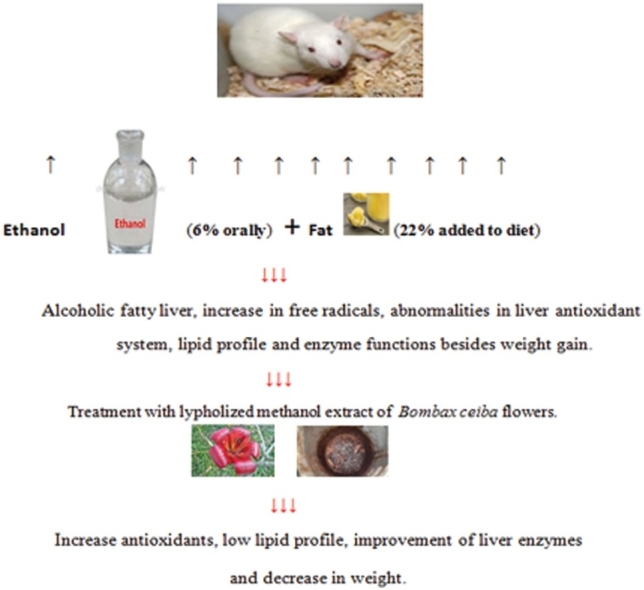
Graphical abstract
Keywords: Alcohol consumption, Hepatosteatosis, B. ceiba flowers extract
Highlights
-
•
Alcohol addiction and ALD are serious problems and no safe treatments are available.
-
•
Administration of fat diet besides alcohol addiction makes the problem more complicated as alcohol helps in fat deposition in the liver.
-
•
Bombax ceiba flowers methanol extract was evaluated as a treatment for alcoholic liver that suffers from steatosis.
-
•
The extract succeeded in ameliorating the ALD complications by potentiating the antioxidant defenses in the liver and getting rid of excess fat.
Abstract
Chronic excessive alcohol consumption could induce serious liver injury. In this study, therapeutic effect of aqueous methanol extract of Bombax ceiba L. flowers (BCE) (Family: Bombacaceae) was investigated against hepatic steatosis. This study included seven groups, and the research period was eight weeks. The first group served as control. The six remaining groups were divided into two categories, three groups in each. The first category was fed fat diet. The second category was fed fat diet and orally administrated ethanol, which was given in graduate doses from 2 g/kg/d to 6 g/kg/d. Then, one group from each category was orally treated with the standard drug fluvastatin (2 mg/Kg/d). Another group was orally treated with BCE (200 mg/kg/d). The third group left untreated. The results revealed that BCE significantly decrease both the body and liver weight. The treatment with BCE extract also ameliorates the effect of alcohol induced increase of liver enzyme activities. In addition, the extract was significantly increased hepatic liver antioxidants and decreased malondialdehyde (MDA) level. Also, serum lipid profiles: triglycerides (TG), total cholesterol (TC) and low density lipoprotein (LDL) were significantly decreased after BCE treatment. Histopathological study showed fatty changes induced by alcohol which were improved by BCE treatment. These data suggest that the BCE has anti-inflammatory, anti-oxidant and anti-steatosis potential properties against alcohol induced liver damage. This may be due to the presence of flavonoids and other phenol compounds.
1. Introduction
Excessive alcohol consumption causes a wide spectrum of liver and other organ diseases. Alcoholic liver disease (ALD) has a complex and incompletely known pathogenic. The liver dysfunction ranges from fatty change to alcoholic hepatitis, cirrhosis and hepatocellular carcinoma [1]. In addition to the cumulative quantity of alcohol intake and alcohol addiction patterns, other factors such as gender, age, geographic area, nutritional factors, genetic background, immunological mechanisms and energy metabolism abnormalities also play a key role in the progression of alcoholic liver damage [2]. Dietary intake of fat and the development of fatty infiltration are important factors in the pathogenic of hepatosteatosis. The degree of fatty infiltration found in liver biopsy of alcoholic patients is a risk factor for the development of liver cirrhosis [3].
Ethanol is the principle ingredient in most of the syrups, mouthwash, tincture, and alcoholic beverages. It is also a common ingredient in a range of products, from cosmetics and beauty products to paints and varnishes to fuel. Ethanol is effective in killing microorganisms like bacteria, fungi and viruses, so it is used in many hand sanitizes and personal care. Alcohol chemotherapy is the first choice treatment for cystic lesions and benign solid thyroid venous malformations [4]. In small doses it has a great medicinal value because of its effectiveness, ease of use, low cost and long shelf life. But some people tend to have ethanol abuse. In excessive dose, it induces severe liver injury in experimental animals and humans [5].
Three obvious enzymatic pathways shared in the process of ethanol oxidation [6]. The first pathway for the ethanol metabolism is dehydrogenase system. It is initiated by alcohol dehydrogenase (ADH), which oxidizes ethanol to acetaldehyde. Then, acetaldehyde enters the mitochondria where it is oxidized to acetate by aldehyde dehydrogenases (ALDH) giving rise to reactive oxygen species (ROS). Increased oxidative stress is well recognized to be an essential factor in the pathogenic of hepatic injury [7]. The second major pathway to oxidize ethanol is the microsomal ethanol oxidizing system (MEOS), which involves the cytochrome P450 enzyme CYP2E1. The MEOS pathway is stimulated in individuals who consume alcohol chronically. In addition, ethanol can be oxidized and this oxidation pathway requires the presence of hydrogen peroxide [8].
Since oxidative stress and metabolite induced inflammatory factors are incorporated in the development of ALD, specific antioxidants potentially are useful for alleviation of ethanol induce oxidative stress and prevent this pathogenesis [9].
In mammals, a sophisticated antioxidant system, both enzymatic and non-enzymatic, has been developed to remain the redox homeostasis in the liver and deals with oxidative stress under physiological conditions. Therefore, the enzymatic antioxidants such as catalase, superoxide dismutase, and glutathione peroxidase and non-enzymatic antioxidant such as reduced glutathione are affected and used as indicators to evaluate the level of oxidative stress [10]. Among alcoholics, the altered lipid profile is one of the crucial factors of fatty liver. Fluctuation in the gene expressions that are involved in the synthesis or degradation of fatty acids, triglycerides, and/or cholesterol may be as a result of differential regulation at the post-transcriptional levels [11].
Bombax ceiba Linn. is known as silk cotton tree and it belongs to family Bombacacae. The plant is widely cultivated in China, Pakistan, India, and Austria and was introduced into Egypt several decades ago as a shade tree and an ornamental plant [12]. For use in foods, plant extracts are nutritionally more relevant and have obvious advantages in safety [13]. It is well-reputed in traditional systems of medicine for treatment of diarrhea, fever, and ulceration of bladder as well as kidney [14]. Different parts of Bombax ceiba (B. ceiba) has shown to possess many biological properties predominantly antioxidant, antimicrobial, anti-inflammatory, antihyperlipidemic, and hypoglycemic activities [15].
The correct pathological mechanisms of alcoholic liver disease remain ambiguous and no new accepted drugs are now on the market. In spite of that, ameliorating hepatic steatosis and oxidative stress are considered to be a promising strategy for ALD treatment. So, the aim of this study is to evaluate the therapeutic effect of the Egyptian BCE flowers extract upon chronic alcohol consumption induced hepatosteatosis in rats fed fat diet.
2. Materials and methods
2.1. Chemicals and reagents
All chemicals used in the experiments were of analytical grade. Kits preformed for the quantitative determination of different parameters were purchased from Greiner Diagnostic Gmbh- Germany and Bio-diagnostic Company, Egypt. Lescol XL drug (fluvastatin as the active ingredient) is a product purchased by Novartis, Switzerland.
2.2. Plant materials
Bombax ceiba (B. ceiba) flowers were collected from Orman botanical garden, Giza, Egypt, in January, 2016. The flowers were kindly authenticated by a botanist at the National Research Centre. A voucher specimen (No. 3241) was deposited in the herbarium of National Research Centre, Doki, Giza.
2.3. Preparation of aqueous methanol extracts of BCE
Shade dried B. ceiba flowers (1 Kg) were refluxed with aqueous methanol (70%W/V). The extracted solutions were combined, filtered, and then concentrated by a rotary evaporator under reduced pressure to remove the methanol solvent. The extract was then subjected to lyophilization for removing the excess water yielding 100 g of crude dark-brown sticky BCE and stored at −20 °C for use.
2.4. Experimental design
2.4.1. Animals
Fifty six adult female Wistar rats weighing 130 ± 5 g were obtained from the animal house of National research Centre. Animals were maintained in clean cages under normal laboratory conditions (22 ± 50C, 12 h light/dark cycle). They were allowed for a standard pellet diet and free access to water. The experimental work on rats was performed with the approval of the Animal care and Experimental Committee, National Research Centre, Cairo, Egypt (Approval no: 18085).
The animals were divided into seven groups of eight animals each. Group1: control and given only standard pellet diet. Group2: Fat diet (Fed 22% fat/kg diet). Group3: Ethanol-fat diet (fed fat diet 22% fat/kg diet + Ethanol). Group 4: Fat diet group treated with standard drug fluvastatin. Group 5: Fat diet group treated with BCE. Group 6: Ethanol-fat diet treated with standard drug fluvastatin. Group 7: Ethanol-fat diet treated with BCE.
During the duration of the experiment, daily food intake and weekly body weights were recorded. At the end of treatment period, blood samples were drawn from the animals via puncturing the retro-orbital venous plexus with a thin sterilized capillary tube. Blood specimens were subjected to serum separation and stored at −20 °C for biochemical analysis. Then, rats were sacrificed by decapitation, livers dissected out, cleaned and weighed. Some samples were rinsed and homogenized in a phosphate buffer (pH, 7.4) and centrifuged at 4000 rpm at 4 °C for 15 min. The obtained supernatants were used for biochemical assays. Other liver tissues were immediately removed and fixed with neutral formalin solution 10% for histological examination.
2.4.2. Doses and route of administration
The oral daily administration route was used in the study.–The starting dose was 2 g.kg−1.d−1 and increased up to 6 g.kg−1.d−1 [16]. The dose of BCE was selected to be 200 mg/kg/day for 30 consecutive days according to the previous study [14].Whereas; fluvastatin dose (2 mg/Kg/day) was selected based on the clinical application from previous studies on human and experimental animals [17].
2.4.3. Biochemical analysis
2.4.3.1. Measurements of blood alcohol concentration
Blood alcohol levels were carried out on samples obtained from tail veins of animals using a commercial kit (Bio STC- Science and Technology Center, Mohandeseen, Giza). This enzymatic test for alcohol utilizes the coenzyme nicotinamide adenine dinucleotide (NAD) and alcohol dehydrogenase (ADH). The formation of NADH can be measured quantitatively by the increase in absorbance at 340 nm.
2.4.3.2. Assays of serum liver enzyme activities
Serum transaminases (AST&ALT) activities were determined by a colormetric method according to Reitman and Frankel [18]. Serum alkaline phasphatase (ALP) activity was determined by enzymatic colorimertic method according to Belfield and Goldberg [19].
2.4.3.3. Assays of some antioxidant defense markers
Liver glutathione (GSH) content and glutathione-S-transferase (GST) activity were determined according to the methods of Beutler et al. [20] and Habig and Pabst [21], respectively. The activity of enzymatic antioxidant (SOD) was assayed by the method of Kakkar et al. [22]. The activity of (CAT) was assayed by the method of Aebi [23]. Liver Lipid peroxidation was measured by estimating the levels of malondialdehyde (MDA) using a thiobarbituric acid reaction method [24].
2.4.3.4. Assays of serum lipid profile
Triglycerides (TG) were determined according to the method of Rifai et al. [25]. Total cholesterol (TC) and low density lipoprotein (LDL) were determined by the method of Deeg and Ziegenhorn [26].
2.4.3.5. Liver histological examination
Sections of 6 μm thickness were prepared and stained with hematoxylin and eosin as described by Afifi [27]. The stained sections were observed under the light microscope, cytoplasm stained in shades of pink to red and the nuclei gave blue color.
2.5. Statistical analysis
Data were analyzed by with SPSS (Statistical Package for the Social Sciences) version 9.05 software (USA). All values were expressed as the mean ±SD. Significant differences between the groups were statistically analyzed by one way analysis of variance (ANOVA) followed by Turkey’s multiple comparison post hoc test. A statistical difference of P < 0.05 was considered significant.
3. Results
3.1. Effect of BCE on body weight, absolute liver weight and food intake
At the end of the experiment, it was found that ethanol-fat diet group recorded significant increase in the percentage of body weight gain compared to all other groups. While, there was no significant difference in the percentage of body weight gain between ethanol-fat diets BCE treated group and ethanol fat diet-standard drug treated group. The absolute liver weight was significantly increased in both fat diet and ethanol-fat diet groups compared to the control group. A significant decrease was also observed in absolute liver weight in both BCE treated groups compared to their correspondence; fat diet and ethanol-fat diet untreated ones, respectively. However, slight changes were recorded in relative liver weights among major groups. In addition, there was no significant difference in food intake between fat diet group and control. While, a significant difference was observed in food intake among major groups compared to control (Table 1).
Table 1.
Changes in body weight, absolute liver weight and food intake of control, fat diet, ethanol-fat diet and treated rat groups.
| Parameters | Groups |
||||||
|---|---|---|---|---|---|---|---|
| 1- Control | 2- Fat diet | 3- Ethanol-fat diet | 4- Fat diet- Fluvastatin | 5- Fat diet- BCE | 6- Ethanol fat diet-Fluvastatin | 7- Ethanol fat diet BCE | |
| Initial body weight (g) | 133.33 ± 5.50 | 132.67 ± 3.19 | 133.33 ± 5.39 | 133.67 ± 4.46 | 132.50 ± 6.09 | 132.00 ± 5.10 | 132.17 ± 4.96 |
| Final body weight (g) | 236.00 ± 16.32(2,3,4,5,6,7) | 309.33 ± 18.19(1,3,4,5,6,7) | 390.50 ± 17.40(1,2,4,5,6,7) | 279.83 ± 13.29(1,2,3,6,7) | 274.17 ± 18.23(1,2,3,6,7) | 361.83 ± 14.16(1,2,3,4,5) | 354.00 ± 10.08(1,2,3,4,5) |
| Body weight gain (%) | 43.50 ± 0.84(2,3,4,5,6,7) | 57.11 ± 0.57(1,3,4,5,6,7) | 65.86 ± 0.76(1,2,4,5,6,7) | 52.23 ± 1.21(1,2,3,6,7) | 51.67 ± 1.35(1,2,3,6,7) | 63.52 ± 1.57(1,2,3,4,5) | 62.66 ± 0.71(1,2,3,4,5) |
| Absolute liver weight (g) | 4.95 ± 0.24(2,3,4,5,6,7) | 6.75 ± 0.43(1,3,4,5,6,7) | 7.72 ± 0.52(1,2,4,5,6,7) | 5.93 ± 0.43(1,2,3) | 5.72 ± 0.44(1,2,3) | 5.75 ± 0.40(1,2,3) | 5.92 ± 0.36(1,2,3) |
| Relative liver weight (g/100 gbw) | 2.09 ± 0.13(6,7) | 2.18 ± 0.17(6,7) | 1.98 ± 0.14(6,7) | 2.11 ± 0.14(6,7) | 2.08 ± 0.15(6,7) | 1.59 ± 0.15(1,2,3,4,5) | 1.67 ± 0.16(1,2,3,4,5) |
| Food intake (g/day/rat) | 27.35 ± 3.12(3,4,5,6,7) | 29.15 ± 2.73(3,6) | 33.24 ± 3.43(1,2,4,7) | 29.76 ± 2.47(1,3) | 29.85 ± 2.19(1,3) | 31.32 ± 2.15(1,2) | 30.94 ± 3.23(1,3) |
Data are represented as Mean ± SD of 8 rats in each group. Significant at P < 0.05.
3.2. Effect of BCE on some biochemical markers in serum
A more significant increase in the percentage change of AST, ALT and ALP levels was observed in ethanol-fat diet group than fat diet one as compared to control as shown in Table 2. However, ethanol-fat diet BCE treated group recorded higher percentage of improvement in the activities of the AST, ALT and ALP enzymes than that recorded in fat diet BCE treated group. Similar results were observed in the percentage of improvement of the AST, ALT and ALP enzyme activities upon treatment with standard drug.
Table 2.
Effect of BCE on hepatic enzyme activities of control, fat diet, ethanol-fat diet and treated rat groups.
| Parameters | Groups |
||||||
|---|---|---|---|---|---|---|---|
| 1- Control | 2- Fat diet | 3- Ethanol-fat diet | 4- Fat diet- Fluvastatin | 5- Fat diet- BCE | 6- Ethanol fat diet-Fluvastatin | 7- Ethanol fat diet- BCE | |
| AST (U/ml) % of change % of improvement |
42.55 ± 3.28(2,3,4,7) – – |
68.95 ± 4.69(1,3,4,5,6) 62.04 – |
93.10 ± 6.39(1,2,4,5,6,7) 118.80 – |
55.21 ± 3.68(1,2,3,5,6,7) 29.75 32.29 |
46.19 ± 4.05(2,3,4,7) 7.88 53.49 |
47.70 ± 4.83(2,3,4,7) 12.10 106.70 |
66.68 ± 6.40(1,3,4,5,6) 56.71 62.09 |
| ALT(U/ml) % of change % of improvement |
15.39 ± 1.90(2,3) – – |
21.87 ± 1.48(1,3,4,5,6,7) 42.11 – |
25.91 ± 3.55(1,2,4,5,6,7) 68.36 – |
13.78 ± 0.87(2,3,6) 10.46 52.57 |
14.56 ± 2.17(2,3) 5.39 47.50 |
17.38 ± 1.68(2,3,4) 12.93 55.43 |
16.85 ± 2.60(2,3) 9.49 58.87 |
| ALP (U/L) % of change % of improvement |
154.45 ± 15.47(2,3,4,5,6,7) – – |
205.83 ± 13.53(1,3) 33.27 – |
241.56 ± 16.84(1,2,4,5,6,7) 56.40 |
185.11 ± 14.28(1,3) 19.85 13.41 |
183.79 ± 12.19(1,3) 18.99 14.24 |
191.67 ± 21.22(1,3) 24.10 32.30 |
194.27 ± 15.22(1,3) 25.78 30.62 |
Data are represented as Mean ± SD of 8 rats in each group. Significant at P < 0.05.
3.3. Effect of BCE on enzymatic antioxidant status in liver
The ethanol fat diet and Fat diet groups showed significant decrease in the activities of GST, SOD and CAT enzymes and reduced the content of GSH as compared to control group. This decreasing was accompanied by a significant increase in MDA level in these groups. Treatment of fat diet group with BCE recorded more significant percentage of improvement in the levels of GSH, GST and SOD than that recorded in the ethanol fat diet BCE treated group. While, ethanol fat diet BCE treated group showed a significant increase in the percentage of improvement in CAT and MDA levels more than the values recorded in Fat diet BCE treated group as shown in Table 4.
Table 4.
Effect of BCE extract on lipid profiles of control, fat diet, ethanol fat diet and treated rat groups.
| Parameters | Groups |
||||||
|---|---|---|---|---|---|---|---|
| 1- Control | 2- Fat diet | 3- Ethanol-fat diet | 4- Fat diet- Fluvastatin | 5- Fat diet- BCE | 6-Ethanol fat diet-Fluvastatin | 7- Ethanol fat diet- BCE | |
| TG (mg/d) % change % of improvement |
198.75 ± 21.57(2,3) – – |
235.13 ± 18.82(1,4,5,6,7) 18.30 – |
254.25 ± 16.99(1,4,5,6,7) 27.92 – |
205.86 ± 14.59(2,3) 3.58 14.73 |
210.95 ± 14.88(2,3) 6.14 12.17 |
202.73 ± 12.34(2,3) 2.02 25.92 |
210.45 ± 9.33(2,3) 5.89 22.04 |
| TC (mg/d) % change % of improvement |
50.56 ± 6.76(2,3,6,7) – – |
63.98 ± 8.49(1,3) 26.54 – |
87.66 ± 7.06(1,2,4,5,6,7) 73.38 – |
59.41 ± 4.58(3,6,7) 17.50 9.03 |
57.20 ± 6.80(3,6,7) 13.13 13.41 |
69.22 ± 6.76(1,3,4,5) 36.91 36.47 |
69.67 ± 4.63(1,3,4,5) 37.80 35.58 |
| LDL (mg/d) % change % of improvement |
21.53 ± 1.64(2,3,4,5) – – |
32.94 ± 4.34(1,3,4,5,6,7) 53.00 – |
37.58 ± 3.53(1,2,4,5,6,7) 74.55 – |
28.18 ± 3.85(1,2,3,6,7) 30.89 22.11 |
26.78 ± 2.47(1,2,3,6) 24.38 26.52 |
21.85 ± 2.10(2,3,4,5) 1.49 73.06 |
22.41 ± 2.25(2,3,4) 4.09 70.46 |
Data are represented as Mean ± SD of 8 rats in each group. Significant at P < 0.05.
3.4. Effect of BCE on lipid profiles
In Table 3, the ethanol-fat diet group showed more significant percentage changes in the levels of the TG, TC and LDL than that of fat diet group as compared to the control one. However, the BCE as a treatment agent showed a more significant increase in the percentage of improvement on the levels of the TG, TC and LDL in ethanol-fat diet treated group than the fat diet BCE treated group. Also, the standard drug treatment groups recorded the same results of percentage of improvement in the levels of TG, TC and LDL.
Table 3.
Effect of BCE on some antioxidant enzymes of control, fat diet, ethanol -fat diet and treated rat groups.
| Parameters | Groups |
||||||
|---|---|---|---|---|---|---|---|
| 1- Control | 2- Fat diet | 3- Ethanol-fat diet | 4- Fat diet- Fluvastatin | 5- Fat diet- BCE | 6-Ethanol fat diet-Fluvastatin | 7- Ethanol fat diet BCE | |
| GSH (mmol/g tissue) % change % of improvement |
257.53 ± 10.48(2,3,4,5,7) – – |
205.28 ± 9.35(1,4,5,6,7) −20.29 – |
201.97 ± 11.49(1,4,5,6,7) −28.56 – |
238.22 ± 6.77(1,2,3) 16.05 12.8 |
242.73 ± 4.43(1,2,3) 18.24 14.54 |
249.95 ± 10.63(2,3) 23.76 18.63 |
239.89 ± 5.48(1,2,3) 18.78 14.72 |
| GST (U/g tissue) % change % of improvement |
16.06 ± 2.36(2,3,6,7) – – |
10.57 ± 1.28(1,4,5,6,7) −34.18 – |
10.22 ± 0.87(1,4,5,6,7) −36.36 – |
14.32 ± 0.95(2,3) 35.48 23.35 |
15.19 ± 1.41(2,3,6) 43.71 28.77 |
12.97 ± 1.26(1,2,3,5) 26.91 17.12 |
13.76 ± 1.56(1,2,3) 34.64 22.04 |
| SOD (U/g tissue) % change % of improvement |
170.29 ± 12.65(2,3) – – |
145.46 ± 8.39(1,7) −14.58 – |
143.59 ± 14.65(1,5,7) −15.68 – |
155.36 ± 15.73 6.81 5.81 |
166.28 ± 6.43(2,3,) 14.31 12.23 |
159.91 ± 15.42 11.37 9.58 |
163.61 ± 7.03(2,3) 13.76 11.76 |
| MDA (nmol/g tissue) % change % of improvement |
845.08 ± 38.98(2,3) – – |
1106.51 ± 59.65(1,3,4,5,6,7) 30.94 – |
1210.06 ± 57.86(1,2,4,5,6,7) 43.19 – |
839.16 ± 43.35(2,3) −24.16 31.63 |
842.07 ± 32.75(2,3) −23.90 31.29 |
818.12 ± 33.80(2,3) −32.39 46.38 |
832.19 ± 31.03(2,3) −31.23 44.71 |
| CAT (U/g tissue) % change % of improvement |
9.02 ± 1.24(2,3,4) – – |
4.14 ± 0.48(1,4,5,6,7) −54.10 – |
3.64 ± 1.37(1,4,5,6,7) −59.65 |
7.18 ± 1.16(1,2,3) 73.43 33.70 |
8.22 ± 0.93(2,3) 98.55 45.23 |
7.96 ± 0.45(2,3) 118.68 47.89 |
8.00 ± 0.45(2,3) 119.78 48.34 |
Data are represented as Mean ± SD of 8 rats in each group. Significant at P < 0.05.
3.5. Effect of BCE on histopathological study
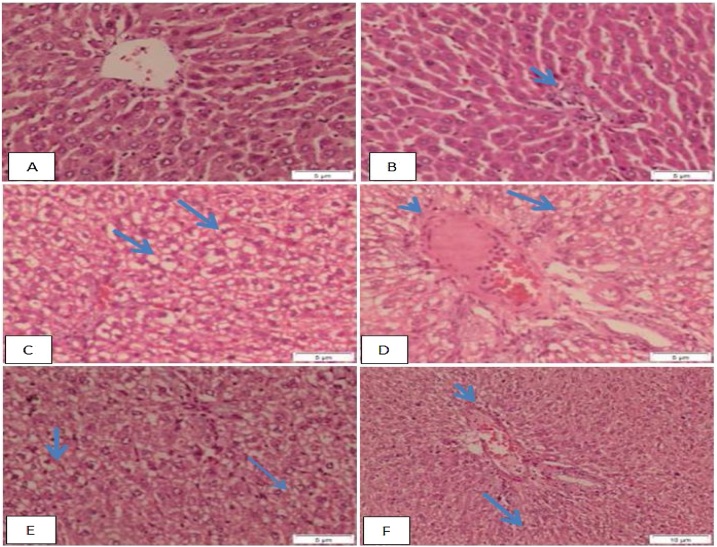
Liver sections of control group show the normal architecture of the hepatic lobules and normal portal tract. The central vein lies at the center of the lobule surrounded by cords of hepatocytes. The hepatocytes show strongly eosinophilic granulated cytoplasm, round nuclei. Sinusoids are often seen between the strands of hepatocytes (Fig.1) (A&B). Fat-diet group shows hydropic degeneration, congested portal tract associated with mild inflammatory infiltration, fatty change and vacuoles in the hepatocytes (Fig.1) (C&D). Ethanol fat-diet group shows disturbance of the hepatic lobules associated with hydropic degeneration, vacuoles of fatty change in the hepatocytes, the hepatocytes around the dilated congested vessels appeared variable in shape and size, congested portal tract associated with mild inflammatory infiltration and necrosis of the hepatocytes degeneration (Fig. 1) (E&F).
Fig. 1.
Micrograph of liver sections of A) control group shows normal architecture of the hepatic lobules, central vein lies at the center, hepatocytes shows strongly eosinophilic granulated cytoplasm, round nuclei and sinusoids are often seen between strands of hepatocytes., B) control rat shows the normal architecture of the portal tract (arrow)., C) Fat-diet group shows hydropic degeneration, and vacuoles in the hepatocytes (arrow)., D) Fat-diet group shows congested portal tract associated with mild inflammatory infiltration (arrowhead), fatty change and vacuoles in the hepatocytes (arrow)., E) Ethanol fat-diet group shows disturbance of the hepatic lobule associated with hydropic degeneration (thick arrow), and vacuoles of fatty change the hepatocytes (thin arrow) and the hepatocytes around the dilated congested vessels appeared variable in shape and size., F) Ethanol fat- diet group shows congested portal tract associated with mild inflammatory infiltration (arrowhead), necrosis of the hepatocytes degeneration, and vacuoles in the hepatocytes (arrow) (H&E stain, bar: 10 μm).
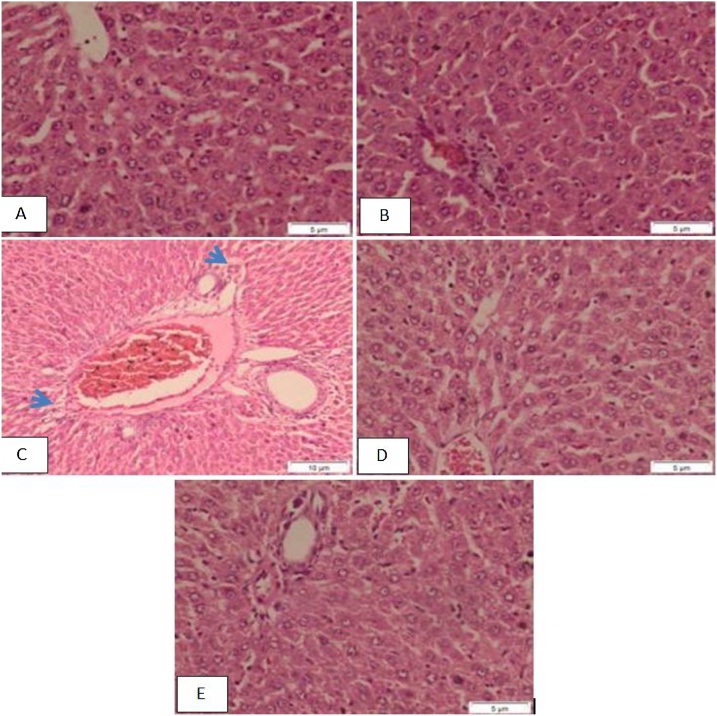
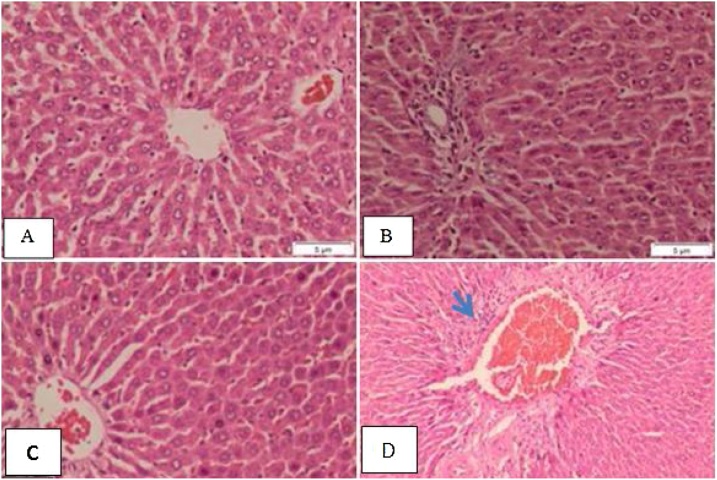
However, liver sections of fat diet-standard treated group show structure of the hepatic and portal lobules appeared more or less like normal, congested portal tract associated with mild inflammatory infiltration and the hepatocytes around the dilated congested vessels appeared variable in shape and size (Fig. 2) (A, B&C). Fat diet BCE treated group shows normal structure of the hepatic and portal lobules (Fig. 2) (D&E). While, Ethanol-fat diet standard treated group shows the structure of the hepatic and portal lobules appeared more or less like normal (Fig. 3) (A&B). Ethanol- fat diet BCE treated group shows the structure of the hepatic lobule appeared more or less like normal, congested portal tract associated with mild inflammatory infiltration (Fig. 3) (C&D).
Fig. 2.
A, B) Fat-diet standard treated group shows structure of the hepatic and portal lobules appeared more or less like normal., C) Fat-diet standard treated group shows congested portal tract associated with mild inflammatory infiltration (arrowhead) and the hepatocytes around the dilated congested vessels appeared variable in shape and size., D, E) Fat-diet BCE treated group shows the normal structure of the hepatic and portal lobules (H&E stain, bar: 5 μm).
Fig. 3.
Micrograph of liver section of A, B) Ethanol fat-diet standard treated group show the structure of the hepatic and portal lobules appeared more or less like normal., C) Ethanol fat-diet BCE treated group shows the structure of the hepatic lobule appeared more or less like normal., D) Ethanol fat-diet BCE treated group shows congested portal tract associated with mild inflammatory infiltration (arrowhead) (H&E stain, bar: 5 μm).
4. Discussion
Alcohol addiction represents the main causes of chronic liver diseases and is associated with high mortality in many developed countries [28]. Chronic ethanol consumption results in decreasing mitochondrial protein synthesis, impairment of the oxidative phosphorylation system, decline in ATP synthesis, leakage of ROS which induce the activation of intrinsic apoptotic pathways [29,30]. In vitro, HL7702 hepatic cell was establishing of steatosis model to evaluate the lipid-lowering effects of certain polyphenols [31]. Also, 4-nonylphenol is correlated with hepatic steatosis as non alcoholic models in rats [32].
The Phytochemical compositions of methanol extract of the Egyptian B. ceiba flowers indicate the presence of alkaloids, flavonoids, glycosides, saponine, polyphenols, tannins, proteins, carbohydrates, lipids, amino acids and coumarins [33,34]. These valuable compounds offer the potent antioxidant and hypolipedimic properties of BCE in the liver tissues [35]. In our study, we examined the efficacy of these constituents in curing the alcohol addiction that were accompanied by fat intake in comparison to the effect of the standard drug; Fluvastatin.
After one month of ethanol ingestion, the body weight of both fat diet and ethanol-fat diet groups were significantly higher than control. These observations may be due to hyperlipidemia and the effect of extra calories as ingesting excessive ethanol [36]. Treatment with BCE reduces the percentage of body weight gain of both fat-diet and ethanol-fat diet treated groups compared with the untreated ones. This explains that the extract may be preventing the pathological mechanisms susceptive for excessive fat accumulation and weight gain due to the presence of bioactive components like alkaloids and glycosides. In addition, the extract is possibly increasing leptin sensitivity, providing anorexic effect and increasing energy expenditure [35,37]. The liver is the primary organ responsible for ethanol metabolism and it is susceptible to alcohol's toxic effects. In the present study, the elevated levels of serum enzymes such as AST, ALT and ALP were observed after consumption of alcohol. The leakage of these enzymes into the blood reflected liver damage [38]. However, BCE treatment significantly decreased the content of these serum parameters in both treated groups. This illustrates the integration of plasma membrane, as well as repair of hepatic tissue damage.
In our study GSH, GR, SOD and CAT enzyme levels depleted by ethanol and fat intake as compared to control group. Treatment with BCE restored the normal hepatic concentrations of these studied antioxidant enzymes, which suggests the ability of active constituent of the extract to interact directly with ROS and scavenge free radicals [39]. Also, treatment with BCE significantly decreased MDA level by stopping the formation of lipid peroxide from fatty acids [40]. Our results were in line with the previous studies which have shown that the watermelon juice had hepatoprotective effects after excessive ethanol consumption in rats [41].
Many factors such as ethanol consumption, nutritional status, ethanol metabolism and genetic makeup can lead to hepatostestosis [42]. Also, hepatostestosis can be induced in people without alcohol abuse history and may appear in those who have metabolic disorders especially obese ones [43].
In our study, alcohol induced liver steatosis was confirmed by significant increase in TG, TC and LDL in fat-diet and ethanol fat-diet groups compared to control group. This increase may be due to disturbance in lipid metabolism and lipid homeostasis [44]. These changes are the result of an adaptive mechanism to resist the fluidizing effect of ethanol [45]. Treatment with BCE significantly lowered the levels of lipid profiles due to the inactivation of acetyl-coA carboxylase as a result of adenosine monophosphate kinase activation that mediates thermogenesis and fatty acid synthesis inhibition [46]. Mangiferin, which is a polyphenol compound found in BCE upregulates proteins for mitochondrial bioenergetics and down regulates proteins controlling de novo lipogenesis [47].
Histopathological results indicated that in control group, there were neither lipid droplets nor steatosis distributed in the hepatic lobules, which approved the maintenance of the lobular structure integrity. On the other hand, liver sections of fat-diet and ethanol fat-diet groups showed different extents of steatosis in the hepatic lobules with mild inflammatory infiltration. Treatment with BCE alleviated the hepatic steatosis and reduced infiltrations of inflammatory cells in the hepatocyte.
5. Conclusion
BCE can be a safe treatment for the alcohol addiction cases accompanied by fat intake. The extract succeeded in decreasing the disease complications in addition to potentiating the antioxidant defenses in the liver. Further studies are needed for applying the extract as a recommended drug for ALD.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- 1.Zhou Y., Zheng J., Li S., Zhou T., Zhang P., Li H.B. Alcoholic beverage consumption and chronic diseases. Int. J. Environ. Res. Public Health. 2016;13(6):522–531. doi: 10.3390/ijerph13060522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kendrick S. Natural history and factors influencing the course of alcoholic- related liver disease. Clin. Liver Disease. 2013;2(2):61–63. doi: 10.1002/cld.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stewart S., Jones D., Day C.P. Alcoholic liver disease: new insights into mechanisms and preventative strategies. Trends Mol. Med. 2001;7:408–413. doi: 10.1016/s1471-4914(01)02096-2. [DOI] [PubMed] [Google Scholar]
- 4.Legiehn G.M., Heran M.K. Venous malformations: classification, diagnosis and interventional radiologic management. Radiol. Clin. North Am. 2008;46:545–597. doi: 10.1016/j.rcl.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Zakhari S., Li T.K. Determinants of alcohol use and abuse: impact of quantity and frequency patterns on liver disease. Hepatology. 2007;46:2032–2039. doi: 10.1002/hep.22010. [DOI] [PubMed] [Google Scholar]
- 6.Wang H., Chen X., Su Y., Paueksakon P., Hu W., Zhang M.Z., Harris R.C., Blackwell T.S., Zent R., Pozzi A. p47phox contributes to albuminuria and kidney fibrosis in mice. Kidney Int. 2015;87:948–962. doi: 10.1038/ki.2014.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albano E. Oxidative mechanisms in the pathogenesis of alcoholic liver disease. Mol. Aspects Med. 2008;29:9–16. doi: 10.1016/j.mam.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Dey A., Lakshmanan J. The role of antioxidants and other agents in alleviating hyperglycemia mediated oxidative stress and injury in liver. Food Funct. 2013;4:1148–1184. doi: 10.1039/c3fo30317a. [DOI] [PubMed] [Google Scholar]
- 9.Liu H., Qi X., Cao S., Li P. Protective effect of flavonoid extract from Chinese bayberry (Myrica rubra Sieb. Et Zucc.) fruit on alcoholic liver oxidative injury in mice. J. Nat. Med. 2014;68:521–529. doi: 10.1007/s11418-014-0829-9. [DOI] [PubMed] [Google Scholar]
- 10.Mallikarjuna K., Shanmugam K.R.K., Nishanth M.C., Wu C.W., Hou C.H., Kuo, Reddy K.S. Alcohol-induced deterioration in primary antioxidant and glutathione family enzymes reversed by exercise training in the liver of old rats. Alcohol. 2010;44:523–529. doi: 10.1016/j.alcohol.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Lucero López V.R., Razzeto G.S., Escudero N.L., Gimenez M.S. Biochemical and molecular study of the influence of Amaranthus hypochondriacus flour on serum and liver lipids in rats treated with ethanol. Plant Foods Hum. Nutr. 2013;68:396–402. doi: 10.1007/s11130-013-0388-3. [DOI] [PubMed] [Google Scholar]
- 12.Shahat A.A., Hasan R.A., Nazif N.M., Miert S.V., L.U.C Pieter, Hammuda F.M., Vlietinck A.J. Isolation of mangiferin from Bombax malabaricum and structure revision of shamimin. Planta Med. 2003;69:1066–1078. doi: 10.1055/s-2003-45161. [DOI] [PubMed] [Google Scholar]
- 13.Wong S.P., Leong L.P., Koh J.H.W. Antioxidant activities of aqueous extracts of selected plants. Food Chem. 2006;99:775–783. [Google Scholar]
- 14.Ravi V., Patel S.S., Verma N.K., Dutta D., Saleem T.S.M. Hepatoprotective activity of Bombax ceiba Linn against isoniazid and rifampicin-induced toxicity in experimental rats. Int. J. Appl. Res. Nat. Prod. 2010;3(3):19–26. [Google Scholar]
- 15.Guang-Kai X.U., Xia-Ying Q.I.N., Guo-Kai W.A.N.G., Guo-Yong X.I.E., Xu-Sen L.I., Chen-Yu S.U.I.V., Bao-Lin L.I.U., Min-Jian Q.I.N. Antihyperglycemic, antihyperlipidemic and antioxidant effects of standared ethanol extract of Bombax ceiba leaves in high fat diet and streptozotocin-induced type 2 diabetic rats. Chin. J. Nat. Med. 2017;15(3):168–177. doi: 10.1016/S1875-5364(17)30033-X. [DOI] [PubMed] [Google Scholar]
- 16.Polavarapu R., Spitz D.R., Sim J.E., Follansbee M.H., Oberley L.W., Rahemtulla A., Nanli A.A. Increased lipid peroxidation and impaired antioxidant enzyme function is associated with pathological liver injury in experimental alcoholic liver disease in rats fed diets high in corn oil and fish oil. Hepatology. 1998;27(5):1317–1323. doi: 10.1002/hep.510270518. [DOI] [PubMed] [Google Scholar]
- 17.Mnafgui K., Derbali A., Sayadi S., Gharsallah N., Elfeki A., Allouche N. Anti-obesity and cardioprotective effects of Cinnamic acid in high fat diet –induced obese rats. J. Food Sci. Technol. 2015;52(7):4369–4377. doi: 10.1007/s13197-014-1488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reitman S., Frankel S. A colorimeteric method for the determination of serum glutamic oxaloacetic, glutamic pyruvic transaminases. J. Clin. Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 19.Belfield A., Goldberg D.M. Revised assay for serum phenyl phosphatase activity using 4-amino-antipyrine. Enzyme. 1971;12(5):561–573. doi: 10.1159/000459586. [DOI] [PubMed] [Google Scholar]
- 20.Beutler E., Duron O., Kefly B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963;61(5):882–888. [PubMed] [Google Scholar]
- 21.Habig W., Pabst M.J. Glutathione-S-transferase (human placenta) Methods Enzymol. 1974;249:7130–7139. [Google Scholar]
- 22.Kakkar P., Das B., Viswanathan P.N. A modified spectrophotometric assay of superoxide dismutase. Ind. J. Biochem. Biophys. 1984;21:130–132. [PubMed] [Google Scholar]
- 23.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 24.Ohkawa H., Ohishi W., Yagi K. Assay of lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 25.Rifai N., Bachorik P.S., Albers J.J. Lipids, lipoproteins and apolipoproteins. In: Burtis C.A., Ashwood E.R., editors. Tietz Text Book of Clin. Chemistry. 3rd ed. 1999. pp. 809–861. Philadelphia. [Google Scholar]
- 26.Deeg R., Ziegenhorn R. Kinetic enzymatic method for automated determination of total cholesterol in serum. Clin. Chem. 1983;29:1798–1802. [PubMed] [Google Scholar]
- 27.Afifi A. UCL, Louvain La Neuve; Belgium: 1986. Contribution to the Chemical Carcinogensis in Xenopus, ph. D.THesis; pp. 90–91. [Google Scholar]
- 28.Altamirano J., Bataller R. Alcoholic liver disease: pathogenesis and new targets for therapy. Nat. Rev. Gastroenterol. Hepatol. 2011;8:491–501. doi: 10.1038/nrgastro.2011.134. [DOI] [PubMed] [Google Scholar]
- 29.Sun Q., Zhang W., Zhong W., Sun X., Zhou Z. Pharmacological inhibition of NOX4 ameliorates alcohol-induced liver injury in mice through improving oxidative stress and mitochondrial function. Biochim. Biophys. Acta. 2017;1861(1):2912–2921. doi: 10.1016/j.bbagen.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie X., Xu X., Sun X., Yu Z. Protective effects of cilostazol on ethanol-induced damage in primary cultured hepatocytes. Cell Stress Chaperones. 2018;23:201–211. doi: 10.1007/s12192-017-0828-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao W., Li J., He X., Lv O., Cheng Y., Liu R. In vitro steatosis hepatic cell model to compare the lipid-lowering effects of pomegranate peel polyphenols with several other plant polyphenols as well as its related cholesterol efflux mechanisms. Toxicol. Rep. 2014;1:945–954. doi: 10.1016/j.toxrep.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kourouma A., Keita H., Duan P., Quan C., Bilivogui K.K., Qi S., Osamuyimen A., Yang K. Effects of 4-nonylphenol on oxidant/antioxidant balance system inducing hepatic steatosis in male rat. Toxicol. Rep. 2015;2:1423–1433. doi: 10.1016/j.toxrep.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Said A., Aboutabl E.A., Nofal S.M., Tokuda H., Raslan M. Phytoconstituents and bioctivity evaluation of Bombax ceiba L. Flowers. J. Tradit. Med. 2011;28(2):55–62. [Google Scholar]
- 34.Mir M.A., Mir B.A., Bisht A., Rao Z., Singh D. Antidiabetic properties and metal analysis of Bombax ceiba flower extracts. Global J. Addict. Rehabil. Med. 2017;1(3):1–7. [Google Scholar]
- 35.Gupta P., Goyal R., Chauhan Y., Sharma P.L. Possible modulation of FAS and PTP-1B signaling in ameliorative potential of Bombax Ceiba against high fat diet induced obesity. Complement. Altern. Med. 2013;13:281–289. doi: 10.1186/1472-6882-13-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pyun C.W., Han K.H., Hong G.E., lee C.H. Effect of curcumin on the increase in hepatic or brain phosphatidylcholine hydroperoxide levels in mice after consumption of excessive alcohol. Bio. Med. Res. Inter. 2013;2013:1–6. doi: 10.1155/2013/242671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ambadath V., Venu R.G., Madambath I. Comparative study of the efficacy of ascorbic acid, quercetin, and thiamine for reversing ethanol-induced toxicity. J. Med. Food. 2010;13(6):1485–1489. doi: 10.1089/jmf.2009.1387. [DOI] [PubMed] [Google Scholar]
- 38.Zeng T., Guo F.F., Zhang C.L., Zhao S., Dou D.D., Gao X.C., Xie K.Q. The anti-fatty liver effects of garlic oil on acute ethanolexposed mice. Chem. Biol. Interact. 2008;176:234–242. doi: 10.1016/j.cbi.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 39.Wanijari M.M., Gangoria R., Dey Y.N., Gaidhani S.N., Pandey Nk., Jadhav A.D. Hepatoprotective and antioxidant activity of Bombax ceiba flowers against carbon tetrachloride-induced hepatotoxicity in rats. Hepatoma Res. 2016;2:144–155. [Google Scholar]
- 40.Hamid A., Ibrahim F.W., Ming T.H., Nasrom M.N., Eusoff N., Husain K., Latif M.A., Zingiber Zerumbet L. (Smith) extract alleviates the ethanol-induced brain damage via its antioxidant activity. Complement. Altern. Med. 2018;18:101–110. doi: 10.1186/s12906-018-2161-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oyenihi O.R., Afolabi B.A., Oyenihi A.B., Ogunmokun O.J. Hepato- and neuro-protective effects of watermelon juice on acute ethanol-induced oxidative stress in rats. Toxicol. Rep. 2016;3:288–294. doi: 10.1016/j.toxrep.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhopale K.K., Kondraganti S., Fernando H., Boor P.J., Kaohalia B.S., Ansari G.A.S. Alcoholic steatosis in different strains of rat: a comparative study. J. Drug Alcohol Res. 2015:1–11. doi: 10.4303/jdar/235912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cioboata R., Gaman A., Trasca D., Ungureanu A., Oanadocea A., Tomescu P., Gherghina F., Arsene A.L., Badiu C., Tsatsakis A.M., Spandidos D.A., Drakoulis N., Calina D. Pharmacological management of non-alcoholic fatty liver disease: atorvastatin versus Pentoxifylline. Exp. Ther. Med. 2017;13:2375–2381. doi: 10.3892/etm.2017.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ojeda M., Delgado-Villa M., Llopis R., Murillo M., Carreras O. Lipid metabolism in ethanol-treated rat pups and adults: effects of folic acid. Alcohol. 2008;43:544–550. doi: 10.1093/alcalc/agn044. [DOI] [PubMed] [Google Scholar]
- 45.Jain S.A., Shetty T., Ray R., Janakiramaiah N. Erythrocyte lipids in alcohol dependence. Indian J. Med. Res. 1988;88:530–535. [PubMed] [Google Scholar]
- 46.Chen J., Zhuang D., Cai W., Xu I., Li F., Wu Y., Sugiyama K. Inhibitory effects of four plants flavonoids extract on fatty acid synthase. J. Environ. Sci. China (China) 2009;21:131–134. doi: 10.1016/S1001-0742(09)60056-5. [DOI] [PubMed] [Google Scholar]
- 47.Wang Z., Yao T., Song Z. Chronic alcohol consumption disrupted cholesterol homeostasis in rats: downregulation of low density lipoprotein receptor and enhancement of cholesterol biosynthesis pathway in the liver. Alcohol. Clin. Exp. Res. 2010;34(3):471–478. doi: 10.1111/j.1530-0277.2009.01111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]