Abstract
The pituitary gland has an important role in homeostasis and disorders of it can result in endocrine dysfunction and/or produce mass effect on surrounding structures, including the hypothalamus, which can cause significant morbidity and mortality. A definitive clinical diagnosis may be delayed or remain elusive and lead to life-threatening conditions. Specifically, pituitary adenomas, pituitary necrosis, hypophysitis, and abscesses have all been reported in the literature to cause sudden and unexpected death and may only be first encountered at autopsy. Recognition by the forensic pathologist of these rare entities is crucial for appropriate death certification. This review emphasizes the need for a comprehensive, detailed forensic examination, including autopsy and routine histologic sampling of the pituitary gland, in order to ascertain its potential role in sudden unexpected death with no other apparent cause.
Keywords: Forensic pathology, Pituitary gland, Adenohypophysis, Neurohypophysis, Endocrine disturbance
Introduction
Often referred to as the “master gland,” the pituitary orchestrates the complex functions of many endocrine glands, including the adrenal glands and thyroid. Although malfunction of the pituitary gland can cause hormone excess or deficiency, a definitive clinical diagnosis may be delayed or remain elusive and lead to life-threatening conditions. In some of these instances, forensic pathologists may encounter pituitary pathology as the underlying cause of death. Herein, an overview of the pituitary gland and its lesions will be presented, with an emphasis on those that may be encountered by the medical examiner/coroner (ME/C) as a cause or contributor to sudden and unexpected death.
Discussion
Pituitary Gland Overview
The pituitary gland is entirely ectodermal in origin but is anatomically, embryologically, and functionally separated into two portions: the adenohypophysis (anterior pituitary) and neurohypophysis (posterior pituitary). The adenohypophysis develops from an ectodermal outpouching of the oropharynx (Rathke's pouch), which grows dorsally and meets with the infundibulum, a downward extension of the neural ectoderm from the floor of the diencephalon. The infundibulum becomes the stalk and the neurohypophysis. The pituitary gland is mature by approximately 20 weeks gestation (1).
The fully developed pituitary gland normally weighs approximately 0.6 g (2). The anterior lobe accounts for two-thirds of the weight. The pituitary gland resides in the sella turcica, a basket-like structure formed by the sphenoid bone. Structures in close proximity to the gland include the optic chiasm superiorly, separated by a tough dural reflection known as the diaphragma sellae, and the cavernous sinus laterally, within which traverse the internal carotid artery, several cranial nerves (oculomotor, trochlear, abducens, and the ophthalmic [V1] and maxillary [V2] branches of the trigeminal nerve) (Figure 1). The pituitary gland is associated with the hypothalamus via rich vascular and neuronal networks that travel within the stalk, and it is the hypothalamus that controls the functions of both the anterior and posterior pituitary.
Figure 1.
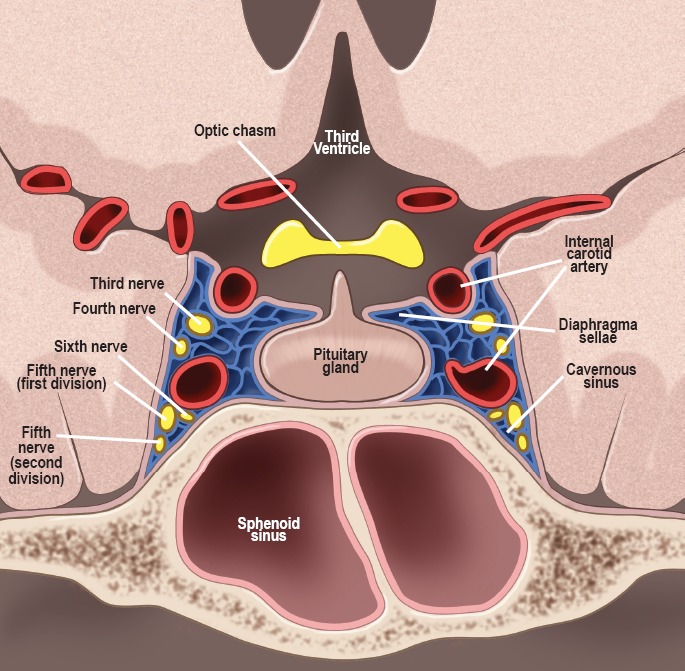
Normal anatomy of the sellar and parasellar regions surrounding the pituitary gland. Drawn under contract with professional medical illustrator Diana Kryski.
The role of the pituitary gland is critical in growth and development. The adenohypophysis regulates several physiological processes and manufactures an array of peptide hormones including growth hormone (GH), prolactin (PRL), thyroid stimulating hormone (TSH), luteinizing hormone (LH), follicle stimulating hormone (FSH), and adrenocorticotropic hormone (ACTH). The release of these hormones is mediated by neurohormones secreted from axonal terminals in the hypothalamus that travel to the adenohypophysis via a portal venous system. The neurohypophysis is responsible for water and osmotic pressure balance, but unlike the adenohypophysis, is not glandular and does not synthesize hormones. Instead, neuronal cell bodies in the hypothalamus produce the hormones that then undergo axonal transport through the pituitary stalk and terminate in the neurohypophysis. The hormones (antidiuretic hormone [ADH] and oxytocin) are stored and released directly into the systemic circulation.
Histologically, the adenohypophysis has a lobulated glandular architecture consisting of interspersed cords and clusters of cuboidal secretory cells that contain hormones stored in cytoplasmic granules (Image 1). Pituitary cells can be classified based on the staining of the granules as acidophils, basophils, or chromophobes. Acidophils contain polypeptide hormones (GH and PRL), basophils contain glycoprotein hormones (ACTH, TSH, and the gonadotrophs), and chromophobes do not contain any identifiable hormone (1). The neurohypophysis consists of unmyelinated axons projecting from neuronal cell bodies in the hypothalamus. Oxytocin and ADH accumulate within the terminal ends within swellings called Herring bodies. In addition, specialized glial cells, or pituicytes, are also interspersed within the posterior pituitary.
Image 1:
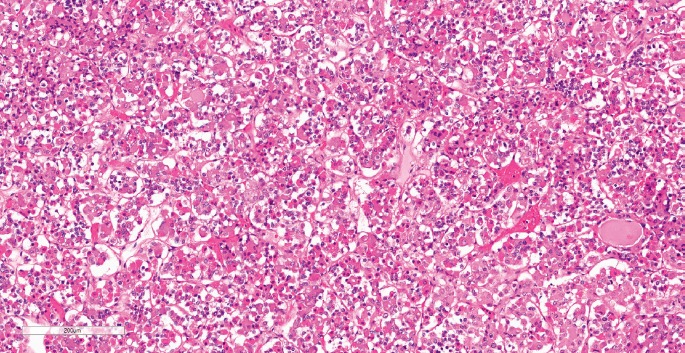
Histology of a normal anterior pituitary gland demonstrating bundles and cords of epithelial cells separated by highly vascularized connective tissue. The gland is formed by three distinctive cell types, including acidophils, basophils, and chromophobes (H&E, x120).
Pituitary gland lesions can lead to a life-threatening imbalance in homeostasis. Specifically, pituitary adenomas, necrosis, hypophysitis, and abscesses can lead to sudden death and may only be encountered at autopsy. Therefore, recognition by the forensic pathologist of these entities and the associated physiologic derangements is critical for appropriate death certification.
Pituitary Adenoma
Pituitary tumors account for 10-20% of all intracranial neoplasms (3). Adenomas account for the majority of these, comprising 90% of sellar and suprasellar lesions (4, 5). The prevalence of pituitary adenomas is difficult to ascertain due to their varied presentation or lack thereof, but one study reported a prevalence of 77.6 per 100 000 people (6). Autopsy studies and magnetic resonance imaging (MRI) series of asymptomatic volunteers have found that prevalence rates in the general population for pituitary adenomas may be as high as 20% (7, 8).
Pituitary adenomas arise from one of five cell types that comprise the adenohypophysis and include lactotrophs, gonadotrophs, somatotrophs, corticotrophs, and thyrotrophs. Prolactinomas are the most common (40-57%), followed by nonfunctioning, or silent, adenomas (28-37%) (9). Adenomas can also be divided by size into microadenomas, which are less than 10 mm, and macroadenomas, which are 10 mm or larger. Studies have indicated, however, that symptoms do not correlate with tumor size (9). The clinical presentation may be a result of endocrine dysfunction, neurologic manifestations from mass effect, or acute hemorrhage and necrosis, which can all contribute to increase the morbidity and mortality in these patients (10-14).
Hyperpituitarism
The oversecretion of hormones by pituitary adenomas leads to classic clinical syndromes. Hyperprolactinemia is the most common, accounting for approximately half of all adenomas, and can lead to oligomenorrhea, amenorrhea, or infertility, as well as galactorrhea. This is followed by GH-producing adenomas causing acromegaly (11-13%) and ACTH-producing adenomas resulting in Cushing disease (1-2%). Those secreting LH, FSH, or TSH are rare (6, 15). Although these classic hypersecretion syndromes increase patient morbidity, the comorbidities associated with acromegaly in particular compromise the quality of life and result in elevated mortality rates.
Acromegaly is often characterized by an increase in hand and foot size, change in facial features, carpal tunnel symptoms, fatigue, proximal muscle weakness, hypertension, and left ventricular hypertrophy (9). Patients are also at an increased risk for cardiovascular and cerebrovascular events, respiratory complications, and malignant neoplasms (16-18). Cardiovascular complications are regarded as the most common cause of death, accounting for 60% of cases (19). Sudden death is often associated with ventricular tachyarrhythmias and may be related to myocardial interstitial fibrosis (20). The risk of cardiovascular disease is directly associated with elevated serum levels of GH and a prolonged clinical course, which affects cardiac morphology and performance, thereby inducing cardiomyopathy specific to the disease (21, 22). Left untreated or poorly controlled for many years, concentric hypertrophy and diastolic dysfunction develop, which may then progress further to valvular disease and impaired systolic performance (19). Histologically, acromegalic cardiomyopathy demonstrates interstitial fibrosis, myocyte apoptosis, and lymphocytic infiltrates (23, 24). Ventricular tachyarrhythmias, however, may still occur in those with an apparently normal heart (22, 25). Therefore, if a cause of death is not identified and cardiac pathology is lacking, it is still reasonable to consider the possibility of acromegaly-associated cardiovascular complications.
Mass Effect
The most common neurologic symptoms in patients with pituitary adenomas are headaches and visual disturbances (9). Mass effect may also result in destruction or compression of the gland itself and cause full or partial hypopituitarism (9). This most often results in hypogonadism by direct compression or inhibition of the pulsatile secretion of LH leading to inadequate gonadal stimulation, and hyperprolactinemia (9). In addition to mass effect on the pituitary gland itself, macroadenomas have also been reported to disrupt the hypothalamic-pituitary axis and can lead to a fatal sequence of events. Suzuki et al. reported a case in which a pituitary adenoma played a direct role in sudden death attributed to hypothermia. A 56-year-old woman was found dead in her cold home during winter with evidence of paradoxical undressing (27). The autopsy revealed a large pituitary adenoma. Chemical analysis revealed low concentrations of thyroid hormones and slightly elevated levels of thyroid stimulating hormone. Thyroid hormone is an important regulator of thermogenesis, and hypothermia is one of the clinical signs of advanced thyroid hormone deficiency. Patients with central hypothyroidism can exhibit low, normal, or even slightly elevated TSH levels (26). Given the circumstances and the chemical analysis, the authors proposed that adenoma-induced dysregulation of the hypothalamic-pituitary-thyroid axis resulted or contributed to the hypothermia (27). Mass effect from an enlarging tumor may not only cause neurologic manifestations, but may also interfere with normal hormone secretion by direct compression.
Pituitary Necrosis (Apoplexy)
Ischemic necrosis and hemorrhage of the pituitary gland is encountered most frequently in the setting of a pituitary adenoma (pituitary apoplexy), but can also result from a number of insults, including trauma, hemorrhagic shock, coagulopathies, and stroke (2).
Pituitary apoplexy is an uncommon acute clinical syndrome of sudden onset headache, visual disturbance, and altered mental status due to sudden hemorrhage and/or infarction of the pituitary gland (1) (Image 2). Most often, this occurs in a preexisting adenoma, but infarction of an apparently normal gland has also been reported (28, 29). The presentation may be dramatic and acute with rapidly developing neurologic deficits, coma, and death. If left untreated, mortality reaches 45% (30).
Image 2:
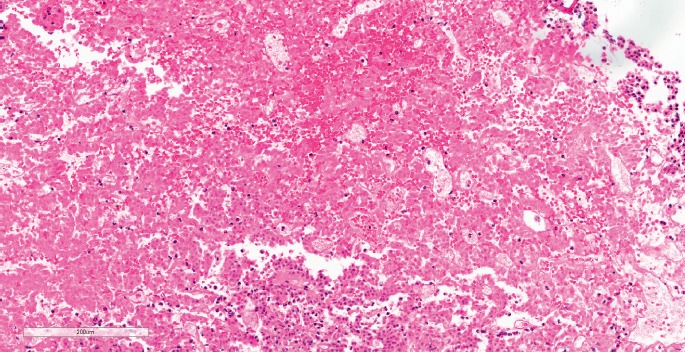
Pituitary apoplexy. Histological view of a pituitary adenoma composed of small, uniform polygonal cells arranged in sheets and cords that were focally acutely infarcted with “ghost cells” and acute hemorrhage (H&E, x120).
Pituitary apoplexy was a well-recognized diagnosis before the clinical syndrome was defined. Bailey was the first to describe a fatal case of hemorrhage into a pituitary adenoma in 1898 (31), followed by Bleibtreu two decades later (32). It was not until 1950, however, that Brougham et al. coined the term “pituitary apoplexy” and summarized the clinical and pathological findings in individuals who died suddenly and whose autopsies revealed hemorrhagic necrosis of a pituitary adenoma (33).
Hemorrhage and infarction in a non-adenomatous pituitary gland is rare, and usually occurs in the setting of severe hypotension with subsequent spasm or thrombosis of the pituitary arteries (28, 34). In 1937, Sheehan described panhypopituitarism following obstetric hemorrhage and defined what we now know as postpartum pituitary necrosis, or “Sheehan syndrome” (34). Due to improvements in obstetric care in developed countries, the incidence of Sheehan syndrome is extremely low and when it does occur, is often identified clinically in the early stages. With treatment, patients may survive the infarction. At autopsy, the pathologist may observe a small pituitary, which should lead the pathologist to investigate endocrine-related causes of death. In these instances, the proximate cause of death may be the disease or injury that caused the infarction/hemorrhage.
The propensity for hemorrhage and necrosis in pituitary adenomas is not entirely clear. Large-scale studies have demonstrated an increased rate of hemorrhage in pituitary adenomas (9.5-15.8%) compared to other intracranial tumors (1.3-9.6%) (35-37). In fact, these tumors are more than 5.4 times more likely to bleed than other central nervous system tumors as a whole (37). The pituitary gland is a highly vascularized structure with a complex blood supply. The anterior lobe is supplied by the superior hypophyseal arteries, which are derived from the internal carotid arteries and traverse the diaphragma sellae along the pituitary stalk, as well as by a system of portal veins that originate in the hypothalamic floor. The posterior lobe is supplied via the inferior hypophyseal arteries, which also arise from the internal carotid artery, but travel inferior to the gland rather than within the stalk. The venous drainage from both lobes flows into the cavernous sinus. The separate circulation between the two lobes may allow one lobe to remain intact while the blood supply to the other is disrupted (38). Two hypotheses exist as to why apoplexy occurs: 1) rapid tumor growth outstrips its blood supply, resulting in ischemic necrosis followed by hemorrhage (37); and 2) direct compression of the infundibulum by the tumor renders the entire lobe ischemic with hemorrhage as a secondary consequence (1). The result can be hypopituitarism, meningism from leakage of blood into the subarachnoid space, and direct compression of surrounding neural structures, including the hypothalamus, which may lead to the commonly observed clinical findings, including headache, nausea, vomiting, visual disturbances, a decreased level of consciousness, electrolyte imbalance, impaired thermal regulation, hypotension, cardiac arrhythmia, and death (39).
The factors that precipitate hemorrhage are not exactly known. Pituitary apoplexy occurs across all age groups and does not preferentially affect males or females. In addition, no histologic subtype confers a greater risk. Although once presumed to occur more often in macroadenomas, it is now known that apoplectic events occur in tumors of any size (1). Numerous case reports have made associations with apoplexy, including medications, procedures, diseases, head trauma, elevated estrogen, diabetes mellitus, radiation, and intravenous drug abuse (35, 37, 40-43), but the majority of cases do not have an identifiable precipitant. Up to 65% of cases of pituitary apoplexy occur in patients with undiagnosed adenomas (44). If left untreated, pituitary apoplexy can be rapidly fatal. Thus, if there is no antemortem suspicion of an intracranial neoplasm, these cases may be referred to the forensic pathologist. DiMaio et al. described a 47-year-old woman who has found dead in bed. She had a history of headaches for weeks prior to her death, but never sought medical attention. At autopsy, a hemorrhagic pituitary adenoma with extension into the left basal ganglia and lateral ventricle was identified (45). Sun et al. reported a 49-year-old man with no past medical history who was in custody for questioning and restrained in a seated position for four days. On the fourth day, he became weak, subsequently unconscious, and died despite resuscitation efforts. Autopsy revealed a large pituitary tumor with hemorrhage and necrosis, and it was presumed to result in pressure on important functional zones such as the hypothalamus and brainstem, resulting in death (46).
In other instances, patients may seek medical attention in the weeks or days prior to their demise, but may be misdiagnosed or experience a delay in appropriate management. Bauer et al. reported a 41-year-old man who was found dead in his apartment, and was last seen alive 24 hours earlier. Six months prior to his death, he presented to a medical provider with headaches and intermittent blurred vision. A physical examination was normal, and imaging was not performed. He was diagnosed with cervical tension syndrome and treated with local anesthetics with temporary improvement, but not complete cessation of his symptoms. A postmortem examination revealed a 3.0 cm encapsulated mass in the pituitary fossa with extension into the skull base and compression of the optic chiasm and hypothalamic region, with hemorrhage. Microscopy was consistent with an apoplectic adenoma. No injuries or other pathology was identified (47). Shields et al. described a 46-year-old woman who sought medical care, but appropriate management was delayed. She suddenly collapsed at home, and was pronounced dead shortly thereafter. The coroner initially attributed her death to myocardial infarction. Records, however, indicated that the patient had been seen by medical personnel on three separate occasions in the week prior to her death for severe headaches, nausea, vomiting, and photophobia. Imaging was performed and a pituitary mass was diagnosed, but it was not thought to account for her symptoms or need immediate intervention, so she was discharged. Autopsy demonstrated a hemorrhagic infarction of the pituitary adenoma and expansion from the sella turcica (48).
Failure of the medical community to recognize and manage pituitary apoplexy may result in sudden and unexpected death of the patient and necessitate a forensic investigation. Forensic pathologists should not only be capable of diagnosing pituitary hemorrhage and infarction, but also thoroughly review the clinical history in order to accurately classify the proximate cause of death.
Hypophysitis
Inflammatory lesions of the pituitary gland, known as hypophysitis, may clinically and radiologically mimic pituitary tumors, causing mass effect and/or hypothalamic-pituitary dysfunction. Hypophysitis may occur secondarily as a result of systemic inflammation, infection, or from localized pituitary/sellar masses, and has been reported to cause sudden death (49, 50). However, primary or idiopathic hypophysitis is more common and can be classified histologically into three categories: lymphocytic hypophysitis, granulomatous hypophysitis, and xanthomatous hypophysitis. These conditions are usually confined to the pituitary gland and their pathogenesis is currently poorly understood.
Lymphocytic hypophysitis was first described in 1962 and is histologically characterized by diffuse infiltration of the pituitary gland by inflammatory cells, predominantly lymphocytes that form lymphoid follicles, with variable reactive fibrosis (51) (Image 3). Females are eight times more frequently affected than males, and classically present in late pregnancy or in the early postpartum period (2). The anterior lobe is predominantly affected and common manifestations are hypopituitarism (63%), mass effects (56%), and hyperprolactinemia (38%) (52). Left untreated, lymphocytic hypophysitis can result in death. Cuyar et al. described a 23-year-old woman diagnosed at autopsy with lymphocytic hypophysitis and concomitant infiltrates in the thyroid gland and adrenal gland who died suddenly and unexpectedly with no other apparent cause of death. Histology showed a diffuse infiltration of the adenohypophysis by a dense lymphoplasmacytic infiltrate, with only focal extension into the neurohypophysis (53). Rare case reports of patients presenting with diabetes insipidus and inflammation confined to the posterior lobe have been described and termed infundibular neurohypophysitis (54-56). In fact, Blisard et al. reported a 37-year-old with intellectual disability who died suddenly with premortem signs of diabetes insipidus (57). At autopsy, her pituitary gland was infiltrated and damaged by a lymphoplasmacytic infiltrate, affecting the posterior pituitary much more severely than the anterior pituitary. Vitreous electrolytes demonstrated a pattern of hypertonic dehydration, compatible with diabetes insipidus.
Image 3:
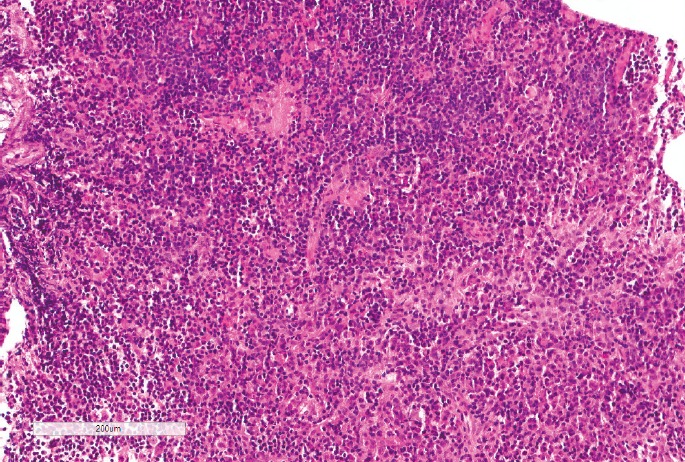
Histological view of lymphocytic hypophysitis demonstrating extensive cellular infiltration of the adenohypophysis with lymphocytes and plasma cells defacing the normal pituitary architecture (H&E, x120).
Granulomatous and xanthomatous hypophysitis are much less common, and it is unclear whether these conditions are truly distinct entities from lymphocytic hypophysitis or merely different manifestations of the same disease. Granulomatous hypophysitis was first described in 1917 and has an incidence of one in ten million (52). Most reported instances are diagnosed at autopsy. Histologically, the disease is characterized by collections of histiocytes, multinucleated giant cells, and variable numbers of lymphocytes and plasma cells (2). Xanthomatous hypophysitis is the least common form of primary hypophysitis and was initially described in 1998 (58). It is characterized by lipid-rich foamy histiocytes with variable numbers of lymphocytes. Very few case reports are available in the literature, thus making it difficult to further characterize.
Pituitary Abscess
Pituitary abscess is a rare but potentially life-threatening disease, accounting for fewer than 1% of all pituitary lesions (59). Since the first description in 1848 by Heslop, only a few hundred cases, of which most are single cases, have been reported in the literature (60). Presenting manifestations are nonspecific and include endocrine disturbances, headache, and visual impairment (61). Radiologic findings cannot always distinguish it from other pituitary lesions (62). In addition, 70-80% of instances do not present with infectious manifestations, such as fever, leukocytosis, or meningismus (61, 62), further complicating the diagnosis, and rapid deterioration to death within several days can occur. The diagnosis is usually made postoperatively or at autopsy.
The disease can develop de novo in normal pituitary tissue (approximately 70%) or in preexisting pituitary pathology (approximately 30%), of which pituitary adenomas are most common, followed by Rathke's cleft cysts, and craniopharyngiomas (63). Some authors have suggested that tumor vulnerability to abscess may depend on impaired circulation, areas of necrosis, and local immunological impairment (64). The abscess is often a result of direct extension of an adjacent infection such as sphenoid sinusitis, meningitis, contaminated cerebrospinal fluid fistula, or cavernous sinus thrombophlebitis (62). Involvement of the pituitary gland in systemic sepsis is rare due to the effective blood-brain barrier. The most common infectious agents are Gram-positive cocci (50%), Gram-negative bacilli, fungi, amoeba, and yeast (61). In the majority of instances, however, an organism is not identified.
Kotani et al. reported a 60-year-old man with a pituitary abscess associated with a pituitary adenoma who died five days after the onset of clinical symptoms without a definitive diagnosis (65). Postmortem imaging and autopsy findings revealed a sellar mass with cystic change and extension toward the optic chiasm. Histopathology of the lesion revealed an abscess with suppurative meningitis and encephalitis. Whalley reported a similar case in 1952 without an underlying adenoma or other pituitary lesion (64). The cause of death in both cases was determined to be hypothalamic involvement of the pituitary abscess, resulting in fatal arrhythmias by disturbance of the cardiac autonomic nervous system.
Pituitary abscess is a rare entity, but given its high mortality risk and the reported cases of unexpected death, the ME/C must be aware of the clinical and pathological findings, as well as the mechanism of death in such cases. If the etiology of the abscess can be discerned (e.g., extension from sinusitis), this disease should be included as the proximate cause of death.
Conclusion
The pituitary gland is often referred to as the “master gland,” and lesions disturbing its function can result in life-threatening complications and sudden, unexpected death. Specifically, pituitary adenomas, pituitary necrosis, hypophysitis, and abscesses have all been reported to contribute to death and have come under the purview of the medical examiner/coroner. This review stresses the importance of greater awareness of these rare entities by the forensic pathologist. A comprehensive, detailed forensic examination, including autopsy accompanied by histological examination and potential biochemical analysis, is of utmost importance to ascertain the role of the pituitary gland in sudden and unexpected death with no apparent cause.
Footnotes
Ethical Approval: As per Journal Policies, ethical approval was not required for this manuscript
Statement of Human and Animal Rights: This article does not contain any studies conducted with animals or on living human subjects
Statement of Informed Consent: No identifiable personal data were presented in this manuscript
Disclosures & Declaration of Conflicts of Interest: The authors, reviewers, editors, and publication staff do not report any relevant conflicts of interest
Financial Disclosure: The authors have indicated that they do not have financial relationships to disclose that are relevant to this manuscript
References
- 1.Rolih C.A., Ober K.P. Pituitary apoplexy. Endocrinol Metab Clin North Am. 1993. Jun; 22(2): 291–302. PMID: 8325288. [PubMed] [Google Scholar]
- 2.Asa S.L. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; c2000-2016. Pituitary histopathology in man: normal and abnormal. 2002. [updated 2007 Jun 10; cited 2016 Mar 31]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK279003/. [Google Scholar]
- 3.Terada T., Kovacs K., Stefaneanu L., Horvath E. Incidence, pathology, and recurrence of pituitary adenomas: study of 647 unselected surgical Cases. Endocr Pathol. 1995. Winter; 6(4): 301–310. PMID: 12114812. 10.1007/bf02738730. [DOI] [PubMed] [Google Scholar]
- 4.Freda P.U., Wardlaw S.L., Post K.D. Unusual causes of sellar/parasellar masses in a large transsphenoidal surgical series. J Clin Endocrinol Metab. 1996; 81(10): 3455–9. PMID: 8855784. 10.1210/jc.81.10.3455. [DOI] [PubMed] [Google Scholar]
- 5.Valassi E., Biller B.M., Klibanski A., Swearingen B. Clinical features of nonpituitary sellar lesions in a large surgical series. Clin Endocrinol (Oxf). 2010; 73(6): 798–807. PMID: 20874772. PMCID: PMC2982869. 10.1111/j.1365-2265.2010.03881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez A., Karavitaki N., Wass J.A. Prevalence of pituitary adenomas: a community-based, cross-sectional study in Banbury (Oxfordshire, UK). Clin Endocrinol (Oxf). 2010. Mar; 72(3): 377–82. PMID: 19650784. 10.1111/j.1365-2265.2009.03667.x. [DOI] [PubMed] [Google Scholar]
- 7.Ezzat S., Asa S.L., Couldwell W.T. et al. The prevalence of pituitary adenomas: a systematic review. Cancer. 2004. Aug 1; 101(3): 613–9. PMID: 15274075. 10.1002/cncr.20412. [DOI] [PubMed] [Google Scholar]
- 8.Molitch M.E. Pituitary incidentalomas. Endocrinol Metab Clin North Am. 1997. Dec; 26(4): 725–40. PMID: 9429857. [DOI] [PubMed] [Google Scholar]
- 9.Lake M.G., Krook L.S., Cruz S.V. Pituitary adenomas: an overview. Am Fam Physician. 2013. Sep 1; 88(5): 319–27. PMID: 24010395. [PubMed] [Google Scholar]
- 10.Tomlinson J.W., Holden N., Hills R.K. et al. Association between premature mortality and hypopituitarism. West Midlands Prospective Hypopituitary Study Group. Lancet. 2001. Feb 10; 357(9254): 425–31. PMID: 11273062. 10.1016/s0140-6736(00)04006-x. [DOI] [PubMed] [Google Scholar]
- 11.Dekkers O.M., Biermasz N.R., Pereira A.M. et al. Mortality in patients treated for Cushing's disease is increased, compared with patients treated for nonfunctioning pituitary macroadenoma. J Clin Endocrinol Metab. 2007. Mar; 92(3): 976–81. PMID: 17200171. 10.1210/jc.2006-2112. [DOI] [PubMed] [Google Scholar]
- 12.Kauppinen-Mäkelin R., Sane T., Sintonen H. et al. Quality of life in treated patients with acromegaly. J Clin Endocrinol Metab. 2006. Oct; 91(10): 3891–6. PMID: 16849407. 10.1210/jc.2006-0676. [DOI] [PubMed] [Google Scholar]
- 13.Matta M.P., Couture E., Cazals L. et al. Impaired quality of life of patients with acromegaly: control of GH/IGF-I excess improves psychological subscale appearance. Eur J Endocrinol. 2008. Mar; 158(3): 305–10. PMID: 18299462. 10.1530/eje-07-0697. [DOI] [PubMed] [Google Scholar]
- 14.Webb S.M., Badia X. Quality of life in growth hormone deficiency and acromegaly. Endocrinol Metab Clin North Am. 2007. Mar; 36(1): 221–32. PMID: 17336742. 10.1016/j.ecl.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Famini P., Maya M.M., Melmed S. Pituitary magnetic resonance imaging for sellar and parasellar masses: ten-year experience in 2598 patients. J Clin Endocrinol Metab. 2011. Jun; 96(6): 1633–41. PMID: 21470998. PMCID: PMC3100749. 10.1210/jc.2011-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dekkers O.M., Biermasz N.R., Pereira A.M. et al. Mortality in acromegaly: a metaanalysis. J Clin Endocrinol Metab. 2008. Jan; 93(1): 61–7. PMID: 17971431. 10.1210/jc.2007-1191. [DOI] [PubMed] [Google Scholar]
- 17.Melmed S. Acromegaly pathogenesis and treatment. J Clin Invest. 2009. Nov; 119(11): 3189–202. PMID: 19884662. PMCID: PMC2769196. 10.1172/jci39375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sherlock M., Ayuk J., Tomlinson J.W. et al. Mortality in patients with pituitary disease. Endocr Rev. 2010. Jun; 31(3): 301–42. PMID: 20086217. 10.1210/er.2009-0033. [DOI] [PubMed] [Google Scholar]
- 19.An Z., He Y.Q., Liu G.H. et al. Malignant ventricular tachycardia in acromegaly: a case report. Sao Paulo Med J. 2015. Feb; 133(1): 55–9. PMID: 25250797. 10.1590/1516-3180.2012.6410005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leclerq F., Fille A., Albat B. et al. Congestive heart failure worsening with octreotide in acromegalic patient. Lancet. 1991. Nov 16; 338(8777): 1272–3. PMID: 1682667. 10.1016/0140-6736(91)92139-s. [DOI] [PubMed] [Google Scholar]
- 21.Abe N., Matsunaga T., Kameda K. et al. Increased level of pericardial insulin-like growth factor-1 in patients with left ventricular dysfunction and advanced heart failure. J Am Coll Cardiol. 2006. Oct 3; 48(7): 1387–95. PMID: 17010800. 10.1016/j.jacc.2006.06.048. [DOI] [PubMed] [Google Scholar]
- 22.Jayasena C.N., Comninos A.N., Clarke H. et al. The effects of long-term growth hormone and insulin-like growth factor-1 exposure on the development of cardiovascular, cerebrovascular and metabolic co-morbidities in treated patients with acromegaly. Clin Endocrinol (Oxf). 2011. Aug; 75(2): 220–5. PMID: 21521288. 10.1111/j.1365-2265.2011.04019.x. [DOI] [PubMed] [Google Scholar]
- 23.Kahaly G., Olshausen K.V., Mohr-Kahaly S. et al. Arrhythmia profile in acromegaly. Eur Heart J. 1992. Jan; 13(1): 51–6. PMID: 1577031. [DOI] [PubMed] [Google Scholar]
- 24.Rodrigues E.A., Caruana M.P., Lahiri A. et al. Subclinical cardiac dysfunction in acromegaly: evidence for a specific disease of heart muscle. Br Heart J. 1989. Sep; 62(3): 185–94. PMID: 2528980. PMCID: PMC1216761. 10.1136/hrt.62.3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colao A., Pivonello R., Grasso L.F. et al. Determinants of cardiac disease in newly diagnosed patients with acromegaly: results of a 10 year survey study. Eur J Endocrinol. 2011. Nov; 165(5): 713–21. PMID: 21868601. 10.1530/eje-11-0408. [DOI] [PubMed] [Google Scholar]
- 26.Clemens K., Payne W., Van Uum S.H. Central hypothyroidism. Can Fam Physician. 2011. Jun; 57(6): 677–80. PMID: 21673213. PMCID: PMC3114669. [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki H., Hayashi K., Fukunaga T. Two forensic autopsy cases of death from unexpected lesions of the pituitary gland. Leg Med (Tokyo). 2014. Jan; 16(1): 36–9. PMID: 24269073. 10.1016/j.legalmed.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Kovacs K., Yao J. Pituitary necrosis following major heart surgery. Z Kardiol. 1975. Jan; 64(1): 52–7. PMID: 1114863. [PubMed] [Google Scholar]
- 29.Reid R.L., Quigley M.E., Yen S.S. Pituitary apoplexy. A review. Arch Neurol. 1985. Jul; 42(7): 712–9. PMID: 4015472. 10.1001/archneur.1985.04060070106028. [DOI] [PubMed] [Google Scholar]
- 30.Jacobi J.D., Fishman L.M., Daroff R.B. Pituitary apoplexy in acromegaly followed by partial pituitary insufficiency. Arch Intern Med. 1974. Sep; 134(3): 559–61. PMID: 4853653. 10.1001/archinte.134.3.559. [DOI] [PubMed] [Google Scholar]
- 31.Bailey P. Pathological report of a case of acromegaly, with especial reference to the lesions in the hypophysis cerebri and in the thyroid gland; and a case of hemorrhage into the pituitary. Philadelphia Medical J. 1898; 1: 789. [Google Scholar]
- 32.Bleibtreu L. Ein Fall von Akoremegalie (Zerstoerung der Hypophysis durch Blutung). Muench Med Wohschr. 1905; 52: 2079–80. German. [Google Scholar]
- 33.Brougham M., Heusner A.P., Adams R.D. Acute degenerative changes in adenomas of the pituitary body–with special reference to pituitary apoplexy. J Neurosurg. 1950. Sep; 7(5): 421–39. PMID: 14774761. 10.3171/jns.1950.7.5.0421. [DOI] [PubMed] [Google Scholar]
- 34.Sheehan H.L. Postpartum necrosis of the anterior pituitary. J Pathol Bacteriol. 1937; 45: 189–214. 10.1002/path.1700450118. [DOI] [Google Scholar]
- 35.Mohr G., Hardy J. Hemorrhage, necrosis, and apoplexy in pituitary adenomas. Surg Neurol. 1982. Sep; 18(3): 181–9. PMID: 7179072. 10.1016/0090-3019(82)90388-3. [DOI] [PubMed] [Google Scholar]
- 36.Müller-Jensen A., Lüdecke D. Clinical aspects of spontaneous necrosis of pituitary tumors (pituitary apoplexy). J Neurol. 1981; 224(4): 267–71. PMID: 6162928. 10.1007/bf00313290. [DOI] [PubMed] [Google Scholar]
- 37.Wakai S., Yamakawa K., Manaka S., Takakura K. Spontaneous intracranial hemorrhage caused by brain tumor: its incidence and clinical significance. Neurosurgery. 1982. Apr; 10(4): 437–44. PMID: 7099393. 10.1097/00006123-198204000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Flerko B. Hypothalamic control of the hypophyseal gonadotrophic function in the rat. Acta Physiol Pharmacol Neerl. 1959; 8: 545–9. PMID: 13823470. [PubMed] [Google Scholar]
- 39.Lubina A., Olchovsky D., Berezin M., Ram Z., Hadani M., Shimon I. Management of pituitary apoplexy: clinical experience with 40 patients. Acta Neurochir (Wien). 2005. Feb; 147(2): 151–7; discussion 157. PMID: 15570437. 10.1007/s00701-004-0413-2. [DOI] [PubMed] [Google Scholar]
- 40.Brennan C.F., Malone R.G., Weaver J.A. Pituitary necrosis in diabetes mellitus. Lancet. 1956. Jul; 271(6932): 12–6. PMID: 13333136. 10.1016/s0140-6736(56)91387-3. [DOI] [PubMed] [Google Scholar]
- 41.Holness R.O., Ogundimu F.A., Langille R.A. Pituitary apoplexy following closed head trauma. Case report. J Neurosurg. 1983. Oct; 59(4): 677–9. PMID: 6886789. 10.3171/jns.1983.59.4.0677. [DOI] [PubMed] [Google Scholar]
- 42.Lufkin E.G., Reagan T.J., Doan D.H., Yanagihara. Acute cerebral dysfunction in diabetic ketoacidosis: survivial followed by panhypopituitarism. Metabolism. 1977. Apr; 26(4): 363–9. PMID: 403389. 10.1016/0026-0495(77)90103-2. [DOI] [PubMed] [Google Scholar]
- 43.Nagulesparan M., Roper J. Haemorrhage into the anterior pituitary during pregnancy after induction of ovulation with clomiphene. Br J Obstet Gynaecol. 1978. Feb; 85(2): 153–5. PMID: 626726. 10.1111/j.1471-0528.1978.tb10471.x. [DOI] [PubMed] [Google Scholar]
- 44.Wakai S., Fukushima T., Teramoto A., Sano K. Pituitary apoplexy: its incidence and clinical significance. J Neurosurg. 1981. Aug; 55(2): 187–93. PMID: 7252541. 10.3171/jns.1981.55.2.0187. [DOI] [PubMed] [Google Scholar]
- 45.DiMaio S.M., DiMaio V.J., Kirkpatrick J.B. Sudden, unexpected deaths due to primary intracranial neoplasms. Am J Forensic Med Pathol. 1980. Mar; 1(1): 29–45. PMID: 6263083. 10.1097/00000433-198003000-00007. [DOI] [PubMed] [Google Scholar]
- 46.Sun T., Liu L., Sunnassee A. et al. Sudden death in custody due to pituitary apoplexy during long restriction in a sitting position: a case report and review of the literature. J Forensic Leg Med. 2013. Oct; 20(7): 812–5. PMID: 24112326. 10.1016/j.jflm.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 47.Bauer M., Lang C., Patzelt D. Sudden death due to pituitary apoplexy. Leg Med (Tokyo). 2001. Sep; 3(3): 183–6. PMID: 12935525. 10.1016/s1344-6223(01)00026-8. [DOI] [PubMed] [Google Scholar]
- 48.Shields L.B., Balko M.G., Hunsaker J.C. 3rd. Sudden and unexpected death from pituitary tumor apoplexy. J Forensic Sci. 2012. Jan; 57(1): 262–6. PMID: 21854388. 10.1111/j.1556-4029.2011.01906.x. [DOI] [PubMed] [Google Scholar]
- 49.Johnston P.C., Chew L.S., Hamrahian A.H., Kennedy L. Lymphocytic infundibulo-neurohypophysitis: a clinical overview. Endocrine. 2015. Dec; 50(3): 531–6. PMID: 26219407. 10.1007/s12020-015-0707-6. [DOI] [PubMed] [Google Scholar]
- 50.Woywodt A., Knoblauch H., Kettritz R., Schneider W., Gobel U. Sudden death and Wegener's granulomatosis of the pituitary. Scand J Rheumatol. 2000; 29(4): 264–6. PMID: 11028850. 10.1080/030097400750041433. [DOI] [PubMed] [Google Scholar]
- 51.Goudie R.B., Pinkerton P.H. Anterior hypophysitis and Hashimoto's disease in a young woman. J Pathol Bacteriol. 1962. Apr; 83(2): 584–5. PMID: 13900798. 10.1002/path.1700830241. [DOI] [PubMed] [Google Scholar]
- 52.Sautner D., Saeger W., Lüdecke D.K. et al. Hypophysitis in surgical and autoptical specimens. Acta Neuropathol. 1995; 90(6): 637–44. PMID: 8615086. 10.1007/bf00318578. [DOI] [PubMed] [Google Scholar]
- 53.Gonzalez-Cuyar L.F., Tavora F., Shaw K. et al. Sudden unexpected death in lymphocytic hypophysitis. Am J Forensic Med Pathol. 2009. Mar; 30(1): 61–3. PMID: 19237858. 10.1097/paf.0b013e3181873851. [DOI] [PubMed] [Google Scholar]
- 54.Hashimoto K., Takao T., Makino S. Lymphocytic adenohypophysitis and lymphocytic infundibuloneurohypophysitis. Endocr J. 1997. Feb; 44(1): 1–10. PMID: 9152609. 10.1507/endocrj.44.1. [DOI] [PubMed] [Google Scholar]
- 55.Imura H., Nakao K., Shimatsu A. et al. Lymphocytic infundibuloneurohypophysitis as a cause of central diabetes insipidus. N Engl J Med. 1993. Sep 2; 329(10): 683–9. PMID: 8345854. 10.1056/nejm199309023291002. [DOI] [PubMed] [Google Scholar]
- 56.Kamel N., Ilgin S.D., Corapçioğlu D. et al. Lymphocytic infundibuloneurohypophysitis presenting as diabetes insipidus in a man. J Endocrinol Invest. 1998. Sep; 21(8): 537–40. PMID: 9801996. 10.1007/bf03347341. [DOI] [PubMed] [Google Scholar]
- 57.Blisard K.S., Pfalzgraf R.R., Balko M.G. Sudden death due to lympho plasmacytic hypophysitis. Am J Forensic Med Pathol. 1992. Sep; 13(3): 207–10. PMID: 1476123. 10.1097/00000433-199209000-00007. [DOI] [PubMed] [Google Scholar]
- 58.Folkerth R.D., Price D.L. Jr., Schwartz M. et al. Xanthomatous hypophysitis. Am J Surg Pathol. 1998. Jun; 22(6): 736–41. PMID: 9630181. 10.1097/00000478-199806000-00011. [DOI] [PubMed] [Google Scholar]
- 59.Jain K.C., Varma A., Mahapatra A.K. Pituitary abscess: a series of six cases. Br J Neurosurg. 1997. Apr; 11(2): 139–43. PMID: 9156001. 10.1080/02688699746492. [DOI] [PubMed] [Google Scholar]
- 60.Heslop T. A case of hypertrophy with abscess of the pituitary body. Dublin Q J Med Sci. 1848. Nov; 6(2): 466. [Google Scholar]
- 61.Dutta P., Bhansali A., Singh P. et al. Pituitary abscess: report of four cases and review of literature. Pituitary. 2006; 9(3): 267–73. PMID: 16845602. 10.1007/s11102-006-8327-z. [DOI] [PubMed] [Google Scholar]
- 62.Liu F., Li G., Yao Y. et al. Diagnosis and management of pituitary abscess: experiences from 33 cases. Clin Endocrinol (Oxf). 2011. Jan; 74(1): 79–88. PMID: 21039726. 10.1111/j.1365-2265.2010.03890.x. [DOI] [PubMed] [Google Scholar]
- 63.Nelson P.B., Haverkos H., Martinez A.J., Robinson A.G. Abscess formation within pituitary tumors. Neurosurgery. 1983. Mar; 12(3): 331–3. PMID: 6843806. 10.1097/00006123-198303000-00016. [DOI] [PubMed] [Google Scholar]
- 64.Whalley N. Abscess formation in a pituitary adenoma. J Neurol Neurosurg Psychiatry. 1952. Feb; 15(1): 66–7. PMID: 14908619. PMCID: PMC499562. 10.1136/jnnp.15.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kotani H., Abiru H., Miyao M. et al. Pituitary abscess presenting a very rapid progression: report of a fatal case. Am J Forensic Med Pathol. 2012. Sep; 33(3): 280–3. PMID: 22835970. 10.1097/paf.0b013e3182557dcf. [DOI] [PubMed] [Google Scholar]


