Abstract
Introduction: Since 1956 there have been numerous scientific articles about free radical theory of aging which both confirm and deny the theory. Due to oxygen metabolism, there are relatively high concentrations of molecular oxygen in human cells, especially in mitochondria. Under normal physiological conditions, a small fraction of oxygen is constantly converted to ROS, such as superoxide radical (O2−•), H2O2, and related metabolites.
Aim of the study: The aim of this work was to show the relation between the activity of main antioxidative enzymes and the age of the examined patients.
Materials and methods: The analysis of antioxidant defense was performed on the blood samples from 184 “aged“ individuals aged 65–90+ years, and compared to the blood samples of 37 individuals just about at the beginning of aging, aged 55–59 years.
Results: The statistically significant decreases of Zn,Cu-superoxide dismutase (SOD-1), catalase (CAT), and glutathione peroxidase (GSH-Px) activities were observed in elderly people in comparison with the control group. Moreover, an inverse correlation between the activities of SOD-1, CAT, and GSH-Px and the age of the examined persons was found. No age-related changes in glutathione reductase activities and malondialdehyde concentrations were observed.
Conclusion: Lower activities of fundamental antioxidant enzymes in the erythrocytes of elderly people, which indicate the impairment of antioxidant defense in the aging organism and the intensity of peroxidative lipid structures, were observed.
Keywords: aging, antioxidant enzymes, free radicals, oxidative stress
Introduction
One of the main global problems is the aging of society which is supposed to be a combined result of low fertility, low immigration, and prolonged lifespan.1,2 In order to tackle this issue it is necessary to learn more about aging at the biochemical and molecular levels, as well as about the biological, medical, and social consequences of this process. A 30-year gain in life expectancy has been recorded in highly developed countries during the 20th century.2–4 This tendency has also been noticeable in Poland since 1990. According to the projection of Polish Central Statistical Office, the life expectancy in Poland may increase from 70.0 years in 2007 to 77.1 years in 2035 for males and from 79.7–82.9 for females, respectively. In this period of time, the percentage of Polish population at post-working age (60+ years for women and 65+ years for men) may change from 16%–26.7%.
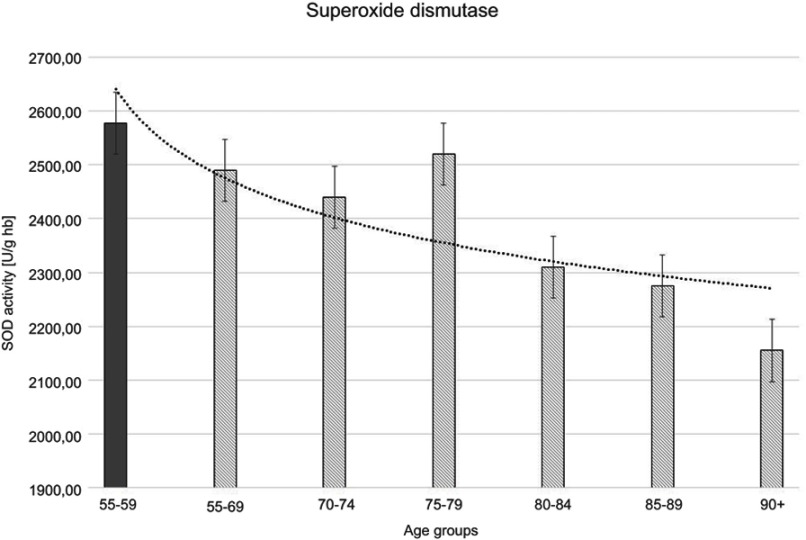
Figure 2.
Superoxide dismutase (SOD) activity with the trend line showing the tendency of change with age.
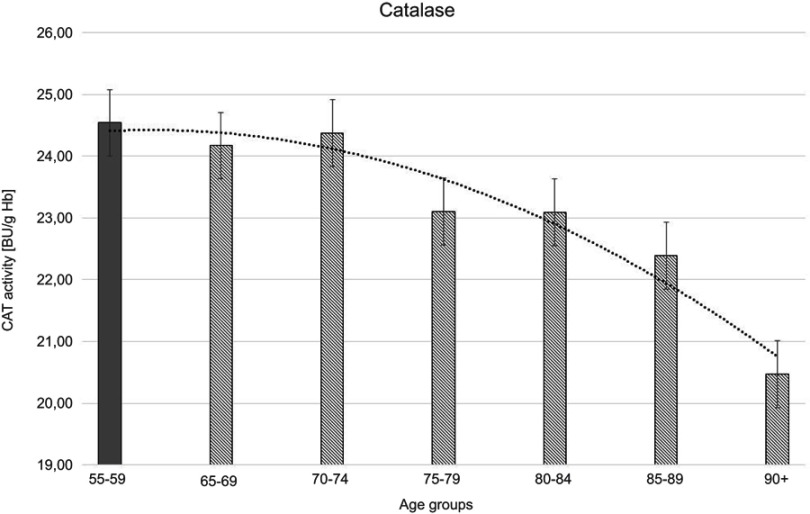
Figure 3.
Catalase (CAT) activity with the trend line showing the tendency of change with age.
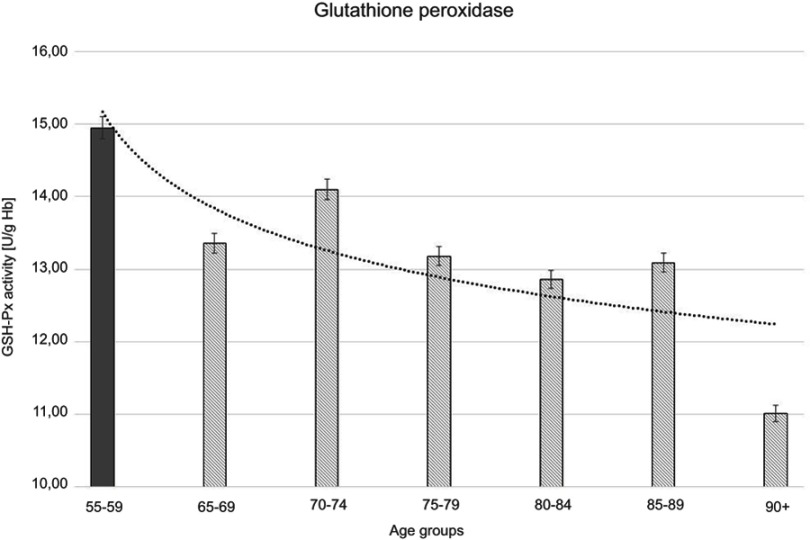
Figure 4.
Glutathione peroxidase (GSH-Px) activity with the trend line showing the tendency of change with age.
An integral part of aerobic metabolism is ROS generation which should be analyzed according to its two main functions. On the one hand, ROS plays an important role in biomodulating and regulating many cellular functions, such as defense against pathogens, signal transduction processes during transmission of intercellular information, and activation of specific transcription factors.5–9 On the other hand, an excessive quantity of ROS has a deleterious effect on cells, reacting with a variety of molecules and thereby interfering with cellular functions.10,11 To cope with the elevated generation of ROS, ROS-scavenging biochemical pathways have been developed in aerobic cells.12–16
One of the more serious objections against free radical theory of aging are results of research performed on mtDNA mutator mice. They have a mutation in exonucleasic catalytic domain of mitochondrial subunit of gamma polymerases.
A consequence of this mutation is an increased expression of proteins which do not have recovery properties. This leads to accumulation of mtDNA mutations.17 The examined mice have a series of symptoms which resemble the natural process of aging, such as kyphosis, anemia, alopecia, greying of the hair, loss of hearing, cardiomyopathy, decreased fertility, loss of weight, and shortened life expectancy. Mutator mice have lack of ROS production and oxidative damages, even though the respiratory chain remains functioning. This observation contradicts the concept in which ROS and mtDNA mutation fuel one another and results in gradual deterioration of mitochondrial function. In the examined mice, an increased level of ROS may cause extension of life expectancy, which is a contradiction of the free radical theory of aging. This leads to a conclusion that the hypothesis of this theory is incorrect.18–20 However, the results from numerous scientific results about the increase of oxidative stress with age suggest the unclear relation between the free radical theory and the aging process.
Due to the peculiar metabolism, the red blood cells are vulnerable to oxidative stress.21 The main source of free radicals in erythrocytes is the process of auto-oxidation of hemoglobin which results in methemoglobin. Furthermore, it may lead to cross-reactions between globin chains which precipitate as so-called Heinz bodies. What is observed is the protein breakdown, creation of large protein complexes, damage of glycoprotein at the surface of blood cells, and disturbance of transport through membrane and membrane potential.22,23 These processes lead to the loss of function and viability of red blood cells. In erythrocytes, there is an exhaustion of glutathione stocks which additionally causes the decrease of antioxidative enzymes' activity, increase peroxidation of cell membrane lipids and oxidative damages of hemoglobin. This may lead to hemolysis of erythrocytes and the release of heme iron and intensification of reaction of free radical among the endothelium.21,24–27
The oxidative stress theory is one of the most popular and controversial explanations of aging pathomechanisms. The free radical theory of aging, proposed in 1956 by Harman, assumed the endogenous generation of oxygen free radicals from normal oxygen-utilizing metabolic processes and their essential role in the aging processes.28 Later, Harman and other researchers modified this theory, in accordance with the findings in this area.29,30 According to this hypothesis, the loss of balance between pro-oxidants and antioxidants leads to accumulation of oxidative damage in macromolecules with age, which results in the disturbances in functional cellular processes and development of aging.31–33 In recent years there have been a lot of studies supporting the role of ROS in molecular aging mechanisms.34,35 The confirmation of oxidative stress increase with age of diverse organisms, and the generation of transgenic invertebrates overexpressing the antioxidant enzymes with increased lifespan were among the most important results of these studies.36–39 Nevertheless, there were no alterations in the lifespan in most of the examined mouse models, which under- or overexpressed a wide variety of genes coding for antioxidant enzymes.40–42 Thus, the role of oxidative stress in aging mammals is not fully understood and still demands further inquiries.43,44
Materials and methods
The study was carried out on 221 persons (100 males, 121 females), divided into seven age subgroups (55–59, 65–69, 70–74, 75–79, 80–84, 85–89, and 90+ years). The subgroup of 55–59 year-old persons was the control group. The participants were recruited according to a multi-stage procedure designed for the study performed in the Department and Clinic of Geriatrics in order to choose a representative sample of elderly people.
The selected anthropometric parameters such as growth, weight, and total cholesterol had a normal distribution. Regarding these parameters, the results of examined patients did not show statistically significant differences (Table 1).
Table 1.
Anthropometric parameters of the examined groups (mean ± SD)
| Parameter | 55–59 years | 65–69 years | 70–74 years | 75–79 years | 80–84 years | 85–89 years | 90+ years |
|---|---|---|---|---|---|---|---|
| Body mass index | 24.2±4.2 | 24.1±3,1 | 24.8±2.5 | 22.30±2.5 | 24.70±3.7 | 22.4±2.1 | 21.1±2.3 |
| Body mass (kg) | 72.2±7.5 | 73.1±4.5 | 75.4±6.5 | 74.7±4.5 | 69.8±5.5 | 71.2±3.5 | 69.2±7.5 |
| Body height (cm) | 170±4.3 | 172±3.3 | 173±4.3 | 169±5.3 | 168±2.3 | 169±5.3 | 167±6.3 |
| Total cholesterol (mg/dL) | 175±8 | 182±4.8 | 168±5.2 | 164±3.1 | 172±6.2 | 161+7.1 | 171±3.8 |
Blood samples were collected in the morning (08:00 am) after 12 hours of fasting from the cubital vein in heparinized tubes (3 mL) to obtain erythrocytes. All samples were centrifuged (2,500 g for 10 minutes). After plasma removal, the hemolysate was prepared by threefold freezing and thawing the washed erythrocytes, suspended in ultrapure water. The hemolysate was used to determine the parameters of oxidative stress and antioxidative defense. Malondialdehyde (MDA) concentration in erythrocytes, as well as erythrocytic activities of Cu-Zn superoxide dismutase (SOD-1; EC 1.15.1.1), catalase (CAT; EC 1.11.1.6), cellular glutathione peroxidase (GSH-Px; EC 1.11.1.9), and glutathione reductase (GR; EC 1.6.4.2)) were assayed by the methods of Placer et al, Misra and Fridovich, Beers and Sizer, Paglia and Valentine, and Flohe and Gunzler, respectively.45–49 MDA level was expressed as a concentration of thiobarbituric acid reactive substances, read at 532 nm. SOD-1 activity was determined at 37°C by recording the increase in absorbance at 480 nm following the auto-oxidation of adrenaline, inhibited by SOD-1. CAT activity was measured at 25°C by recording H2O2 decomposition at 240 nm. GSH-Px activity was determined at 25°C by recording the decrease in absorbance at 340 nm following the oxidation of NADPH in the presence of tert-butyl hydroperoxide as a substrate, GSH, yeast GR, and NaN3 as a CAT inhibitor. GR activity was determined at 37°C by recording the decrease in absorbance at 340 nm following the oxidation of NADPH in the presence of oxidized glutathione. The hemoglobin concentration in the hemolysate was estimated after conversion into cyanmethemoglobin form using a commercial reagent (Biomed, Lublin, Poland), read at 540 nm.
Statistical analysis
All results were expressed as mean ± SD. The one-way analysis of variance followed by the Tukey post hoc test was performed to determine the statistical significance of differences. The Pearson’s correlation coefficient was used to quantify the relationship between the measured parameters. The level of significance was set at P<0.05.
Results
All results of measured biochemical parameters of oxidative stress were shown in Table 2. Figure 1–5 present parameters in particular age groups together with the trend line showing the tendency of change with age.
Table 2.
Selected parameters of pro- and antioxidant balance in the blood of the examined groups (mean ± SD)
| Age groups (years) | Number of subjects (male/female) | Malondialdehyde [µmol/g Hb] | Zn,Cu-superoxide dismutase [U/g Hb] | Catalase [BU/g Hb] | Glutathione peroxidase [U/g Hb] | Glutathione reductase [U/g Hb] |
|---|---|---|---|---|---|---|
| 55–59 (control) | 37 (17/20) | 0.249±0.031 | 2578±170.8* | 24.54±1.76* | 14.95±3.17* | 58.55±7.13 |
| 65–69 | 28 (13/15) | 0.240±0.026 | 2490±220.0 | 24.17±2.11 | 13.36±2.29 | 59.89±12.76 |
| 70–74 | 29 (12/17) | 0.229±0.0350 | 2440 ±206.2 | 24.37±2.46 | 14.10±2.39 | 56.51±12.71 |
| 75–79 | 29 (14/15) | 0.235±0.0329 | 2520±203.3 | 23.10±1.86 | 13.18±2.57 | 57.87±10.69 |
| 80–84 | 32 (16/16) | 0.239±0.036 | 2310±192.7 | 23.09±2.12 | 12.86±2.56 | 54.75±15.89 |
| 85–89 | 32 (12/20) | 0.239±0.0327 | 2275±169.9 | 22.39±2.29 | 13.09±2.14 | 62.97±12.27 |
| 90+ | 34 (16/18) | 0.261±0.0346 | 2155±156.8 | 20.47±1.27 | 11.01±1.71 | 58.42±18.41 |
Note: *Statistically significant differences between control group (55–59 years) and elderly persons, P<0.001.
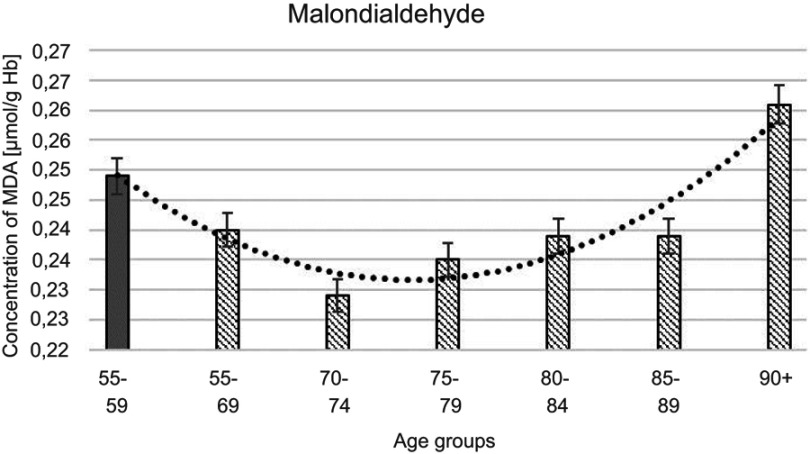
Figure 1.
Maloddialdehyde (MDA) concentration with the trend line showing the tendency of change with age.
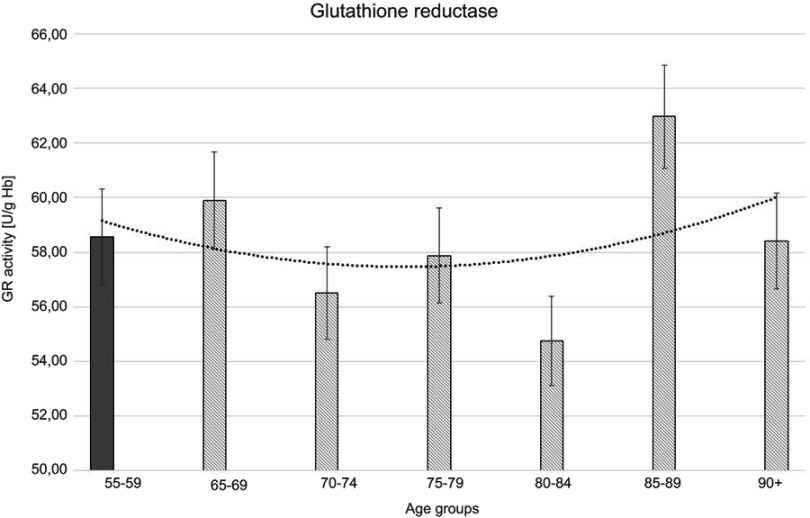
Figure 5.
Glutathione reductase (GR) activity with the trend line showing the tendency of change with age.
The progressive decrease of antioxidant enzymatic defense in the erythrocytes during aging was observed in this study. The activities of SOD-1 were lowered by 17% in elderly subjects over 90 years of age in comparison with the persons aged 55–59 years, whereas the activities of CAT and GSH-Px were reduced by 20% and 27%, respectively. Only GR activities remained unchanged during aging in this study.
Moreover, the strong negative correlations between age and SOD-1, CAT, and GSH-Px activities in the erythrocytes were observed.
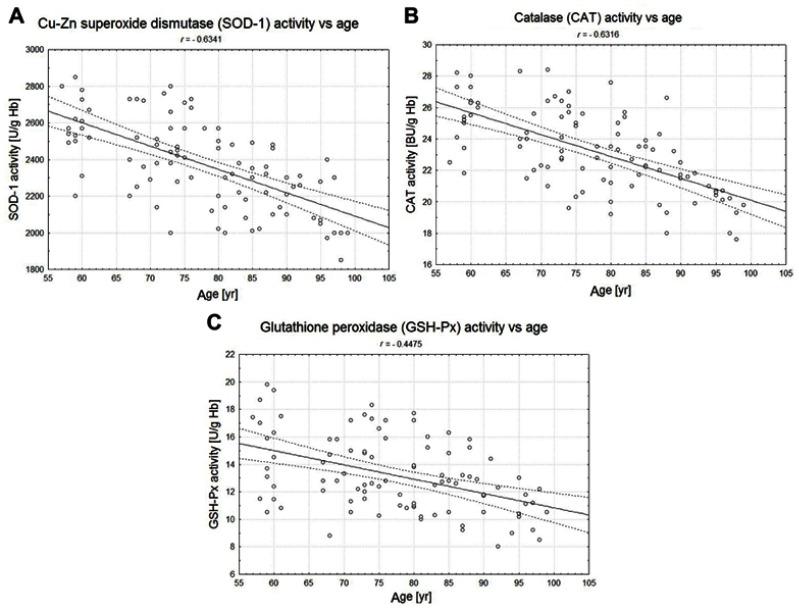
The statistically significant decreases of erythrocytic SOD-1 (P<0.00001), CAT (P<0.00001), and GSH-Px (P<0.001) activities were observed in elderly groups in comparison with the control group. Moreover, strong negative correlations between the activities of SOD-1 (r= –0,6341; Figure 6A), CAT (r= –0,6316; Figure 6B), and GSH-Px (r= –0,4475; Figure 6C) and the age of the examined persons were found. No age-related changes in GR activities and MDA concentrations were noticed.
Figure 6.
The correlations between the erythrocytic Zn-Cu superoxide dismutase (SOD-1) (A), catalase (CAT) (B), and glutathione peroxidase (GSH-Px) (C) activities and age of examined persons (P<0.05).
Discussion
ROS is not only a toxic side product of aerobic metabolism but it may also play an important role as signaling molecules. The most current research confirms the possibility that ROS takes part in different physiological processes as signaling molecules of stress in response to cellular damage.50 Therefore, the physiological level of ROS is most probably the main important issue for maintaining cellular homeostasis, whereas the increased production of ROS at a certain level has a detrimental influence on physiology of the cells. Among model organisms not showing an increase in the level of oxidative damage or cases of life extension among organisms with a high level of oxidative stress, suggest that the free radical theory of aging may be incorrect.51,52 Undoubtedly, mitochondria play an important role in the aging process and the accumulation of mutations and decreasing with age mitochondrial function irreversibly leads to cellular dysfunction. In the context of increasing research on the role of ROS in signaling and cellular regulation, one should not completely reject the role of free radicals in the process of aging.53
These pathways involve a complex antioxidant defense system that is composed of both low molecular weight antioxidants (reduced glutathione [GSH], ascorbic acid, tocopherols, etc) and antioxidant enzymes. The first line in enzymatic antioxidant defense is SOD, which catalyses dismutation of O2−·into H2O2. Subsequently, H2O2 may be dismutated into H2O by CAT. Differently from CAT, GSH-Px may catalyse the reduction of both H2O2 and organic peroxides, using GSH as a hydrogen donor whereby GSH is oxidized to glutathione disulfide (GSSG). GR can reduce GSSG into GSH, just providing with this molecule for antioxidant actions. The activities of antioxidant enzymes, as well as the levels of low molecular weight antioxidants have been observed to be altered in old organisms, which may lead to the intensification of oxidative stress and in this way to the development of aging. Several reports, as well as our own previous results, confirm the weakening of the enzymatic antioxidant defense during the organism's aging.54,55
There are diverse assumptions about the causes of observed age-dependent decline in antioxidant enzymatic defense. The oxidative modifications of enzymatic proteins caused by ROS are supposed to be one of the possible mechanisms of this phenomenon.56 Malnutrition in elderly people, resulting from poor nutritional habits, loss of appetite, or intestinal malabsorption in this group of people, may be the other probable explanation. As a result of malnutrition, deficiencies of some trace elements may occur, such as Zn2+ ions, essential for SOD-1 activity or selenium, essential for the synthesis of selenoenzyme GSH-Px.57,58 Taking into account this explanation, the unchanged activity of GR during aging in this study may point to the sufficient supply of riboflavin in the diet of examined elderly subjects. Nevertheless, among the most interesting hypotheses, there is a possible link between the age-dependent decrease of antioxidant enzymes' activities and the lowered levels of pineal hormone melatonin, which is observed in elderly people. Melatonin may both regulate the expression of genes coding for antioxidant enzymes such as SOD, GSH-Px, and GR and directly influence their activities.59 The amplitude of melatonin production declines with aging until almost total disappearance of its specific day-night cycle in many persons over 65 years of age.60,61 Thus, the decrease of antioxidant enzymes' activities during aging may be at least partly due to the failure of melatonin secretion in elderly subjects.
On the contrary, some authors found an increase of different antioxidant enzymes' activities in elderly subjects, which may implicate the compensatory effect of augmented oxidative stress in aging organisms.62–64 Among the most interesting studies, there are results obtained by Kłapcińska et al, in the examination of oxidative stress parameters in healthy Polish centenarians.65 There were significantly higher erythrocytic CAT and GR activities found in centenarians as compared to young healthy adults in Kłapcińska et al's study. These results indicate that increased capacity for antioxidant defense may contribute to human longevity.
It is still not fully understood whether the augmentation of oxidative stress with advancing age is due to a decrease of antioxidant defense system or an increase in endogenous reactive oxygen production. The oxidative stress intensity may be approximately estimated by the measurement of erythrocytic concentrations of MDA formed from the breakdown of lipid hydroperoxides. Several authors demonstrated the increased MDA concentrations in elderly subjects.54,55 The elevated MDA levels correspond to the intensified rate of lipid peroxidation during aging. Lipid peroxidation processes result in impaired membrane fluidity, increased non-specific membrane permeability, and inactivation of membrane enzymes, which may contribute to the damage occurring in aging organisms.66–70 Surprisingly, there were no age-dependent changes in MDA levels during aging in the present study. It is worth mentioning that some authors found decreased MDA concentrations in healthy centenarians as compared to the aged subjects or even to younger adults.65,68,71 These results may support the hypothesis that reduced oxidative stress may be related to the increase in lifespan of human beings.
Conclusion
Summing up, there are many reports supporting the role of oxidative stress in development of aging processes in human organisms. However, these data are still not consistent and further studies are necessary to confirm the existing hypotheses. Our preliminary study revealed some interesting links between antioxidant enzymatic defense capacity and aging. The subsequent investigations in the framework of this study may lead us to some answers for the questions about the role of oxidative stress in aging processes.
The obtained results clearly show the decrease of crucial activity of antioxidative enzymes with the simultaneous intensity of peroxidative lipids. In this context, it makes sense to continue the research aiming at thoroughly explaining the role of this phenomenon in the aging process. Based on the results of research on mice, it may be stated that free radical reactions may not be the only reason for intensity of the aging process but they certainly have an influence on it.
Acknowledgments
This work was supported by the funds of Ludwik Rydygier Collegium Medicum in Bydgoszcz Nicolaus Copernicus University in Torun.
Data sharing statement
The datasets generated during and/or analyzed during the current study can be obtained from the corresponding author upon request from other scientists.
Ethics statement
This study was approved by the Bioethics Commission of Nicolaus Copernicus University in Torun. All participants gave their written informed consent and the study was conducted in accordance with the Declaration of Helsinki.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Amalberti, R, Vincent C, Nicklin W, Braithwaite J. Coping with more people with more illness. Part 1: the nature of the challenge and the implications for safety and quality. Int J Qual Health Care. 2018;31(2):154–158. doi: 10.1093/intqhc/mzy235 [DOI] [PubMed] [Google Scholar]
- 2.Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet. 2009;374(9696):1196–1208. doi: 10.1016/S0140-6736(09)61460-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reeves D, Pye S, Ashcroft DM, et al. The challenge of ageing populations and patient frailty: can primary care adapt? BMJ. 2018;362:k3349. doi: 10.1136/bmj.k3349 [DOI] [PubMed] [Google Scholar]
- 4.Abbing HR. Health, healthcare and ageing populations in Europe, a human rights challenge for European health systems. Eur J Health Law. 2016;23(5):435–452. [DOI] [PubMed] [Google Scholar]
- 5.Ana Isabel CM, Francisco Ignacio J-R, Margarita R-K, Gill SS, Alicia B-F, Juan Francisco J-B. Down-regulation of arginine decarboxylase gene-expression results in reactive oxygen species accumulation in Arabidopsis. Biochem Biophys Res Commun. 2018;506(4):1071–1077. doi: 10.1016/j.bbrc.2018.10.165 [DOI] [PubMed] [Google Scholar]
- 6.Jardeleza C, Jones D, Baker L, et al. Gene expression differences in nitric oxide and reactive oxygen species regulation point to an altered innate immune response in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2013;3(3):193–198. doi: 10.1002/alr.21114 [DOI] [PubMed] [Google Scholar]
- 7.San Martin A, Du P, Dikalova A, et al. Reactive oxygen species-selective regulation of aortic inflammatory gene expression in type 2 diabetes. Am J Physiol Heart Circ Physiol. 2007;292(5):H2073–82. doi: 10.1152/ajpheart.00943.2006 [DOI] [PubMed] [Google Scholar]
- 8.Akram S, Teong HFC, Fliegel L, Pervaiz S, Clément M-V. Reactive oxygen species-mediated regulation of the Na+-H+ exchanger 1 gene expression connects intracellular redox status with cells‘ sensitivity to death triggers. Cell Death Differ. 2006;13(4):628–641. doi: 10.1038/sj.cdd.4401775 [DOI] [PubMed] [Google Scholar]
- 9.Turpaev KT. Reactive oxygen species and regulation of gene expression. Biochemistry (Mosc). 2002;67(3):281–292. [DOI] [PubMed] [Google Scholar]
- 10.Ikeno Y. New insights and current concepts of the oxidative stress theory of aging. Arch Biochem Biophys. 2015;576:1. doi: 10.1016/j.abb.2015.03.019 [DOI] [PubMed] [Google Scholar]
- 11.Durackova Z. Some current insights into oxidative stress. Physiol Res. 2010;59(4):459–469. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Nishimura T, Teruya K, et al. Protective mechanism of reduced water against alloxan-induced pancreatic beta-cell damage: scavenging effect against reactive oxygen species. Cytotechnology. 2002;40(1–3):139–149. doi: 10.1023/A:1023936421448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu BP. Cellular defenses against damage from reactive oxygen species. Physiol Rev. 1994;74(1):139–162. doi: 10.1152/physrev.1994.74.1.139 [DOI] [PubMed] [Google Scholar]
- 14.Roy J, Galano J-M, Durand T, Le Guennec J-Y, Lee JC-Y. Physiological role of reactive oxygen species as promoters of natural defenses. FASEB J. 2017;31(9):3729–3745. doi: 10.1096/fj.201700170R [DOI] [PubMed] [Google Scholar]
- 15.Barouki R. [Ageing free radicals and cellular stress]. Med Sci (Paris). 2006;22(3):266–272. doi: 10.1051/medsci/2006223266 [DOI] [PubMed] [Google Scholar]
- 16.Poon HF, Calabrese V, Scapagnini G, Butterfield DA. Free radicals: key to brain aging and heme oxygenase as a cellular response to oxidative stress. J Gerontol A Biol Sci Med Sci. 2004;59(5):478–493. [DOI] [PubMed] [Google Scholar]
- 17.Trifunovic A, Wredenberg A, Falkenberg M, et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429(6990):417–423. doi: 10.1038/nature02517 [DOI] [PubMed] [Google Scholar]
- 18.Kujoth GC, Hiona A, Pugh TD, et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309(5733):481–484. doi: 10.1126/science.1112125 [DOI] [PubMed] [Google Scholar]
- 19.Terzioglu M, Larsson NG. Mitochondrial dysfunction in mammalian ageing. Novartis Found Symp. 2007;287:197–208. discussion 208–13. [DOI] [PubMed] [Google Scholar]
- 20.Yamasoba T, Lin FR, Someya S, Kashio A, Sakamoto T, Kondo K. Current concepts in age-related hearing loss: epidemiology and mechanistic pathways. Hear Res. 2013;303:30–38. doi: 10.1016/j.heares.2013.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bardyn M, Rappaz B, Jaferzadeh K, et al. Red blood cells ageing markers: a multi-parametric analysis. Blood Transfus. 2017;15(3):239–248. doi: 10.2450/2017.0318-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zs-Nagy I. Aging of cell membranes: facts and theories. Interdiscip Top Gerontol. 2014;39:62–85. doi: 10.1159/000358900 [DOI] [PubMed] [Google Scholar]
- 23.Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001 [DOI] [PubMed] [Google Scholar]
- 24.Reisz JA, Wither MJ, Dzieciatkowska M, et al. Oxidative modifications of glyceraldehyde 3-phosphate dehydrogenase regulate metabolic reprogramming of stored red blood cells. Blood. 2016;128(12):e32–42. doi: 10.1182/blood-2016-05-714816 [DOI] [PubMed] [Google Scholar]
- 25.Dobi A, Bravo SB, Veeren B, et al. Advanced glycation end-products disrupt human endothelial cells redox homeostasis: new insights into reactive oxygen species production. Free Radic Res. 2019;1–20. doi: 10.1080/10715762.2018.1529866 [DOI] [PubMed] [Google Scholar]
- 26.Vassallo DV, Wiggers GA, Padilha AS, RonacherSimões M. Endothelium:atarget for harmful actions of metals. Curr Hypertens Rev. 2019. doi: 10.2174/1573402115666190115153759 [DOI] [PubMed] [Google Scholar]
- 27.Park SY, Kentish SJ, Wittert GA, Page AJ. Age-related endothelial dysfunction in human skeletal muscle feed arteries: the role of free radicals derived from mitochondria in the vasculature. Acta Physiol (Oxf). 2018;222(1). doi: 10.1111/apha.12884 [DOI] [PubMed] [Google Scholar]
- 28.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11(3):298–300. [DOI] [PubMed] [Google Scholar]
- 29.Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H. Trends in oxidative aging theories. Free Radic Biol Med. 2007;43(4):477–503. doi: 10.1016/j.freeradbiomed.2007.03.034 [DOI] [PubMed] [Google Scholar]
- 30.Perez VI, Bokov A, Van Remmen H, et al. Is the oxidative stress theory of aging dead? Biochim Biophys Acta. 2009;1790(10):1005–1014. doi: 10.1016/j.bbagen.2009.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh S, Singh AK, Garg G, Rizvi SI. Fisetin as a caloric restriction mimetic protects rat brain against aging induced oxidative stress, apoptosis and neurodegeneration. Life Sci. 2018;193:171–179. doi: 10.1016/j.lfs.2017.11.004 [DOI] [PubMed] [Google Scholar]
- 32.Zanetti M, Gortan Cappellari G, Burekovic I, Barazzoni R, Stebel M, Guarnieri G. Caloric restriction improves endothelial dysfunction during vascular aging: effects on nitric oxide synthase isoforms and oxidative stress in rat aorta. Exp Gerontol. 2010;45(11):848–855. doi: 10.1016/j.exger.2010.07.002 [DOI] [PubMed] [Google Scholar]
- 33.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273(5271):59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore A. A new theory of aging based on energy maintenance. Bioessays. 2018;40(8):e1800124. doi: 10.1002/bies.v40.8 [DOI] [PubMed] [Google Scholar]
- 35.Chaudhari SN, Kipreos ET. The energy maintenance theory of aging: maintaining energy metabolism to allow longevity. Bioessays. 2018;40(8):e1800005. doi: 10.1002/bies.v40.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cabello-Verrugio C, Vilos C, Rodrigues-Diez R, Estrada L. Oxidative stress in disease and aging: mechanisms and therapies 2018. Oxid Med Cell Longev. 2018;2018:2835189. doi: 10.1155/2018/2835189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liguori I, Russo G, Curcio F, et al. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018;13:757–772. doi: 10.2147/CIA.S158513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simioni C, Zauli G, Martelli AM, et al. Oxidative stress: role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget. 2018;9(24):17181–17198. doi: 10.18632/oncotarget.24729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pesce M, Tatangelo R, La Fratta I, et al. Aging-related oxidative stress: positive effect of memory training. Neuroscience. 2018;370:246–255. doi: 10.1016/j.neuroscience.2017.09.046 [DOI] [PubMed] [Google Scholar]
- 40.Eleutherio E, Brasil ADA, França MB, de Almeida DSG, Rona GB, Magalhães RSS. Oxidative stress and aging: learning from yeast lessons. Fungal Biol. 2018;122(6):514–525. doi: 10.1016/j.funbio.2017.12.003 [DOI] [PubMed] [Google Scholar]
- 41.Lee JY, Paik IY, Kim JY. Voluntary exercise reverses immune aging induced by oxidative stress in aging mice. Exp Gerontol. 201. 9;115:148–154. doi: 10.1016/j.exger.2018.08.009 [DOI] [PubMed] [Google Scholar]
- 42.Zhou HJ, Zeng C-Y, Yang -T-T, Long F-Y, Kuang X, Du J-R. Lentivirus-mediated klotho up-regulation improves aging-related memory deficits and oxidative stress in senescence-accelerated mouse prone-8 mice. Life Sci. 2018;200:56–62. doi: 10.1016/j.lfs.2018.03.027 [DOI] [PubMed] [Google Scholar]
- 43.Kirkwood TB, Kowald A. The free-radical theory of ageing–older, wiser and still alive: modelling positional effects of the primary targets of ROS reveals new support. Bioessays. 2012;34(8):692–700. doi: 10.1002/bies.201200014 [DOI] [PubMed] [Google Scholar]
- 44.Pomatto LCD, Davies KJA. Adaptive homeostasis and the free radical theory of ageing. Free Radic Biol Med. 2018;124:420–430. doi: 10.1016/j.freeradbiomed.2018.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Placer ZA, Cushman LL, Johnson BC. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal Biochem. 1966;16(2):359–364. [DOI] [PubMed] [Google Scholar]
- 46.Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247(10):3170–3175. [PubMed] [Google Scholar]
- 47.Beers RF Jr., Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195(1):133–140. [PubMed] [Google Scholar]
- 48.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70(1):158–169. [PubMed] [Google Scholar]
- 49.Flohe L, Gunzler WA. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114–121. [DOI] [PubMed] [Google Scholar]
- 50.Roth E, Hejjel L, Jaberansari M, Jancso G. The role of free radicals in endogenous adaptation and intracellular signals. Exp Clin Cardiol. 2004;9(1):13–16. [PMC free article] [PubMed] [Google Scholar]
- 51.Terashvili M, Pratt PF, Gebremedhin D, Narayanan J, Harder DR. Reactive oxygen species cerebral autoregulation in health and disease. Pediatr Clin North Am. 2006;53(5):1029–37.xi. doi: 10.1016/j.pcl.2006.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Afanas‘Ev IB. On mechanism of superoxide signaling under physiological and pathophysiological conditions. Med Hypotheses. 2005;64(1):127–129. doi: 10.1016/j.mehy.2004.05.009 [DOI] [PubMed] [Google Scholar]
- 53.Forman HJ. Redox signaling: an evolution from free radicals to aging. Free Radic Biol Med. 2016;97:398–407. doi: 10.1016/j.freeradbiomed.2016.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kasapoglu M, Ozben T. Alterations of antioxidant enzymes and oxidative stress markers in aging. Exp Gerontol. 2001;36(2):209–220. [DOI] [PubMed] [Google Scholar]
- 55.Inal ME, Kanbak G, Sunal E. Antioxidant enzyme activities and malondialdehyde levels related to aging. Clin Chim Acta. 2001;305(1–2):75–80. [DOI] [PubMed] [Google Scholar]
- 56.Sohal RS. Role of oxidative stress and protein oxidation in the aging process. Free Radic Biol Med. 2002;33(1):37–44. [DOI] [PubMed] [Google Scholar]
- 57.Sato M, Yanagisawa H, Nojima Y, Tamura J, Wada O. Zn deficiency aggravates hypertension in spontaneously hypertensive rats: possible role of Cu/Zn-superoxide dismutase. Clin Exp Hypertens. 2002;24(5):355–370. [DOI] [PubMed] [Google Scholar]
- 58.Holben DH, Smith AM. The diverse role of selenium within selenoproteins: a review. J Am Diet Assoc. 1999;99(7):836–843. doi: 10.1016/S0002-8223(99)00198-4 [DOI] [PubMed] [Google Scholar]
- 59.Maharaj DS, Glass BD, Daya S. Melatonin: new places in therapy. Biosci Rep. 2007;27(6):299–320. doi: 10.1007/s10540-007-9052-1 [DOI] [PubMed] [Google Scholar]
- 60.Yanar K, Simsek B, Cakatay U. Integration of melatonin related redox homeostasis, aging and circadian rhythm. Rejuvenation Res. 2018. doi: 10.1089/rej.2018.2159 [DOI] [PubMed] [Google Scholar]
- 61.Yang Y, Cheung -H-H, Zhang C, Wu J, Chan W-Y. Melatonin as potential targets for delaying ovarian aging. Curr Drug Targets. 2019;20(1):16–28. doi: 10.2174/1389450119666180828144843 [DOI] [PubMed] [Google Scholar]
- 62.Marzani B, Felzani G, Bellomo RG, Vecchiet J, Marzatico F. Human muscle aging: ROS-mediated alterations in rectus abdominis and vastus lateralis muscles. Exp Gerontol. 2005;40(12):959–965. doi: 10.1016/j.exger.2005.08.010 [DOI] [PubMed] [Google Scholar]
- 63.Al-Abrash AS, Al-Quobaili FA, Al-Akhras GN. Catalase evaluation in different human diseases associated with oxidative stress. Saudi Med J. 2000;21(9):826–830. [PubMed] [Google Scholar]
- 64.Flores-Mateo G, Elosua R, Rodriguez-Blanco T, et al. Oxidative stress is associated with an increased antioxidant defense in elderly subjects: a multilevel approach. PLoS One. 2014;9(9):e105881. doi: 10.1371/journal.pone.0105881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Klapcinska B, Derejczyk J, Wieczorowska-Tobis K, Sobczak A, Sadowska-Krepa E, Danch A. Antioxidant defense in centenarians (a preliminary study). Acta Biochim Pol. 2000;47(2):281–292. [PubMed] [Google Scholar]
- 66.Spiteller G. The important role of lipid peroxidation processes in aging and age dependent diseases. Mol Biotechnol. 2007;37(1):5–12. [DOI] [PubMed] [Google Scholar]
- 67.Spiteller G. Lipid peroxidation in aging and age-dependent diseases. Exp Gerontol. 2001;36(9):1425–1457. [DOI] [PubMed] [Google Scholar]
- 68.Girotti AW. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J Lipid Res. 1998;39(8):1529–1542. [PubMed] [Google Scholar]
- 69.Yavuzer H, Yavuzer S, Cengiz M, et al. Biomarkers of lipid peroxidation related to hypertension in aging. Hypertens Res. 2016;39(5):342–348. doi: 10.1038/hr.2015.156 [DOI] [PubMed] [Google Scholar]
- 70.Pratico D. Lipid peroxidation and the aging process. Sci Aging Knowledge Environ. 2002;2002(50):re5. [DOI] [PubMed] [Google Scholar]
- 71.Salminen LE, Paul RH. Oxidative stress and genetic markers of suboptimal antioxidant defense in the aging brain: a theoretical review. Rev Neurosci. 2014;25(6):805–819. doi: 10.1515/revneuro-2014-0046 [DOI] [PMC free article] [PubMed] [Google Scholar]