Abstract
Purpose: This study aimed to investigate the efficacy and safety of combining cinobufotalin and chemotherapy for advanced gastric cancer (GC).
Patients and methods: Literature retrieval was performed in Cochrane Library, Web of Science, PubMed, Embase, China National Knowledge Infrastructure (CNKI), Chinese Biological Medicine Database (CBM), Wanfang database and Chinese Scientific Journal Database (VIP) before September 2018. The primary reported outcomes including therapeutic efficacy, quality of life (QoL), and adverse events were systematically evaluated.
Results: Data from 27 trials including 1,939 advanced GC patients were included. The results indicated that, compared with chemotherapy alone, the combination of chemotherapy and cinobufotalin significantly improved patients' overall response rate (odds ratio [OR] =1.88, 95% confidence interval [CI] =1.54–2.31, P<0.00001) and disease control rate (OR =2.05, 95% CI =1.63–2.58, P<0.00001). The QoL of patients also evidently improved after chemotherapy and cinobufotalin combined treatment, as indicated by increased QoL improved rate (OR =2.39, 95% CI =1.81–3.15, P<0.00001), Karnofsky Performance Score (OR =7.00, 95% CI =2.25–11.75, P=0.004) and pain relief rate (OR =7.00, 95% CI =2.25–11.75, P=0.004). Adverse events including nausea and vomiting, diarrhea, leukopenia, hand-foot syndrome, anemia, gastrointestinal side effects and peripheral neurotoxicity caused by chemotherapy were evidently alleviated (P<0.05) when cinobufotalin was administered to GC patients.
Conclusion: Evidence from the meta-analysis suggested that the combination of chemotherapy and cinobufotalin is more effective in treating GC than chemotherapy alone. It alleviates the adverse effects associated with chemotherapy and improves the QoL of GC patients.
Keywords: cinobufotalin, traditional Chinese medicine, chemotherapy, gastric cancer, meta-analysis
Introduction
Gastric cancer (GC) represents the second leading cause of death among all cancer types and caused 782,685 deaths worldwide in 2018.1 Currently, the incidence of GC has significantly increased, with about 1,033,701 new cases every year.1 China has a high risk for GC, and the new cases of GC in this region account for about 43% in the world.2 Despite the improvement of diagnostic and therapeutic methods in the past decades,3,4 the prognosis of GC is still poor (5-year survival rate <20%) since it is mostly diagnosed at advanced stage.3,4 Therefore, effective therapeutic approaches should be developed.
Traditional Chinese medicine has an extensive history and has been more widely used as an effective adjuvant drug for cancer treatment.5–10 Cinobufotalin is a cardiotonic steroid or bufotalin, which is extracted from the skin secretion of the giant toad.10–14 Many in vitro studies have shown that cinobufotalin has antitumor activity and enhanced chemotherapeutic effect.7,10,13,14 Cinobufotalin can inhibit the growth and metastasis of the tumor by inhibiting the expression of vascular endothelial growth factor and epidermal growth factor receptor.15 Additionally, it can also kill tumor cells by inducing non-apoptotic death possibly depending on cyclophilin-D involved pathway.12
Several studies have indicated that chemotherapy combined with cinobufotalin exhibits more prominent therapeutic effects than chemotherapy alone for advanced GC.16–42 Despite the intensive clinical studies using cinobufotalin and chemotherapy combined therapy in treating GC, its clinical efficacy and safety have not been systematically evaluated. In this study, we conducted a meta-analysis to investigate the treatment efficacy and safety of chemotherapy combined with cinobufotalin in comparison with chemotherapy alone for advanced GC to provide scientific reference for the design of future clinical trials.
Materials and methods
Search strategy and selection criteria
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and Cochrane Handbook. Original articles were searched across eight electronic databases, including Cochrane Library, Web of Science, PubMed, Embase, China National Knowledge Infrastructure (CNKI), Chinese Biological Medicine Database (CBM), Wanfang database and Chinese Scientific Journal Database (VIP) before September 2018, with key terms “huachansu” or “cinobufotalin,” “cinobufacini,” or ”cinobufagin” combined with “gastric carcinoma” or “gastric cancer.” No language limits were applied.
Selection Criteria: The inclusion criteria were as follows: (1) controlled trials concerning advanced GC patients, (2) literature comparing the clinical outcomes of chemotherapy plus cinobufotalin adjuvant therapy (experimental group) with chemotherapy treatments alone (control group) and (3) articles involving more than 40 GC patients. On the other hand, the exclusion criteria were as follows: (1) non-contrast articles, case studies and review papers and (2) patients with mixed malignancies.
Data extraction and quality assessment
Data were independently extracted by two investigators (Sun HL, and Bai MH) following the same inclusion criteria; disagreements were adjudicated by the third reviewer (Liu DL). The extracted characteristics were summarized as follows: (I) first author’s names, (II) years of publication, (III) study locations, (IV) tumor stages, (V) Karnofsky Performance Score (KPS), (VI) number of cases, (VII) patient ages, (VIII) study parameter types, (IX) therapeutic regimens, (X) enrollment period and (XI) dosage of cinobufotalin. The included trial’s quality was evaluated according to the Cochrane Handbook.43
Outcome definition
Clinical responses include treatment efficacy, quality of life (QoL) and adverse events. Treatment efficacy was assessed in terms of the overall survival rates (OS rates, defined as the length of time from the start of treatment to death from any cause), complete response (CR) rates, partial response (PR) rates, stable disease (SD) rates, progressive disease (PD) rates, overall response rates (ORRs, ORR=CR + PR) and disease control rates (DCRs, DCR=CR + PR + SD). Patients’ QoL was evaluated using QoL improved rate (QIR), KPS and pain relief rate (PRR). Adverse events including nausea and vomiting, diarrhea, leucopenia, thrombocytopenia, hepatotoxicity, nephrotoxicity, oral mucositis, alopecia, hand-foot syndrome, anemia, gastrointestinal adverse effects, peripheral neurotoxicity, neutropenia and myelosuppression were also assessed.
Statistical analysis
RevMan 5.3 (Nordic Cochran Centre, Copenhagen, Denmark) and Stata 13.0 (Stata Corp., College Station, TX, USA) software were the main statistical analysis tools in this study. P<0.05 was considered statistically significant. Analysis model was determined by heterogeneity among studies assessed using Cochran’s Q test, and publication bias was analyzed using Begg’s and Egger’s regression asymmetry tests and presented using funnel plots.44 I2<50% or P>0.1 indicated that the studies were homogenous. Treatment effects were mainly represented by odds ratio (OR) presented with a 95% confidence interval (CI). Pooled analysis with publication bias determined that trim and fill method would be applied to coordinate the estimates of unpublished studies, and the adjusted results were compared with the original pooled OR.45 Sensitivity analysis was performed to evaluate the impact of different therapeutic regimens, drug forms of cinobufotalin, sample sizes and research types on clinical efficacy.
Results
Search results
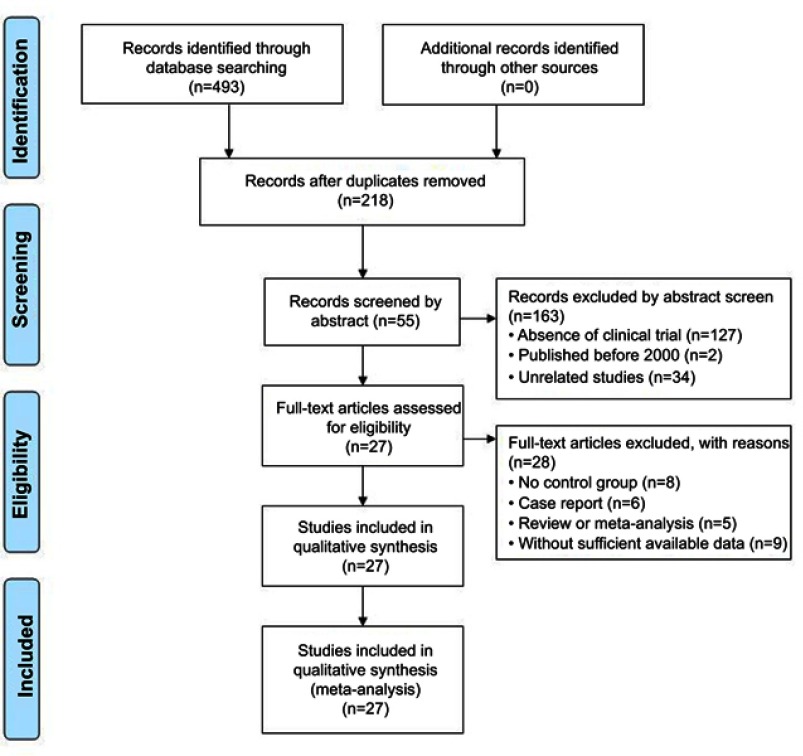
A total of 493 articles were identified and initially retrieved, and 275 papers were excluded due to duplication. After title and abstract review, 163 articles were further excluded because they did not include clinical trials (n=127) and were unrelated studies (n=34) or published before 2000 (n=2), leaving 55 studies as potentially relevant. After detailed assessment of full texts, articles without control group (n=8), studies with case reports (n=6), reviews or meta-analysis (n=5), and trials with insufficient data (n=9) were excluded. Finally, 27 trials16–42 involving 1,939 advanced GC patients were included in this analysis (Figure 1).
Figure 1.
Flow diagram of the selection process.
Patient characteristics
After selection, all included studies were performed in different medical centers of China since 2000. In total, 972 advanced GC patients were treated with chemotherapy in combination with cinobufotalin adjuvant therapy, while 967 patients were treated with chemotherapy alone. Detailed information of the involved studies and GC patients is shown in Tables 1 and 2.
Table 1.
Clinical information from the eligible trials in the meta-analysis
| Included studies | Nation | Tumor stage | KPS | Patients Con/Exp | Age (year) | Parameter types | |
|---|---|---|---|---|---|---|---|
| Con | Exp | ||||||
| Cha XT (2016)16 | China | ND | >70 | 20/20 | ND | ND | ORR, DCR, AE |
| Chen GF (2012)17 | China | IV | ND | 86/62 | 71.8±18.6 (mean) | 73.1±22.3 (mean) | ORR, DCR, QoL, AE |
| Chen HM(2009)18 | China | III–IV | KPS≥60 | 33/34 | 49.6 (median) | 50.6 (median) | ORR, DCR |
| Cui P (2009)19 | China | ND | 65 (mean) | 23/32 | ND | ND | ORR, DCR, QoL, AE |
| Guo CJ (2011)20 | China | IV | ≥50 | 43/43 | ND | ND | ORR, DCR, QoL |
| Guo XY (2013)21 | China | III–IV | ≥65 | 38/42 | 64.8±3.7 (mean) | 66.4±4.2 (mean) | ORR, DCR, QoL |
| Huang Q (2014)22 | China | ND | ≥60 | 20/26 | 55.8±4.9 (mean) | 57.4±5.6 (mean) | ORR, DCR, QoL |
| Li W (2016)23 | China | ND | ND | 74/76 | 66.8±1.4 (mean) | 66.6±1.5 (mean) | ORR, DCR, AE |
| Li YX (2012)24 | China | ND | ND | 74/74 | ND | ND | ORR, DCR, QoL, AE |
| Lu B (2016)25 | China | ND | >60 | 30/30 | 74.8±6.2 (mean) | 73.7±5.1 (mean) | ORR, DCR, QoL, AE |
| Lu CH (2014)26 | China | ND | 71 (mean) | 31/31 | ND | ND | ORR, DCR |
| Tian B (2012)27 | China | III–IV | KPS>60 | 22/23 | ND | ND | ORR, DCR, AE |
| Wang F (2014)28 | China | ND | ND | 58/58 | 58.8 (mean) | 58.4 (mean) | ORR, DCR, QoL, AE |
| Wang WM (2010)29 | China | ND | >60 | 23/20 | ND | ND | ORR, DCR, AE |
| Wang YH (2009)30 | China | III–IV | >60 | 32/36 | ND | ND | ORR, DCR, AE |
| Wang ZF (2012)31 | China | ND | >60 | 24/24 | 59.1 (median) | 58.7 (median) | ORR, DCR, QoL |
| Xiao XN (2018)32 | China | III–IV | 58 (mean) | 31/34 | ND | ND | ORR, DCR, QoL, AE |
| Xu DM (2015)33 | China | ND | >60 | 30/30 | 65.0±3.9 (mean) | 66.3±4.6 (mean) | ORR, DCR, QoL, AE |
| Xu YM (2016)34 | China | ND | ≥60 | 30/30 | 49.9 (median) | 45.8 (median) | ORR, DCR, QoL, AE |
| Yang B (2017)35 | China | ND | >60 | 34/34 | 53 (mean) | 51 (mean) | ORR, DCR |
| Yang F (2018)36 | China | ND | ND | 25/25 | 50 (median) | 54 (median) | ORR, DCR, QoL, AE |
| Zhang CW (2001)37 | China | III–IV | >70 | 32/35 | 66 (median) | 64 (median) | ORR, DCR, AE |
| Zhang RG (2004)38 | China | IV | ≥40 | 43/43 | 48 (median) | 49 (median) | OS, ORR, DCR, AE |
| Zhang Y (2005)39 | China | IV | ≥40 | 29/28 | 54 (median) | 57 (median) | OS, ORR, AE |
| Zheng YL (2007)40 | China | III–IV | 68 (mean) | 20/20 | ND | ND | OS, QoL |
| Zhu WK (2012)41 | China | III–IV | ≥70 | 32/32 | 62.8 (mean) | 61.7 (mean) | ORR, DCR, QoL, AE |
| Zou HP (2012)42 | China | III–IV | ND | 30/30 | 56.5 (median) | 59.1 (median) | ORR, DCR, AE |
Notes: Con, control group (chemotherapy alone group); Exp, experimental group (chemotherapy and cinobufotalin combined group).
Abbreviations: ND, non determined; KPS, karnofsky performance score; ORR, overall response rate; DCR, disease control rate; Qol, quality of life; AE, adverse events.
Table 2.
Information on cinobufotalin combined with chemotherapy
| Included studies | Therapeutic regimen | Enrollment Period | Dosage of cinobufotalin | |
|---|---|---|---|---|
| Experimental group | Control group | |||
| Cha XT (2016)16 | Oxaliplatin+Tegafur+CF/SF+Cinobufotalina | Oxaliplatin+Tegafur+CF/SF | January 2013–March 2016 | 750 mg/time, 3 times/day |
| Chen GF (2012)17 | Capecitabine+Cinobufotalinb | Capecitabine | October 2006–October 2010 | 10 ml/time, 3 times/day |
| Chen HM (2009)18 | Paclitaxel+Cisplatin+5-Fu+Cinobufotalinb | Paclitaxel+Cisplatin+5-Fu | October 2005–December 2007 | 30 ml/time, 1 time/day |
| Cui P (2009)19 | FOLFOX+Cinobufotalinb | FOLFOX | 2004–2006 | 30 ml/time, 1 time/day |
| Guo CJ (2011)20 | Docetaxel+Cinobufotalinb | Docetaxel | March 2005–March 2010 | 20 ml/time, 1 time/day |
| Guo XY (2013)21 | FOLFOX+Cinobufotalinc | FOLFOX | January 2009–May 2010 | 1200 mg/time, 4 times/day |
| Huang Q (2014)22 | XELOX+Cinobufotalinb | XELOX | August 2009–August 2013 | 50 ml/time, 1 time/day |
| Li W (2016)23 | Capecitabine+Cinobufotalinb | Capecitabine | January 2012–January 2015 | 10-20 ml/time, 1 time/day |
| Li YX (2012)24 | Capecitabine+Cinobufotalinb | Capecitabine | January 2006–July 2011 | 10 ml/time, 1 time/day |
| Lu B (2016)25 | Capecitabine+Cinobufotalina | Capecitabine | January 2010–December 2012 | 500 mg/time, 3 times/day, |
| Lu CH (2014)26 | FOLFOX+Cinobufotalinb | FOLFOX | 2009–2013 | 20 ml/time, 1 time/day |
| Tian B (2012)27 | FOLFOX+Cinobufotalinb | FOLFOX | 2004–2006 | 30 ml/time, 1 time/day |
| Wang F (2014)28 | Cisplatin+5-Fu+Cinobufotalina | Cisplatin+5-Fu | ND | 500 mg/time, 3 times/day, |
| Wang WM (2010)29 | S-1+Cinobufotalina | S-1 | October 2011–October 2013 | 500 mg, 3 times/day |
| Wang YH (2009)30 | FOLFOX+Cinobufotalinb | FOLFOX | December 2004–May 2008 | 10-20 ml/time, 1 time/day |
| Wang ZF (2012)31 | FOLFOX+Cinobufotalinb | FOLFOX | December 2003–May 2008 | 20 ml/time, 1 time/day |
| Xiao XN (2018)32 | FOLFOX+Cinobufotalinb | FOLFOX | January 2008–December 2010 | 10-20 ml/time, 1 time/day |
| Xu DM (2015)33 | Docetaxel+Cisplatin+Cinobufotalinb | Docetaxel+Cisplatin | 2013–2016 | 20 ml/time, 1 time/day |
| Xu YM (2016)34 | Capecitabine+Cinobufotalinb | Capecitabine | June 2010–June 2011 | 20 ml/time, 1 time/day |
| Yang B (2017)35 | FOLFOX+Cinobufotalina | FOLFOX | January 2014–April 2015 | 750 mg/time, 3 times/day, |
| Yang F (2018)36 | XELOX+Cinobufotalinb | XELOX | January 2015–June 2017 | 20 ml/time, 1 time/day |
| Zhang CW (2001)37 | EOF+Cinobufotalina | EOF | May 2014–May 2015 | 200-500 mg/time, 3 times/day |
| Zhang RG (2004)38 | Etoposide+Leucovorin+5-Fu+Cinobufotalinb | Etoposide+Leucovorin+5-Fu | March 1999–December 2000 | 20 ml/time, 1 time/day |
| Zhang Y (2005)39 | HCPT+CF+5-Fu+Cinobufotalinb | HCPT+CF+5-Fu | July 1998–June 2003 | 20 ml/time, 1 time/day |
| Zheng YL (2007)40 | FOLFOX+Cinobufotalinb | FOLFOX | March 2002–February 2003 | 50 ml/time, 1 time/day |
| Zhu WK (2012)41 | Oxaliplatin+Capecitabine+Cinobufotalinb | Oxaliplatin+Capecitabine | March 2010–MArch 2011 | 30 ml/time, 1 time/day |
| Zou HP (2012)42 | EOF+Cinobufotalinb | EOF | May 2008–May 2011 | 20 ml/time, 1 time/day |
Notes: Control group, chemotherapy alone group; Experimental group, chemotherapy and cinobufotalin combined group. a, cinobufotalin capsule; b, cinobufotalin injection; c, cinobufotalin tablet; S-1, Gimeracil and Oteracil Porassium Capsules.
Abbreviations: ND, non determined; CF, Calcium folinate; SF, Sodium folinate; Fu, Fluorouracil; HCPT, Hydroxycamptothecin; FOLFOX, Oxaliplatin+CF+5-Fu; XELOX, Oxaliplatin+Capecitabine; EOF, Epirubicin+Oxaliplatin+5-Fu.
Quality assessment
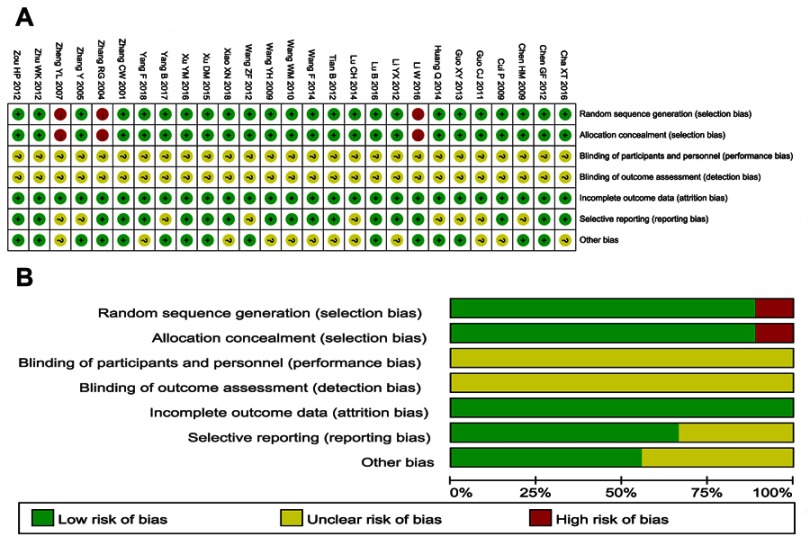
The assessment of bias risk is shown in Figure 2. A total of 24 studies were determined as having low risk, and the remaining 3 studies were not true randomized controlled trials. All included trials did not provide clear description of performance and detection risks. The attrition risks of involved trials were low; 9 trials were considered as having unclear risk owing to selective reporting.
Figure 2.
(A) Risk of bias summary: review of authors’ judgments about each risk of bias item for included studies. (B) Risk of bias graph: review of authors’ judgments about each risk of bias item presented as percentages across all included studies. Each color represents a different level of bias: red for high-risk, green for low-risk and yellow for unclear-risk of bias.
Therapeutic efficacy assessment
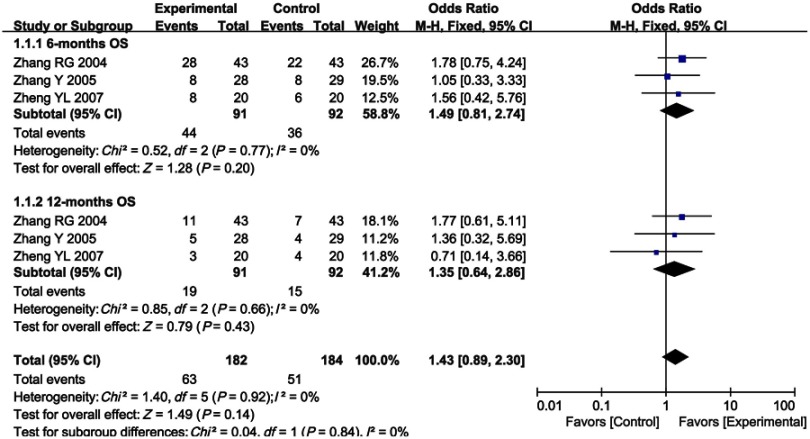
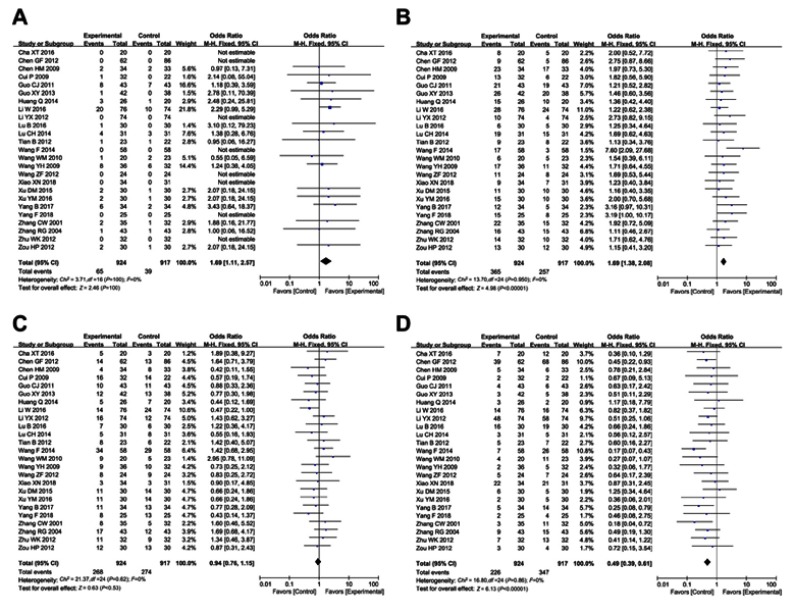
As shown in Figures 3 and 4, Figure S1 and Table 3, the pooled results showed that patients who underwent combined therapy had significantly improved CR, PR, ORR and DCR (CR, OR =1.69, 95% CI =1.11–2.57, P=0.01; PR, OR =1.69, 95% CI =1.38–2.08, P<0.00001; ORR, OR =1.88, 95% CI =1.54–2.31, P<0.00001; DCR, OR =2.05, 95% CI =1.63–2.58, P<0.00001) and significantly decreased PD (OR =0.49, 95% CI =0.39–0.61, P<0.00001), whereas SD and 6- and 12-months OS rates had no significant differences in patients who received chemotherapy alone (SD, OR =0.94, 95% CI =0.76–1.15, P=0.53; 6-months OS, OR =1.49, 95% CI =0.81–2.74, P=0.20; 12-months OS, OR =1.35, 95% CI =0.64–2.86, P=0.43). Fixed effect models were used to analyze OR rate because of low heterogeneity.
Figure 3.
Forest plot of the comparison of 6-months (A) and 12-months (B) overall survival (OS) between the experimental and control group. Control group, chemotherapy alone group; Experimental group, chemotherapy and cinobufotalin combined group. The fixed-effects meta-analysis model (Mantel–Haenszel method) was used.
Figure 4.

Forest plot of the comparison of overall response rate (ORR, A) and disease control rate (DCR, B) between the experimental and control group. Control group, chemotherapy alone group; Experimental group, chemotherapy and cinobufotalin combined group. The fixed-effects meta-analysis model (Mantel–Haenszel method) was used.
Figure S1.
Forest plot of the comparison of complete response rates (CR, A), partial response rates (PR, B), stable disease rates (SD, C) and progressive disease rates (PD, D) between the experimental and control group. Control group, chemotherapy alone group; Experimental group, chemotherapy and cinobufotalin combined group. The fixed-effects meta-analysis model (Mantel–Haenszel method) was used.
Table 3.
Comparison of CR, PR, SD, PD, ORR and DCR between the experimental and control group
| Parameter | Experimental group | Control group | Analysis method | Heterogeneity | Odds Ratio (OR) | 95% CI | P-value | |
|---|---|---|---|---|---|---|---|---|
| No. patients (n) | No. patients (n) | I2 (%) | P-value | |||||
| CR | 924 | 917 | Fixed | 0 | 1.00 | 1.69 | 1.11 to 2.57 | 0.01 |
| PR | 924 | 917 | Fixed | 0 | 0.95 | 1.69 | 1.38 to 2.08 | <0.00001 |
| SD | 924 | 917 | Fixed | 0 | 0.62 | 0.94 | 0.76 to 1.15 | 0.53 |
| PD | 924 | 917 | Fixed | 0 | 0.86 | 0.49 | 0.39 to 0.61 | <0.00001 |
| ORR | 952 | 946 | Fixed | 0 | 0.96 | 1.88 | 1.54 to 2.31 | <0.00001 |
| DCR | 924 | 917 | Fixed | 0 | 0.86 | 2.05 | 1.63 to 2.58 | <0.00001 |
Notes: Control group, chemotherapy alone group; Experimental group, chemotherapy and cinobufotalin combined group.
Abbreviations: CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, overall response rate; DCR, disease control rate.
Quality of life assessment
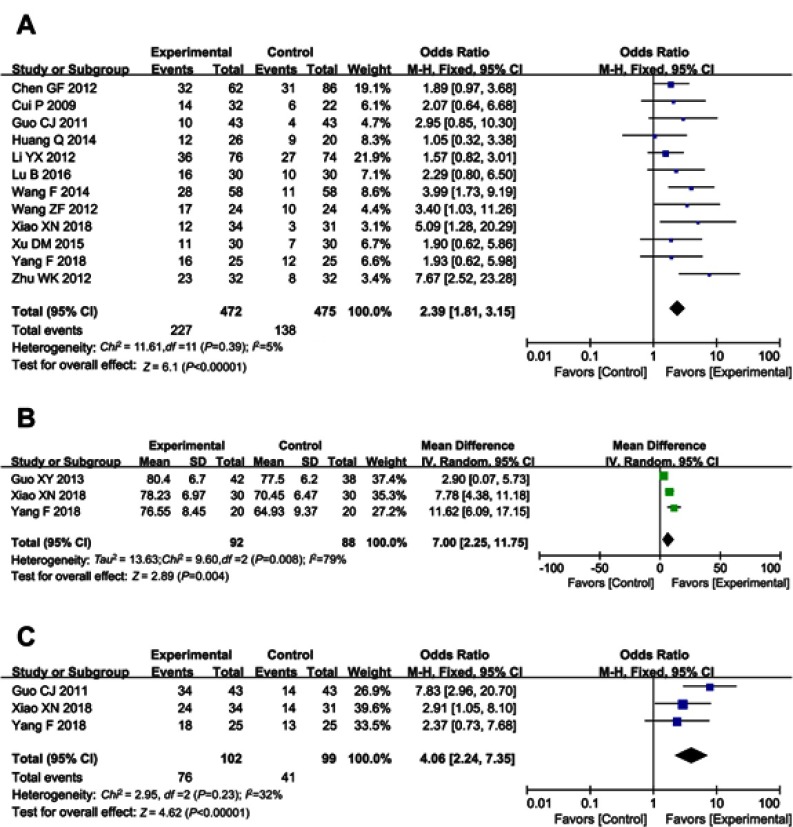
QoL was evaluated in this analysis. Result showed that QoL of patients in the combined group was significantly better than that of the control group, indicated by increased QIR, KPS and PRR (Figure 5, QIR, OR =2.39, 95% CI =1.81–3.15, P<0.00001; KPS, OR =7.00, 95% CI =2.25–11.75, P=0.004; PRR, OR =4.06, 95% CI =2.24–7.35, P<0.00001).
Figure 5.
Forest plot of the comparison of quality of life improved rate (QIR, A), karnofsky performance score (KPS, B) and pain relief rate (PRR, C) between the experimental and control group. Control group, chemotherapy alone group; Experimental group, chemotherapy and cinobufotalin combined group. The fixed-effects meta-analysis model (Mantel–Haenszel method) was used.
Adverse event assessment
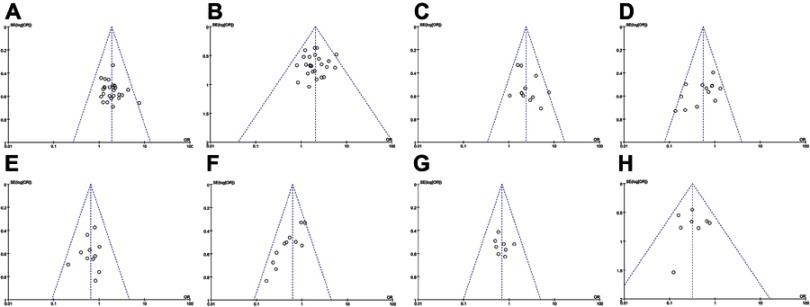
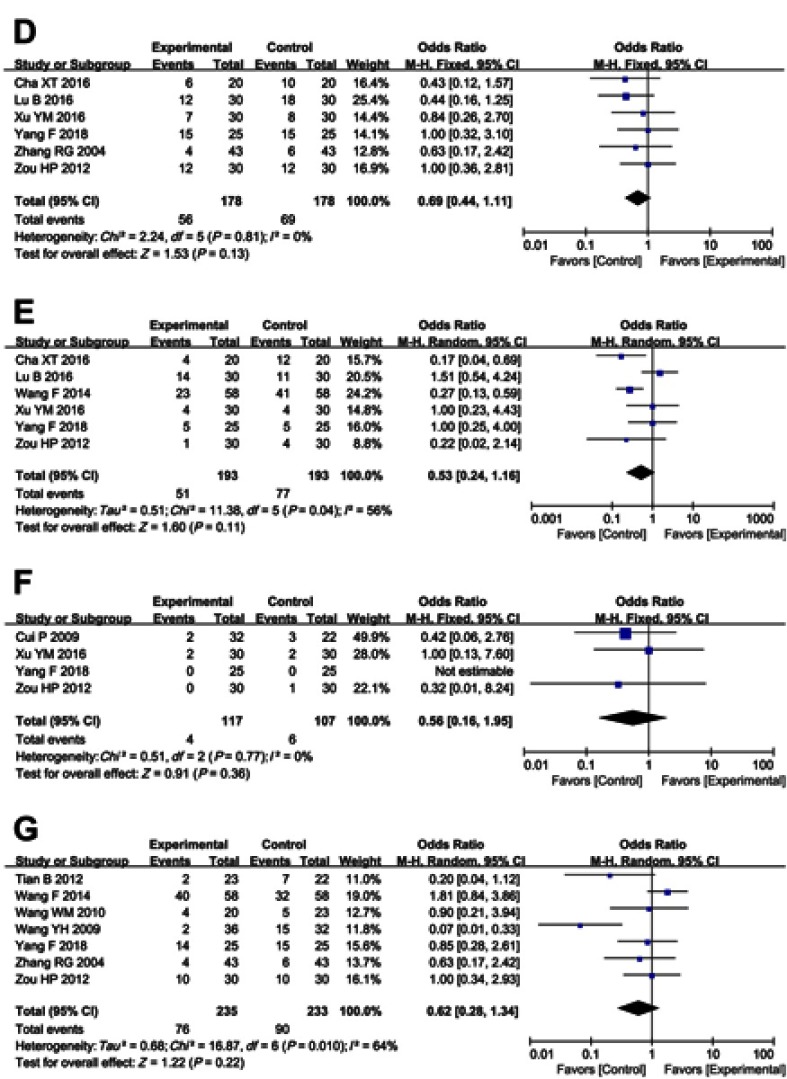
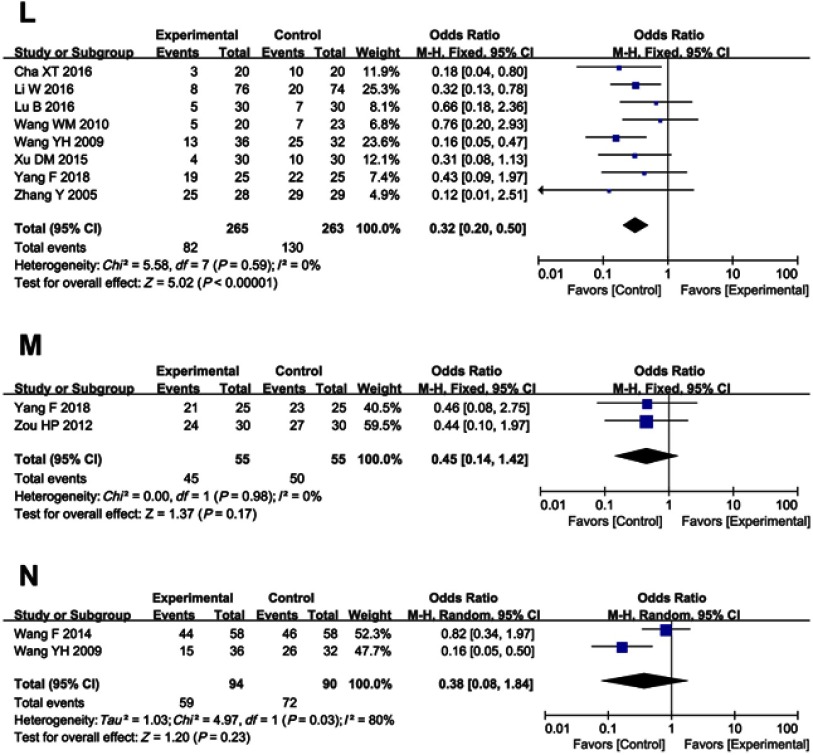
As shown in Table 4 and Figure S2, patients treated with cinobufotalin and chemotherapy combined therapy showed lower incidences of nausea and vomiting, diarrhea, leucopenia, hand-foot syndrome, anemia, gastrointestinal side effects and peripheral neurotoxicity (nausea and vomiting, OR =0.55, 95% CI =0.41–0.74, P<0.0001; diarrhea, OR =0.65, 95% CI =0.46–0.90, P=0.010; leucopenia, OR =0.62, 95% CI =0.47–0.82, P=0.0008; hand-foot syndrome, OR =0.57, 95% CI =0.41–0.79, P=0.0007; anemia, OR =0.69, 95% CI =0.48–0.99, P=0.05; gastrointestinal side effects, OR =0.56, 95% CI =0.32–1.00, P=0.05; peripheral neurotoxicity, OR =0.32, 95% CI =0.20–0.50, P<0.00001), whereas analysis on thrombocytopenia, hepatotoxicity, nephrotoxicity, oral mucositis, alopecia, neutropenia and myelosuppression (thrombocytopenia, OR =0.69, 95% CI =0.44–1.11, P=0.13; hepatotoxicity, OR =0.53, 95% CI =0.24–1.16, P=0.11; nephrotoxicity, OR =0.56, 95% CI =0.16–1.95, P=0.36; oral mucositis, OR =0.62, 95% CI =0.28–1.34, P=0.22; alopecia, OR =0.61, 95% CI =0.24–1.56, P=0.30; neutropenia, OR =0.45, 95% CI =0.14 −1.42, P=0.17; myelosuppression, OR =0.38, 95% CI =0.08–1.84, P=0.23) did not differ significantly between the two groups.
Table 4.
Comparison of adverse events between the experimental and control group
| Adverse events | Experimental group | Control group | Analysis method | Heterogeneity | Odds Ratio (OR) | 95% CI | P-value | |
|---|---|---|---|---|---|---|---|---|
| No. of patients (n) | No. of patients (n) | I2 (%) | P-value | |||||
| Nausea, vomiting | 452 | 437 | Fixed | 37 | 0.09 | 0.55 | 0.41 to 0.74 | <0.0001 |
| Nausea, vomiting I+II | 292 | 279 | Fixed | 0 | 0.50 | 0.83 | 0.59 to 1.16 | 0.27 |
| Nausea, vomiting III+IV | 292 | 279 | Fixed | 4 | 0.41 | 0.41 | 0.23 to 0.75 | 0.003 |
| Diarrhea | 395 | 379 | Fixed | 0 | 0.88 | 0.65 | 0.46 to 0.90 | 0.010 |
| Diarrhea I+II | 235 | 221 | Fixed | 0 | 0.69 | 0.84 | 0.56 to 1.27 | 0.41 |
| Diarrhea III+IV | 235 | 221 | Fixed | 0 | 1.00 | 0.27 | 0.10 to 0.75 | 0.01 |
| Leucopenia | 420 | 429 | Fixed | 34 | 0.13 | 0.62 | 0.47 to 0.82 | 0.0008 |
| Leucopenia I+II | 250 | 238 | Fixed | 0 | 0.86 | 0.57 | 0.39 to 0.83 | 0.003 |
| Leucopenia III+IV | 250 | 238 | Fixed | 0 | 0.77 | 0.36 | 0.17 to 0.75 | 0.007 |
| Thrombocytopenia | 178 | 178 | Fixed | 0 | 0.81 | 0.69 | 0.44 to 1.11 | 0.13 |
| Thrombocytopenia I+II | 178 | 178 | Fixed | 0 | 0.84 | 0.70 | 0.43 to 1.13 | 0.14 |
| Thrombocytopenia III+IV | 178 | 178 | Fixed | 0 | 0.83 | 0.91 | 0.39 to 2.14 | 0.83 |
| Hepatotoxicity | 193 | 193 | Random | 56 | 0.04 | 0.53 | 0.24 to 1.16 | 0.11 |
| Hepatotoxicity I+II | 193 | 193 | Fixed | 26 | 0.24 | 0.61 | 0.38 to 0.97 | 0.04 |
| Hepatotoxicity III+IV | 193 | 193 | Fixed | 0 | 0.70 | 0.14 | 0.02 to 0.81 | 0.03 |
| Nephrotoxicity | 117 | 107 | Fixed | 0 | 0.77 | 0.56 | 0.16 to 1.95 | 0.36 |
| Nephrotoxicity I+II | 117 | 107 | Fixed | 0 | 0.54 | 0.63 | 0.16 to 2.46 | 0.51 |
| Nephrotoxicity III+IV | 117 | 107 | Fixed | 0.32 | 0.01 to 8.24 | 0.49 | ||
| Oral mucositis | 235 | 233 | Random | 64 | 0.010 | 0.62 | 0.28 to 1.34 | 0.22 |
| Oral mucositis I+II | 179 | 178 | Fixed | 44 | 0.13 | 1.08 | 0.68 to 1.72 | 0.74 |
| Oral mucositis III+IV | 179 | 178 | Fixed | 0 | 0.58 | 0.54 | 0.15 to 1.96 | 0.35 |
| Alopecia | 133 | 130 | Fixed | 0 | 0.58 | 0.61 | 0.24 to 1.56 | 0.30 |
| Alopecia I+II | 133 | 130 | Fixed | 0 | 0.93 | 0.93 | 0.48 to 1.81 | 0.83 |
| Alopecia III+IV | 133 | 130 | Fixed | 0 | 0.97 | 0.72 | 0.30 to 1.75 | 0.47 |
| Hand foot syndrome | 334 | 356 | Fixed | 0 | 0.52 | 0.57 | 0.41 to 0.79 | 0.0007 |
| Hand foot syndrome I+II | 92 | 92 | Fixed | 12 | 0.32 | 0.64 | 0.33 to 1.24 | 0.18 |
| Hand foot syndrome III+IV | 92 | 92 | Fixed | 0.48 | 0.04 to 5.63 | 0.56 | ||
| Anemia | 292 | 291 | Fixed | 0 | 0.91 | 0.69 | 0.48 to 0.99 | 0.05 |
| Anemia I+II | 186 | 187 | Fixed | 0 | 0.65 | 0.92 | 0.60 to 1.42 | 0.71 |
| Anemia III+IV | 186 | 187 | Fixed | 0 | 0.87 | 0.34 | 0.12 to 0.96 | 0.04 |
| Gastrointestinal adverse effects | 277 | 295 | Random | 57 | 0.04 | 0.56 | 0.32 to 1.00 | 0.05 |
| Gastrointestinal adverse effects I+II | 71 | 72 | Fixed | 0 | 0.75 | 0.71 | 0.35 to 1.42 | 0.33 |
| Gastrointestinal adverse effects III+IV | 71 | 72 | Fixed | 0.39 | 0.09 to 1.60 | 0.19 | ||
| Peripheral neurotoxicity | 265 | 263 | Fixed | 0 | 0.59 | 0.32 | 0.20 to 0.50 | <0.00001 |
| Peripheral neurotoxicity I+II | 103 | 104 | Fixed | 29 | 0.24 | 0.52 | 0.26 to 1.03 | 0.06 |
| Peripheral neurotoxicity III+IV | 103 | 104 | Fixed | 0 | 0.53 | 0.58 | 0.21 to 1.63 | 0.30 |
| Neutropenia | 55 | 55 | Fixed | 0 | 0.98 | 0.45 | 0.14 to 1.42 | 0.17 |
| Neutropenia I+II | 55 | 55 | Fixed | 0 | 0.35 | 0.93 | 0.44 to 1.96 | 0.85 |
| Neutropenia III+IV | 55 | 55 | Fixed | 0 | 0.40 | 0.73 | 0.34 to 1.59 | 0.43 |
| Myelosuppression | 94 | 90 | Random | 80 | 0.03 | 0.38 | 0.08 to 1.84 | 0.23 |
| Myelosuppression I+II | 58 | 58 | Fixed | 1.09 | 0.48 to 2.49 | 0.83 | ||
| Myelosuppression III+IV | 58 | 58 | Fixed | 0.24 | 0.03 to 2.19 | 0.20 | ||
Notes: Control group, chemotherapy alone group; Experimental group, chemotherapy and cinobufotalin combined group.
Figure S2.

Forest plot of the comparison of adverse effects including nausea and vomiting (A), diarrhea (B), leukopenia (C), thrombocytopenia (D), hepatotoxicity (E), nephrotoxicity (F), oral mucositis (G), alopecia (H), hand-foot syndrome (I), anemia (J), gastrointestinal adverse effects (K), peripheral neurotoxicity (L), neutropenia (M) and myelosuppression (N) between the experimental and control group. Control group, chemotherapy alone group; Experimental group, chemotherapy and cinobufotalin combined group.
Publication bias
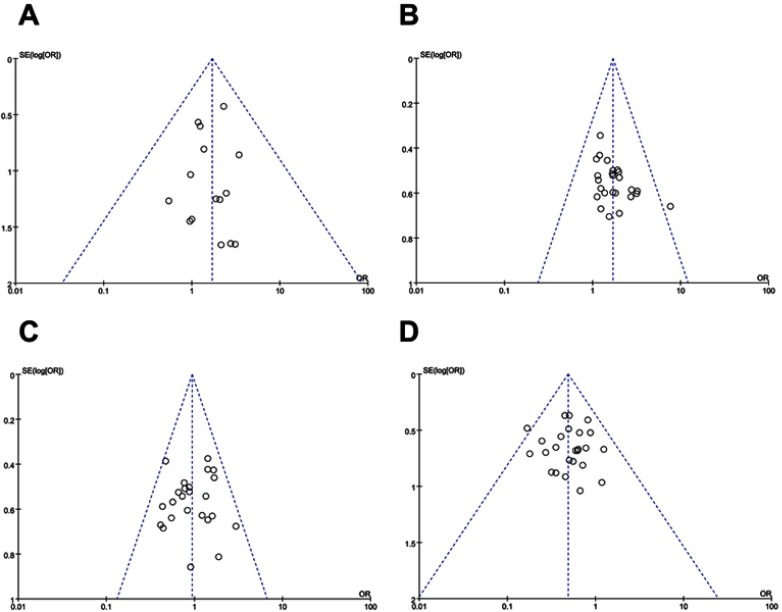
Funnel plots drawn for the studies on primary outcomes (CR, PR, SD, PD, ORR, DCR and adverse events) were approximately symmetrical, which indicated generally controlled publication bias and reliability of our primary conclusions (Figure 6 and S3).
Figure 6.
Funnel plot of overall response rate (ORR, A), disease control rate (DCR, B), quality of life improved rate (QIR, C), Nausea and vomiting (D), Diarrhea (E), Leukopenia (F), Anemia (G) and neurotoxicity (H).
Figure S3.
Funnel plot of percentage of complete response rates (CR, A), partial response rates (PR, B), stable disease rates (SD, C) and progressive disease rates (PD, D).
We also assessed publication bias using Begg’s and Egger’s regression asymmetry tests (Table 5), and PR and leucopenia were found with bias (PR, Begg, 0.038; Egger, 0.015; leucopenia, Begg, 0.003; Egger, <0.0001). To determine if the bias affects the pooled risk, we conducted a trim and fill analysis. The adjusted OR rate indicated the same trend with the result of the primary analysis (PR [before, P<0.0001; after, P<0.0001], leukopenia [before, P=0.0002; after. P=0.0002]), reflecting the reliability of our primary conclusions, except those based on a few number of trials.
Table 5.
Publication bias on therapeutic efficacy indexes (CR, PR, SD, PD, ORR, DCR and QIR) and adverse events indexes (Nausea and vomiting, Diarrhea, Leucopenia, Anemia and Neurotoxicity)
| Publication Bias | Therapeutic efficacy | Adverse events | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CR | PR | SD | PD | ORR | DCR | QIR | Nausea and vomiting | Diarrhea | Leucopenia | Anemia | Neurotoxicity | |
| Begg | 0.742 | 0.038 | 0.513 | 0.870 | 0.280 | 0.870 | 0.304 | 0.161 | 0.755 | 0.003 | 0.454 | 1.000 |
| Egger | 0.833 | 0.015 | 0.721 | 0.905 | 0.331 | 0.905 | 0.235 | 0.069 | 0.623 | <0.0001 | 0.528 | 0.894 |
Note: Parameters discussed in over 8 papers were conducted bias analyses.
Abbreviations: CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, overall response rate; DCR, disease control rate; QIR, quality of life improved rate.
Sensitivity analysis
We performed subgroup analysis to explore the source of heterogeneity in ORR and DCR with respect to therapeutic regimens, drug forms of cinobufotalin, sample sizes and research types. As shown in Table 6, our analysis results showed that no significant difference was found between different forms of cinobufotalin, sample sizes and research types. Moreover, cinobufotalin combined with FOLFOX/XELOX/capecitabine chemotherapy regimens was found to be more effective for GC treatment.
Table 6.
Subgroup analyses of ORR and DCR between the experimental and control group
| Parameter | Factors at study level | Experimental group No. of patients (n) | Control group No. of patients (n) | Analysis method | Heterogeneity | Odds Ratio (OR) | 95% CI | P-value | |
|---|---|---|---|---|---|---|---|---|---|
| I2 (%) | P-value | ||||||||
| ORR | Therapeutic regimen | ||||||||
| Cinobufotalin+FOLFOX | 215 | 200 | Fixed | 0 | 0.99 | 1.84 | 1.23 to 2.76 | 0.003 | |
| Cinobufotalin+XELOX | 92 | 86 | Fixed | 0 | 0.41 | 2.43 | 1.30 to 4.53 | 0.005 | |
| Cinobufotalin+EOF | 55 | 55 | Fixed | 22 | 0.26 | 1.93 | 0.91 to 4.10 | 0.09 | |
| Cinobufotalin+Capecitabine | 272 | 294 | Fixed | 0 | 0.85 | 1.98 | 1.29 to 3.04 | 0.002 | |
| Drug form of cinobufotalin | |||||||||
| Cinobufotalin capsule | 186 | 185 | Fixed | 9 | 0.36 | 2.47 | 1.54 to 3.98 | 0.0002 | |
| Cinobufotalin injection | 724 | 723 | Fixed | 0 | 0.98 | 1.78 | 1.41 to 2.25 | <0.00001 | |
| Study sample size | |||||||||
| >60 | 634 | 641 | Fixed | 0 | 0.67 | 2.05 | 1.58 to 2.65 | <0.00001 | |
| ≤60 | 318 | 305 | Fixed | 0 | 0.99 | 1.64 | 1.18 to 2.28 | 0.003 | |
| Type of control trials | |||||||||
| RCT | 833 | 829 | Fixed | 0 | 0.96 | 1.93 | 1.55 to 2.41 | <0.00001 | |
| Overall | 952 | 946 | Fixed | 0 | 0.96 | 1.88 | 1.54 to 2.31 | <0.00001 | |
| DCR | Therapeutic regimen | ||||||||
| Cinobufotalin+FOLFOX | 215 | 200 | Fixed | 0 | 0.97 | 2.26 | 1.26 to 4.04 | 0.006 | |
| Cinobufotalin+XELOX | 92 | 86 | Fixed | 0 | 0.39 | 2.55 | 1.24 to 5.23 | 0.01 | |
| Cinobufotalin+EOF | 55 | 55 | Fixed | 0 | 0.71 | 1.70 | 0.52 to 5.57 | 0.38 | |
| Cinobufotalin+Capecitabine | 272 | 294 | Fixed | 0 | 0.63 | 1.63 | 1.11 to 2.38 | 0.01 | |
| Drug form of cinobufotalin | |||||||||
| Cinobufotalin capsule | 186 | 185 | Fixed | 0 | 0.49 | 2.78 | 1.69 to 4.58 | <0.0001 | |
| Cinobufotalin injection | 696 | 694 | Fixed | 0 | 0.87 | 1.88 | 1.45 to 2.45 | <0.00001 | |
| Study sample size | |||||||||
| >60 | 634 | 641 | Fixed | 0 | 0.53 | 2.21 | 1.68 to 2.90 | <0.00001 | |
| ≤60 | 290 | 276 | Fixed | 0 | 0.94 | 1.73 | 1.13 to 2.64 | 0.01 | |
| Type of control trials | |||||||||
| RCT | 805 | 800 | Fixed | 0 | 0.86 | 2.16 | 1.69 to 2.77 | <0.00001 | |
| Overall | 924 | 917 | Fixed | 0 | 0.86 | 2.05 | 1.63 to 2.58 | <0.00001 | |
Notes: Control group, chemotherapy alone group; Experimental group, chemotherapy and cinobufotalin combined group.
Abbreviations: ORR, overall response rate; DCR, disease control rate; FOLFOX, Oxaliplatin+Calcium folinate+5-Fluorouracil; XELOX, oxaliplatin+capecitabine; EOF, epirubicin+oxaliplatin+calcium folate+fluorouracil; RCT, randomized controlled trial.
Discussion
In view of the limitations of the current chemotherapy for malignancies such as drug resistance and toxic side effects, clinicians have been exploring complementary and alternative medicine treatments to improve patients’ survival time or QoL and reduce side effects caused by chemotherapy.6,10,46,47 Traditional Chinese medicine, particularly cinobufotalin, has been clinically applied as an adjuvant therapy for decades.7,10,11 Several studies have been reported that the addition of cinobufotalin could be beneficial to advanced GC patients.16–42 Even though there was a statistical analysis of published clinical trials, the exact therapeutic effects were still not systematically evaluated because of small sample sizes and different applied protocols in different studies. Therefore, in this analysis, we conducted a wide range of online search according to strict inclusion and exclusion criteria to provide clear and systematical conclusion.
Our meta-analysis revealed that cinobufotalin and chemotherapy combined therapy for GC patients achieved more beneficial effects in comparison with those treated with chemotherapy alone. Combined therapy-treated patients broadly exhibited increased ORR and DCR (P<0.05) and also significantly improved their QoL. These results indicated that using cinobufotalin could improve the curative effects of chemotherapy.
Safety is the top priority of the clinical treatment. One trial7 that was conducted at Fudan University Cancer Hospital showed that cinobufotalin is well tolerated by hepatocellular carcinoma, non-small-cell lung cancer and pancreatic cancer patients (only mild adverse events were observed in cancer patients who received cinobufotalin therapy; no grade III and IV toxicities were observed). Our analysis showed that most of the adverse events caused by chemotherapy, including nausea and vomiting, diarrhea, leucopenia, hand-foot syndrome, anemia, gastrointestinal side effects and peripheral neurotoxicity, were alleviated with cinobufotalin combination therapy (P<0.05). Therefore, cinobufotalin is a safe auxiliary antitumor medicine for GC and can effectively alleviate the adverse events associated with chemotherapy.
The analysis on therapeutic effects may be influenced by several factors. In our study, no difference was found between different drug forms of cinobufotalin, sample sizes and research types. Cinobufotalin combined with FOLFOX/XELOX/capecitabine chemotherapy regimens was more effective for GC treatment (Table 6). However, a comparative analysis of the above-mentioned individual chemotherapy regimens should be performed in the future to rule out the possibility that the therapeutic advantage of cinobufotalin combined with FOLFOX, XELOX or capecitabine is due to the better therapeutic effect of them alone compared to that of EOF. As a summary, recent studies on the impact of these factors on the curative effects of cinobufotalin adjuvant therapy remain insufficient, and hence, further investigations should be performed.
There are some limitations in our analysis. First, although traditional Chinese medicine has been exported to 185 countries and regions, its main markets still remained in Asia.48 As a traditional medicine, cinobufotalin was mainly applied in China, which may bring the unavoidable regional bias and subsequently influence the clinical application of cinobufotalin worldwide. Second, according to the Cochrane Handbook for systematic reviews of interventions, the most appropriate way of summarizing survival outcomes is to use methods of survival analysis and express the intervention effect as a hazard ratio (HR) because this method takes into consideration the time factor and censored participants. However, the included articles that reported the OS rate only provided the survival number and the total number of patients at 6 months and 12 months, and none of them provided HR with 95% CI and Kaplan–Meier survival curves. Therefore, there were insufficient data to perform a statistical analysis using HR, which almost certainly will introduce bias. Third, treatment/medical history is very important in evaluating the efficacy of cinobufotalin-mediated therapy. However, our data were partly extracted from published papers rather than from the original patient records; therefore, analytical bias would possibly exist. Moreover, the therapeutic effects of the combined therapy may be influenced by numerous variables such as dosage of cinobufotalin, tumor stage and patient’s age. However, based on currently available literature, there are insufficient data to perform more statistical analysis to evaluate the correlation. We will keep following up with upcoming clinical trials to obtain relevant data when available. Finally, the follow-up durations of the included studies were short, and the long-term efficacy of cinobufotalin for advanced GC remains to be further evaluated.
Conclusion
In summary, this meta-analysis indicated that cinobufotalin and chemotherapy combined therapy was effective in treating advanced GC. Clinical application of cinobufotalin not only evidently improved the therapeutic effects of chemotherapy but also effectively alleviated most of the side effects caused by chemotherapy. However, the long-term efficacy of cinobufotalin-mediated adjuvant therapy for advanced GC still needs methodologically rigorous trials to verify its efficacy.
Acknowledgments
The risk bias assessment in this study was helped and guided by Dr. Ma J (Statistician, Department of Science and Education, Liaocheng People’s Hospital). No funding was received for conducting out this study.
Author contributions
All authors contributed to study design, data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Supplementary materials
Figure S2.
(Continued).
Figure S2.

(Continued).
Figure S2.
(Continued).
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 3.Cheng H, Sun A, Guo Q, Zhang Y. Efficacy and safety of apatinib combined with chemotherapy for the treatment of advanced gastric cancer in the Chinese population: a systematic review and meta-analysis. Drug Des Devel Ther. 2018;12:2173–2183. doi: 10.2147/DDDT.S170678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mu Y, Zhou CH, Chen SF, et al. Effectiveness and safety of chemotherapy combined with cytokine-induced killer cell/dendritic cell-cytokine-induced killer cell therapy for treatment of gastric cancer in China: a systematic review and meta-analysis. Cytotherapy. 2016;18(9):1162–1177. doi: 10.1016/j.jcyt.2016.05.015 [DOI] [PubMed] [Google Scholar]
- 5.Xue JX, Zhu ZY, Bian WH, Yao C. The traditional Chinese medicine Kangai injection as an adjuvant method in combination with chemotherapy for the treatment of breast cancer in Chinese patients: a meta-analysis. Evid Based Complement Alternat Med. 2018;2018:6305645. doi: 10.1155/2018/9567061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Zhang G, Chen X, et al. Jianpi Bushen, a traditional Chinese medicine therapy, combined with chemotherapy for gastric cancer treatment: a meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2018;2018:4924279. doi: 10.1155/2018/9567061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meng Z, Yang P, Shen Y, et al. Pilot study of huachansu in patients with hepatocellular carcinoma, nonsmall-cell lung cancer, or pancreatic cancer. Cancer. 2009;115(22):5309–5318. doi: 10.1002/cncr.24602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen YZ, Feng XB, Li ZD, Zheng WX, Sun H, Li PP. Clinical study on long-term overall survival of advanced non-small-cell lung cancer patients treated with Chinese medicine and Western medicine. Chin J Integr Med. 2014;20(3):179–183. doi: 10.1007/s11655-014-1770-6 [DOI] [PubMed] [Google Scholar]
- 9.Jiang Y, Liu LS, Shen LP, et al. Traditional Chinese medicine treatment as maintenance therapy in advanced non-small-cell lung cancer: a randomized controlled trial. Complement Ther Med. 2016;24:55–62. doi: 10.1016/j.ctim.2015.12.006 [DOI] [PubMed] [Google Scholar]
- 10.Shi Z, Song T, Wan Y, et al. A systematic review and meta-analysis of traditional insect Chinese medicines combined chemotherapy for non-surgical hepatocellular carcinoma therapy. Sci Rep. 2017;7(1):4355. doi: 10.1038/s41598-017-04351-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kai S, Lu JH, Hui PP, Zhao H. Pre-clinical evaluation of cinobufotalin as a potential anti-lung cancer agent. Biochem Biophys Res Commun. 2014;452(3):768–774. doi: 10.1016/j.bbrc.2014.08.147 [DOI] [PubMed] [Google Scholar]
- 12.Emam H, Zhao QL, Furusawa Y, et al. Apoptotic cell death by the novel natural compound, cinobufotalin. Chem Biol Interact. 2012;199(3):154–160. doi: 10.1016/j.cbi.2012.07.005 [DOI] [PubMed] [Google Scholar]
- 13.Chen KK, Anderson RC, Henderson FG. Comparison of cardiac action of bufalin, cinobufotalin, and telocinobufagin with cinobufagin. Proc Soc Exp Biol Med. 1951;76(2):372–374. [DOI] [PubMed] [Google Scholar]
- 14.Cheng L, Chen YZ, Peng Y, et al. Ceramide production mediates cinobufotalin-induced growth inhibition and apoptosis in cultured hepatocellular carcinoma cells. Tumour Biol. 2015;36(8):5763–5771. doi: 10.1007/s13277-015-3245-1 [DOI] [PubMed] [Google Scholar]
- 15.Li QW, Sun T, Hu KW. Research progress on anti-tumor mechanism of cinobufagin. China J Tradit Chin Med Pharm. 2010;25(12):2075–2078. [Google Scholar]
- 16.Cha XT, Hang ZK. Efficacy of cinobufotalin capsule combined with oxaliplatin and tegafur in the treatment of advanced gastric cancer. Psychol Doctor. 2016;22(18):126–127. [Google Scholar]
- 17.Chen GF, Jin DX, Li MJ. Clinical observation of cinobufotalin combined with capecitabine in the treatment of 62 cases of senile advanced gastric cancer. Zhejiang J Tradit Chin Med. 2012;47(6):462–463. [Google Scholar]
- 18.Chen HM. Efficacy of cinobufotalin combined with TPF regimen in the treatment of advanced gastric cancer. J Emergency Tradit Chin Med. 2009;18(1):35–36. [Google Scholar]
- 19.Cui P. Clinical efficacy observation of cinobufotalin in the treatment of advanced gastric cancer. J Liaoning Med Univ. 2009;30(4):333–334. [Google Scholar]
- 20.Guo CJ, Yu TH, Zhang HP, Xing JH. The observation of clinical therapeutic effect of cinobufacini combined with docetaxel on advanced stomach cancer. Chin Med Guides. 2011;8(28):54–55. [Google Scholar]
- 21.Guo XY, Sun T, Wang XX, Wang Y. Curative efficacy of cinobufacini adjuvant FOLFOX6 regimen in treatment of non operative elderly patients with advanced gastric cancer. J Liaoning Univ Tradit Chin Med. 2013;15(12):190–192. [Google Scholar]
- 22.Huang Q, Dong J. Efficacy observation of cinobufotalin injection via hepatic artery perfusion combined with XELOX chemotherapy regimen in the treatment of gastric cancer with liver metastasis. Chin J Tradit Med Sci Technol. 2014;21(3):311–312. [Google Scholar]
- 23.Li W, Li HZ. Clinical efficacy analysis of cinobufotalin combined with capecitabine in the treatment of elderly patients with gastric cancer. Med J Chin People‘S Health. 2016;28(04):82–83. [Google Scholar]
- 24.Li YX. Clinical treatment comparison of 148 elderly patients with advanced gastric cancer. J Front Med. 2012;35:204–205. [Google Scholar]
- 25.Lu B, Wu J, Tong RM, Zhang JF. Clinical observation for the combination of capecitabine and cinobufacin capsule on treating elderly advanced gastric cancer. J Liaoning Univ Tradit Chin Med. 2016;18(9):84–87. [Google Scholar]
- 26.Lu CH, Hong M, Liu KH, You J. Efficacy observation of neoadjuvant chemotherapy with cinobufotalin in advanced gastric cancer. Tradit Chin Med Jl. 2014;13(3):41–49. [Google Scholar]
- 27.Tian B. The enteric capsule of cinobufotalin efficacy combined with chemotherapy in the treatment of gastric cancer. Med Aesthetics Cosmetology. 2012;20(11):33. [Google Scholar]
- 28.Wang F, Wu LG, Le XY, Chen XD. Clinical effect of gimeracil and oteracil porassium capsules combined with Huachan vegetarian capsules in the treatment of patients with gastric cancer. Chin J Clin Oncol Rehabil. 2014;21(12):1485–1488. [Google Scholar]
- 29.Wang WM, Li CF, Yao RJ. Clinical observation of cinobufotalin injection combined with chemotherapy in the treatment of advanced gastric cancer. Clin J Tradit Chin Med. 2010;22(4):314–315. [Google Scholar]
- 30.Wang YH. Cinobufotalin injection combined with chemotherapy in the treatment of 36 cases of advanced gastric cancer. Jiangxi J Traditional Chin Med. 2009;40(4):31–32. [Google Scholar]
- 31.Wang ZF, Wang P. Efficacy observation of cinobufotalin combined with chemotherapy in the treatment of advanced gastric cancer. Chin J Primary Med Pharm. 2012;19(13):1991–1992. [Google Scholar]
- 32.Xiao XN, Lin CH, Li Q, Lin ZJ, Xiao HB. Effect of chemotherapy of DC combined with cinobufacini in the treatment of advanced stomach cancer. Chin J Primary Med Pharm. 2018;25(3):322–324. [Google Scholar]
- 33.Xu DM, Liu LJ. Clinical observation of cinobufotalin combined with capecitabine for gastric cancer in elderly patients. Pract J Cancer. 2015;30(3):405–407. [Google Scholar]
- 34.Xu YM, Liu S. Efficacy observation of huachansu capsule combined with chemotherapy in treating advanced gastric cancer. World Chin Med. 2016;11(7):1212–1214. [Google Scholar]
- 35.Yang B. Efficacy observation of cinobufotalin injection combined with chemotherapy in the treatment of advanced gastric cancer. World Clin Med. 2017;11(23):92. [Google Scholar]
- 36.Yang F, Zhang T. Clinical study of cinobufotalin capsule combined with chemotherapy in the treatment of advanced gastric cancer. Chin Remedies Clin. 2018;18(2):266–268. [Google Scholar]
- 37.Zhang CW, Wang QH. Cinobufotalin combined with chemotherapy in the treatment of 35 cases of advanced gastric cancer. J Anhui Tradit Chin Med Coll. 2001;20(4):18–19. [Google Scholar]
- 38.Zhang RG, Cheng CH, Shen B, Zhou DM, Zhuang GX. Clinical observation of cinobufotalin combined with chemotherapy in the treatment of advanced gastric cancer. Chin Clin Oncol. 2004;9(3):269–270. [Google Scholar]
- 39.Zhang Y, Zhu M, Cao Y, Zhang P, Yao LG, Huang HX. Efficacy observation of cinobufotalin combined with chemotherapy in the treatment of intermediate and late stage gastric cancer. Henan J Oncol. 2005;18(5):359–360. [Google Scholar]
- 40.Zheng YL, Ma BH, Yang F. Observation of cinobufotalin combined with chemotherapy for intermediate and late stage gastric cancer. Qingdao Med J. 2007;39(4):260–261. [Google Scholar]
- 41.Zhu WK, Li Y, Hou FG, Chen M, Zhou YY. Efficacy of cinobufacini combined with CapeOX regimen in treatment of advanced gastric cancer. Chin Med Guides. 2012;9(5):35–36. [Google Scholar]
- 42.Zou HP, Guo XZ, Zhu YF. Clinical research on huachansu with EOF regimen in patients with advanced gastric cancer. Chin J Clin Med. 2012;19(2):140–141. [Google Scholar]
- 43.Zeng X, Zhang Y, Kwong JS, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8(1):2–10. doi: 10.1111/jebm.12141 [DOI] [PubMed] [Google Scholar]
- 44.Jackson D, White IR, Riley RD. Quantifying the impact of between-study heterogeneity in multivariate meta-analyses. Stat Med. 2012;31(29):3805–3820. doi: 10.1002/sim.5453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. [DOI] [PubMed] [Google Scholar]
- 46.Yan Z, Lai Z, Lin J. Anticancer properties of traditional Chinese medicine. Comb Chem High Throughput Screen. 2017;20(5):423–429. doi: 10.2174/1386207320666170116141818 [DOI] [PubMed] [Google Scholar]
- 47.Xu J, Song Z, Guo Q, Li J. Synergistic effect and molecular mechanisms of traditional Chinese medicine on regulating tumor microenvironment and cancer cells. Biomed Res Int. 2016;2016:1490738. doi: 10.1155/2016/1490738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin AX, Chan G, Hu Y, et al. Internationalization of traditional Chinese medicine: current international market, internationalization challenges and prospective suggestions. Chin Med. 2018;13:9. doi: 10.1186/s13020-018-0167-z [DOI] [PMC free article] [PubMed] [Google Scholar]