; 109: 1089–1094. [DOI] [PubMed] [Google Scholar]
- 5.Takasu J, Mao S, Budoff MJ. Aortic atherosclerosis detected with electron-beam CT as a predictor of obstructive coronary artery disease. Acad Radiol 2003; 10: 631–637. [DOI] [PubMed] [Google Scholar]
- 6.Danielsen R, Sigvaldason H, Thorgeirsson G, et al. Predominance of aortic calcification as an atherosclerotic manifestation in women: the Reykjavik study. J Clin Epidemiol 1996; 49: 383–387. [DOI] [PubMed] [Google Scholar]
- 7.Elias-Smale SE, Wieberdink RG, Odink AE, et al. Burden of atherosclerosis improves the prediction of coronary heart disease but not cerebrovascular events: the Rotterdam Study. Eur Heart J 2011; 32: 2050–2058. [DOI] [PubMed] [Google Scholar]
- 8.Peters SA, den Ruijter HM, Bots ML, et al. Improvements in risk stratification for the occurrence of cardiovascular disease by imaging subclinical atherosclerosis: a systematic review. Heart 2012; 98: 177–184. [DOI] [PubMed] [Google Scholar]
- 9.Ruiz JL, Hutcheson JD, Aikawa E. Cardiovascular calcification: current controversies and novel concepts. Cardiovasc Pathol 2015; 24: 207–212. [DOI] [PubMed] [Google Scholar]
- 10.Aikawa E, Aikawa M, Libby P, et al. Arterial and aortic valve calcification abolished by elastolytic cathepsin S deficiency in chronic renal disease. Circulation 2009; 119: 1785–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cecelja M, Jiang B, Bevan L, et al. Arterial stiffening relates to arterial calcification but not to noncalcified atheroma in women. A twin study. J Am Coll Cardiol 2011; 57: 1480–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cecelja M, Chowienczyk P. Arterial stiffening: cause and prevention. Hypertension 2010; 56: 29–30. [DOI] [PubMed] [Google Scholar]
- 13.Cecelja M, Hussain T, Greil G, et al. Multimodality imaging of subclinical aortic atherosclerosis: relation of aortic stiffness to calcification and plaque in female twins. Hypertension 2013; 61: 609–614. [DOI] [PubMed] [Google Scholar]
- 14.Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006; 27: 2588–2605. [DOI] [PubMed] [Google Scholar]
- 15.Cecelja M, Chowienczyk P. Molecular mechanisms of arterial stiffening. Pulse (Basel) 2016; 4: 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irkle A, Vesey AT, Lewis DY, et al. Identifying active vascular microcalcification by (18)F-sodium fluoride positron emission tomography. Nature Commun 2015; 6: 7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frost ML, Compston JE, Goldsmith D, et al. (18)F-fluoride positron emission tomography measurements of regional bone formation in hemodialysis patients with suspected adynamic bone disease. Calcif Tissue Int 2013; 93: 436–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frost ML, Moore AE, Siddique M, et al. (1)(8)F-fluoride PET as a noninvasive imaging biomarker for determining treatment efficacy of bone active agents at the hip: a prospective, randomized, controlled clinical study. J Bone Miner Res 2013; 28: 1337–1347. [DOI] [PubMed] [Google Scholar]
- 19.Puri T, Blake GM, Frost ML, et al. Comparison of six quantitative methods for the measurement of bone turnover at the hip and lumbar spine using 18F-fluoride PET-CT. Nucl Med Commun 2012; 33: 597–606. [DOI] [PubMed] [Google Scholar]
- 20.Dweck MR, Chow MW, Joshi NV, et al. Coronary arterial 18F-sodium fluoride uptake: a novel marker of plaque biology. J Am Coll Cardiol 2012; 59: 1539–1548. [DOI] [PubMed] [Google Scholar]
- 21.Shankar LK, Hoffman JM, Bacharach S, et al. Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Cancer Institute Trials. J Nucl Med 2006; 47: 1059–1066. [PubMed] [Google Scholar]
- 22.Cecelja M, Frost ML, Spector TD, et al. Abdominal aortic calcification detection using dual-energy X-ray absorptiometry: validation study in healthy women compared to computed tomography. Calcif Tissue Int 2013; 92: 495–500. [DOI] [PubMed] [Google Scholar]
- 23.Dweck MR, Jenkins WS, Vesey AT, et al. 18F-sodium fluoride uptake is a marker of active calcification and disease progression in patients with aortic stenosis. Circ Cardiovasc Imaging 2014; 7: 371–378. [DOI] [PubMed] [Google Scholar]
- 24.Popovic ZB, Thomas JD. Assessing observer variability: a user’s guide. Cardiovasc Diagn Ther 2017; 7: 317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Derlin T, Richter U, Bannas P, et al. Feasibility of 18F-sodium fluoride PET/CT for imaging of atherosclerotic plaque. J Nucl Med 2010; 51: 862–865. [DOI] [PubMed] [Google Scholar]
- 26.Derlin T, Wisotzki C, Richter U, et al. In vivo imaging of mineral deposition in carotid plaque using 18F-sodium fluoride PET/CT: correlation with atherogenic risk factors. J Nucl Med 2011; 52: 362–368. [DOI] [PubMed] [Google Scholar]
- 27.Dweck MR, Jones C, Joshi NV, et al. Assessment of valvular calcification and inflammation by positron emission tomography in patients with aortic stenosis. Circulation 2012; 125: 76–86. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Heber D, Cal-Gonzalez J, et al. Association between osteogenesis and inflammation during the progression of calcified plaque evaluated by 18F-fluoride and 18F-FDG. J Nucl Med 2017; 58: 968–974. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Berenji GR, Shaba WF, et al. Association of vascular fluoride uptake with vascular calcification and coronary artery disease. Nucl Med Commun 2012; 33: 14–20. [DOI] [PubMed] [Google Scholar]
- 30.Ishiwata Y, Kaneta T, Nawata S, et al. Quantification of temporal changes in calcium score in active atherosclerotic plaque in major vessels by 18F-sodium fluoride PET/CT. Eur J Nucl Med Mol Imaging 2017; 44: 1529–1537. [DOI] [PubMed] [Google Scholar]
- 31.Oliveira-Santos M, Castelo-Branco M, Silva R, et al. Atherosclerotic plaque metabolism in high cardiovascular risk subjects – A subclinical atherosclerosis imaging study with 18F-NaF PET-CT. Atherosclerosis 2017; 260: 41–46. [DOI] [PubMed] [Google Scholar]
- 32.Morbelli S, Fiz F, Piccardo A, et al. Divergent determinants of 18F-NaF uptake and visible calcium deposition in large arteries: relationship with Framingham risk score. Int J Cardiovasc Imaging 2014; 30: 439–447. [DOI] [PubMed] [Google Scholar]
- 33.Fiz F, Morbelli S, Piccardo A, et al. 8)F-NaF uptake by atherosclerotic plaque on PET/CT imaging: inverse correlation between calcification density and mineral metabolic activity. J Nucl Med 2015; 56: 1019–1023. [DOI] [PubMed] [Google Scholar]
- 34.Hecht HS, de Siqueira ME, Cham M, et al. Low- vs. standard-dose coronary artery calcium scanning. Eur Heart J Cardiovasc Imaging 2015; 16: 358–363. [DOI] [PubMed] [Google Scholar]