Abstract
Background
Poor medication adherence is a major factor in the secondary prevention of cardiovascular diseases (CVD) and contributes to increased morbidity, mortality, and costs. Interventions for improving medication adherence may have limited effects as a consequence of self selection of already highly adherent participants into clinical trials.
Methods
In this retrospective cohort study, existing levels of medication adherence were examined in self-decided participants and non-participants prior to inclusion in a randomized controlled study (RCT), evaluating the effect of an intervention to improve adherence. In addition, the non-participants were further divided into ‘responders’ and ‘non responders’. All individuals had manifest cardiovascular disease and completed a questionnaire with baseline characteristics, the Beliefs about Medicines Questionnaire (BMQ) and the Modified Morisky Scale® (MMS®) as part of a regular screening program. A logistic regression was conducted to examine the relationship between study participation willingness, adherence level and the beliefs about medication.
Results
According to the MMS® the adherence level was comparable in all groups. In both (non)-participants groups, 36% was classified as high adherent; 46% participants versus 44% non-participants were classified as medium adherent and 19% of the participants versus 20% of the non-participants were low adherent (p = 0.91. The necessity concern differential (NCD) from the BMQ was 3.8 for participants and 3.4 for non-participants (p = 0.32).
Conclusion
This study shows that adherence to medication and beliefs about medication do not differ between participants and non-participants before consenting to participate in an RCT. The study design seems not to have led to greater adherence in the study group.
Keywords: Randomized controlled trials, Informed consent, Participation, Selection bias, Adherence
Background
Cardiovascular risk reduction is predominantly based on lipid and blood pressure lowering treatment, inhibition of platelet aggregation, smoking cessation and control of obesity [1]. A limitation in lowering cardiovascular risk is poor adherence to prescribed medication [2] which may consequently can lead to increased morbidity, mortality and costs [3–6]. Nevertheless, a recent review with mostly cohort studies, showed that only 60% of people who use cardiovascular medication, were adherent to their cardiovascular medication [7]. In view of that there is a need for interventions to improve medication adherence in this population. Although there is a considerable amount of research in the field of interventions to improve medication adherence in cardiovascular patients, they often show only small effects [8]. It is suggested that patient recruitment methods in randomized controlled trials (RCT) to improve patient adherence to medication may influence outcome [8, 9]. An important observation is that patients participating in RCTs generally have higher adherence rates at baseline than could be expected based on observational studies [10–14]. It is conceivable that the informed consent procedure results in a selection of patients with higher adherence rates [12]. Willingness to participate is positively influenced by patients’ engagement with their medical condition, high level of education and the influence of an important person [11, 15]. These characteristics are also considered as positive determinants for medication adherence [16]. Although a recent review showed that the inclusion of non adherent patients was the single feature significantly associated with effective adherence interventions, most studies seem to include patients because they are willing to participate not because they are poor adherent [11]. It is suggested that patients participating in these RCTs already have a pre-existing high adherence level at baseline [10, 12–14]. Selection of participants with high levels of adherence at baseline, makes it difficult to show an improving intervention effect (ceiling effect) [8]. More understanding about the medication adherence of participants as well as non-participants before the start of these RCT’s may contribute to a better understanding of why so many studies show no improvement in medication adherence. One possible explanatory determinant for (non) adherent behaviour is medication beliefs. Personal beliefs about need for treatment (necessity beliefs) and concerns about several potential adverse consequences (concern beliefs) could explain a large part of (non) adherent behaviour [16–18]. If patients perceive that the need for medication outweighs the concerns, they are more likely to be adherent to their medication(s) [19].
Methods
Aim
The aim of this study is to explore possible differences in adherence to existing prescribed medication in cardiovascular patients who did or did not consent to participate in an RCT which expressly explored the effects of an intervention to improve adherence. We hypothesized that patients who are willing to participate in a clinical trial are more likely to be medication adherent and have more ‘necessity beliefs’ about their medication compared to patients who are not willing to participate.
Study design and setting
In this retrospective cohort study we included patients who participated or declined participation in the (MIRROR) trial (a Multifaceted nurse -and web-based Intervention for impRoving adheRence to treatment in patients with cardiOvasculaR disease) [20]. In brief, the MIRROR trial was a prospective, randomized controlled trial in which patients aged ≥18 years and diagnosed with a manifest cardiovascular disease (i.e. acute coronary syndrome, peripheral arterial disease or stroke/Transient Ischemic Attack (TIA)) after providing written informed consent, were included. The MIRROR trial aimed to study the effect of different adherence enhancing strategies on cardiovascular medication adherence. Within this context, patients were randomized to usual care, an e-health intervention, and an e-health intervention combined with motivational technique consultations.
Participants
All patients referred to the Radboud University Medical Center with a new diagnosis of acute coronary syndrome, myocardial infarction, peripheral arterial disease, an aneurysm of the aorta or TIA or stroke over the prior 6 weeks were included into the hospital CVD screening program. This screening program aims to identify cardiovascular risk factors and consists of screening for lifestyle (smoking, diet and exercise), medication adherence by the self reported questionnaires Modified Morisky Scale® (MMS®) and the Beliefs about Medication Questionnaire (BMQ), blood lipid levels, blood pressure, waist circumference, body mass index (BMI), glucose blood levels and a family history of cardiovascular diseases. If indicated, preventive therapies (medication and lifestyle interventions) are initiated and followed over time [1]. All patients referred to this screening program were asked to participate in the MIRROR- trial. ‘Participants’ were patients who were willing to participate in the intervention study and ‘non-participants’ were patients who declined informed consent for the MIRROR trial. Because adherence to medication may also differ between responders and non- responders to surveys, with responders having higher adherence levels [21] we divided the group of non-participants further. Retrospectively of the MIRROR trial, a letter was sent to all non-participants for a different study not subject to this paper. For this study, a signed informed consent was requested from the non-participants. Non-responders were patients who did not sign this letter. Responders were patients who signed the informed consent letter. We aimed to explore if the non-responding subgroup of the non-participants differed from the responders with respect to their level of medication adherence on the basis of prior MMS® from the screening program.
Ethical approval
The Ethical Committee waived the need for a formal informed consent for this study. The study was conducted according to the good clinical practice protocol and we used usual care data considering the research question of this study. Data was anonymized according to the research protocols of the Ethical Committee.
Outcomes
Participation or declining to participate to the RCT was the independent variable in this study. Adherence to medication and the beliefs about medication the dependent variables. Adherence to cardiovascular medication was calculated by the MMS® [22–24]. It consists of eight items aimed at measuring adherence. Each item accounts for 0 or 1 in the case questions are answered by no or yes respectively. Consequently, total MMS® scores range between 0 and 8. These scores were divided into three levels of adherence: low adherence (sum score < 6), medium adherence (sum score 6 to < 8) and high adherence (sum score of 8) [25]. To evaluate patients’ beliefs and perceptions about their medication, BMQ [26] was used. This validated questionnaire provides information about the beliefs, perceived necessity and concerns the patient has regarding their illness and prescribed medication. There are five statements regarding “necessity beliefs” and five regarding “concern beliefs”. Patients indicated their degree of agreement with each individual statement about the use of their medicines on a five-point Likert scale. Thus, total scores for the necessity and concerns scales could range from 5 to 25. The necessity– concerns differential (NCD) was then calculated as the difference between necessity and concerns scores and had a possible range of − 20 to 20 [19, 27]. To differentiate between patients on the basis of their beliefs about the necessity of their medication and their concerns about taking medication, the total necessity and concern scores [5−25] were split at midpoint (thus 5–12 was considered as low and 13 t/m 25 was considered as high). Patients were then classified into four different categories: accepting (high necessity and low concerns), ambivalent (high necessity and high concerns), skeptical (high concerns and low necessity) and indifferent (low concerns and low necessity) [28–30].
From all patients the type of CVD (acute coronary syndrome, myocardial infarction, peripheral arterial disease, an aneurysm of the aorta or TIA) was recorded. Also, the following baseline and clinical characteristics were collected: age, sex, level of education, employment status, the country of origin and the type of cardiovascular medication used.
Data collection and timeline
Data were derived from the screening program. Data were registered in a secure website which could only be accessed by nurses involved in the screening program. Within, on average, six weeks after the CVD-event, baseline characteristics and the questionnaires were collected for all patients as part of the screening program.
Statistical analyses
Data were analyzed and evaluated using SPSS version 22. Descriptive statistics (mean, median, standard deviation) were used for all variables. A Mann-Whitney Test was used to compare groups (participants and non-participants) with the non parametric outcome, the MMS®. Confounders were explored by performing a logistic linear test of all the characteristics in the case the groups significantly differed (including the NCD). The same Mann-Whitney test was performed to compare the NCD between the groups. A logistic regression was used to explore differences between (non) participants and the four belief groups. The same statistical analyses were performed for the responders and non-responders. A Kruskal-Wallis test was performed to explore the relationship between the NCD and the MMS® for all groups.
Results
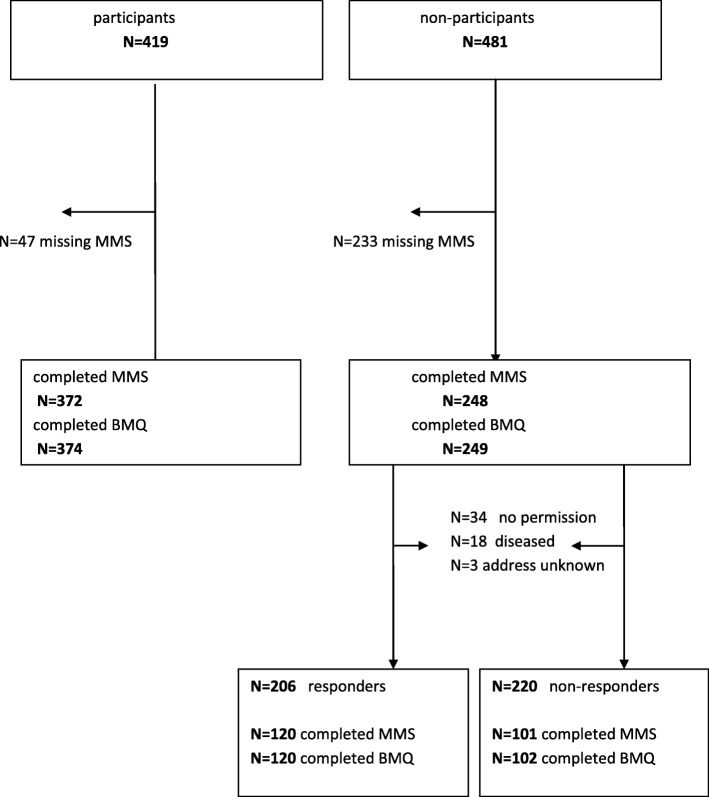
In total, 900 patients with a new cardiovascular event between October 2011 and October 2013 were eligible for participation into the MIRROR trial. Of these, 419 agreed and 481 refused participation. Of all the non-participants who received a letter for another study, 220 did not respond. Consequently, 261 non-participants were classified as responders (Fig. 1).
Fig. 1.
Participants, non-participants,responders and non responders and MMS and BMQ
Patient characteristics
The total cohort (participants and non-participants) had a mean age of 62 years and was predominantly male (67%). Participants significantly differed from non-participants with respect to age (61 years versus 63 years, p = 0.001), male sex (71% versus 58%, p = 0.001), systolic blood pressure (136 mmHg versus 142 mmHg, p = 0.001). Participants were more frequently diagnosed with an acute coronary syndrome (36% versus 16%, p < 0.001]), were using more beta blocking agents or agents acting on the renin-angiotensin system (58% versus 46% p = 0.001 and 59% versus 44% p = 0.001, respectively), and were using more lipid lowering medication (94% versus 82%, p < 0.001). Among the non-participants, responders were older (64 versus 60 years, p = 0.002) and used more agents acting on the renin-angiotensin system (48% versus 37%, p = 0.02) than non-responders (Tables 1 and 2).
Table 1.
Differences in patient characteristics between participants and non participants in a RCT-trial on adherence
| Participants [n = 419] | Non-participants [n = 481] | P-value | |
|---|---|---|---|
| Age [mean, ±SD) | 60.5 [±10] | 63 [11] | 0.001 |
| Gender (N [%]) | |||
| Male | 296 [7] | 279 [58] | < 0.001 |
| Female | 123 [29 | 202 [42] | |
| Education level (N[%]) | < 0.001 | ||
| Primary | 66 [18] | 141 [31] | |
| Secondary | 185 [49] | 180 [39] | |
| University | 124 [33] | 140 [30] | |
| Labour (N[%]) | 0.08 | ||
| Paid labour | 137 [37] | 123 [27] | |
| Unemployed | 98 [26.3] | 138 [30] | |
| Retired | 138 [37] | 199 [43.3] | |
| Country of origin is the Netherland (N[%]) | 0.66 | ||
| Yes | 327 [90] | 398 [86] | |
| No | 37 [10] | 64 [14] | |
| Reason referral (N[%]) | < 0.001 | ||
| acute coronary syndrome | 150 [36] | 79 [16] | |
| peripheral arterial disease | 71 [17] | 101 [21] | |
| troke/TIA | 198 [47] | 301 [63] | |
| Blood pressure (mmHg; mean ± SD) | < 0.001 | ||
| Systolic | 136 [±18] | 142 [±20] | 0.23 |
| Diastolic | 77 [±11] | 78 [±11] | |
| Body Mass Index (mean ± SD) | 27 [±4] | 26 [±4] | 0.30 |
| Waist (mean ± SD) | |||
| Male | 99.5 [±9] | 98.4 [±12] | 0.10 |
| Female | 92 [±14] | 90 [±13] | 0.07 |
| Lipids (mmol/ltr, mean SD) | |||
| Totaal cholesterol | 4.5 [±1.1] | 4.6 [±1] | 0.7 |
| Triglyceriden | 1.8 [±1] | 1.7 [±1] | 0.01 |
| HDL | 1.2 [±0.3] | 1.2 [±0.3] | 0.002 |
| LDL | 2.5 [±0.9] | 2.6 [±0.9] | 0.66 |
| Medication (N [%]) | |||
| Antithrombotic agents [ATC B01] | 404 [98] | 461 [98] | 0.78 |
| Diuretics [ATC C03] | 109 [26] | 135 [29] | 0.44 |
| Beta Blocking agents [ATC C07] | 239 [58] | 218 [46] | 0.001 |
| Calcium channel blockers [ATCC08] | 65 [16] | 72 [15] | 0.86 |
| Agents acting on [..] system [ATC C09] | 244 [59] | 206 [44] | 0.001 |
| Lipid modifying agents [ATC C10] | 387 [94] | 384 [82] | < 0.001 |
Table 2.
Differences in patient characteristics between responders and non-responders
| responder [n = 206] | Non-responder [n = 220] | P-value | |
|---|---|---|---|
| Age (mean ± SD) | 64 [10] | 60 [12] | 0.002 |
| Gender (N [%]) | |||
| Male | 120 [58] | 129 [59] | 0.93 |
| Female | 86 [42] | 91 [41] | |
| Education level (N[%]) | |||
| Primary | 52 [26] | 67 [32] | 0.43 |
| Secondary | 80 [41] | 81 [39] | |
| University | 66 [33] | 62 [29] | |
| Labour (N [%]) | |||
| Paid labour | 49 [25] | 68 [33] | 0.08 |
| Unemployed | 55 [28] | 64 [31] | |
| Retired | 94 [47] | 77 [36] | |
| Country of origin is the Netherlands(N[%]) | |||
| Yes | 174 [88] | 181 [86] | 0.62 |
| No | 24 [12] | 29 [14] | |
| Reason referral (N[%]) | 0.89 | ||
| acute coronary syndrome | 34 [16] | 37 [17] | |
| peripheral arterial disease | 47 [23] | 46 [21] | |
| stroke/TIA | 125 [61] | 137 [62] | |
| Blood pressure (mmHg; mean ± SD) | |||
| Systolic | 140 [±19] | 142 [±20] | 0.30 |
| Diastolic | 78 [±11] | 79 [±10] | 0.23 |
| Body Mass Index (mean ± SD) | 26 [±4] | 26 [±4] | 0.22 |
| Waist (mean ± SD) | |||
| Male | 97 [±11] | 99 [±12] | 0.16 |
| Female | 91 [±13] | 89 [±13] | 0.36 |
| Lipids (mmol/ltr; mean ± SD) | |||
| Totaal cholesterol | 4.6 [±1] | 4.6 [±0.9] | 0.73 |
| Triglyceriden | 1.7 [±1] | 1.7 [±0.9] | 0.01 |
| HDL | 1.3 [±0.3] | 1.2 [±0.3] | 0.06 |
| LDL | 2.5 [±0.9] | 2.6 [±0.9] | 0.78 |
| Medication(N [%]) | |||
| Antithrombotic agents [ATC B01] | 196 [97] | 213 [98] | 0.27 |
| Diuretics [ATC C03] | 62 [31] | 56 [26] | 0.28 |
| Beta Blocking agents [ATC C07] | 93 [46] | 96 [44] | 0.74 |
| Calcium channel blockers [ATCC08] | 30 [15] | 34 [16] | 0.80 |
| Agents acting on [..] system [ATC C09] | 98 [48] | 80 [37] | 0.02 |
| Lipid modifying agents [ATC C10] | 166 [82] | 173 [80] | 0.59 |
Medication adherence
We did not observe differences in adherence measured by the MMS® between both groups (p = 0.99). According to the MMS® 19% of the participants was classified as low adherers compared to 20% in the non-participants group. Forty-six percent of the participants and 44% of the non-participants were classified as medium adherers, whereas 36 and 37% were classified as high adherers, respectively. There were no differences in adherence according to the MMS® between responders and non-responders (p = 0.47). In both the responders and non-responders group, 36% scored high on adherence. Sixteen percent of the responders were low adherent compared to 24% in the non-responder group. Forty-eight percent of the responder group scored a medium adherence and 41% of the non-responders. Compared to study participation all characteristics that significantly differed between both groups were separately analyzed by a logistic regression analyses. None of the variables significantly influenced the association between study participation and adherence according to the MMS® (Tables 3 and 4).
Table 3.
Differences participants and non-participants in adherence and beliefs about medication
| Totaal | Non-participants | Participants | P-value | |
|---|---|---|---|---|
| Adherence according to the MMS N [%] | 0.99 | |||
| Low adherence | 119 [19] | 49 [20] | 70 [19] | |
| Medium adherence | 279 [45] | 109 [44] | 170 [46] | |
| High adherence | 222 [36] | 90 [36] | 132 [35] | |
| NCD mean [SD] | 3.65[±4.8] | 3.4 [±5] | 3.8 [±4.9] | 0.13 |
| Belief Groups [N%] | 0.23 | |||
| Accepting | 160 [26] | 61 [24] | 100 [27] | |
| Ambivalent | 418 [67] | 165 [67] | 255 [68] | |
| Sceptical | 19 [3] | 10 [4] | 9 [2] | |
| Indifferent | 23 [4] | 13 [10] | 10 [3] | |
Table 4.
Differences responders and non-responders in adherence and belief about medication
| Totaal | Non-responders | Responders | P-value | |
|---|---|---|---|---|
| Adherence according to the MMS N [%] | 0.47 | |||
| Low adherence | 43 [20] | 24 [24] | 19 [16] | |
| Medium adherence | 99 [45] | 41 [40] | 58 [48] | |
| High adherence | 79 [36] | 36 [36] | 43 [36] | |
| NCD mean [SD] | 3.6 [±4.9] | 3.1 [±5] | 4 [±4.9] | 0.17 |
| Belief Groups [N%] | 0.001 | |||
| Accepting | 56 [25] | 24 [24] | 32 [27] | |
| Ambivalent | 148 [67] | 62 [61] | 86 [72] | |
| Sceptical | 8 [4] | 6 [6] | 2 [2] | |
| Indifferent | 10 [4] | 10 [9] | 0 [0] | |
Beliefs about medication
Based on the BMQ the necessity concerns differential (NCD) was 3.8 among participants compared to 3.4 among non-participants (p = 0.13). Of all the participating and non-participating patients 26% were in the accepting group, 67% in the ambivalent group, 3% in the skeptical and 4% in the indifferent group. No differences were observed between the two groups (p = 0.23). The mean score of the NCD in the responders and non-responders groups was 3.6 and 3.1 respectively (p = 0.21). Among the non-responders 24% were in the accepting group, 61% in the ambivalent group, 6% in the skeptical group and 9% in the indifferent group. For the responders this was 27, 72, 2 and 0% respectively. Differences between both groups were statistically significant (p < 0.01). Logistic regression analysis on NCD did not significantly influence the association between study participation and adherence according to the MMS®.
Discussion
To our knowledge this is the first study exploring the differences in medication adherence in patients who did or did not consent to participate in an RCT evaluating the effect of an intervention to improve medication adherence. Our study showed that patients willing to participate in an RCT evaluating the effect of an intervention to improve medication adherence, have a comparable adherence level to patients who declined participation. Even by further exploring the non-participant group in responders and non-responders, we did not observe differences in adherence between the groups. Consequently, the results of this study suggest that a population representative in adherence level participated in an RCT evaluating the effect of an intervention to improve medication adherence.
Previous studies suggested that patients not participating in RCTs to improve medication adherence have a different pre-existing adherence level from patients who participate [10–14]. This was supported by the observed differences in adherence levels between these RCTs and observational studies [10–14]. Typically, adherence levels among patients in RCTs were higher than in observational studies. Although not different among participants and non-participants, adherence in this study was also high. An explanation for the high adherence rate in both groups could be that we started inclusion for the RCT within six weeks after the cardiovascular event. For cardiovascular patients who just had an event, the need for adherent behaviour is emerging [31, 32]. Yet, as the event fades and symptoms subside, adherence levels can also decline [33]. Research with a long follow up is needed to establish if there will be a difference in adherence between participants and non-participants over time.
In all groups, we observed significant differences in patient characteristics. Compared to non-participants, participants were younger and more were highly educated. This was also observed among responders and non-responders. These are known as prognostic characteristics for patients who are willing to participate in a clinical trial [15] and for a high adherence level [34, 35]. Although the relationship between socio-demographic variables and adherence is mainly weak and inconsistent [34, 36, 37] it was expected that these characteristics could have been an explanation for the assumed higher adherence rates in the participant groups. However, we could not support this hypothesis. Also, next to the high adherence rate, a high mean NCD score was present in all groups. This only confirmed the adherent behaviour in both groups [19]. It is also congruent with earlier studies showing that medication beliefs can be a more powerful predictor of medication adherence than clinical and socio-demographic factors [19, 38]. However, we did not observe a relationship between NCD and trial participation. We did observe a significant difference in the beliefs about medication groups in the (non) responder groups. More patients of the non-responder group were also in the indifferent and skeptical group. Non-responders of surveys are known for more negative evaluations of healthcare [39]. This could be associated with higher concern beliefs in medication as these are partly influenced by the prescriber-patient relationship in healthcare [40]. The number of patients who differed in these groups was very small and the NCD did not differ. More research is needed to draw any conclusions on this point.
This study had some limitations. We had to deal with missing data especially in the self reported questionnaires BMQ and MMS®. There were fewer missing in the participants group compared to the non-participants group. The questionnaires were just implemented in the screening program. As the questionnaires were also part of the MIRROR trial, more attention could have been paid to participants for documenting these questionnaires. So there were more patients in the non-participant group who did not fill out the MMS®. These patients could very well be non-adherent [21]. There are different methods available to measure adherence. Each method has advantages and disadvantages. The MMS® [22, 24, 41] is a validated questionnaire that can be easily applied to large populations. As MMS® is a subjective measure, adherence levels may be higher than what is expected in real life. Refill data from the out-patient pharmacy on the other hand has been used extensively to provide insight into drug acquisition and dispensing [42]. However, to use the pharmacy refill data we need an informed consent from patients. This study however used data from patients collected only in standard care because we wanted to include patients who declined participation in a RCT. Other methods, such as MEMS or pill count, seem to influence patient’s behavior through direct confrontation. Moreover, application of MEMS is relatively expensive, especially when applied in standard care [42]. The BMQ was used because, to our knowledge, is the only validated questionnaire that evaluates patients’ beliefs, necessity and concerns the patient has according to his illness and prescribed medication. This in contrast to other validated adherence questionnaires that measure specific medication-taking behavior of patients [26, 43]. The high NCD score confirmed the prediction of adherent behaviour in both groups [19, 38] .
Conclusion
This study showed no differences in medication adherence between participants and non-participants prior to the inclusion of the MIRROR trial. A representative group seems to have participated in this randomized controlled trial designed to improve medication adherence [20].
Acknowledgements
Not applicable.
Funding
There was no funding for this study.
Availability of data and materials
The datasets generated and analyzed during this study are not publicly available due to the Dutch privacy laws. But are available from the corresponding author on reasonable request.
Abbreviations
- BMI
Body mass index
- BMQ
Beliefs about Medicines Questionnaire
- CVD
Cardiovascular diseases
- MIRROR trial
A Multifaceted nurse -and web-based Intervention for impRoving adheRence to treatment in patients with cardiOvasculaR disease
- MMS®
Modified Morisky Scale®
- NCD
Necessity concern differential
- RCT
Randomized controlled study
- TIA
Transient Ischemic Attack
Authors’ contributions
AS, SB, HvO have agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. AS and JL have made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data. SvD and KvL have been involved in drafting the manuscript or revising it critically for important intellectual content. All authors have given final approval of the version to be published. Each author has participated sufficiently in the work to take public responsibility for appropriate portions of the content.
Ethics approval
The Ethical Committee (the human related research committee of the Arnhem-Nijmegen region) waived the need for a formal informed consent for this study. The study was conducted according to the good clinical practice protocol and we used usual care data considering the research question of this study. Data was anonymised according to the research protocols of the Ethical Committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.De Backer G, Ambrosioni E, Borch-Johnsen K, Brotons C, Cifkova R, Dallongeville J, et al. European guidelines on cardiovascular disease prevention in clinical practice; third joint task force of European and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of eight societies and by invited experts) Eur J Cardiovasc Prev Rehabil. 2003;10(4):S1–S10. doi: 10.1097/00149831-200308000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Munger MA, Van Tassell BW, LaFleur J. Medication nonadherence: an unrecognized cardiovascular risk factor. MedGenMed. 2007;9(3):58. [PMC free article] [PubMed] [Google Scholar]
- 3.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA. 2007;297(2):177–186. doi: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- 4.Simpson RJ, Jr, Mendys P. The effects of adherence and persistence on clinical outcomes in patients treated with statins: a systematic review. J Clin Lipidol. 2010;4(6):462–471. doi: 10.1016/j.jacl.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 5.De Vera MA, Bhole V, Burns LC, Lacaille D. Impact of statin adherence on cardiovascular disease and mortality outcomes: a systematic review. Br J Clin Pharmacol. 2014;78(4):684–698. doi: 10.1111/bcp.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bansilal S, Castellano JM, Garrido E, Wei HG, Freeman A, Spettell C, et al. Assessing the impact of medication adherence on long-term cardiovascular outcomes. J Am Coll Cardiol. 2016;68(8):789–801. doi: 10.1016/j.jacc.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Chowdhury R, Khan H, Heydon E, Shroufi A, Fahimi S, Moore C, et al. Adherence to cardiovascular therapy: a meta-analysis of prevalence and clinical consequences. Eur Heart J. 2013;34(38):2940–2948. doi: 10.1093/eurheartj/eht295. [DOI] [PubMed] [Google Scholar]
- 8.Nieuwlaat R, Wilczynski N, Navarro T, Hobson N, Jeffery R, Keepanasseril A, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2014;11:Cd000011. doi: 10.1002/14651858.CD000011.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeffery RA, Navarro T, Wilczynski NL, Iserman EC, Keepanasseril A, Sivaramalingam B, et al. Adherence measurement and patient recruitment methods are poor in intervention trials to improve patient adherence. J Clin Epidemiol. 2014;67(10):1076–1082. doi: 10.1016/j.jclinepi.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 10.van Onzenoort HA, Menger FE, Neef C, Verberk WJ, Kroon AA, de Leeuw PW, et al. Participation in a clinical trial enhances adherence and persistence to treatment: a retrospective cohort study. Hypertension. 2011;58(4):573–578. doi: 10.1161/HYPERTENSIONAHA.111.171074. [DOI] [PubMed] [Google Scholar]
- 11.Allemann SS, Nieuwlaat R, Navarro T, Haynes B, Hersberger KE, Arnet I. Congruence between patient characteristics and interventions may partly explain medication adherence intervention effectiveness: an analysis of 190 randomized controlled trials from a Cochrane systematic review. J Clin Epidemiol. 2017;91:70–79. doi: 10.1016/j.jclinepi.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Wetzels GE, Nelemans P, Schouten JS, Prins MH. Facts and fiction of poor compliance as a cause of inadequate blood pressure control: a systematic review. J Hypertens. 2004;22(10):1849–1855. doi: 10.1097/00004872-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Horne R, Clatworthy J, Hankins M. High adherence and concordance within a clinical trial of antihypertensives. Chronic Illn. 2010;6(4):243–251. doi: 10.1177/1742395310369018. [DOI] [PubMed] [Google Scholar]
- 14.Atar D, Ong S, Lansberg PJ. Expanding the evidence base: comparing randomized controlled trials and observational studies of statins. Am J Ther. 2015;22(5):e141–e150. doi: 10.1097/MJT.0b013e318245ce94. [DOI] [PubMed] [Google Scholar]
- 15.Ross S, Grant A, Counsell C, Gillespie W, Russell I, Prescott R. Barriers to participation in randomised controlled trials: a systematic review. J Clin Epidemiol. 1999;52(12):1143–1156. doi: 10.1016/S0895-4356(99)00141-9. [DOI] [PubMed] [Google Scholar]
- 16.Horne RWJ, Barber N, et al. Concordance, adherence and compliance in medicine taking. London: national Co-ordinating Centre for NHS Service and Organisation R&D (NCCSDO); 2006. [Google Scholar]
- 17.Hugtenburg JG, Timmers L, Elders PJ, Vervloet M, van Dijk L. Definitions, variants, and causes of nonadherence with medication: a challenge for tailored interventions. Patient Prefer Adherence. 2013;7:675–682. doi: 10.2147/PPA.S29549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kardas P, Lewek P, Matyjaszczyk M. Determinants of patient adherence: a review of systematic reviews. Front Pharmacol. 2013;4:91. doi: 10.3389/fphar.2013.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47(6):555–567. doi: 10.1016/S0022-3999(99)00057-4. [DOI] [PubMed] [Google Scholar]
- 20.Sieben A, van Onzenoort HA, van Laarhoven KJ, Bredie SJ. A multifaceted nurse- and web-based intervention for improving adherence to treatment in patients with cardiovascular disease: rationale and design of the MIRROR trial. JMIR Res Protoc. 2016;5(3):e187. doi: 10.2196/resprot.5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gadkari AS, Pedan A, Gowda N, McHorney CA. Survey nonresponders to a medication-beliefs survey have worse adherence and persistence to chronic medications compared with survey responders. Med Care. 2011;49(10):956–961. doi: 10.1097/MLR.0b013e3182204503. [DOI] [PubMed] [Google Scholar]
- 22.Krousel-Wood M, Islam T, Webber LS, Re RN, Morisky DE, Muntner P. New medication adherence scale versus pharmacy fill rates in seniors with hypertension. Am J Manag Care. 2009;15(1):59–66. [PMC free article] [PubMed] [Google Scholar]
- 23.Krousel-Wood MA, Muntner P, Islam T, Morisky DE, Webber LS. Barriers to and determinants of medication adherence in hypertension management: perspective of the cohort study of medication adherence among older adults. Med Clin North Am. 2009;93(3):753–769. doi: 10.1016/j.mcna.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. The Journal of Clinical Hypertension. 2008;10(5):348–354. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Korb-Savoldelli V, Gillaizeau F, Pouchot J, Lenain E, Postel-Vinay N, Plouin P-F, et al. Validation of a French version of the 8-item Morisky medication adherence scale in hypertensive adults. The Journal of Clinical Hypertension. 2012;14(7):429–434. doi: 10.1111/j.1751-7176.2012.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health. 1999;14(1):1–24. doi: 10.1080/08870449908407311. [DOI] [Google Scholar]
- 27.Clifford S, Barber N, Horne R. Understanding different beliefs held by adherers, unintentional nonadherers, and intentional nonadherers: application of the necessity-concerns framework. J Psychosom Res. 2008;64(1):41–46. doi: 10.1016/j.jpsychores.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Menckeberg TT, Bouvy ML, Bracke M, Kaptein AA, Leufkens HG, Raaijmakers JAM, et al. Beliefs about medicines predict refill adherence to inhaled corticosteroids. J Psychosom Res. 2008;64(1):47–54. doi: 10.1016/j.jpsychores.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 29.Aikens JE, Nease DE, Jr, Nau DP, Klinkman MS, Schwenk TL. Adherence to maintenance-phase antidepressant medication as a function of patient beliefs about medication. Ann Fam Med. 2005;3(1):23–30. doi: 10.1370/afm.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clatworthy J, Bowskill R, Parham R, Rank T, Scott J, Horne R. Understanding medication non-adherence in bipolar disorders using a necessity-concerns framework. J Affect Disord. 2009;116(1–2):51–55. doi: 10.1016/j.jad.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Jackevicius CA, Li P, Tu JV. Prevalence, predictors, and outcomes of primary nonadherence after acute myocardial infarction. Circulation. 2008;117(8):1028–1036. doi: 10.1161/CIRCULATIONAHA.107.706820. [DOI] [PubMed] [Google Scholar]
- 32.Miller NH. Adherence behavior in the prevention and treatment of cardiovascular disease. Journal of Cardiopulmonary Rehabilitation and Prevention. 2012;32(2):63–70. doi: 10.1097/HCR.0b013e318235c729. [DOI] [PubMed] [Google Scholar]
- 33.Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119(23):3028–3035. doi: 10.1161/CIRCULATIONAHA.108.768986. [DOI] [PubMed] [Google Scholar]
- 34.Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc. 2011;86(4):304–314. doi: 10.4065/mcp.2010.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mauskop A, Borden W. Predictors of statin adherence. Curr Cardiol Rep. 2011;13(6):553–558. doi: 10.1007/s11886-011-0221-2. [DOI] [PubMed] [Google Scholar]
- 36.Schedlbauer A, Davies P, Fahey T. Interventions to improve adherence to lipid lowering medication. Cochrane Database Syst Rev. 2010;3:CD004371. doi: 10.1002/14651858.CD004371.pub3. [DOI] [PubMed] [Google Scholar]
- 37.Miller NH, Hill M, Kottke T, Ockene IS, Compliance FtEPo The multilevel compliance challenge: recommendations for a call to action: a statement for healthcare professionals. Circulation. 1997;95(4):1085–1090. doi: 10.1161/01.CIR.95.4.1085. [DOI] [PubMed] [Google Scholar]
- 38.Foot H, La Caze A, Gujral G, Cottrell N. The necessity-concerns framework predicts adherence to medication in multiple illness conditions: a meta-analysis. Patient Educ Couns. 2016;99(5):706–717. doi: 10.1016/j.pec.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 39.Perneger TV, Chamot E, Bovier PA. Nonresponse bias in a survey of patient perceptions of hospital care. Med Care. 2005;43(4):374–380. doi: 10.1097/01.mlr.0000156856.36901.40. [DOI] [PubMed] [Google Scholar]
- 40.Horne R, Chapman SC, Parham R, Freemantle N, Forbes A, Cooper V. Understanding patients’ adherence-related beliefs about medicines prescribed for long-term conditions: a meta-analytic review of the necessity-concerns framework. PLoS One. 2013;8(12):e80633. doi: 10.1371/journal.pone.0080633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morisky DE, DiMatteo MR. Improving the measurement of self-reported medication nonadherence: response to authors. J Clin Epidemiol. 2011;64(3):255–257. doi: 10.1016/j.jclinepi.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 43.Horne R, Buick D, Fisher M, Leake H, Cooper V, Weinman J. Doubts about necessity and concerns about adverse effects: identifying the types of beliefs that are associated with non-adherence to HAART. Int J STD AIDS. 2004;15(1):38–44. doi: 10.1258/095646204322637245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during this study are not publicly available due to the Dutch privacy laws. But are available from the corresponding author on reasonable request.